Abstract
Over-expression of mutant copper, zinc superoxide dismutase (SOD) in mice induces ALS and has become the most widely used model of neurodegeneration. However, no pharmaceutical agent in twenty years has extended lifespan by more than a few weeks. The Copper-Chaperone-for-SOD (CCS) protein completes the maturation of SOD by inserting copper, but paradoxically human CCS causes mice co-expressing mutant SOD to die within two weeks of birth. Hypothesizing that co-expression of CCS created copper deficiency in spinal cord, we treated these pups with the PET-imaging agent CuATSM, which is known to deliver copper into the CNS within minutes. CuATSM prevented the early mortality of CCSxSOD mice, while markedly increasing Cu,Zn SOD protein in their ventral spinal cord. Remarkably, continued treatment with CuATSM extended the survival of these mice by an average of 18 months. When CuATSM treatment was stopped, these mice developed ALS-related symptoms and died within three months. Restoring CuATSM treatment could rescue these mice after they became symptomatic, providing a means to start and stop disease progression. All ALS patients also express human CCS, raising the hope that familial SOD ALS patients could respond to CuATSM treatment similarly to the CCSxSOD mice.
Keywords: SOD1, Lou Gehrig, amyotrophic lateral sclerosis, superoxide dismutase, CCS
Graphical abstract
Introduction
Mouse and rat models of ALS produced by the overexpression of mutant copper, zinc superoxide dismutase (SOD) recapitulate the human disease with higher fidelity than other models based on more recently discovered ALS-associated mutations. Mutant SODs also confer a toxic gain-of-function that leads to motor neuron degeneration in humans, dogs, zebrafish and Drosophila. The first mouse model based on high-expression SODG93A (1) has now become the most widely used model for any neurodegenerative disease. The symptoms of ALS become obvious in these mice around 100 days of age as the mice lose weight and their hind limbs atrophy. Recent studies have established that subtle symptoms start earlier than 30 days (2, 3).
However, frustration with the SOD models of ALS has grown because twenty years of testing in ALS-mutant SOD mice have failed to yield an effective therapy in mice or man. Few pharmaceutical treatments have been able to extend life by even 10–15% in the high-expressing SODG93A mice and no agent has been successfully translated into human treatments. The Prize4Life.org renewed its offer of $1 million for a therapeutic solution that extends life by 25% in the high-expressing SODG93A mice. The goal may seem modest because simply breeding from the standard B6SJL hybrid of these mice to a homogenous B6 background extends life by 21% from 130 ± 12 to 157 ± 9 days (4). Yet, the 25% extension of life has not been achieved reproducibly by any pharmaceutical approach and the treatment of any SOD-based model of ALS has proven to be a formidable and elusive challenge.
To approach this problem from a different angle, we began exploring what processes accelerate the development of motor neuron disease in SOD mice. The most rapid acceleration of ALS ever described results when the human Copper-Chaperone-for-SOD (CCS) is co-expressed with human SOD; low-expressing SODG93A mice co-expressing CCS die of ALS in 30–50 days, about eight times faster than those without expression of human CCS (5).
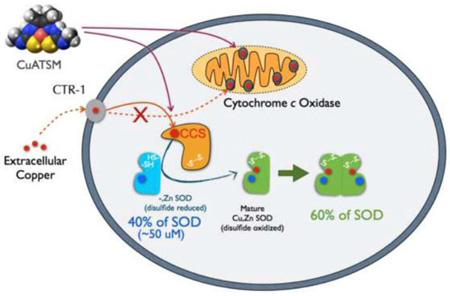
Understanding why CCS accelerates toxicity of SODG93A in mice is important because ALS patients express human CCS in a much higher ratio to SOD compared to the ratio of mouse CCS to human SODG93A expressed in transgenic mice (6). Son and Elliott (7) clearly showed that copper-dependent cytochrome c oxidase activity is greatly reduced in the low-expressing SODG93AxCCS mice, consistent with an apparent copper deficiency within the spinal cord. Copper distribution in vivo is determined by affinity gradients with SOD having the strongest affinity for copper (8). Thus, overexpression of CCS has been proposed to impair copper-import into mitochondria in low-expressing SODG93AxCCS mice (5, 9).
Among the most promising therapeutics for ALS is the copper complex CuATSM. It is protective in the low expressing SODG93A and SODG37R ALS mouse models, extending life by 15% and 26% respectively (10, 11). CuATSM is used as a PET-imaging agent for hypoxic tumors in humans (12), has low toxicity, and penetrates the blood-brain barrier of ALS patients in minutes (13). The ATSM moiety is a small hydrophobic ligand with a high affinity for copper in the 2+ oxidation state (14). The rationale for the use of CuATSM as a PET scanning agent comes from selective release of copper in hypoxic tissues that are able to reduce copper to the 1+ oxidation state (15). The unusually low reduction potential of CuATSM prevents copper release in most tissues (14), but allows selective release of copper in cells with damaged mitochondria (15).
We have recently shown that CuATSM can deliver copper to SOD in SODG37R mice (16). Because of its ability to deliver copper safely, we hypothesized that CuATSM could protect ALS transgenic models expressing human CCS by providing copper to partially folded intermediates of SOD. The toxic gain-of-function appears to involve partially unfolded intermediates of SOD, which often lack the metal cofactors critical for stabilizing SOD (17). To quantify binding of copper and zinc to SOD, we have developed a mass spectrometric method to measure the entire SOD protein directly from ventral spinal cord of ALS-affected tissues. This method revealed that nearly half of the SOD protein in the spinal cord of mutant SOD mice was Cu,Zn SOD, while the remaining half of SOD protein predominately contained zinc but not copper (18, 19). Based upon these observations, we therefore investigated how CuATSM might modulate copper binding to SOD and affect the survival of transgenic SOD mice co-expressing CCS.
Results
Without CuATSM treatment, pups expressing SODG93A with CCS were clearly identifiable as runts in the litter as early as 4 days. They lagged littermates in development by several days and died in 8–13 days (Fig. 1A). Because the early mortality was most likely due to copper deficiency affecting mitochondria during development (7), we tested whether CuATSM would rescue the early death. CuATSM dissolved in DMSO applied dermally to the back of the pup’s neck was found to be absorbed within ten minutes, which provided a simple means to treat young pups. Remarkably, CuATSM-treated pups could respond within hours with increased mobility. We then tested a variety of CuATSM-dosing schemes to rescue the pups and found that the best protection came from first treating mothers during pregnancy and then treating pups starting at 5 days of age with 30 mg/kg/dose CuATSM twice daily. The prenatal CuATSM treatment allowed all treated pups to grow normally and completely prevented early mortality. Once a treatment regime for CuATSM was found that was safe and avoided the early mortality, we organized blinded randomized trials to compare how CuATSM affected the survival of SODG93A mice with and without human CCS co-expression.
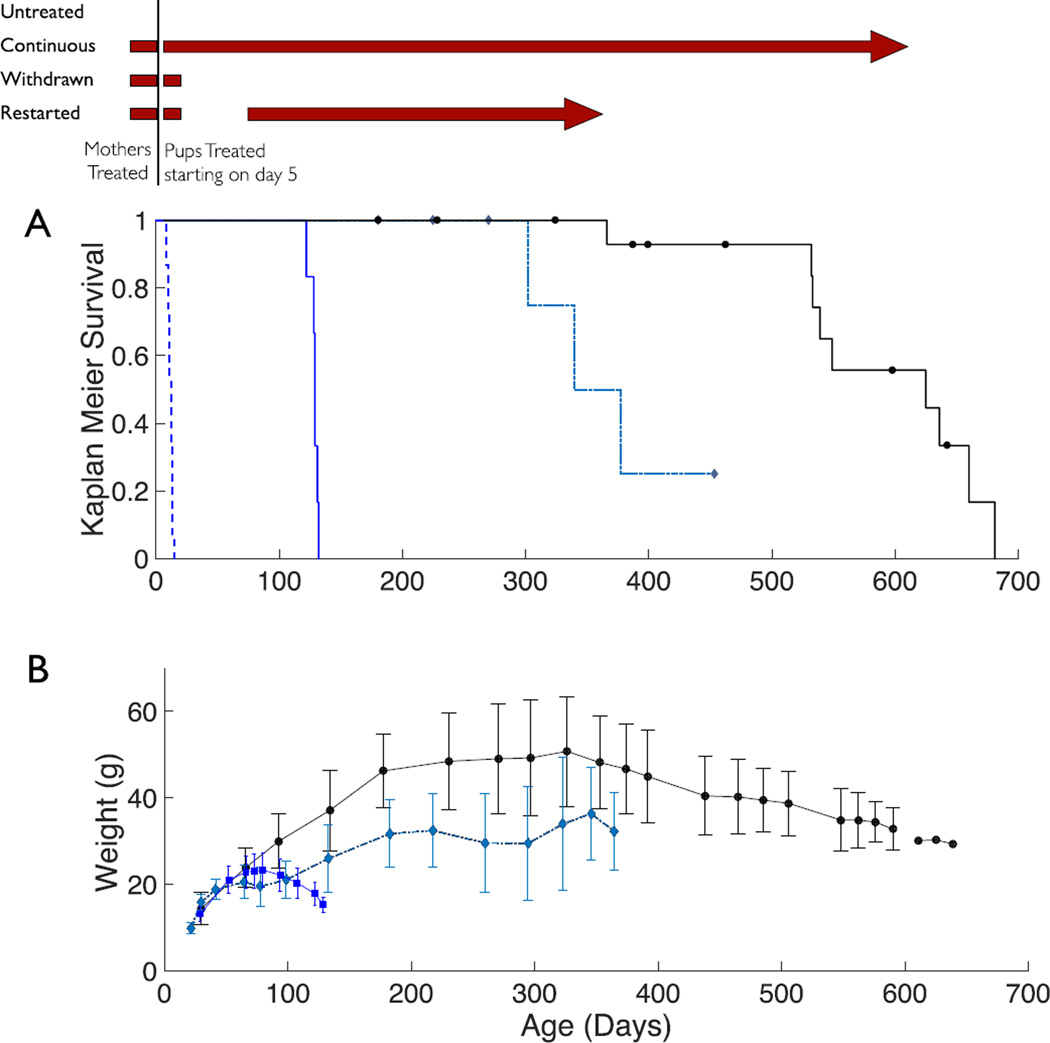
Figure 1. CuATSM treatment and survival of SODG93AxCCS mice.
