Abstract
Metastatic associated proteins (MTA) are integrators of upstream regulatory signals with the ability to act as master coregulators for modifying gene transcriptional activity. The MTA family includes three genes and multiple alternatively spliced variants. The MTA proteins neither have their own enzymatic activity nor have been shown to directly interact with DNA. However, MTA proteins interact with a variety of chromatin remodeling factors and complexes with enzymatic activities for modulating the plasticity of nucleosomes, leading to the repression or derepression of target genes or other extra-nuclear and nucleosome remodeling and histone deacetylase (NuRD)-complex independent activities. The functions of MTA family members are driven by the steady state levels and subcellular localization of MTA proteins, the dynamic nature of modifying signals and enzymes, the structural features and post-translational modification of protein domains, interactions with binding proteins, and the nature of the engaged and resulting features of nucleosomes in the proximity of target genes. In general, MTA1 and MTA2 are the most upregulated genes in human cancer and correlate well with aggressive phenotypes, therapeutic resistance, poor prognosis and ultimately, unfavorable survival of cancer patients. Here we will discuss the structure, expression and functions of the MTA family of genes in the context of cancer cells.
Keywords: MTA1, MTA2, MTA3, coregulators, chromatin remodeling, cancer, metastasis
Introduction
The metastatic associated protein (MTA) family consists of three genes - MTA1, MTA2 and MTA3, as well as resulting alternatively spliced variants of MTA1 and MTA3. The MTA1, MTA2 and MTA3 genes in human cells are localized on chromosomes 14q32, 11q12-q13.1, and 2p21, respectively. Corresponding murine homologues are localized on chromosomes 12F, 19B and 12, respectively. Although MTA family members are evolutionary conserved, most of our current understanding of the MTA genes come from the genomic organization of the murine genes and the founding family member MTA1 (Kumar, 2014). Such genomic details also provide us with clues about the potential regulation of MTA genes by upstream pathways, and the nature of events involved in the generation of differentially spliced variants. In general, the MTA genes and protein products are ubiquitously expressed in various organ-systems with a certain degree of differential expression in rapidly proliferating versus differentiated tissues (Liu et al., 2014a).
The founding family member, Mta1 in mice, was cloned by Garth Nicholson’s laboratory and published in September 1994 at the University of Texas MD Anderson Cancer Center (Toh et al., 1994). The putative role of MTA1 in chromatin remodeling was inferred due to the presence of MTA1 polypeptides in the nucleosome remodeling and histone deacetylase (NuRD) complex (Xue et al., 1998). As opposed to MTA1, MTA2 was initially recognized as MTA1-like 1 gene, named as MTA1-L1, as a randomly selected clone from a large scale sequencing effort of human cDNAs by Takashi Tokino’s laboratory (Futamura M et al., 1999). MTA2’s suspected role in chromatin remodeling was inferred from the prevalence of MTA2 polypeptides with the NuRD complex in a proteomic study (Zhang et al., 1999). This was followed by targeted cloning of murine Mta2 (Xia and Zhang, 2001). Murine Mta3 was initially cloned as a partial cDNA while screening a mouse keratinocyte cDNA library with human MTA1 partial fragment as a probe, and followed by cloning of the full length Mta3 cDNA through 5’ RACE of the RNA from C57B1/6J mouse skin by 5’ RACE methodology by Mỹ G Mahoney laboratory (Simpson et al., 2001).
Over the years, the MTA family members have emerged as one of the most important regulatory molecules with both redundant and non-redundant activities in both physiological and cancer cells (Sen et al., 2014a, Sen et al., 2014b). A large body of work over the years suggests that the MTA family members regulate numerous cellular pathways with roles in cancer progression and metastasis (Li and Kumar, 2015). However, because MTA family members are ubiquitously expressed and natural levels of MTA family members are equally important for several essential physiological functions (Sen et al., 2014a), the authors believe the MTA family members may also follow a nomenclature that is based on their structural elements, rather than the current nomenclature derived from the historical cloning of the founding member MTA1 - as a differential expressed gene from rat metastatic mammary gland tumors (Toh et al., 1994). However, this is more of a philosophical point of continuing scientific debate as the functions of MTA family members are independent of what we name these proteins.
Since the discovery of the MTA family, the scientific community has witnessed a prolific growth in the number of MTA-research articles as evident by the existence of over 500 articles in PubMed since the first publication in 1994, with MTA1 leading the field with over 350 articles followed by MTA2 and MTA3. Because MTA family members are not found to exhibit any enzymatic activity or directly bind to DNA so far, the functions of MTA proteins, are derived by their unique structural features and domains, particularly from the structure functional relationship of MTA1 (Fig. 1), allowing MTA proteins to interact with a large number of proteins to manifest its functions. In general, MTA proteins are components of multi-protein regulatory complexes with roles in chromatin remodeling and modifying gene expression through interactions with histone- or non-histone proteins and modified nucleosomes while recognizing specific post-translational modifications (Kumar, Gururaj, 2008; Nair et al., 2013; Millard et al., 2014; Alqarni et al., 2014; Liu et al., 2015; Li, Kumar, 2015).
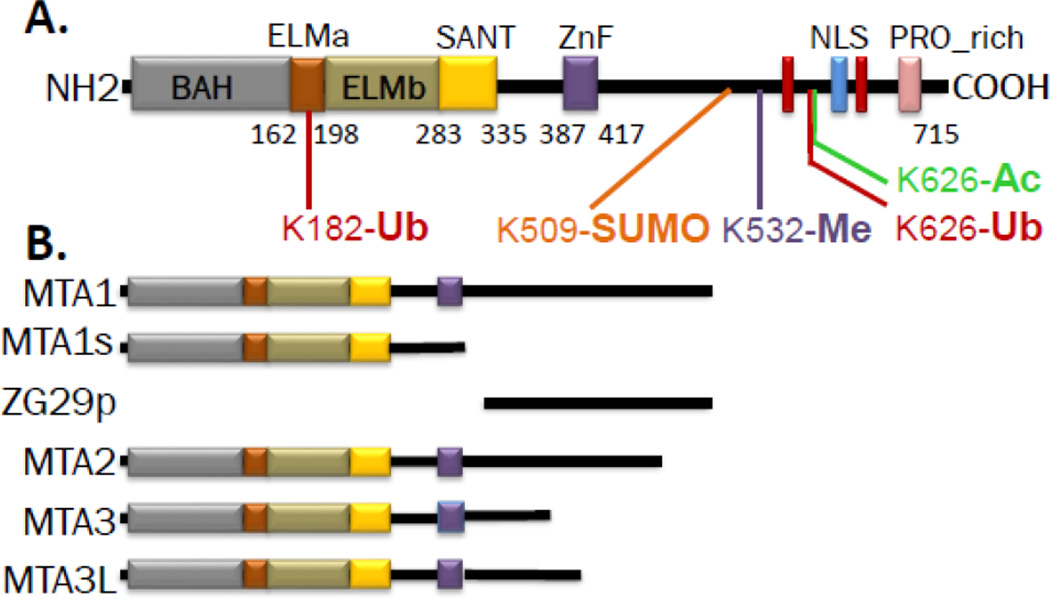
Figure 1.
Schematic representation of the MTA family members. A, MTA1 domains and motifs: BAH (Bromo-Adjacent Homology), ELM2 (egl-27 and MTA1 homology), SANT (SWI, ADA2, N-CoR, TFIIIB-B), ZnF (GATA-like zinc finger), NLS (nuclear localization signal), and PRO_rich (proline rich region). Known post-translational modifications on specific amino acids are depicted underneath. B, Illustrations of major isoforms of the MTA family.
MTA proteins are one of the most widely deregulated molecules in human cancer and contribute to cancer progression and metastasis and maintenance of cancerous phenotypes (Kumar, 2014). In addition, MTA proteins represent a converging nodule for a large number of upstream pathways and influence cellular functions through their interacting proteins, target genes, or modifying the biological functions of downstream effector components. As several excellent reviews have extensively summarized the details of upstream modifiers and downstream effectors of MTA proteins (Li et al., 2012; Kumar, 2014; Li, Kumar, 2015), these will not be extensively discussed here. Here we will discuss the genomic organization and expression of MTA genes and functions of MTA proteins.