The dosing schedule with CuATSM (30 mg/kg given twice a day) is shown on the top as maroon bars. (A) Kaplan-Meier plot of ALS-survival probability of SODG93AxCCS mice receiving either no treatment (black dashed line, n=15), CuATSM treatment stopped at weaning (blue, n=6), treatment stopped at weaning and restarted at symptom onset (dashed grey-blue, n=7), and continuous CuATSM treatment (black, n=17). Censored mice are shown as black circles or grey-blue diamonds. (B) Weights with standard deviations for the three treated groups are show using the same symbols as in 1A. Only means are shown after age 600 days because three or fewer mice survived.
CuATSM Treatment of CCSxSODG93A mice
This trial had three treatment arms as shown in the Scheme at the top of Fig. 1. The first arm assessed the efficacy of continuous treatment. The second arm assessed the effects of stopping CuATSM treatment at 21 days of age when the critical window for copper in perinatal development had passed (20, 21). The third arm assessed whether restoring CuATSM treatment after symptom development could inhibit further ALS progression.
Continuous treatment
Continuous treatment with CuATSM kept 17 of 17 high-expressing SODG93AxCCS mice alive for past a year (Fig. 1A; p = 1.6e-9 versus the untreated group using the Cox proportional hazard model with the likelihood ratio test). The continuously treated mice remained remarkably active even after a year (Supplemental Video 1), but most developed motor issues in their hind limbs by 600 days. The continuously treated mice gained excessive weight during the first year, but subsequently lost weight starting around 300 days (Fig. 1B). The first mouse reached end-stage motor neuron symptoms at 366 days while five others reached end stage between 530 to 660 days. Seven of the 17 mice were censored due to unrelated health issues that arose mostly in their second year of life (Supplemental Table S1).
Symptoms for SODG93AxCCS mice who started to progress were milder than in the standard SODG93A mice, with the hind limbs suffering less atrophy while the forelimbs remained largely unaffected. The mice exhibited spasticity in their hind limbs with their toes extended. Considerable strength remained in the extensor muscles. The mice lost weight, but far more gradually than SODG93A mice reaching end stage. The long survival could not be explained by a loss of SOD gene copy number, because all mice retained their full SOD copy number as shown by qPCR (Supplemental Fig. S1) and as described below accumulated substantially more SOD protein in ventral spinal cord than SODG93A mice without CCS.
Withdrawal of CuATSM
In the second treatment arm, CuATSM treatment was stopped at 21 days. These SODG93AxCCS mice continued to grow normally for 6–8 weeks, but then developed hind limb spasticity between 70–90 days. All became end stage between 122 to 132 days with motor symptoms primarily affecting the hind limbs (Fig. 1A; n=6; p = 1.3e-5 versus the untreated group). Their survival was similar to that of the standard SODG93A mice.
Restarting CuATSM
In the third treatment arm, CuATSM treatment was stopped at 21 days (n=7; p = 4.3e-6 versus the untreated group) until the mice exhibited ataxia at approximately 80 days. Twice daily treatments with 30 mg/kg CuATSM were then resumed. The mice gradually recovered over the next two months and gained weight (Fig. 1B). The severity of symptoms was reduced, but three of the mice eventually developed progressive ALS symptoms 120–180 days later and were sacrificed at 302–377 days of age. Three others were sacrificed due to other issues unrelated to ALS as marked on Fig 1A and described in Supplemental Table S1.
Protection obtained with related experimental treatments
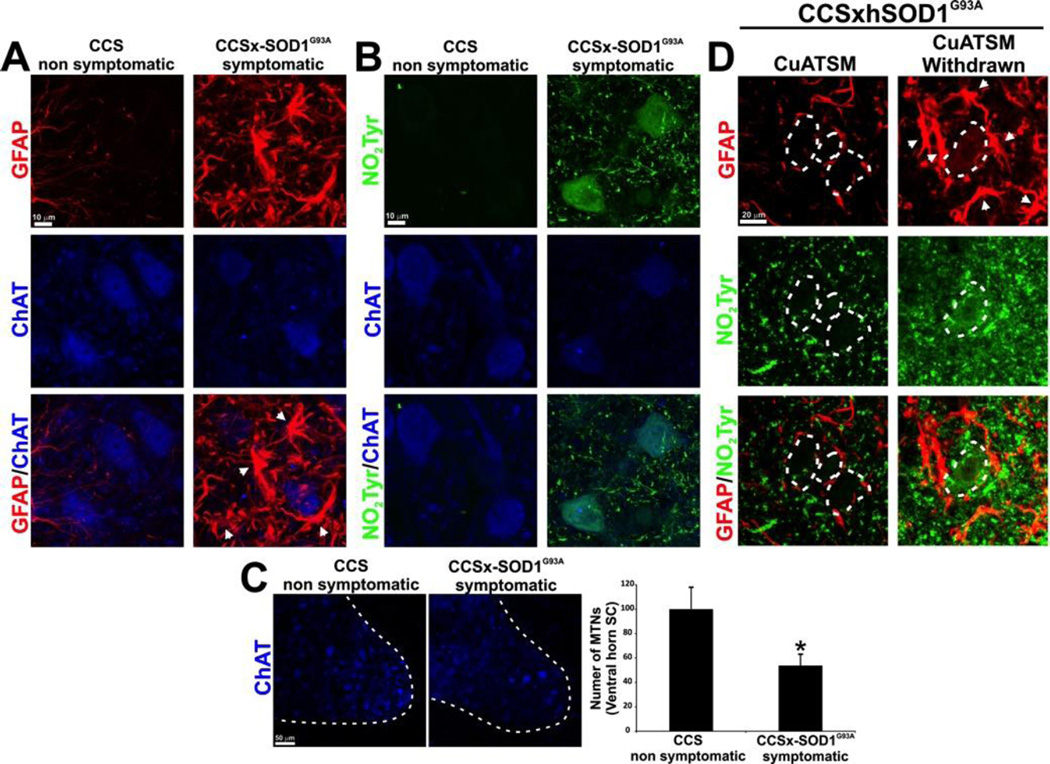
Additional test groups of SODG93AxCCS mice have been treated with different dosages of CuATSM, including mice used to test different dosing methods. Aggregating all of these groups receiving continuous treatment with CuATSM revealed that 36 of 36 SODG93AxCCS mice remained healthy well past 180 days. This age is 38% longer than the average 130-day survival of untreated SODG93A mice. Some of SODG93AxCCS mice receiving low dosages of CuATSM in pilot experiments became symptomatic between 230–380 days of age. These mice were examined by immunohistology and exhibited reactive astrocytosis and nitrotyrosine accumulation in motor neurons (Fig. 2A–B). They had 50% fewer motor neurons compared to CCS littermates or asymptomatic mice remaining on CuATSM treatment (Fig. 2C). Intense nitration of the motor neurons was found relative to CCS-control spinal cord, particularly in the environment of motor neurons in the symptomatic spinal cord. Withdrawal of CuATSM resulted in increased nitrotyrosine accumulation and a pronounced astrogliosis when the mice became end stage (Fig. 2D). The hippocampus of CuATSM-treated mice appeared unaffected even in symptomatic mice surviving as long as 650 days (Supplemental Figure S2).