Genomic structure and splicing of MTA genes
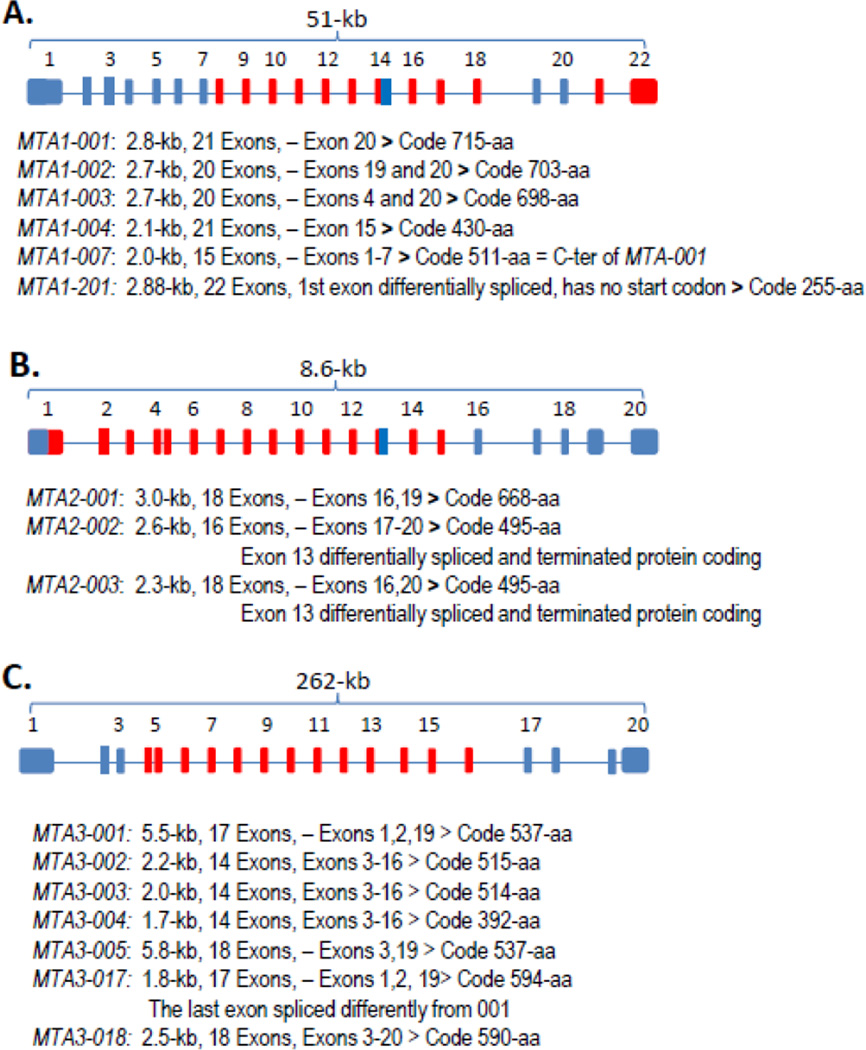
The human MTA1 gene has 21 exons, spanning a length of around 51-kb in humans, but only 39-kb in murine cells (Fig. 2A). In humans, there are 20 transcripts generated by alternative splicing from these exons. The lengths of these transcripts range from 416-bp to 2.9-kb. However, only eight predicted spliced transcripts contain open-reading frames, coding six proteins and two polypeptides. The lengths of the other 12 transcripts range from 561-bp to 1.9-kb, belong to the non-coding long RNA and of them, ten transcripts retain intron sequences. The murine Mta1 gene has six transcripts, including three transcripts coding for three proteins, and three non-coding RNA transcripts. The protein coding transcripts are around 2.7-kb to 2.8-kb in size, and longer than the non-coding transcripts, which range from 584-bp to 2.1-kb.
Figure 2.
Structure and partial transcripts of MTA genes. The diagram and transcripts of MTA1, MTA2 and MTA3 genes (A–C) are based on the ENSEMBL database (MTA1 ENSG00000182979, MTA2 ENSG00000149480, MTA3 ENSG00000057935). The blues are alternatively spliced exons. The diagrams are based on the known protein coding transcripts, not including non-coding RNAs.
The MTA2 gene is much shorter than the MTA1 gene. The human MTA2 gene is about 8.6-kb, while the murine Mta2 gene is 10.4-kb (Fig. 2B). The human MTA2 gene has 20 exons and has seven transcripts, including three protein-coding transcripts but code two proteins of 688 amino acids and 495 amino acids, respectively, while four non-coding RNA transcripts run from 532-bp to 627-bp. The murine Mta2 gene has six transcripts, but only a 3.1-kb transcript is expected to code a protein of 668 amino acids. The remaining five Mta2 transcripts are non-coding RNAs, ranging from 620-bp to 839-bp.
The MTA3 gene is rather long as compared to other family members (Fig. 2C). The human MTA3 gene is 262-kb while the murine Mta3 gene is 109-kb. The human MTA3 gene contains 20 exons, and 19 transcripts generated by alternative splicing. Nine MTA3 transcripts are protein coding RNAs and predicted to code six proteins of 392, 514, 515, 537, 590 and 594 amino acids long, and two small 18 amino acids and 91 amino acids polypeptides. The other 10 transcripts are non-coding RNAs. The murine Mta3 gene has 17 exons, but only nine transcripts, seven of which are predicted to code six proteins, ranging from 251 amino acids to 591 amino acids, and one 40 amino acids polypeptide. There are only two predicted non-coding RNA in murine Mta3, with a length of about 500-bp. The large variety of predicted transcripts and coded proteins from three MTA genes highlights the potential functional complexity of MTA genes and the broader impact of this family on the biology of living systems. It would be worthwhile to point out that most of the reported studies are about the full lengths proteins of the three MTA genes and the roles of other transcripts remain predictive at this time. In this context, considering the significance of non-coding RNAs and small polypeptides in the biology, the future of the MTA field of research will be much more rewarding in the coming years and expected to modulate the biology of three full length MTA proteins.
Domains of MTA Proteins
The MTA1 protein is the longest among the human family members with 715 amino acids as compared to MTA2 and MTA3 which contain 668 and 594 amino acids, respectively. Alignment of amino acid sequences across human MTA proteins using the Pblast-NCBI tools suggests that MTA2 shares 58% homology with MTA1 while MTA3 shares 69% homology with MTA1. The MTA proteins contain both conserved N-terminal and divergent C-terminal regions. The conserved domains of MTA proteins include: the BAH (Bromo-Adjacent Homology), the ELM2 (egl-27 and MTA1 homology), the SANT (SWI, ADA2, N-CoR, TFIIIB-B), and the GATA-like zinc finger domains (Fig. 1). The divergent regions of MTA proteins partially share a Src homology 3-binding domain, acidic regions, and one predicted bipartite nuclear localization signal (NLS) sequence. These structural domains provide clues about their potential functions of MTA proteins. For example, the BAH domain is generally found in proteins with abilities to interact with nucleosomes and methylated histones and contribute to protein-protein interactions and gene modulation (Kuo et al., 2012; Norris et al., 2008; Yang and Xu, 2013); the ELM2 domain interacts with histone deacetylases to remodel chromatin and regulate gene transcription (Ding et al., 2003); and while ELM2-SANT domains serve as scaffolds for a number of DNA-binding transcription factors for chromatin remodeling (Wang et al., 2008). The presence of the SH3 motif suggests that the functions MTA proteins could also be regulated by SH3-domain containing signaling molecules as Mta3 has been shown to interact with Fyn and Grb2 (Simpson et al., 2001). These structural features suggest that the MTA family members are likely to be involved in chromatin remodeling, modulation of transcription, and expression of target genes – which turn out to be true as this family of chromatin remodeling proteins have emerged as one of the major modifiers of gene transcription in both physiological and disease settings.
Subcellular localization of MTA proteins
The functions of MTA proteins are expected to be regulated by the nature of its binding proteins, subcellular localization and upstream signaling cascades in both normal and cancer cells. Although MTA proteins contain bipartite nuclear localization signals (NLS) and predominantly reside in the nuclear compartment, MTA1 and MTA3 are also found to be present in other extra-nuclear compartments (Liu et al., 2014a). For example, MTA1 has been shown to localize in the cytoplasm of embryonic mouse tissues, as well as in the cytoplasm and nucleus in colon, liver and endometrial cancer cells (Liu et al., 2014b), in hepatocytes (Li et al., 2008) as well as normal breast tissues (Sharma et al., 2011). More recently, cytoplasmic MTA1 has been found to also localize on cytoplasmic microtubules and onto the nuclear envelope, with cytoplasmic levels of MTA1 correlating well with tumor progression (Liu et al., 2014b).
Similarly, while MTA1 is expressed in both the nucleus and cytoplasm, the status of the nuclear MTA1 overexpression defines prognostic value of MTA1 in prostate cancer (Dias et al., 2013). In contrast to MTA1, ZG29p (Kleene et al., 1999) and MTA1s (Kumar et al., 2002) spliced variants localize in the cytoplasm due to the lack of NLS motifs. Similarly, MTA3 also localizes in both nuclear and cytoplasmic compartments in keratinocytes (Simpson et al., 2001) and murine epithelial cells (Zhang et al., 2006a), while MTA3 staining in cancer cells is largely diffused (Liu et al., 2014a). The nature of the underlying mechanisms and a full significance of extra-nuclear localization of MTA proteins are not fully understood at the moment and must await further studies.
Basis of functionality of MTA proteins
The expression of MTA1 and MTA2 is believed to be distinct from the expression of MTA3 in cancer cells with some degree of exceptions as all three genes are also upregulated in certain human cancers. Although MTA1 and MTA2 generally exhibit overlapping expression patterns in human cancer, the functions of the two proteins are not always overlapping. The best documented example of overlapping functions of MTA1 and MTA2 is the repression of ER-alpha transactivation functions by corresponding NuRD-complexes (Sen et al., 2014b; Li and Kumar, 2015). Similarly, non-redundant functions of MTA1 and MTA2 could be exemplified by MTA targeting of p53 tumor suppressor leading to two distinct functional outcomes. For example, MTA1 stabilizes p53 by preventing its ubiquitination by Mdm2 and COP1 in cells exposed to ionizing radiation and that MTA1-p53 pathway is a component of the DNA damage response (Li et al., 2009a); while MTA2 deacetylate p53 and inhibits its transactivation functions, inhibiting p53’s growth suppressive activity (Luo et al., 2000).
Functions of MTA proteins are affected by signaling-dependent post-translational modifications (PTM) which, in-turn, will modulate the nature of interactions and the formation of intermediate sub-complexes, before MTA-containing complexes are engaged with target gene chromatin (Li, Kumar, 2015) (Fig. 3A). Most of our current understanding of MTA’s PTM is derived from the work on MTA1 which is subjected to acetylation at lysine 626, ubiquitination at lysine 182 and lysine 626, SUMOylation at lysine 509, and methylation at lysine 532 (Ohshiro et al., 2010; Li et al., 2009b; Cong et al., 2011; Nair et al., 2013). The MTA2 protein is acetylated at lysine 152 by p300 histone acetylase enzyme (Zhou et al., 2014). In addition, functions of MTA proteins are influenced by the ability of MTA 1 and MTA2 to interact with histone H3 and chromatin remodeling components (Nair, et al., 2013; Wu et al., 2013).