Figure 2. Motor neuron death, astrogliosis and tyrosine nitration during the symptomatic phase in CCSxSODG93A mice.
All of the mice shown have been treated with CuATSM, which was necessary to allow the SODG93AxCCS mice to survive the first two weeks after birth. (A) Representative images of glial fibrillary acid protein (GFAP red) and choline acetyl transferase (ChAT blue) in lumbar spinal cord sections from transgenic CCS and symptomatic SODG93AxCCS mice illustrate how reactive astrocytes surround motor neurons during the symptomatic phase in the SODG93AxCCS mice. In particular, swollen GFAP(+) astrocytes (white arrows) surround choline acetyl transferase-positive (ChAT) motor neurons in the symptomatic spinal cord (scale bar = 10 µm). (B) Higher expression of nitrotyrosine in the spinal cord is observed during the symptomatic phase in the SODG93AxCCS mice as shown by a representative image of nitrotyrosine (NO2Tyr – green) costained with ChAT (blue) in lumbar spinal cord sections from transgenic CCS and symptomatic SODG93AxCCS mice. Scale bar, 10 µm. (C) Representative ChAT staining (blue) in the ventral horn of the spinal cord (the white dotted line demarcates the border between white and grey matter). The bar graph reports the number of neuronal somas located in Rexed lamina IX (mean ± SEM from 30 slices per group *p<0.05: scale bar = 50 µm). (D) Withdrawal of CuATSM from treated SODG93AxCCS increased nitration and astrocytic reactivity. The left panel shows nitrotyrosine (Green) and GFAP (red) in the ventral horn of the spinal cord of a 281 day-old continuously CuATSM-treated mouse for comparison. The right panel shows that CuATSM withdrawal at weaning significant increases tyrosine nitration as well as GFAP expression that labeled swollen astrocytes that surround motor neurons in the degenerating spinal cord (a white dotted line indicates motor neuron bodies; scale bar = 10 µm).
Rescue of symptomatic low-expressing SODG93AxCCS mice
Co-expression of CCS in low-expressing SODG93A mice decreased survival to the 20–50 day range as previously reported (5). Starting treatment with CuATSM at 24 days of age rescued low-expressing SODG93AxCCS mice (n=5) from the early death initially described (5). These mice regained weight, showed improved movement within a week, and continued to survive with minimal residual symptoms. At the time treatment was started, these mice had hind limb weakness with impaired coordination and would typically succumb if untreated after another week. All 5 mice remain alive and healthy at 480 days. Untreated litters of low-expressing SODG93AxCCS mice with similar symptoms became end stage within a week. Low-expressing SODG93A mice without CCS normally reach end-stage at 240–260 days (5, 9)
CuATSM and survival in SODG93A mice without CCS co-expression
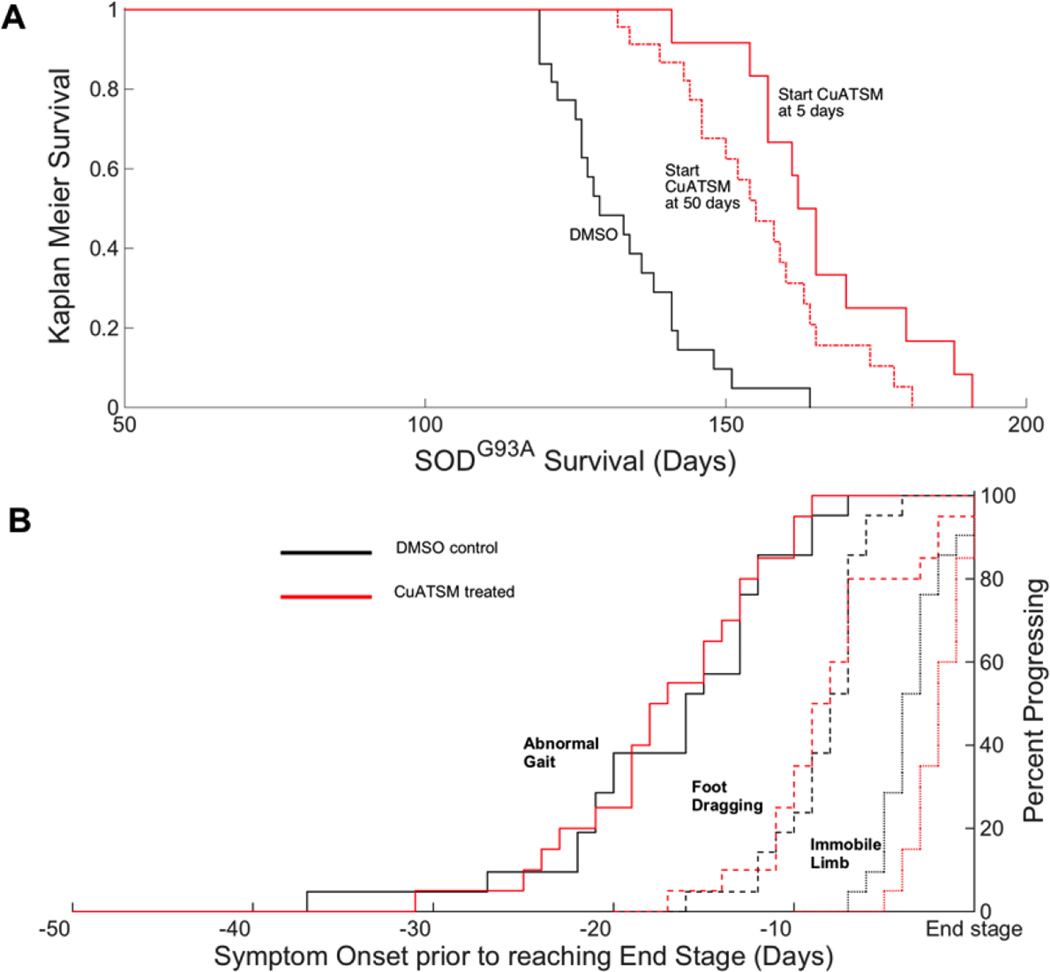
Without CCS co-expression, CuATSM still provided substantial protection to high-expressing SODG93A mice (Fig. 3). Based upon prior results (11) that showed a strong dose-dependence on survival in SODG37R mice, we chose to use the maximum possible dosage of CuATSM (100 mg/kg/dose) that could be practically delivered via dermal application in DMSO. This dose is approximately three times higher than used to treat the CCS co-expressing mice. It was similarly administered twice daily. When started at 50 days of age, CuATSM extended the median survival of SODG93A mice by 22 days, a 16% increase relative to DMSO-treated mice (Fig. 3A; p = 1.6e-5). CuATSM-treated mice had a median survival of 155 ± 14 days, while the DMSO-treated control mice had a median survival of 133 ± 12 days.
Figure 3. Survival probability and symptom onset of SODG93A mice with CuATSM treatment.
(A) Kaplan-Meier survival probabilities of SODG93A mice treated with DMSO starting at day 50 (solid black, n=21), CuATSM given twice daily at a dose of 100 mg/kg starting at day 50 (dashed dot orange, n=20), or 100 mg/kg starting at day 5 (solid orange, n=12). The end stage was determined when the mice could no longer pass the right test in 15 seconds. (B) Three stages of disease progression for SODG93A mice plotted relative to when each mouse reached end stage. CuATSM treatment increased survival by over 3 weeks as shown in panel A by delaying onset rather than affecting progression. Treatments were DMSO (black, n=21) or 100 mg/kg CuATSM (red, n=20) starting at day 50. The three measures for the onset of symptoms in relative order of severity are 1) an abnormal gait (solid lines), 2) dragging of at least one hind limb (dashed lines), and 3) failure to use one limb for forward motion (dotted lines).
While the delay of symptom onset and extension of survival were highly significant, CuATSM did not affect the progression of the disease as measured by development of abnormal gait, by dragging of hind limbs, or by use of hind limbs for forward propulsion, relative to the time when mice became end stage (Fig. 3B). Thus, the time of onset was delayed by CuATSM treatment, but disease progression was unaffected for both early and late-stage symptoms. Nine mice in total were censored during these studies and are described in Supplemental Table S2.
When both mothers and SODG93A pups were treated with 100 mg /kg/dose of CuATSM twice daily, the median survival of the SODG93A mice increased by 25% to 166 ± 14 days compared to the DMSO treated group (Fig. 3; p = 3.2e-6). The additional protection from early treatment was consistent with motor neuron pathology starting much earlier than commonly thought (2, 3, 22).