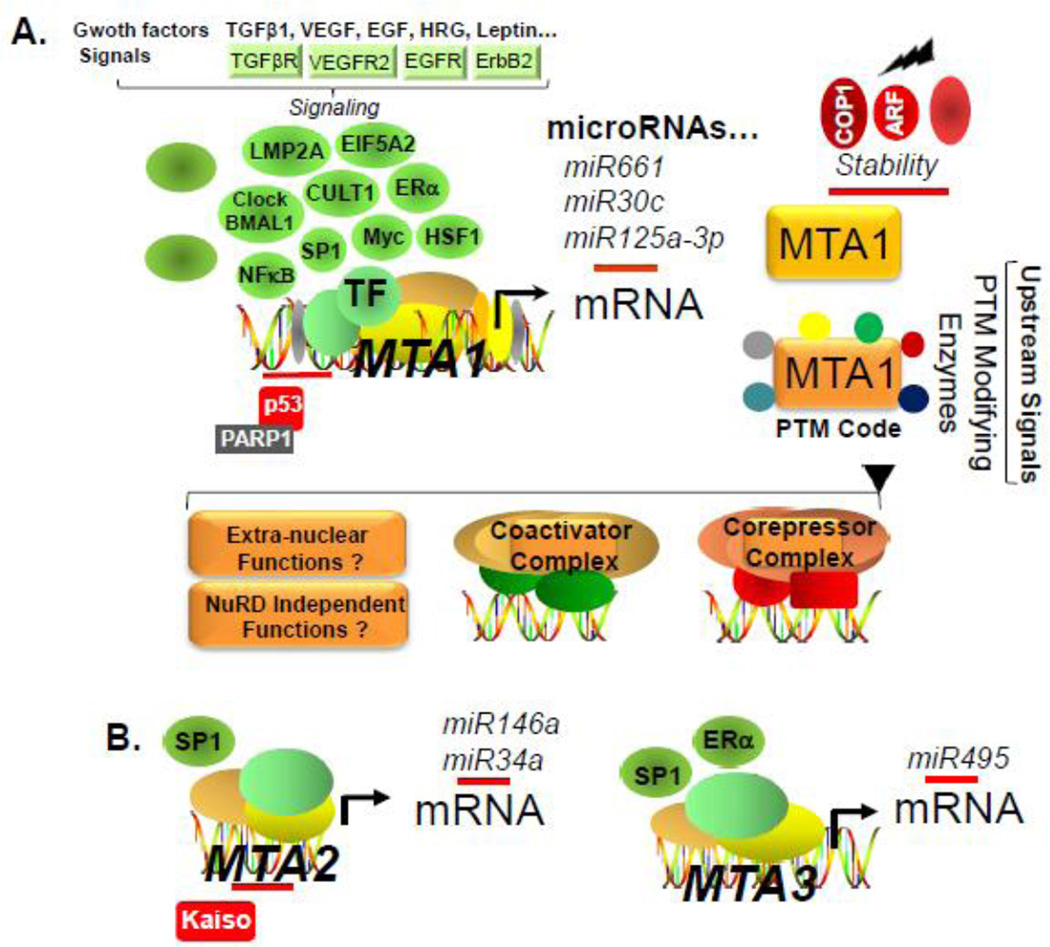
Figure 3.
Regulation of expression and functions of MTA genes and MTA proteins. A, Regulation of transcription of MTA1 gene by growth factors and receptors, oncogenes and transcription factors (round and oval shapes), level of MTA1 mRNA by microRNAs, and stability MTA1 protein by ligases. Post-translational modifications of MTA1 lead to combinatorial assembly of MTA1-containing corepressor or coactivator complexes to repress or stimulate the expression of target genes or other less understood extra-nuclear and NuRD complex independent functions of MTA1. B, Regulation of transcription of MTA2 and MTA3 genes and MTA2/3 mRNAs by microRNAs.
In general, the functions of MTA-containing chromatin-remodeling complexes are governed by the notion that MTA proteins do not co-exists within the same NuRD complex and one family member may not always functionally compensate the other family member (Kumar, 2014; Li and Kumar, 2015). Increased expression of MTA1 and MTA2 during the development of mammary tumors in a multi-stage model of tumor progression (from normal duct to premalignant lesions to hyperplasia to ductal carcinoma to invasive carcinoma) is often accompanied by a gradual decrease in the level of MTA3 (Zhang et al., 2006a). Both MTA1 and MTA2 supports tumor progression and epithelial-to-mesenchymal transition (EMT) (Sen et al., 2014b), while alternatively, MTA3 inhibits EMT (Fujita et al., 2003). These representative observations suggest that the functions of MTA3 in breast cancer are different from MTA1 and MTA2 and that MTA1 and MTA2 could compensate each other in-principle. However, this is not always true and may be target gene chromatin context-dependent (see below). The mechanism of these contrasting functions of MTA1 and MTA2 as compared to that of MTA3, at-least, in breast cancer remained unexplained until Si et al discovered that ZEB2 regulates its target genes by recruiting the MTA1-NuRD complex while GATA3 utilizes the MTA3-NuRD complex to its target genes; Thus, GATA3 and ZEB2 selectively control respective downstream targets (Si et al., 2015). During the course of this study, Si et al also provided a mechanistic basis of generally noticed opposing functions of MTA1 and MTA3 (Si et al., 2015). Another remarkable feature of this study was the observation that the biochemical and cellular activities of MTA1 cannot be compensated by MTA2, highlighting non-redundant functions of MTA1 and MTA2. The existence of non-redundancy among MTA proteins in the chromatin remodeling complexes explains, in-part, the mechanism through which MTA-containing regulatory complexes may manifest their functional specificity through binding with distinct target genes (Fig. 3).
Regulation of expression of MTA genes
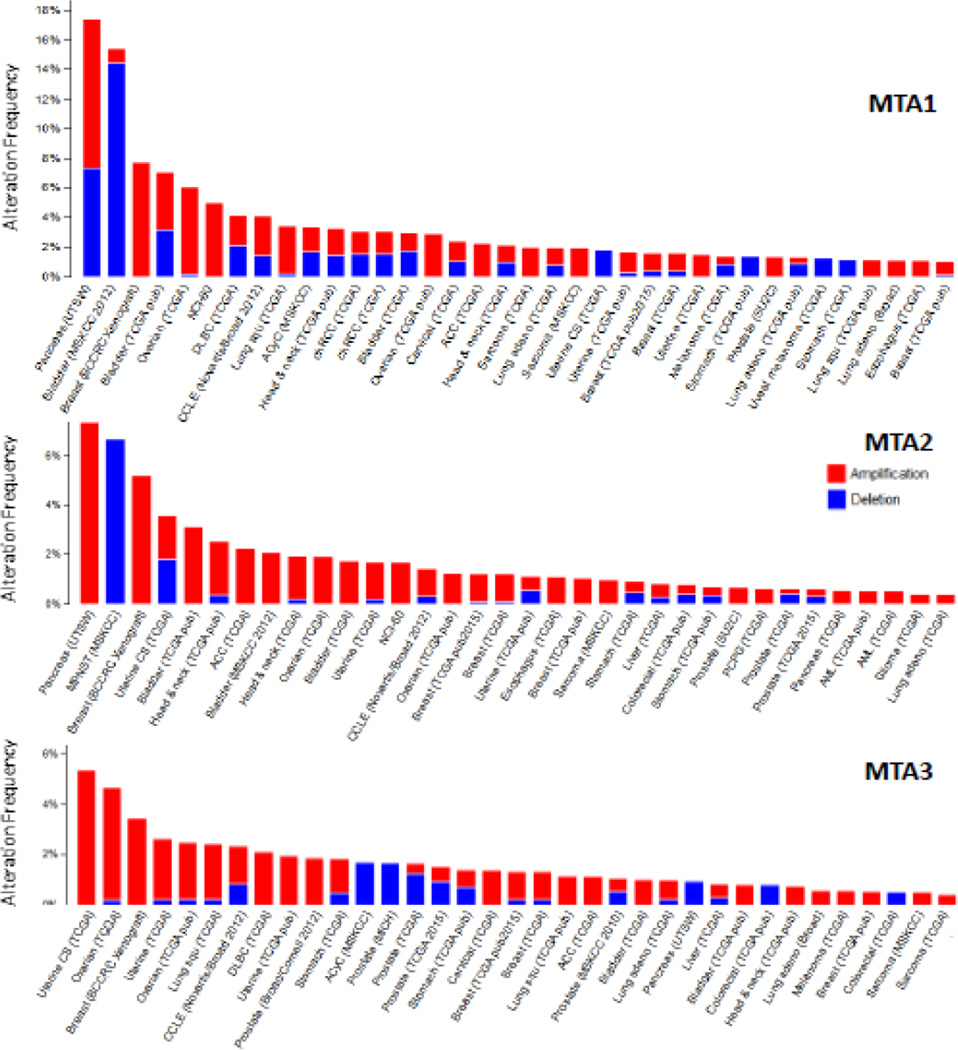
The overexpression of MTA family members in human cancer is generally accompanied by increased mRNA and/or protein and infrequently associated with copy number amplification (Figs. 4, 5). The expression of MTA gene products is regulated through both transcription and post-transcriptional mechanisms in cancer cells. A closer examination of the promoters of MTA genes reveals the presence of multiple predicted binding sites for a number of transcription factors, both shared or selective, among three MTA genes (Table 1). This provides us with clues about the nature of pathways that might potentially modulate the expression of MTA genes. However, available experimental data provides support the regulation of MTA genes by only a few transcriptional factors. For example, although c-Myc binding sites are present in both MTA1 and MTA2 promoters, this has been only investigated for the MTA1 gene: c-Myc stimulates MTA1 transcription and in-turn, MTA1 is an integral part of c-Myc-mediated cellular transformation (Zhang et al., 2005). In addition, the eukaryotic initiation factor 5A2 oncogene also utilizes c-Myc to upregulate the expression of MTA1 in colon cancer cells (Zhu et al., 2012a). The SP1 transcription factor stimulates the transcription of MTA1 (Li et al., 2011) as well as of MTA2 (Xia and Zhang, 2001; Zhou et al., 2013) and MTA3 (Fujita et al, 2004a; Fujita et al., 2004a). The expression of MTA1 is also induced by CUTL1 homeodomain in TGF-p1 stimulated (Pakala et al., 2011), NF- κB transcription factor in stress-exposed tumor cells (Pakala et al., 2010), heat shock factor 1-dependent manner (Khaleque et al., 2008), hypoxia inducible factor 1α in breast cancer cells (Yoo et al., 2006), Clock/BMAL1 binding to E-box in murine Mta1 promoter (Li et al. 2013a). The estrogen and estrogen receptor-α system stimulates MTA1 expression in endometrial cancer cells (Kong et al., 2014) and MTA3 in breast cancer cells (Fujita et al., 2003; Fujita et al., 2004b; Mishra et al., 2004). More recently, Epstein-Barr virus-encoded latent membrane protein 2A oncogene has been shown to induce MTA1 expression during nasopharyngeal carcinoma pathogenesis (Lin et al., 2014).