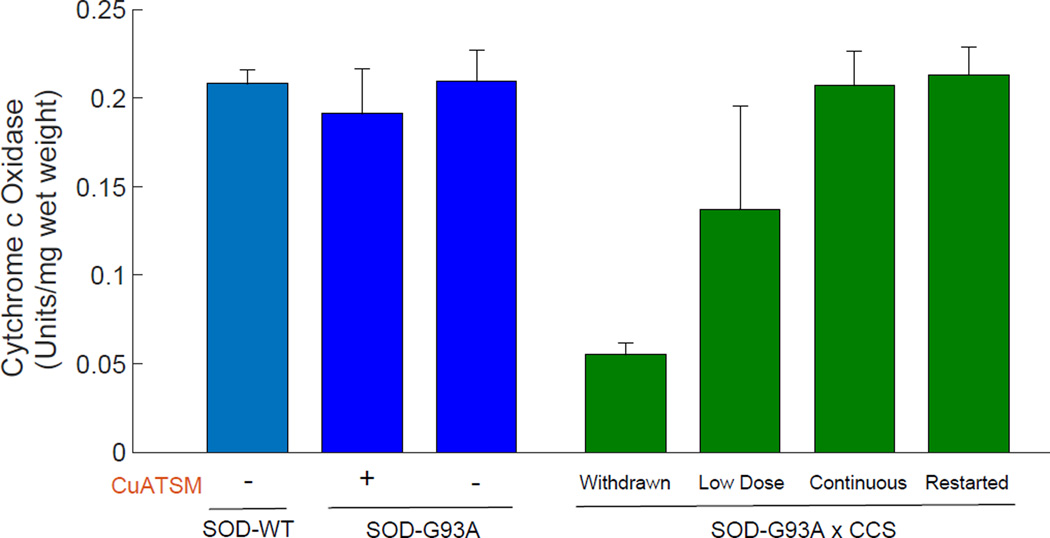
Impact of CuATSM and CCS on Cytochrome c Oxidase Activity
The copper-dependent activity of cytochrome c oxidase in ventral spinal cord was nearly equal in mice expressing wild-type SOD, mice expressing just SODG93A with or without CuATSM treatment, and mice expressing SODG93AxCCS that were continuously treated with 30 mg/kg of CuATSM (Fig. 4). This measured activity is consistent with previously reported activities in nontransgenic mice using the same assay methodology (23). When CuATSM was withdrawn from SODG93AxCCS mice, cytochrome c oxidase activity in spinal cord was reduced by 75% when these mice reached end stage around 130 days. In the SODG93AxCCS mice used for our initial dose-finding experiments, cytochrome c oxidase activity in spinal cord was highly variable but reduced by an average of 30%. These mice received a fifth of the CuATSM dosage and survived for 280–370 days.
Figure 4.
Cytochrome c oxidase activity in cerebral cortex from the different treated mouse groups shown in Figs. 1 and 3. The Low Dose group was from pilot studies where the mice were treated with five lower dosages of CuATSM and developed end-stage symptoms between 240 and 370 days. Similar cytochrome c oxidase activities were observed in spinal cords of these mice, but fewer samples were available due to use in SOD assays and for immunohistology. Activities are reported as the mean ± STD (n=3).
Impact of CuATSM and CCS on SODG93A protein and metal content
Human SODG93A protein is well known to be highly expressed in SODG93A mice based upon activity measurements (24). Using mass spectrometry methods that kept copper and zinc bound to the SOD protein, we found that nontransgenic mice contained a total of 3–4 µM of the endogenous mouse Cu,Zn SOD in ventral spinal cord (all concentrations here refer to the monomer). In contrast, human SODG93A mice expressed nearly 50 times more human SOD protein (210±18 µM) when they reached end-stage. Approximately 50% of total SOD was present as mature Cu,Zn SOD, which is the most stable and enzymatically active form of SOD. Less than 7% was apoSOD that lacked both copper and zinc. The remaining 43% contained zinc but not copper. This copper-deficient SOD will readily form a heterodimer with CCS where the copper from CCS is transferred to form Cu,Zn SOD (25, 26). However, the slow import of copper into the CNS apparently limited the maturation of copper-deficient SOD by CCS to form Cu,Zn SOD in CCSxSODG93A mice.
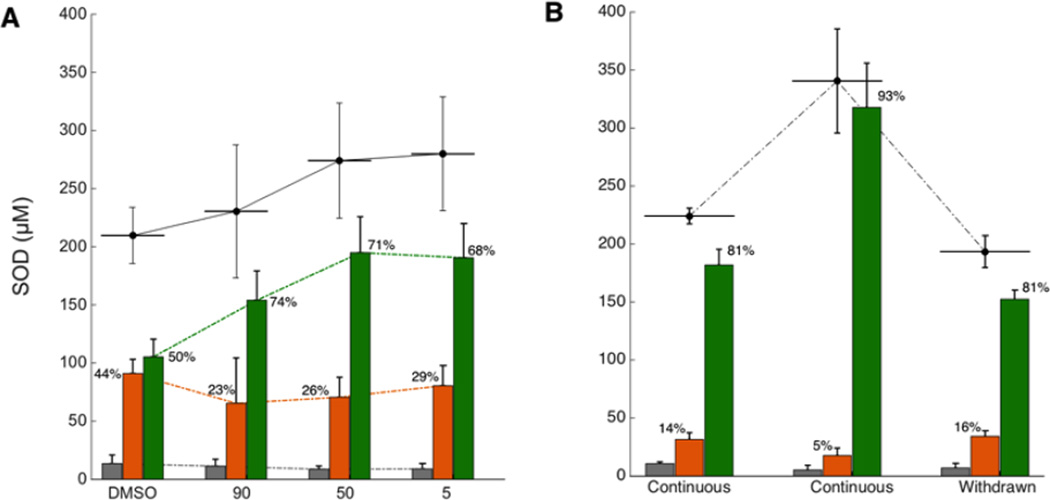
Using CuATSM to deliver copper into the CNS, we found that the concentration of Cu,Zn SOD increased in SODG93A mice without CCS co-expression. This increase amounted to 85–90 µM additional Cu,Zn SODG93A protein accumulating in treated mice (Fig. 5; p=.003 by ANOVA). This increase was consistent with treated mice surviving 20 days to 60 days longer than untreated mice, allowing more time for Cu,Zn SOD to accumulate. CuATSM treatment reduced the percentage of copper-deficient SOD by 15–21%, but this percentage remained relatively constant whether the treatment lasted for 1–2 months or for as long as 5–6 months (Fig. 5A). These results suggest that copper loading into SOD in the CNS of SODG93A mice reached a rate-limiting process that was too slow to keep up with SOD synthesis, which we hypothesized was due to the limited amount of endogenous mouse CCS relative to human SOD protein.
Figure 5. Effect of CuATSM treatment on metal content of SOD.
Concentration of apoSOD (gray), Zn-containing, Cu-deficient SOD (orange) and Cu,Zn SOD (green) are shown as bars with total SOD concentration as a black line above. The percentages of zinc-containing SOD and Cu,Zn SOD are also reported for each treatment. (A) The effects of CuATSM treatment on SODG93A mice, when CuATSM was started at 90 days (30 mg/kg/dose, n=4), 50 days (100 mg/kg/dose, n=4), or 5 days (100 mg/kg/dose, n=4) versus a DMSO control (n=4). (B) Effect of CCS co-expression on maturation of SOD with CuATSM treatment starting at 5 days old. The first group shows SOD concentrations from SODG93AxCCS mice continuously treated with CuATSM sacrificed for biochemical assays at 130 days (n=2). The middle group reports SOD concentrations for continuous CuATSM-treated SODG93AxCCS mice that are >200 days old (n=6). The final group shows concentrations from ~130 day old SODG93AxCCS mice that were taken off CuATSM at 21 days (n=5).
This hypothesis was tested using SODG93AxCCS mice. With the increased expression of human CCS, CuATSM treatment of these mice resulted in the lowest percentages of copper-deficient SOD that we have ever observed in ventral spinal cord (Fig. 5B). Combined CuATSM treatment with CCS overexpression also led to a remarkable accumulation of Cu,Zn SOD, which reached 340±45 µM in the long surviving CCSxSODG93A mice. This is a hundred fold more SOD protein compared to the endogenous mouse SOD and nearly twice as much SOD protein as found in SODG93A mouse spinal cord without CCS coexpression.
Withdrawal of CuATSM when the mice were 21 days of age resulted in a slight decrease in total SOD compared with continuous treatment at the same age. Withdrawal did not result in subsequent accumulation of copper-deficient SOD when the mice reached end stage 110 days later. Thus, CuATSM may have other actions in addition to copper-delivery to allow SOD to mature to its fully active and stable form.
Discussion
The remarkable protection by CuATSM in SODG93AxCCS mice reveals the complex interplay of copper in survival of motor neurons during development and in their demise from mutant SOD expression. Without CCS co-expression, CuATSM is still among the most effective pharmaceutical agents in high-expressing SODG93A mice among the hundreds tested in this model so far (27). With CCS co-expression, CuATSM helped meet the apparent early demand for copper during development resulting from overexpression of human CCS. In addition, CuATSM essentially increased survival of high expressing SODG93A mice to beyond 600 days while the doubling amount of SOD protein (Fig. 1). Furthermore, withdrawal of CuATSM initiated motor neuron disease progression, while restarting treatment arrested further progression of symptomatic mice for 180–270 days. This makes possible the pharmacological control of disease progression in SOD-transgenic mice with CuATSM.