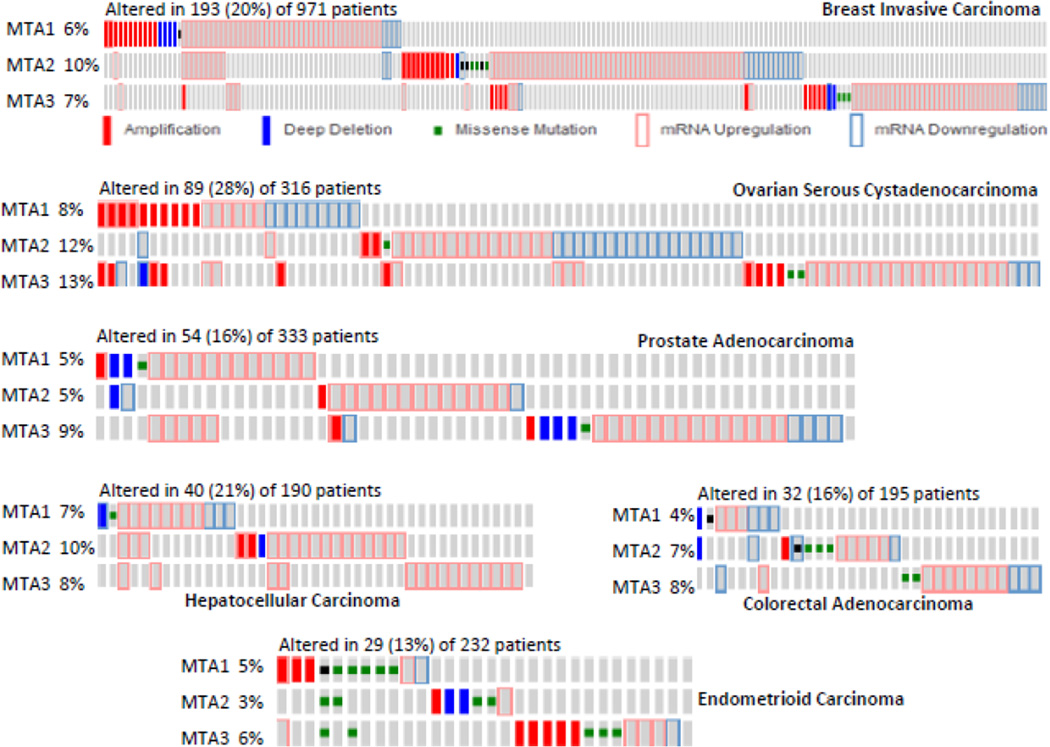
Figure 4.
Genomic changes in the status of MTA1, MTA2 and MTA3 in the same tumors as analyzed using cBioPortal genomic database.
Figure 5.
Status of copy number variation (CNV) in MTA1, MTA2 and MAT3 in human cancer as analyzed using cBioPortal genomic database.
Table 1.
Representative transcription factor binding sites in human MTA gene promoters.
| Genes | Binding Sites (numbers) |
|---|---|
| MTA 1 | P53 (3), YY1 (15), Arnt (1), CUTL1 (1), FOXC1 (1), MZF1 (1), Max (1), MAX1 (1), C-Myc (1), N-Myc (1), Pax5 (1), SREBP1a (1), SREBP1b (1), SREBP1c (1), USF1 (1), XBP1 (1) |
| MTA 2 | NF-κB (2), NF-κB1 (10), CREB (18), δCREB (14), P53 (7), YY1 (9) |
| MTA 3 | SP1(6), ERa(1) |
In addition to positive regulation of MTA transcription, there is also example of negative regulation of MTA genes by transcription factors: p53 inhibits MTA1 transcription in conjunction with p53-poly(ADP-ribose)ylation by poly(ADP-ribose) polymerase 1 (Lee et al., 2012a), while Kaiso transcription factor that binds to methylated CpG locus to represses MTA2 transcription (Yoon et al., 2003).
The expression of the MTA1 gene is also influenced by growth factors and receptors such as VEGF and VEGF receptor 2 (Nagaraj et al., 2015), EGFR in HaCaT keratinocytes (Mahoney et al., 2002), heregulin beta 1 and ErbB2 in breast cancer cells (Mazumdar et al., 2001), and leptin, a fat cell derived growth factor, in breast cancer cells (Yan et al., 2012).
In addition to genomic mechanisms, the levels of MTA proteins are also subject to post-transcriptional regulation by acetylation, ubiquitination, microRNAs, and other pathways. The functionality of MTA1 and MTA2 proteins is profoundly affected by their acetylation status by histone acetylase p300 (Ohshiro et al., 2010, Zhou et al., 2014). The RING-finger ubiquitin-protein ligase COP1 regulates the level of MTA1 protein by controlling its degradation via the ubiquitin-proteasome pathway in the context of DNA damage response (Li et al., 2009b); and MTA1 stability is modulated by its physical association with the tumor suppressor protein ARF (Li et al., 2011). The levels of MTA2 are inhibited by the Rho GDIa in breast cancer cells (Barone et al., 2011) and by human β-defensins in colorectal cancer cells (Uraki et al., 2014).Similarly micro-RNAs such as miR-661, miR-30c and miR-125a-3p target MTA1 expression (Kong et al., 2014, Zhang et al., 2015), while miR-146a and miR-34a targets MTA2 (Li et al., 2010, Kaller et al., 2011). Micro-RNA-495 targets MTA3 in cancer cell lines (Chu et al., 2014) and β-elemene stimulates the expression of MTA3 in breast cancer cells (Zhang et al., 2013).
Mutations in MTA genes
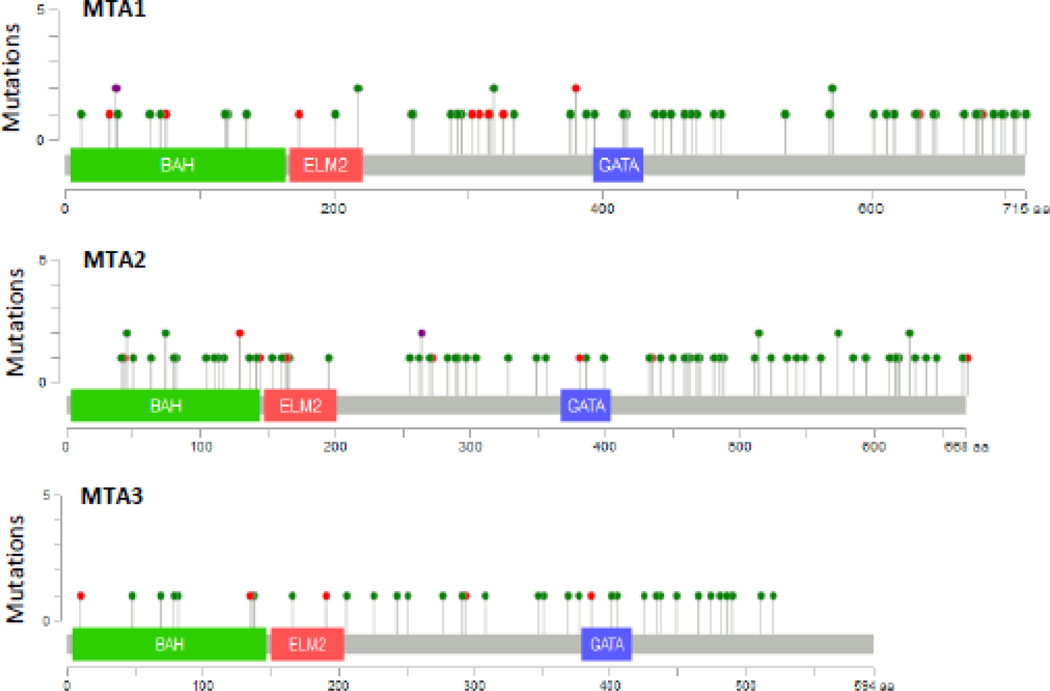
A widespread upregulation of MTA family members in human cancer is now fully confirmed by the available cancer sequencing databases. Among the MTA family, MTA1 and MTA2 are widely overexpressed followed by MTA3 as evident in the cBioPortal database (Cerami et al., 2012; Gao et al., 2013). As illustrated in Fig. 4, upregulation of MTA in human cancer is not always accompanied by an increased copy number and thus, genomic amplification of MTA genes does not constitute the major cause of MTA’s upregulation in human cancer. In fact, upregulation of MTA mRNAs is much more prevalent than increased copy numbers. Further, the spectrum of expression of MTA family members varies across cancer types and within a given cancer-type (Figs. 4, 5). The genomic data also suggests that MTA genes are frequently mutated in human cancer: a total of 98 mutations in MTA1, 114 mutations in MTA2, and 58 mutations in MTA3 across cancer-types (Fig. 6). Interestingly, many of these mutations are also harbored within the BAH, ELM, and GATA domains and could potentially impair the roles of these proteins in chromatin remodeling. Although available sequencing data has shown a large number of mutations in cancer tissues, the significance and experimental validation of these mutations on the functions of MTA gene products remains unclear at this time and waits future research. There are also examples of patient polymorphism in MTA1 and MTA3 genes in the context of estrogen-receptor alpha pathway and the risk of developing breast cancer (Yu et al., 2006). In addition, a single nucleotide polymorphism at position 81 (G81>A) in MTA1 in patients with liver cancer has been shown to be associated with MTA1-overexpressing (Lee et al., 2012b).
Figure 6.
Mutational landscape of MTA1, MTA2 and MTA3 as analyzed using cBioPortal genomic database.
Targets of MTA proteins
The MTA family members exert their cellular effects by regulating the expression of target genes through NuRD-dependent or -independent coregulatory complexes, and/or modifying the functions of its downstream effector molecules. In general, functions of MTA proteins are largely governed by combinatorial post-translational modifications, and distinct protein-protein interactions (Fig. 3). The target specificity of MTA-containing chromatin remodeling complexes is influenced by dynamic interactions with nucleosomes (Nair, Kumar, 2012; Nair et al., 2013).
ERα represents the first direct target of the MTA1-NuRD complex (Mazumdar et al., 2001). Both MTA1 and MTA2 inhibit ERα transactivation activity and promote the development of hormone-independent phenotypes in breast cancer cells (Mazumdar et al., 2001; Cui et al., 2006). Further MTA1 regulation of hormone action is also modulated by MTA1’s binding to coregulatory factors [i.e. MAT1 (Talukder et al., 2003); MICoA (Mishra et al., 2003); NRIF3 (Talukder et al., 2004); and LMO4 (Singh et al., 2005)] and via recruitment of distinct transacting factor complexes to the target genes (Kang et al., 2014). In addition to blocking ER-transactivation, MTA1 also inhibits the transcription of breast cancer type 1 susceptibility gene (Molli et al., 2008), PTEN (Reddy et al., 2012), p21WAF1(Li and Kumar, 2010), guanine nucleotide-binding protein G(i) subunit alpha-2 (Ohshiro et al., 2010), SMAD family member 7 (SMAD7) (Salot and Gude, 2013), nuclear receptor subfamily 4 group A member 1 (Yu et al., 2013), and homeobox protein SIX3 (Manavathi et al., 2007), and represses BCL11B during T-cell leukemia (Cismasiu et al., 2005).
MTA1 and MTA2 also regulate the invasive behavior of breast cancer cells, esophageal squamous cell carcinoma, and non-small cell lung cancer cells via targeting E-cadherin expression (Pakala et al., 2011; Weng et al., 2014; Zhang et al., 2015). Interestingly, MTA3 also targets E-cadherin expression through directly repressing the expression of Snail – EMT master regulator, and accordingly, loss of MTA3 promotes during breast cancer progression contributes to EMT due to Snail upregulation which inhibits the expression of E-cadherin adhesion molecule (Fujita e al., 2003). MTA3-corepressor suppresses Wnt4 expression in mammary epithelial cells (Zhang et al., 2006b) and MTA2 represses IL-4 in T-cells (Lu et al., 2008). Further targets of MTA3-NuRD complex also participate in primitive hematopoietic and angiogenesis in a zebrafish model system (Li et al., 2009c), and MTA3 contributes to BCL6-dependent repression program during differentiation of B-cells (Fujita, N. et al., 2004a; Parekh, S., et al., 2007). The MTA3 also interacts with GATA3 and regulates the expression of GATA3 downstream targets (Si et al., 2015).
In addition to acting as a corepressor, MTA1 also acts as a coactivator due to NuRD-independent functions (Sen et al., 2014b; Li and Kumar, 2015). In fact, genetic depletion of Mta1 in mouse embryonic fibroblasts is accompanied by differential down- and up-regulation of a large number of genes as compared to mouse embryonic fibroblasts with Mta1 (Ghanta et al. 2011b), reinforcing a potential broader role of MTA1 in gene stimulation. Examples of MTA1 stimulation of its targets include TWIST and transcription factor STAT3 (Pakala et al., 2013), breast cancer-amplified sequence 3 (BCAS3) (Gururaj et al., 2006), FosB (Pakala et al., 2011), paired box gene 5 (Pax5) (Balasenthil et al., 2007), proto-oncogene Wnt1 due to derepession of its transcription from Six3 (Kumar et al., 2010), and transglutaminase 2 (TG2) (Ghanta et al., 2011a), myeloid differentiation primary response 88 (Pakala et al., 2010), rhodopsin due to derepression of its transcription from Six3 (Manavathi et al., 2007), tumor suppressorp14/p19ARF (Li et al., 2011), tyrosine hydroxylase (Reddy et al., 2011), clock gene CRY1(Li et al.a, 2013), and SUMO2 (Cong et al., 2011). MTA family member MTA2 supports the growth of gastric cancer cells via upregulating the expression of IL-11 (Zhou et al., 2015). There are also examples wherein the MTA-NuRD complex functions by de-acetylation of its target proteins: MTA1 and MTA2 deacetylate p53 (Moon et al., 2007; Luo et al., 2000); MTA1-mediated deacetylation of HIF1α modulates its transactivation activity (Moon et al., 2006; Yoo et al., 2006), and MTA1 deacetylation of Pten de-acetylation (Dhar et al, 2015). Interestingly, expression of HIF1α and its ability to stimulate transcription from heat-shock responsive element-based reporter system are upregulated by MTA1 and MTA3 in trophoblast cells upon exposure to hypoxic condition (Wang et al., 2013).
Similarly, the MTA2 corepressor complex modulates the circadian rhythm by regulating transcription activity of CLOCK-BMAL1 (Kim et al., 2014). In addition, MTA1 may also influence the status of cellular genes indirect through a microRNA network as depletion of MTA1 in cancer cells alters the levels of miR-210, miR-125b, miR-194, miR-103, and miR-500 (Zhu et al., 2012b; Li et al., 2013c).
Cellular functions of MTA family
The functions of MTA proteins are largely driven by their ability to undergoes post-translational modifications, interacts with histones and non-histone proteins, and modify the expression of target genes (Fig. 3). Due to a broad spectrum of target genes, the MTA family, particularly, MTA1 hub regulates a large number of pathways involved in cellular transformation, motility and invasion, ability to grow in soft-agar, antagonize apoptosis, angiogenesis, DNA damage response, therapeutic resistance including hormone-independence, tumor aggressiveness, stem cell-like properties, epithelial-to-mesenchymal transition, and metastasis (Li and Kumar, 2015). Because expression of MTA1 and MTA2 may be different from that of MTA3, MTA1 and MTA2 have emerged as one of the most upregulated genes in human cancer including breast, gastric, esophageal, endometrial, pancreatic, thymoma, lung, liver, prostate, colon, ovarian, and cervical cancers. In general, overexpression of MTA1 (and also MTA2) correlates well with increased invasion, lymph-node positivity, increased tumor size, aggressive phenotypes, and poor prognosis of cancer patients. However, information about the impact of MTA alterations on the survival rates of cancer patients is only available for a few cancer-types such as hepatocellular carcinomas wherein MTA1 overexpression has been shown to be associated with a poorer survival (reviewed by Toh and Nicholson, 2014). While MTA3 may be either downregulated in cancer such as in breast cancer and endometrioid adenocarcinomas (Brüning, A. et al., 2014) or upregulated in human cancer such as in non-small cell lung cancer (Li et al., 2013b), human placenta and chorionic carcinoma cells (Brüning, A. et al., 2009). The nature of relationship between the status of MTA3 and survival of cancer patients is somewhat not fully examined at the moment. The spectrum of the cellular functions of MTA family members in various human cancer-types has been recently summarized in a thematic volume on the MTA proteins in cancer (Kumar 2014).