Overexpression of human SOD in transgenic mice is known to induce widespread changes in copper homeostasis in the spinal cord (28). The basis for these changes likely results from the large demand created for copper by the accumulation of copper-deficient, zinc-bound SOD in spinal cord that we have identified by mass spectrometry (16, 18, 19). The accumulation of copper-deficient SOD is consistent with other reports using indirect assays that show copper depletion from the protein in multiple ALS-associated mutant SODs in transgenic mice (24, 29).
The accumulation of copper-deficient, zinc-bound SOD as the predominant form of misfolded SOD in transgenic mice can be reconciled with recent findings on the importance of zinc in modulating the folding landscape of SOD (17). Zinc has been shown to accelerate the kinetics of folding of the apoSOD protein by 100-fold, in addition to stabilizing the SOD monomer thermodynamically by 13 kcal/monomer (17). Because zinc strongly stabilizes unfolded SOD protein, zinc-containing SOD accumulates in the spinal cord awaiting copper from CCS to complete its maturation to Cu,Zn SOD. Copper transport into other organs is much faster than into the spinal cord and brain, which is consistent with the accumulation of copper-deficient SOD in the CNS (30).
There are only about a dozen enzymes in humans that bind copper (31). The vast majority of intracellular copper is bound primarily by two enzymes, cytochrome c oxidase and Cu,Zn SOD. A complex network of copper transporters and chaperones provides the intracellular delivery of copper to these two enzymes. The net distribution is driven by the relative affinity gradients for copper (8), with CCS having a stronger affinity for copper than the chaperones for cytochrome c oxidase. The expression of human CCS in the SODG93AxCCS mice removes the kinetic barrier for loading copper into zinc-containing SOD. This allows more of the copper in the CNS to be diverted into SOD, thereby exacerbating the copper deficiency created by SOD overexpression. Withdrawal of CuATSM from SODG93AxCCS mice resulted in a 75% decrease in cytochrome c oxidase activity when these mice reached end stage at 130 days (Fig. 4), where as SODG93A mice reaching end stage at about the same age had no apparent decrease in cytochrome c oxidase activity. These data support the role of human CCS overexpression in increasing delivery of copper to SOD at the expense of depleting copper from mitochondrial cytochrome c oxidase (5, 7).
Mitochondria in the spinal cord are well-known to be a target in motor neuron diseases (32) and both CCS and SOD enter as metal-deficient forms in the mitochondrial intermembrane space in SOD-transgenic mice (33–37). This mitochondrial-localized fraction of SOD with CCS should be particularly effective at competing with cytochrome c oxidase for copper and potentially can contribute to the accelerated death of SODG93AxCCS mice. The time course for the early death in our double transgenic mice is similar to that observed in pregnant mice that have been given copper-deficient diets in late pregnancy (21), and in rat pups made copper-deficient prenatally; these pups also suffer from life-long motor deficits (20). These results are consistent with the need to treat high-expressing SODG93AxCCS mice early with CuATSM to avoid the consequences of severe copper deficiency during perinatal development.
An eight-fold increase of endogenous mouse CCS expression is one of the major changes observed in copper homeostasis resulting from SOD overexpression (28). In non-transgenic cells, approximately one CCS molecule is expressed per 7–14 SOD monomers (6). In the high-expressing SODG93A mice, there are 27 genomic DNA copies of human SOD plus the two endogenous mouse SOD alleles, compared to just two endogenous copies of mouse CCS. Thus, the eight-fold increase in expression likely represents the upper limit of endogenous CCS possible, but still too low to meet the increased demand created by human SOD overexpression. Our mass spectrometry assays in SODG93A mice show that endogenous mouse CCS cannot catalyze the complete maturation of human SOD in these mice when treated with CuATSM (Fig. 5). Although human and mouse CCS share 86% identity (38), the efficiency of mouse CCS interacting with human SOD might further limit copper delivery. Thus, the accumulation of copper-deficient SOD in the CNS may result from diminished efficiency for mouse CCS delivering copper to human SOD, which is further amplified by the dramatic difference in expression of CCS to SOD. Because co-expression of human CCS in the SODG93AxCCS mice more closely replicates the balance of CCS with SOD found in humans, familial ALS-SOD patients could potentially respond to CuATSM treatment more like SODG93AxCCS mice than the standard SODG93A model.
Familial ALS caused by SOD mutations represent only 2–7% of all ALS cases (39), but there is growing evidence that wild-type SOD can participate in sporadic ALS (40–42). CuATSM might also have additional protective actions, such as restoring mitochondrial function. CuATSM is also known to reduce cytosolic accumulation of TDP43 both in vitro (43) and in vivo (10). A broader potential role for CuATSM beyond ALS is suggested by its efficacy in treating four mouse models of Parkinson’s disease that are unrelated to mutant SOD (44). In addition to supplying copper, CuATSM is also a scavenger of peroxynitrite in these models (44) and this mode of action is consistent with a role in ALS as well (45–47). Tyrosine nitration was reduced in the SODG93AxCCS mice treated with CuATSM (Fig. 2). Characterization of these alternative protective actions of CuATSM may help further improve efficacy as well as identify additional mechanisms underlying the pathogenesis of ALS. Collectively, these studies provide a rationale for testing CuATSM in sporadic as well as familial SOD patients. Because CuATSM also rescues symptomatic low-expressing SODG93AxCCS mice that were never treated as pups, pretreatment of ALS patients may not necessarily be required for CuATSM to be protective.
Multiple lines of evidence now support the importance of copper as a pathogenic factor in ALS (16, 24, 28, 48, 49). The vulnerability of motor neurons to altered copper homeostasis is highlighted by the discovery that specific mutations in the copper transporter ATP7A occur in progressive X-linked spinal muscular atrophy type 3 (SMAX3) (50). A causal linkage is supported by the targeted knockout in motor neurons of ATP7A in mice, which results in motor neuron degeneration (51).
Because copper uptake in the CNS is highly regulated and its turnover is extremely slow, copper supplements cannot replace CuATSM for treating ALS. Copper supplements can damage the liver and are generally toxic at high dosages. CuATSM offers a safe delivery vehicle to bypass the distribution system naturally limiting copper transport into the CNS, providing sufficient copper for CCS to complete the maturation of Cu,Zn SOD while avoiding the toxicity of high dietary copper intake.
Materials and Methods
Mouse breeding and husbandry
All mice procedures were conducted in AAALAC approved facilities following current ethical regulations of the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (USA) international guidelines and were approved by Oregon State University’s Institutional Animal Committee. Male SOD transgenic mice expressing SODG93A, both the high-expressing SODG93A mice (B6SJL-Tg(SOD1*G93A)1Gur/J) and low-expressing SODG93A ((B6SJL-Tg(SOD1*G93A)dl1Gur/J) were obtained from The Jackson Laboratory (Bar Harbor, ME). These animals were bred against non-transgenic hybrid female mice (B6SJL/J) also obtained from Jackson Laboratory to generate additional SODG93A transgenic mice. Transgenic mouse line 17 expressing human CCS under control of the PRP promoter was previously described (5). The CCS mice have been maintained on the same heterozygous background as the SOD mice. Initially, SODG93AxCCS mice were produced by crossing male SODG93A mice with hemizygous CCS+/− mice. To maximize the number of double transgenic mice generated in each litter, we subsequently bred the CCS mice to homozygosity. Subsequent SODG93AxCCS were generated by breeding male SODG93A mice against homozygous female CCS mice to produce litters composed entirely of heterozygous CCS mice. Both SODG93AxCCS and SODG93A mice had their SOD copy number verified by qPCR using mouse tissue obtained directly from The Jackson Laboratory as the reference standard for gene copy number (Fig. S1). The qPCR protocol for SOD was identical to that described by Appendix A from the Jackson Laboratory Manual for SODG93A mice (4).
Study Design
To produce the large numbers of mice needed quickly for therapeutic trials, five male SODG93A mice were purchased from Jackson Labs. When the males were within the breeding age for SODG93A mice (49–75 days of age), each was placed in a cage with two female breeders on the same hybrid background purchased from Jackson Laboratories. After 4–5 days, the males were transferred to new cages containing additional female breeders. The pairs of female breeders produced litters within 1–2 days of each other that were treated as one group for randomization to treatment cages. The pups were genotyped by PCR at 18–20 days of age and weaned at 21 days of age. Mice from these litters were randomized to pairs of cages containing 3–4 mice per cage and balanced as closely as possible for coat color. Males were housed separately from females and equal numbers of both sexes were enrolled in the trials. Groups of matched pairs of cages were blindly allocated to treatment after filling the cages was completed. Several littermates that were nontransgenic for SODG93A were also included and evaluated blindly throughout the study. The SODG93AxCCS mice were produced similarly by transferring male hemizygous SODG93A mice into cages containing two homozygous CCS female breeders.
Statistical Analysis
The survival of SODG93A mice was evaluated by the Cox Proportional Hazards Model using the likelihood ratio to test statistical significance from SAS 9.4 (PHREG procedure). The Wald statistic commonly used to calculate significance in Cox Proportional Hazards statistical test fails with the SODG93AxCCS studies because of the large separation of survival time between groups. Equivalent highly significant differences were obtained with the nonparametric Mantel-Cox (Log-Rank) test (not shown). Power analyses for the SODG93AxCCS studies showed that a group size of 1 mouse surviving past 180 days would be highly significant using either the survival of untreated SODG93AxCCS or SODG93A mice. A minimum group size of six mice was chosen for each group.
Treatment Group Assignments and Randomization
The study was randomized by assigning balanced cages to treatment groups and the treatment groups were gender-balanced. The assignment of treatment for the paired groups of cages was randomized by a coin flip by a technician in another laboratory just prior to initiation of treatment.
Survival Endpoints and Symptom Assessment for SODG93A mice
The failure of SODG93A mice to right themselves after 30 seconds is the de facto standard for end-of-life because death invariably follows within 1–3 days (52). These mice have flaccid hind limbs and have lost significant forelimb strength that prevents them from righting themselves or moving effectively around the cage. In concordance with discussions with our attending veterinarian and the IACUC committee, SODG93A mice were deemed to be end stage when they could no longer right themselves after 15 seconds. The mice were treated morning and evening and assessed at each treatment time for the ability to right themselves on both sides. They were given three opportunities to do so. In our prior experience, mice that failed to right themselves in 15 seconds only rarely could right themselves within 30 seconds (53).
Separate blinded evaluations were conducted for symptom progression. Cages were de-identified by one technician one cage at a time and presented to a blinded observer working in a sterile hood for evaluation. The blinded observer wore red safety goggles to eliminate their ability to see residual red color from CuATSM on the mouse coat. A random number generator was used to determine the order of cages to be evaluated. Each animal was given a score of 0 to 3 (0-Animals had no obvious symptoms; 1- Animal has an abnormal gait; 2- Animals dragged hind limb at least twice in a 12 inch span; 3 – Animal is not using limb for forward motion).
Assessment of SODG93AxCCS mice
These mice did not experience the same disease progression as SODG93A mice and we realized that the end stage determination described for SODG93A mice was inappropriate. The CuATSM-treated SODG93AxCCS mice generally fail the traditional right test when placed on a solid surface, but were still able to right themselves in the cage bedding material, move around the cage, feed themselves and after an initial weight loss maintained their weight until the last week. Notably, these mice maintain their forelimb strength. Their difficulty in righting themselves on a solid surface was due to the rigid extension of the hind limbs that interfered with rolling over when using the front paws. In the cage, the hind limbs are able to sink into the bedding allowing the animal to stay upright and use its forelimbs for movement. In consultation with the head veterinarian and staff, the endpoint was chosen to be the failure of mice after three attempts to move 15 cm on a solid surface covered with an absorbent paper towel. Although the difference in survival assessment added at least 2–4 weeks to the apparent survival, the end stage SODG93AxCCS mice were still far more mobile than their SODG93A counterparts.
Mice censured from trials
All censured mice are described in Supplemental Table 1 and 2. There was one major sudden incidence of mortality when 4 CuATSM-treated mice and 2 DMSO-treated mice at approximately 100 days of age died from a severe gastrointestinal inflammation apparent on autopsy. Several mice undergoing DMSO-based treatments independently of the trials reported here also died unexpectedly at this time. The toxicity apparently resulted from a new staff member sterilizing gloves with Clidox (aqueous chlorine dioxide), which may have been absorbed with DMSO during handling. After switching to 70% ethanol for sterilization of gloves, no deaths have occurred in the following 20 months using the same CuATSM preparation at the same dose.
Applying CuATSM treatments
Mice were treated twice daily by applying a 15 µg/µL solution of CuATSM dissolved in DMSO directly to the skin on the neck and back of each mouse with a pipette. For larger doses, the CuATSM is applied in multiple streaks down the back to maximize absorption. Animals were not shaved as the fur helped to keep the compound on the animal as they moved around the cage following treatment. Doses were adjusted weekly for each cage by weighing all animals and calculating the dose based on the heaviest mouse in the cage. Animals assigned to a DMSO-treatment group had equivalent volumes of DMSO applied.
Animals were anesthetized with isofluorane, followed by transcardial perfusion with heparinized PBS. The spinal column was dissected from mice without isolating the spinal cord. Tissues were then either fixed for immunohistochemical staining as described below or frozen in liquid nitrogen. Frozen tissues were stored at −80 °C until time of use.
Immunohistochemical staining of mouse spinal cords and brains
Animals were deeply anesthetized and transcardial perfusion was performed with 0.9% saline and 4% paraformaldehyde in 0.1 M PBS (pH 7.2–7.4) at a constant flow of 1 mL/min. Fixed spinal cord was removed, post-fixed by immersion in fixative for 24 h, and then transversely sectioned (30–40 µm) on a vibrating microtome (Leica 1000s). Serial sections were collected in 100 mM PBS for immunohistochemistry. Free-floating sections were permeabilized for 30 min at room temperature with 0.3% Triton X-100 in PBS, washed with buffered solutions, blocked with 5% BSA:PBS for 1 hour at room temperature, and incubated overnight at 4 °C in a solution of 0.3% Triton X-100 and PBS containing the primary antibodies, mouse anti-nitrotyrosine (1:200, Millipore), rabbit anti-GFAP (1:500, Sigma), or rabbit anti-ChAT (1:300, Millipore). Nitrotyrosine immunoreactivity was completely blocked by preincubation of the primary antibody with free nitrotyrosine (10 mM at pH 7.4). After washing, sections were incubated in 1:1,000-diluted secondary antibodies conjugated to Alexa Fluor 488 or Alexa Fluor 633. (Invitrogen). Antibodies were detected by confocal microscopy using a confocal Zeiss LSM 510 META. For Nissl and myelin staining, the sections were permeabilized for 10 minutes with PBS+ 0.1% Triton X-100. After washing twice in PBS for 5 minutes, sections were incubated with Nissl NeuroTrace (1:200, Invitrogen) and fluoroMyelin (1:500, Invitrogen) in PBS for 20 minutes and washing for 10 min in PBS plus 0.1% Triton X-100. The stain was removed and sections washed 3 times in PBS over 2 h. To examine the brain for possible changes, 30 brain slices were block for 1 h at room temperature in PBS-Triton 0.25% - BSA 5%. GFAP antibody (Sigma #G9269, 1:500) was incubated overnight at 4 °C. Nissl staining (Thermo Scientific, 1:400) was incubated for 30 minutes at room temperature.
CuATSM synthesis and formulation
ATSM was synthesized by adding 4.8 g 4-methyl-3-thiosemicarbazide to 100 mL dry ethanol heated to 60°C until completely in solution. While stirring, 2 mL of diacetyl (2,3-butadione) was pipetted into the vessel. Ten drops of concentrated sulfuric acid was added to accelerate the reaction. ATSM precipitated as an off-white powder that was collected by filtration. The ATSM precipitate was washed with ethanol and methanol and then vacuum dried. These reaction conditions were the most commonly used, but we were successful preparing ATSM using different concentrations and proportions of precursors. All reagents were obtained from TCI America (Portland, OR).
To incorporate copper, 4 g ATSM was stirred at room temperature in 100 mL of methanol. The vast majority of the ATSM did not dissolve. Both ATSM and CuATSM have limited solubility in methanol, but it is sufficient to facilitate complete copper loading into ATSM. 160 µL of 100 mM copper chloride in water was added drop-wise to the vessel and the mixture stirred for 30 min. To collect the product, 100 mL of water was added to completely precipitate CuATSM. CuATSM was collected via filtration and washed with methanol and water on the filter prior to vacuum drying. Mass spectrometry confirmed stoichiometric metal loading into CuATSM. The most common impurity seen was clusters of CuATSM with excess copper loading, such as two ATSM molecules with three bound copper atoms. Excess bound copper was removed by additional washing of the product with methanol and water.
To prepare CuATSM for dosing mice, 1.5 g CuATSM was added to a volumetric flask, brought to volume with 100 mL of dry pharmaceutical grade DMSO and dissolved in a bath sonicator for 15 min. CuATSM would not dissolve in DMSO contaminated with water. By using fresh bottles of dry DMSO and minimizing exposure to moisture, CuATSM solutions were stable in the freezer for many months and for over a week at room temperature.