Concluding remarks
A large body of work over the last two decades has firmly established that MTA family of proteins are major modifiers of several physiological functions as well as cancer progression and metastasis both in experimental model systems and human tumors. In addition to cancer progression, the MTA family members also regulate several critical physiological pathways in normal cells. In addition, the MTA proteins have emerged as potential biomarkers, prognostic factors, and therapeutic targets for human cancer. In addition to experimental depletion of MTAs in experimental model systems, biology of the MTA1-effector molecules could be also modified by natural compound. In this context, curcumin inhibits the invasiveness of non-small cell lung cancer by impacting the status of MTA1-Wnt/β-catenin axis (Lu et al., 2014); and dietary resveratrol or its synthetic analogue pterostilbene suppresses the levels of MTA1 as well as the growth and metastasis of prostate cancer xenografts (Li et al., 2015). Collectively, an increased understanding of the expression and functions of MTA family has enhanced our appreciation for a critical role of chromatin remodeling factors in gene expression and thus, revealed an additional regulatory layer for cellular processes. Because cellular functions of regulatory hub gene products, such as MTA1, are expected to be regulated by specific modifications or biochemical ability in addition to their levels, Kumar’s laboratory and collaborators are attempting to bring out the roles of trans-regulation of MTA1’s post-translational modifications with a goal to target a specific modification(s) and/or activity of MTA1 as a superior therapeutic approach rather than targeting the net expression. Further, such a therapeutic targeting approach is likely to minimize non-specific effects on the physiological functions of MTA1 in the normal cells. In closing, the MTA family of proteins have attracted a huge amount of attention in the past two decades due to its close association with cancer and roles in chromatin remodeling processes.
Highlights.
Metastatic associated proteins are integrators of upstream signals into gene activity.
MTAs functions are driven by its expression status, localization, and modifications.
Upregulated MTA1 and MTA2 in human cancer associates with tumor aggression.
Acknowledgments
This review and the corresponding Gene Wiki article are written as part of the Gene Wiki Review series--a series resulting from a collaboration between the journal GENE and the Gene Wiki Initiative. The Gene Wiki Initiative is supported by National Institutes of Health (GM089820). Additional support for Gene Wiki Reviews is provided by Elsevier, the publisher of GENE. The authors are sorry for not citing the work of many of our colleagues due to space limitations. The chromatin remodeling and signaling research in Kumar laboratory is supported by grants from the National Institutes of Health grants CA98823 and CA090970, and research in Wang laboratory is supported by the Natural Science Foundation Committee of China grant 30971535. The corresponding Gene Wiki entry for this review can be found here:
Abbreviations
- MTA
metastasis associated proteins
- NuRD
nucleosome remodeling and histone deacetylase
- BAH
Bromo-Adjacent Homology
- ELM2
egl-27 and MTA1 homology
- SANT
SWI, ADA2, N-CoR, TFIIIB-B
- NLS
nuclear localization signal
- PTM
post-translational modifications
- PARP1
poly(ADP-ribose) polymerase 1
- ZnF
zinc finger
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement The authors have no conflicts of interest to declare.
References
- Alqarni SS, et al. Insight into the architecture of the NuRD complex: structure of the RbAp48-MTA1 subcomplex. J Biol Chem. 2014;289:21844–21855. doi: 10.1074/jbc.M114.558940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasenthil S, et al. Identification of Pax5 as a target of MTA1 in B-cell lymphomas. Cancer Res. 2007;67:7132–7138. doi: 10.1158/0008-5472.CAN-07-0750. [DOI] [PubMed] [Google Scholar]
- Barone I, et al. Loss of Rho GDIalpha and resistance to tamoxifen via effects on estrogen receptor alpha. J Natl Cancer Inst. 2011;103:538–552. doi: 10.1093/jnci/djr058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüning A, et al. Function and regulation of MTA1 and MTA3 in malignancies of the female reproductive system. Cancer Metastasis Rev. 2014;33:943–951. doi: 10.1007/s10555-014-9520-6. [DOI] [PubMed] [Google Scholar]
- Brüning A, et al. The metastasis-associated genes MTA1 and MTA3 are abundantly expressed in human placenta and chorionic carcinoma cells. Histochem Cell Biol. 2009;132:33–38. doi: 10.1007/s00418-009-0595-z. [DOI] [PubMed] [Google Scholar]
- Cerami E, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H, et al. MiR-495 regulates proliferation and migration in NSCLC by targeting MTA3. Tumour Biol. 2014;35:3487–3494. doi: 10.1007/s13277-013-1460-1. [DOI] [PubMed] [Google Scholar]
- Cismasiu VB, Adamo K, Gecewicz J, Duque J, Lin Q, Avram D. BCL11B functionally associates with the NuRD complex in T lymphocytes to repress targeted promoter. 2005;24:6753–6764. doi: 10.1038/sj.onc.1208904. [DOI] [PubMed] [Google Scholar]
- Cong L, et al. SUMOylation and SUMO-interacting motif (SIM) of metastasis tumor antigen 1 (MTA1) synergistically regulate its transcriptional repressor function. J Biol Chem. 2011;286:43793–43808. doi: 10.1074/jbc.M111.267237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, et al. Metastasis-associated protein 2 is a repressor of estrogen receptor alpha whose overexpression leads to estrogen-independent growth of human breast cancer cells. Mol Endocrinol. 2006;20:2020–2035. doi: 10.1210/me.2005-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar S, et al. Resveratrol regulates PTEN/Akt pathway through inhibition of MTA1/HDAC unit of the NuRD complex in prostate cancer. Biochimica et biophysica acta. 2015;1853:265–275. doi: 10.1016/j.bbamcr.2014.11.004. [DOI] [PubMed] [Google Scholar]
- Dias SJ, et al. Nuclear MTA1 overexpression is associated with aggressive prostate cancer, recurrence and metastasis in African Americans. Sci Rep. 2013;3:2331. doi: 10.1038/srep02331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z, et al. Human MI-ER1 alpha and beta function as transcriptional repressors by recruitment of histone deacetylase 1 to their conserved ELM2 domain. Mol Cell Biol. 2003;23:250–258. doi: 10.1128/MCB.23.1.250-258.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita N, et al. MTA3, a Mi-2/NuRD complex subunit, regulates an invasive growth pathway in breast cancer. Cell. 2003;113:207–219. doi: 10.1016/s0092-8674(03)00234-4. [DOI] [PubMed] [Google Scholar]
- Fujita N, et al. MTA3 and the Mi-2/NuRD complex regulate cell fate during B lymphocyte differentiation. Cell. 2004a;119:75–86. doi: 10.1016/j.cell.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Fujita N, et al. Hormonal regulation of metastasis-associated protein 3 transcription in breast cancer cells. Mol Endocrinol. 2004b;18:2937–2949. doi: 10.1210/me.2004-0258. [DOI] [PubMed] [Google Scholar]
- Futamura M, et al. Molecular cloning, mapping, and characterization of a novel human gene, MTA1-L1, showing homology to a metastasis-associated gene, MTA1. J Hum Genet. 1999;44:52–56. doi: 10.1007/s100380050107. [DOI] [PubMed] [Google Scholar]
- Gao J, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:l1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanta KS, et al. MTA1 coregulation of transglutaminase 2 expression and function during inflammatory response. J Biol Chem. 2011a;286:7132–7138. doi: 10.1074/jbc.M110.199273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanta KS, et al. Gene profiling of MTA1 identifies novel gene targets and functions. PLoS One. 2011b;6:e17135. doi: 10.1371/journal.pone.0017135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gururaj AE, et al. MTA1, a transcriptional activator of breast cancer amplified sequence 3. Proc Natl Acad Sci U S A. 2006;103:6670–6675. doi: 10.1073/pnas.0601989103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaller M, Liffers ST, Oeljeklaus S, Kuhlmann K, Röh S, Hoffmann R, Warscheid B, Hermeking H. Genome-wide characterization of miR-34a induced changes in protein and mRNA expression by a combined pulsed SILAC and microarray analysis. Mol Cell Proteomics. 2011 Aug;10(8):M111.010462. doi: 10.1074/mcp.M111.010462. PMID: 21566225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HJ, et al. Differential regulation of estrogen receptor alpha expression in breast cancer cells by metastasis-associated protein 1. Cancer Res. 2014;74:1484–1494. doi: 10.1158/0008-5472.CAN-13-2020. [DOI] [PubMed] [Google Scholar]
- Khaleque MA, et al. Heat shock factor 1 represses estrogen-dependent transcription through association with MTA1. Oncogene. 2008;27:1886–1893. doi: 10.1038/sj.onc.1210834. [DOI] [PubMed] [Google Scholar]
- Kim JY, et al. Specificity in circadian clock feedback from targeted reconstitution of the NuRD corepressor. Mol Cell. 2014;56:738–748. doi: 10.1016/j.molcel.2014.10.017. [DOI] [PubMed] [Google Scholar]
- Kleene R, et al. A novel zymogen granule protein (ZG29p) and the nuclear protein MTA1p are differentially expressed by alternative transcription initiation in pancreatic acinar cells of the rat. J Cell Sci. 1999;112(Pt 15):2539–2548. doi: 10.1242/jcs.112.15.2539. [DOI] [PubMed] [Google Scholar]