SOD Quantitation
High-resolution mass spectrometry (ThermoFinnigan 7 Tesla FT-ICR Ultra Mass Spectrometer) was performed on homogenized spinal cord punches as previously described (16, 18, 19). Approximately 200 µg of ventral spinal cord tissue was weighed on a Cahn 25 Automatic Electrobalance (Cerritos, CA, USA) and homogenized for 30 seconds in 10 mM ammonium acetate buffer with an adapted microcentrifuge tube pestle. A hole drilled in the shaft of the pestle allowed for complete sample and buffer recovery by briefly centrifuging the homogenate while the pestle was suspended above the sample by a paper clip. After removing the pestle, a supernatant was generated by centrifuging the sample for 2 minutes at 13,000×g. Aliquots of the supernatant were loaded onto the mass spectrometer via a C4 ZipTip® (Millipore). Bovine SOD was added to aliquots (final concentration of 300 nM) as an internal standard for quantitation. The data were analyzed using a custom Matlab™ program that calculates theoretical isotope distributions of SOD to identify the different metal bound species (19).
Cytochrome c oxidase assays
This activity was determined by following the oxidation of reduced cytochrome c as decreased absorbance at 550 nm. Reduced cytochrome c was prepared by incubating horse heart cytochrome c (Sigma) with ascorbic acid; excess ascorbic acid was removed using a centrifugal filter unit (3,000 MWCO). Tissue punches (~500 µg wet weight) were collected on dry ice from frozen brain motor cortex and then homogenized (10 µg/µl) in 50 mM phosphate buffer pH 7 containing 20 µM EDTA with 24 units/ml catalase and 0.1% Triton X-100 added immediately prior to the time of enzyme assay. Tissue homogenate was diluted 1:250. Enzyme activity defined as one unit oxidizing 1.0 µmole of ferrocytochrome c per minute at 25°C, pH 7.0 per mg wet tissue, using 21.8 mM−1.cm−1 as the differential extinction coefficient for horse heart cytochrome c.
Supplementary Material
Highlights.
The copper ligand CuATSM extends life in high-expressing SODG93A mice by up to 25%.
CuATSM in high-expressing SODG93A mice co-expressing CCS survive for 20 months (500%)!
Motor neuron disease can be restarted and then stopped by controlling CuATSM treatment.
CCS plus copper nearly doubles the amount of SOD protein in mouse spinal cord.
The vast majority of the mutant SOD is found in its mature and active form, Cu,Zn SOD.
Acknowledgments
We thank Kristin Clausing, the vivarium staff and Dr. Helen Diggs, our attending veterinarian for their support and partnership in monitoring the health of the mice and quick identification of emerging health issues affecting the mice. We thank Michael Camillo as well as Burgess and Libby Jamieson for donations to the Linus Pauling Institute that made this work possible. This work was once funded by AT002034 (JSB), NS058628 (JSB), and NS036761 (AGE) and supported by the Amyotrophic Lateral Sclerosis Association. It is currently funded by AL140108 from the Department of Defense (JSB). The SOD analyses were made possible by the Oregon State University Biomolecular Mass Spectrometry Core Facility and the NIEHS ES000210 Core Center (JSB). The Muscular Dystrophy Association supports JLE. The Australian National Health and Medical Research Association provided Project Grant 1061550 (PJC and BRR) and CDF2 1084972 (PJC) from the Australian government. This publication was made possible in part by award number 1337774 from the National Science Foundation, MRI: Acquisition of Confocal and Two-Photon Excitation Microscope in the Confocal Microscopy Facility of the Center for Genome Research and Biocomputing at Oregon State University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: JRW, PRB, NIL, EML and JSB treated the mice. ET, PB and LB performed the immunohistology. JRW, BRR, EJM, CSB and TWR conducted the mass spectrometric assays. JRW, PRB, NIL, PJC, CP, MS, MCF, AGE, LB and JSB contributed to the design of the experiments and to the writing of the manuscript, and also conducted supporting experiments not shown.
Competing interests: PJC and BRR are employees of The University of Melbourne. Collaborative Medicinal Development LLC has licensed intellectual property on this subject from The University of Melbourne. No other conflicts are listed.
References
- 1.Gurney ME, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 2.Vinsant S, et al. Characterization of early pathogenesis in the SOD1(G93A) mouse model of ALS: part II, results and discussion. Brain and behavior. 2013;3(4):431–457. doi: 10.1002/brb3.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saxena S, Cabuy E, Caroni P. A role for motoneuron subtype-selective ER stress in disease manifestations of FALS mice. Nat. Neurosci. 2009;12(5):627–636. doi: 10.1038/nn.2297. [DOI] [PubMed] [Google Scholar]
- 4.Leitner M, Menzies S, Lutz C. Working with ALS Mice, Guidlines for preclinical testing and colony management. The Jackson Laboratory. 2009 (Appendix A) [Google Scholar]
- 5.Son M, et al. Overexpression of CCS in G93A-SOD1 mice leads to accelerated neurological deficits with severe mitochondrial pathology. Proc Natl Acad Sci U S A. 2007;104(14):6072–6077. doi: 10.1073/pnas.0610923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rothstein JD, et al. The copper chaperone CCS is abundant in neurons and astrocytes in human and rodent brain. J Neurochem. 1999;72(1):422–429. doi: 10.1046/j.1471-4159.1999.0720422.x. [DOI] [PubMed] [Google Scholar]
- 7.Son M, et al. Isolated cytochrome c oxidase deficiency in G93A SOD1 mice overexpressing CCS protein. J Biol Chem. 2008;283(18):12267–12275. doi: 10.1074/jbc.M708523200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banci L, et al. Affinity gradients drive copper to cellular destinations. Nature. 2010;465(7298):645–648. doi: 10.1038/nature09018. [DOI] [PubMed] [Google Scholar]
- 9.Son M, Elliott JL. Mitochondrial defects in transgenic mice expressing Cu,Zn superoxide dismutase mutations: the role of copper chaperone for SOD1. J. Neurol. Sci. 2014;336(1–2):1–7. doi: 10.1016/j.jns.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Soon CP, et al. Diacetylbis(N(4)-methylthiosemicarbazonato) coppeRII) (CuII(atsm)) protects against peroxynitrite-induced nitrosative damage and prolongs survival in amyotrophic lateral sclerosis mouse model. J Biol Chem. 2011;286(51):44035–44044. doi: 10.1074/jbc.M111.274407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McAllum EJ, et al. Therapeutic effects of CuII(atsm) in the SOD1-G37R mouse model of amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis and Frontotemporal Dementia. 2013;14:586–590. doi: 10.3109/21678421.2013.824000. [DOI] [PubMed] [Google Scholar]
- 12.Dearling JL, Lewis JS, Mullen GE, Welch MJ, Blower PJ. Copper bis(thiosemicarbazone) complexes as hypoxia imaging agents: structure-activity relationships. J Biol Inorg Chem. 2002;7(3):249–259. doi: 10.1007/s007750100291. [DOI] [PubMed] [Google Scholar]
- 13.Ikawa M, et al. Increased oxidative stress is related to disease severity in the ALS motor cortex: A PET study. Neurology. 2015;84(20):2033–2039. doi: 10.1212/WNL.0000000000001588. [DOI] [PubMed] [Google Scholar]
- 14.Dearling JL, Packard AB. Some thoughts on the mechanism of cellular trapping of Cu(II)-ATSM. Nuclear medicine and biology. 2010;37(3):237–243. doi: 10.1016/j.nucmedbio.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Donnelly PS, et al. An impaired mitochondrial electron transport chain increases retention of the hypoxia imaging agent diacetylbis(4-methylthiosemicarbazonato)copperII. Proc Natl Acad Sci U S A. 2012;109(1):47–52. doi: 10.1073/pnas.1116227108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts BR, et al. Oral Treatment with CuII(atsm) Increases Mutant SOD1 In Vivo but Protects Motor Neurons and Improves the Phenotype of a Transgenic Mouse Model of Amyotrophic Lateral Sclerosis. J Neurosci. 2014;34(23):8021–8031. doi: 10.1523/JNEUROSCI.4196-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kayatekin C, Zitzewitz JA, Matthews CR. Zinc binding modulates the entire folding free energy surface of human Cu,Zn superoxide dismutase. J Mol Biol. 2008;384(2):540–555. doi: 10.1016/j.jmb.2008.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rhoads TW, et al. Measuring copper and zinc superoxide dismutase from spinal cord tissue using electrospray mass spectrometry. Analytical biochemistry. 2011;415(1):52–58. doi: 10.1016/j.ab.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rhoads TW, et al. Using theoretical protein isotopic distributions to parse small-mass-difference post-translational modifications via mass spectrometry. Journal of the American Society for Mass Spectrometry. 2013;24(1):115–124. doi: 10.1007/s13361-012-0500-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Penland JG, Prohaska JR. Abnormal motor function persists following recovery from perinatal copper deficiency in rats. J Nutr. 2004;134(8):1984–1988. doi: 10.1093/jn/134.8.1984. [DOI] [PubMed] [Google Scholar]
- 21.Prohaska JR, Brokate B. The timing of perinatal copper deficiency in mice influences offspring survival. J Nutr. 2002;132(10):3142–3145. doi: 10.1093/jn/131.10.3142. [DOI] [PubMed] [Google Scholar]
- 22.Vinsant S, et al. Characterization of early pathogenesis in the SOD1(G93A) mouse model of ALS: part I, background and methods. Brain and behavior. 2013;3(4):335–350. doi: 10.1002/brb3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prohaska JR. Changes in Cu,Zn-superoxide dismutase, cytochrome c oxidase, glutathione peroxidase and glutathione transferase activities in copper-deficient mice and rats. J Nutr. 1991;121(3):355–363. doi: 10.1093/jn/121.3.355. [DOI] [PubMed] [Google Scholar]
- 24.Jonsson PA, et al. Disulphide-reduced superoxide dismutase-1 in CNS of transgenic amyotrophic lateral sclerosis models. Brain. 2006;129(Pt 2):451–464. doi: 10.1093/brain/awh704. [DOI] [PubMed] [Google Scholar]
- 25.Brown NM, Torres AS, Doan PE, O'Halloran TV. Oxygen and the copper chaperone CCS regulate posttranslational activation of Cu,Zn superoxide dismutase. Proc Natl Acad Sci U S A. 2004;101(15):5518–5523. doi: 10.1073/pnas.0401175101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamb AL, Torres AS, O'Halloran TV, Rosenzweig AC. Heterodimer formation between superoxide dismutase and its copper chaperone. Biochemistry. 2000;39(48):14720–14727. doi: 10.1021/bi002207a. [DOI] [PubMed] [Google Scholar]
- 27.Turner BJ, Talbot K. Transgenics, toxicity and therapeutics in rodent models of mutant SOD1-mediated familial ALS. Progress in neurobiology. 2008;85(1):94–134. doi: 10.1016/j.pneurobio.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Tokuda E, Okawa E, Watanabe S, Ono S, Marklund SL. Dysregulation of intracellular copper homeostasis is common to transgenic mice expressing human mutant superoxide dismutase-1s regardless of their copper-binding abilities. Neurobiol Dis. 2013;54:308–319. doi: 10.1016/j.nbd.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Lelie HL, et al. Copper and zinc metallation status of copper-zinc superoxide dismutase from amyotrophic lateral sclerosis transgenic mice. J Biol Chem. 2011;286(4):2795–2806. doi: 10.1074/jbc.M110.186999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levenson CW, Janghorbani M. Long-term measurement of organ copper turnover in rats by continuous feeding of a stable isotope. Analytical biochemistry. 1994;221(2):243–249. doi: 10.1006/abio.1994.1408. [DOI] [PubMed] [Google Scholar]
- 31.Prohaska JR. Impact of copper deficiency in humans. Ann N Y Acad Sci. 2014;1314:1–5. doi: 10.1111/nyas.12354. [DOI] [PubMed] [Google Scholar]
- 32.Kong J, Xu Z. Massive mitochondrial degeneration in motor neurons triggers the onset of Amyotrophic Lateral Sclerosis in mice expressing a mutant SOD1. J. Neurosci. 1998;18(9):3241–3250. doi: 10.1523/JNEUROSCI.18-09-03241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Igoudjil A, et al. In vivo pathogenic role of mutant SOD1 localized in the mitochondrial intermembrane space. J Neurosci. 2011;31(44):15826–15837. doi: 10.1523/JNEUROSCI.1965-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manfredi G, Xu Z. Mitochondrial dysfunction and its role in motor neuron degeneration in ALS. Mitochondrion. 2005;5(2):77–87. doi: 10.1016/j.mito.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Mattiazzi M, et al. Mutated human SOD1 causes dysfunction of oxidative phosphorylation in mitochondria of transgenic mice. J Biol Chem. 2002;277(33):29626–29633. doi: 10.1074/jbc.M203065200. [DOI] [PubMed] [Google Scholar]
- 36.Deng HX, et al. Conversion to the amyotrophic lateral sclerosis phenotype is associated with intermolecular linked insoluble aggregates of SOD1 in mitochondria. Proc Natl Acad Sci U S A. 2006;103(18):7142–7147. doi: 10.1073/pnas.0602046103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jaarsma D, et al. CuZn superoxide dismutase (SOD1) accumulates in vacuolated mitochondria in transgenic mice expressing amyotrophic lateral sclerosis-linked SOD1 mutations. Acta Neuropathol (Berl) 2001;102(4):293–305. doi: 10.1007/s004010100399. [DOI] [PubMed] [Google Scholar]
- 38.Moore SD, Chen MM, Cox DW. Cloning and mapping of murine superoxide dismutase copper chaperone (Ccsd) and mapping of the human ortholog. Cytogenetics and cell genetics. 2000;88(1–2):35–37. doi: 10.1159/000015480. [DOI] [PubMed] [Google Scholar]
- 39.Andersen PM, et al. Sixteen novel mutations in the Cu/Zn superoxide dismutase gene in amyotrophic lateral sclerosis: a decade of discoveries, defects and disputes. Amyotroph Lateral Scler Other Motor Neuron Disord. 2003;4(2):62–73. doi: 10.1080/14660820310011700. [DOI] [PubMed] [Google Scholar]
- 40.Guareschi S, et al. An over-oxidized form of superoxide dismutase found in sporadic amyotrophic lateral sclerosis with bulbar onset shares a toxic mechanism with mutant SOD1. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(13):5074–5079. doi: 10.1073/pnas.1115402109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bosco DA, et al. Wild-type and mutant SOD1 share an aberrant conformation and a common pathogenic pathway in ALS. Nature neuroscience. 2010;13(11):1396–1403. doi: 10.1038/nn.2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rakhit R, et al. Oxidation-induced misfolding and aggregation of superoxide dismutase and its implications for amyotrophic lateral sclerosis. J Biol Chem. 2002;277(49):47551–47556. doi: 10.1074/jbc.M207356200. [DOI] [PubMed] [Google Scholar]
- 43.Parker SJ, et al. Inhibition of TDP-43 accumulation by bis(thiosemicarbazonato)-copper complexes. PloS one. 2012;7(8):e42277. doi: 10.1371/journal.pone.0042277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hung LW, et al. The hypoxia imaging agent CuII(atsm) is neuroprotective and improves motor and cognitive functions in multiple animal models of Parkinson's disease. The Journal of experimental medicine. 2012;209(4):837–854. doi: 10.1084/jem.20112285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Estevez AG, et al. Induction of nitric oxide-dependent apoptosis in motor neurons by zinc-deficient superoxide dismutase. Science. 1999;286(5449):2498–2500. doi: 10.1126/science.286.5449.2498. [DOI] [PubMed] [Google Scholar]
- 46.Cassina P, et al. Peroxynitrite triggers a phenotypic transformation in spinal cord astrocytes that induces motor neuron apoptosis. J Neurosci Res. 2002;67(1):21–29. doi: 10.1002/jnr.10107. [DOI] [PubMed] [Google Scholar]
- 47.Franco MC, et al. Nitration of Hsp90 induces cell death. Proc Natl Acad Sci U S A. 2013;110:E1102–E1111. doi: 10.1073/pnas.1215177110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trumbull KA, Beckman JS. A role for copper in the toxicity of zinc-deficient superoxide dismutase to motor neurons in amyotrophic lateral sclerosis. Antioxid Redox Signal. 2009;11(7):1627–1639. doi: 10.1089/ars.2009.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tokuda E, et al. Metallothionein proteins expression, copper and zinc concentrations, and lipid peroxidation level in a rodent model for amyotrophic lateral sclerosis. Toxicology. 2007;229(1–2):33–41. doi: 10.1016/j.tox.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 50.Kennerson ML, et al. Missense mutations in the copper transporter gene ATP7A cause X-linked distal hereditary motor neuropathy. American journal of human genetics. 2010;86(3):343–352. doi: 10.1016/j.ajhg.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hodgkinson VL, et al. X-linked spinal muscular atrophy in mice caused by autonomous loss of ATP7A in the motor neuron. The Journal of pathology. 2015;236(2):241–250. doi: 10.1002/path.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scott S, et al. Design, power, and interpretation of studies in the standard murine model of ALS. Amyotrophic lateral sclerosis : official publication of the World Federation of Neurology Research Group on Motor Neuron Diseases. 2008;9(1):4–15. doi: 10.1080/17482960701856300. [DOI] [PubMed] [Google Scholar]
- 53.Trumbull KA, et al. Diapocynin and apocynin administration fails to significantly extend survival in G93A SOD1 ALS mice. Neurobiol Dis. 2012;45(1):137–144. doi: 10.1016/j.nbd.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.