- Kong X, et al. Estrogen regulates the tumour suppressor MiRNA-30c and its target gene, MTA-1, in endometrial cancer. PLoS One. 2014;9:e90810. doi: 10.1371/journal.pone.0090810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Gururaj A. In: Coregulators as Oncogenes and Tumor Suppressors; Book, Nuclear Receptor Coregulators and Human Diseases. Kumar Rakesh, Bert W O’Malley., editors. Hong Kong: World Scientific Publishers; 2008. pp. 195–218. ISBN: 10-9812705368. [Google Scholar]
- Kumar R, et al. A naturally occurring MTA1 variant sequesters oestrogen receptor-alpha in the cytoplasm. Nature. 2002;418:654–657. doi: 10.1038/nature00889. [DOI] [PubMed] [Google Scholar]
- Kumar R, et al. Metastasis-associated protein 1 and its short form variant stimulates Wnt1 transcription through promoting its derepression from Six3 corepressor. Cancer Res. 2010;70:6649–6658. doi: 10.1158/0008-5472.CAN-10-0909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R. Functions and clinical relevance of MTA proteins in human cancer. Cancer Metastasis Rev. 2014;33(4) doi: 10.1007/s10555-014-9509-1. ISSN: 0167–7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo AJ, et al. The BAH domain of ORC1 links H4K20me2 to DNA replication licensing and Meier-Gorlin syndrome. Nature. 2012;484:115–119. doi: 10.1038/nature10956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MH, et al. Poly(ADP-ribosyl)ation of p53 induces gene-specific transcriptional repression of MTA1. Oncogene. 2012a;31:5099–5107. doi: 10.1038/onc.2012.2. [DOI] [PubMed] [Google Scholar]
- Lee SH, et al. Single nucleotide polymorphisms associated with metastatic tumour antigen 1 overexpression in patients with hepatocellular carcinoma. Liver Int. 2012b;32:457–466. doi: 10.1111/j.1478-3231.2011.02648.x. [DOI] [PubMed] [Google Scholar]
- Li DQ, et al. E3 ubiquitin ligase COP1 regulates the stability and functions of MTA1. Proc Natl Acad Sci U S A. 2009a;106:17493–17498. doi: 10.1073/pnas.0908027106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DQ, et al. MTA1 coregulator regulates p53 stability and function. J Biol Chem. 2009b;284:34545–34552. doi: 10.1074/jbc.M109.056499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, et al. Mta3-NuRD complex is a master regulator for initiation of primitive hematopoiesis in vertebrate embryos. Blood. 2009c;114:5464–5472. doi: 10.1182/blood-2009-06-227777. [DOI] [PubMed] [Google Scholar]
- Li DQ, et al. Bidirectional autoregulatory mechanism of metastasis-associated protein 1-alternative reading frame pathway in oncogenesis. Proc Natl Acad Sci U S A. 2011;108:8791–8796. doi: 10.1073/pnas.1018389108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DQ, et al. Metastasis-associated protein 1/nucleosome remodeling and histone deacetylase complex in cancer. Cancer Res. 2012;72:387–394. doi: 10.1158/0008-5472.CAN-11-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DQ, et al. Metastasis-associated protein 1 is an integral component of the circadian molecular machinery. Nat Commun. 2013a;4:2545. doi: 10.1038/ncomms3545. [DOI] [PubMed] [Google Scholar]
- Li DQ, Kumar R. Mi-2/NuRD complex making inroads into DNA-damage response pathway. Cell Cycle. 2010;9:2071–2079. doi: 10.4161/cc.9.11.11735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DQ, Kumar R. Unravelling the Complexity and Functions of MTA Coregulators in Human Cancer. Adv Cancer Res. 2015;127:1–47. doi: 10.1016/bs.acr.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Li H, et al. Overexpression of MTA3 Correlates with Tumor Progression in Non-Small Cell Lung Cancer. PLoS One. 2013b;8:e66679. doi: 10.1371/journal.pone.0066679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, et al. Pterostilbene acts through metastasis-associated protein 1 to inhibit tumor growth, progression and metastasis in prostate cancer. PloS one. 2013;8:e57542. doi: 10.1371/journal.pone.0057542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, et al. Involvement of metastasis tumor antigen 1 in hepatic regeneration and proliferation. Cell Physiol Biochem. 2008;22:315–326. doi: 10.1159/000149810. [DOI] [PubMed] [Google Scholar]
- Li Y, et al. miR-146a suppresses invasion of pancreatic cancer cells. Cancer Res. 2010;70:1486–1495. doi: 10.1158/0008-5472.CAN-09-2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, et al. MTA1 promotes the invasion and migration of non-small cell lung cancer cells by downregulating miR-125b. J Exp Clin Cancer Res. 2013c;32:33. doi: 10.1186/1756-9966-32-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, et al. Epstein-Barr virus-encoded latent membrane protein 2A promotes the epithelial-mesenchymal transition in nasopharyngeal carcinoma via metastatic tumor antigen 1 and mechanistic target of rapamycin signaling induction. J Virol. 2014;88:11872–11885. doi: 10.1128/JVI.01867-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, et al. Subcellular localization of MTA proteins in normal and cancer cells. Cancer Metastasis Rev. 2014a;33:843–856. doi: 10.1007/s10555-014-9511-7. [DOI] [PubMed] [Google Scholar]
- Liu J, et al. The subcellular distribution and function of MTA1 in cancer differentiation. Oncotarget. 2014b;5:5153–5164. doi: 10.18632/oncotarget.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, et al. MTA1 regulates higher-order chromatin structure and histone H1-chromatin interaction in-vivo. Mol Oncol. 2015;9:218–235. doi: 10.1016/j.molonc.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, et al. Inactivation of NuRD component Mta2 causes abnormal T cell activation and lupus-like autoimmune disease in mice. J Biol Chem. 2008;283:13825–13833. doi: 10.1074/jbc.M801275200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, et al. Deacetylation of p53 modulates its effect on cell growth and apoptosis. Nature. 2000;408:377–381. doi: 10.1038/35042612. [DOI] [PubMed] [Google Scholar]
- Mahoney MG, et al. Metastasis-associated protein (MTA)1 enhances migration, invasion, and anchorage-independent survival of immortalized human keratinocytes. Oncogene. 2002;21:2161–2170. doi: 10.1038/sj.onc.1205277. [DOI] [PubMed] [Google Scholar]
- Manavathi B, et al. Repression of Six3 by a corepressor regulates rhodopsin expression. Proc Natl Acad Sci U S A. 2007;104:13128–13133. doi: 10.1073/pnas.0705878104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumdar A, et al. Transcriptional repression of oestrogen receptor by metastasis-associated protein 1 corepressor. Nat Cell Biol. 2001;3:30–37. doi: 10.1038/35050532. [DOI] [PubMed] [Google Scholar]
- Millard CJ, et al. Towards an understanding of the structure and function of MTA1. Cancer Metastasis Rev. 2014;33:857–867. doi: 10.1007/s10555-014-9513-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, et al. MICoA, a novel metastasis-associated protein 1 (MTA1) interacting protein coactivator, regulates estrogen receptor-alpha transactivation functions. J Biol Chem. 2003;278:19209–19219. doi: 10.1074/jbc.M301968200. [DOI] [PubMed] [Google Scholar]
- Mishra SK, et al. Upstream determinants of estrogen receptor-alpha regulation of metastatic tumor antigen 3 pathway. J Biol Chem. 2004;279:32709–32715. doi: 10.1074/jbc.M402942200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molli PR, et al. MTA1-mediated transcriptional repression of BRCA1 tumor suppressor gene. Oncogene. 2008;27:1971–1980. doi: 10.1038/sj.onc.1210839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon HE, et al. Metastasis-associated protein 1 enhances angiogenesis by stabilization of HIF-1alpha. Oncol Rep. 2006;16:929–935. [PubMed] [Google Scholar]
- Moon HE, et al. Metastasis-associated protein 1 inhibits p53-induced apoptosis. Oncol Rep. 2007;18:1311–1314. [PubMed] [Google Scholar]
- Nagaraj SR, et al. Crosstalk between VEGF and MTA1 signaling pathways contribute to aggressiveness of breast carcinoma. Mol Carcinog. 2015;54:333–350. doi: 10.1002/mc.22104. [DOI] [PubMed] [Google Scholar]
- Nair SS, Kumar R. Chromatin remodeling in cancer: a gateway to regulate gene transcription. Mol Oncol. 2012;6:611–619. doi: 10.1016/j.molonc.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SS, et al. A core chromatin remodeling factor instructs global chromatin signaling through multivalent reading of nucleosome codes. Mol Cell. 2013;49:704–718. doi: 10.1016/j.molcel.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris A, et al. Compensatory interactions between Sir3p and the nucleosomal LRS surface imply their direct interaction. PLoS Genet. 2008;4:e1000301. doi: 10.1371/journal.pgen.1000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshiro K, et al. Acetylation-dependent oncogenic activity of metastasis-associated protein 1 co-regulator. EMBO Rep. 2010;11:691–697. doi: 10.1038/embor.2010.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakala SB, et al. Regulation of NF-kappaB circuitry by a component of the nucleosome remodeling and deacetylase complex controls inflammatory response homeostasis. J Biol Chem. 2010;285:23590–23597. doi: 10.1074/jbc.M110.139469. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Pakala SB, et al. TGF-beta1 signaling targets metastasis-associated protein 1, a new effector in epithelial cells. Oncogene. 2011;30:2230–2241. doi: 10.1038/onc.2010.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakala SB, et al. MTA1 promotes STAT3 transcription and pulmonary metastasis in breast cancer. Cancer Res. 2013;73:3761–3770. doi: 10.1158/0008-5472.CAN-12-3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh S, et al. BCL6 programs lymphoma cells for survival and differentiation through distinct biochemical mechanisms. Blood. 2007;110:2067–2074. doi: 10.1182/blood-2007-01-069575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy SD, et al. MicroRNA-661, a c/EBPalpha target, inhibits metastatic tumor antigen 1 and regulates its functions. Cancer Res. 2009;69:5639–5642. doi: 10.1158/0008-5472.CAN-09-0898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy SD, et al. Metastasis-associated protein 1/histone deacetylase 4-nucleosome remodeling and deacetylase complex regulates phosphatase and tensin homolog gene expression and function. J Biol Chem. 2012;287:27843–27850. doi: 10.1074/jbc.M112.348474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy SD, et al. Multiple coregulatory control of tyrosine hydroxylase gene transcription. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:4200–4205. doi: 10.1073/pnas.1101193108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salot S, Gude R. MTA1-mediated transcriptional repression of SMAD7 in breast cancer cell lines. Eur J Cancer. 2013;49:492–499. doi: 10.1016/j.ejca.2012.06.019. [DOI] [PubMed] [Google Scholar]
- Sen N, et al. Physiological functions of MTA family of proteins. Cancer Metastasis Rev. 2014a;33:869–877. doi: 10.1007/s10555-014-9514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen N, et al. Role of MTA1 in cancer progression and metastasis. Cancer Metastasis Rev. 2014b;33:879–889. doi: 10.1007/s10555-014-9515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma G, et al. Clinical significance of Maspin promoter methylation and loss of its protein expression in invasive ductal breast carcinoma: correlation with VEGF-A and MTA1 expression. Tumour Biol. 2011;32:23–32. doi: 10.1007/s13277-010-0087-8. [DOI] [PubMed] [Google Scholar]
- Si W, et al. Dysfunction of the Reciprocal Feedback Loop between GATA3- and ZEB2-Nucleated Repression Programs Contributes to Breast Cancer Metastasis. Cancer Cell. 2015;27:822–836. doi: 10.1016/j.ccell.2015.04.011. [DOI] [PubMed] [Google Scholar]
- Simpson A, et al. Differential expression and subcellular distribution of the mouse metastasis-associated proteins Mta1 and Mta3. Gene. 2001;273:29–39. doi: 10.1016/s0378-1119(01)00563-7. [DOI] [PubMed] [Google Scholar]
- Singh RR, et al. Negative regulation of estrogen receptor alpha transactivation functions by LIM domain only 4 protein. Cancer Res. 2005;65:10594–10601. doi: 10.1158/0008-5472.CAN-05-2268. [DOI] [PubMed] [Google Scholar]
- Talukder AH, et al. MTA1 interacts with MAT1, a cyclin-dependent kinase-activating kinase complex ring finger factor, and regulates estrogen receptor transactivation functions. J Biol Chem. 2003;278:11676–11685. doi: 10.1074/jbc.M209570200. [DOI] [PubMed] [Google Scholar]
- Talukder AH, et al. Metastasis-associated protein 1 interacts with NRIF3, an estrogen-inducible nuclear receptor coregulator. Mol Cell Biol. 2004;24:6581–6591. doi: 10.1128/MCB.24.15.6581-6591.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh Y, et al. A novel candidate metastasis-associated gene, mta1, differentially expressed in highly metastatic mammary adenocarcinoma cell lines. cDNA cloning, expression, and protein analyses. J Biol Chem. 1994;269:22958–22963. [PubMed] [Google Scholar]
- Toh Y, Nicolson GL. Properties and clinical relevance of MTA1 protein in human cancer. Cancer Metastasis Reviews. 2014;33:891–900. doi: 10.1007/s10555-014-9516-2. [DOI] [PubMed] [Google Scholar]
- Uraki S, et al. Human β-defensin-3 inhibits migration of colon cancer cells via downregulation of metastasis-associated 1 family, member 2 expression. Int J Oncol. 2014;45:1059–1064. doi: 10.3892/ijo.2014.2507. [DOI] [PubMed] [Google Scholar]
- Wang K, et al. MTA1 and MTA3 Regulate HIF1a Expression in Hypoxia-Treated Human Trophoblast Cell Line HTR8/Svneo. Med J Obstet Gynecol. 2013;1(3) pii: 1017. PMID: 25705708. [PMC free article] [PubMed] [Google Scholar]
- Wang L, et al. Atrophin recruits HDAC1/2 and G9a to modify histone H3K9 and to determine cell fates. EMBO Rep. 2008;9:555–562. doi: 10.1038/embor.2008.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng W, et al. Metastasis-associated protein 1 promotes tumor invasion by downregulation of E-cadherin. Int J Oncol. 2014;44:812–818. doi: 10.3892/ijo.2014.2253. [DOI] [PubMed] [Google Scholar]
- Si W, et al. Dysfunction of the Reciprocal Feedback Loop between GATA3- and ZEB2-Nucleated Repression Programs Contributes to Breast Cancer Metastasis. Cancer Cell. 2015;27:822–836. doi: 10.1016/j.ccell.2015.04.011. [DOI] [PubMed] [Google Scholar]
- Wu M, et al. The MTA family proteins as novel histone H3 binding proteins. Cell Biosci. 2013;3(1) doi: 10.1186/2045-3701-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, Zhang Y. Sp1 and ETS family transcription factors regulate the mouse Mta2 gene expression. Gene. 2001;268:77–85. doi: 10.1016/s0378-1119(01)00429-2. [DOI] [PubMed] [Google Scholar]
- Xue Y, et al. NURD, a novel complex with both ATP-dependent chromatin-remodeling and histone deacetylase activities. Mol Cell. 1998;2:851–861. doi: 10.1016/s1097-2765(00)80299-3. [DOI] [PubMed] [Google Scholar]
- Yan D, et al. Leptin-induced epithelial-mesenchymal transition in breast cancer cells requires beta-catenin activation via Akt/GSK3- and MTA1/Wnt1 protein-dependent pathways. J Biol Chem. 2012;287:8598–8612. doi: 10.1074/jbc.M111.322800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, Xu RM. Structure and function of the BAH domain in chromatin biology. Crit Rev Biochem Mol Biol. 2013;48:211–221. doi: 10.3109/10409238.2012.742035. [DOI] [PubMed] [Google Scholar]
- Yoo YG, et al. Metastasis-associated protein 1 enhances stability of hypoxia-inducible factor-1alpha protein by recruiting histone deacetylase 1. EMBO J. 2006;25:1231–1241. doi: 10.1038/sj.emboj.7601025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon HG, et al. N-CoR mediates DNA methylation-dependent repression through a methyl CpG binding protein Kaiso. Mol Cell. 2003;12:723–734. doi: 10.1016/j.molcel.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Yu JC, et al. Breast cancer risk associated with genotypic polymorphism of the genes involved in the estrogen-receptor-signaling pathway: a multigenic study on cancer susceptibility. J Biomed Sci. 2006;13:419–432. doi: 10.1007/s11373-006-9069-7. [DOI] [PubMed] [Google Scholar]
- Yu L, et al. Repression of NR4A1 by a chromatin modifier promotes docetaxel resistance in PC-3 human prostate cancer cells. FEBS Lett. 2013;587:2542–2551. doi: 10.1016/j.febslet.2013.06.029. [DOI] [PubMed] [Google Scholar]
- Zhang B, Zhang H, Shen G. Metastasis-associated protein 2 (MTA2) promotes the metastasis of non-small-cell lung cancer through the inhibition of the cell adhesion molecule Ep-CAM and E-cadherin..Jpn. J Clin. Oncol. 2015;45:755–766. doi: 10.1093/jjco/hyv062. [DOI] [PubMed] [Google Scholar]
- Zhang H, et al. Metastasis tumor antigen family proteins during breast cancer progression and metastasis in a reliable mouse model for human breast cancer. Clin Cancer Res. 2006a;12:1479–1486. doi: 10.1158/1078-0432.CCR-05-1519. [DOI] [PubMed] [Google Scholar]
- Zhang H, et al. Metastatic tumor antigen 3 is a direct corepressor of the Wnt4 pathway. Genes Dev. 2006b;20:2943–2948. doi: 10.1101/gad.1461706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, et al. miR-125a-3p targets MTA1 to suppress NSCLC cell proliferation, migration, and invasion. Acta Biochim Biophys Sin (Shanghai) 2015;47:496–503. doi: 10.1093/abbs/gmv039. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhang Y, Li Y. β-elemene decreases cell invasion by upregulating E-cadherin expression in MCF-7 human breast cancer cells. Oncol Rep. 2013;30:745–750. doi: 10.3892/or.2013.2519. [DOI] [PubMed] [Google Scholar]
- Zhang XY, et al. Metastasis-associated protein 1 (MTA1) is an essential downstream effector of the c-MYC oncoprotein. Proc Natl Acad Sci U S A. 2005;102:13968–13973. doi: 10.1073/pnas.0502330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, et al. Analysis of the NuRD subunits reveals a histone deacetylase core complex and a connection with DNA methylation. Genes Dev. 1999;13:1924–1935. doi: 10.1101/gad.13.15.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, et al. MTA2 promotes gastric cancer cells invasion and is transcriptionally regulated by Sp1. Mol Cancer. 2013;12:102. doi: 10.1186/1476-4598-12-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, et al. MTA2 enhances colony formation and tumor growth of gastric cancer cells through IL-11. BMC Cancer. 2015;15:343. doi: 10.1186/s12885-015-1366-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, et al. P300 binds to and acetylates MTA2 to promote colorectal cancer cells growth. Biochem Biophys Res Commun. 2014;444:387–390. doi: 10.1016/j.bbrc.2014.01.062. [DOI] [PubMed] [Google Scholar]
- Zhu W, et al. Overexpression of EIF5A2 promotes colorectal carcinoma cell aggressiveness by upregulating MTA1 through C-myc to induce epithelial-mesenchymaltransition Gut. 2012a;61:562–575. doi: 10.1136/gutjnl-2011-300207. [DOI] [PubMed] [Google Scholar]
- Zhu X, et al. MTA1 gene silencing inhibits invasion and alters the microRNA expression profile of human lung cancer cells. Oncol Rep. 2012b;28:218–224. doi: 10.3892/or.2012.1770. [DOI] [PubMed] [Google Scholar]