Summary
Background
Recombinant factor VIIa (rFVIIa) has been used widely for treating hemophilia patients with inhibitory autoantibodies against factor VIII or IX. Its mechanism of action is not entirely known. A majority of in vitro studies suggested that pharmacological concentrations of rFVIIa restore hemostasis in hemophilia in a phospholipid-dependent mechanism, independent of tissue factor (TF). However, a few studies suggested that a TF-dependent mechanism plays a primary role in rFVIIa correction of bleeding in hemophilia patients. Here, we investigated the potential contribution of TF in rFVIIa-induced hemostasis in hemophilia employing a model system of FVIII antibody-induced hemophilia in TF transgenic mice.
Methods
Mice expressing low levels of human TF (LTF mice), relatively high levels of human TF (HTF mice) or wild-type mice (WT mice) were administered with neutralizing anti-FVIII antibodies to induce hemophilia in these mice. The mice were then treated with varying concentrations of rFVIIa. rFVIIa-induced hemostasis was evaluated with the saphenous vein bleeding model.
Results
Administration of FVIII inhibitory antibodies induced the hemophilic bleeding phenotype in all three genotypes. rFVIIa administration rescued the bleeding phenotype in all three genotypes. No significant differences were observed in rFVIIa-induced correction in the bleeding of LTF and HTF mice administered with FVIII antibodies.
Conclusions
Our results provide strong evidence supporting that the hemostatic effect of pharmacological doses of rFVIIa stems from a TF-independent mechanism.
Keywords: Factor VIIa, Thromboplastin, Hemostasis, Hemorrhage, Hemophilia A, acquired
Introduction
Recombinant FVIIa is used successfully to treat hemophilia patients with inhibitory antibodies against FVIII or FIX [1,2]. Despite its successful and widespread use, the mechanism of rFVIIa action in treating hemophilia patients with inhibitors is not entirely clear. In early 1990s, before the commercialization of rFVIIa, we provided in vitro data supporting the hypothesis that the hemostatic effectiveness of infusion of high doses of FVIIa in patients with hemophilia stems from FVIIa-catalyzed activation of FX requiring phospholipid, but independent of TF [3]. Later, studies from Monroe and colleagues using cell model systems supported this hypothesis. They postulated that direct activation of FX by FVIIa bound to phospholipids exposed on the activated platelets is responsible for the hemostatic effect of rFVIIa in hemophilia patients, and it is entirely independent of TF [4,5]. However, studies of Mann and colleagues suggested that the therapeutic efficacy of rFVIIa in the treatment of hemophiliacs with inhibitors is dependent on TF, in part, based on overcoming the inhibitory effect of zymogen FVII [6,7]. The most recent studies failed to resolve the above conflicting conclusions. From in vitro data and mathematical modeling, Shibeko et al. concluded that action of rFVIIa at therapeutic doses is dominated by the TF-dependent pathway with a minor contribution from a phospholipid-dependent mechanism [8]. Recently, Feng et al. showed that a chimera of murine FIX (Gla and EGF1 domain) and FVIIa (EGF2 and catalytic domain), which does not bind TF, was as effective as murine FVIIa in controlling bleeding in hemophilia B mice, indicating that the hemostatic effect of pharmacological doses of rFVIIa is TF-independent [9]. In vitro experiments of Augustsson and Persson also suggested that rFVIIa treatment of hemophilia works primarily through a TF-dependent mechanism [10].
In the present study, we tested the role of TF in rFVIIa-induced hemostasis in hemophilia directly using mice expressing low or relatively normal levels of TF by inducing hemophilia in these mice with administration of FVIII inhibitory antibodies. Data of these studies clearly show that pharmacological doses of rFVIIa restored hemostasis in Ab-induced hemophilia in LTF mice as effectively as in HTF mice. These data provide a strong evidence for that the therapeutic effect of high doses of rFVIIa in hemophilia stems from TF-independent mechanism.
Materials and methods
Reagents
Recombinant FVIIa was provided by the late Walter Kisiel, University of New Mexico, Albuquerque, NM. Preparation and characterization of monospecific polyclonal antibodies against human TF was described previously [11]. TF mAb 5G9 hybridoma was kindly provided by James H. Morrissey, University of Illinois, College of Medicine, Urbana, IL, USA. The 5G9 mAb was purified from the ascites using the Affi-Gel Protein A MAPS II Kit from Bio-Rad (Hercules, CA, USA). hFVIII mAb that cross reacts with murine FVIII and inhibits murine FVIII activity (GMA 8015) was obtained from Green Mountain Antibodies (Burlington, VT).
Mice
Breeding pairs for LTF and HTF mice were obtained from Nigel Mackman, University of North Carolina, Chapel Hill, NC, and bred in-house. Generation of these mice and description of their phenotype were given in earlier publications [12,13]. Wild-type mice (C57BL) and FVIII−/− were obtained from Jackson Laboratories, Bar Harbor, ME. The age of mice was ~16 to 24 weeks. The average weight of mice was: LTF mice, 23.2 ± 3.2; HTF, 25 ± 3.6; C57BL 25.3 ± 1.3 gm. Animal experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at Tyler.
Saphenous vein bleeding
We adopted the saphenous vein bleeding model originally described by Buyue et al. [14]. Before the saphenous vein incision, mice were administered with saline, TF mAb, FVIII mAb or TF mAb plus FVIII mAb (1 mg/kg body weight, in 100 μl volume) intravenously via the tail vein. Two hours later, saline or varying doses of rFVIIa (0.25, 1, or 4mg/kg) were given to the mice via the tail vein in 100 μl volume. Five minutes following rFVIIa administration, mice were subjected to the saphenous vein incision. Briefly, mice were anesthetized with ketamine (100 mg/Kg ketamine plus 8.5 mg/Kg xylazine, ~100 μl volume, i.p) and placed in the supine position on a heating mat. Saphenous vein from the ventral hind limb of the right leg of the mouse was exposed by dissecting the skin lengthwise over the saphenous neurovascular bundle. The exposed vein was overlaid with warm saline. Then, at the midway point of the exposed vein, an entry hole in the vein was made by inserting the tip of a 23-G needle into the vein. Blood was adsorbed on a Kim-wipe by gently touching the blood drop away from the puncture site. Immediately following the initial hemostasis, an approximately 1 mm longitudinal distal cut was made using a Student Vannas spring scissors by inserting one blade into the vessel using the needle hole as the entry point. Bleeding was observed for 30 min from this cut. After each hemostasis Incident, the clot was disrupted gently by stroking the clot with a blunted 30-G needle in the direction of the blood flow to reinitiate a new bleeding episode. Duration of each bleeding episode in the 30-min period was noted and the average time to achieve hemostasis (ATH) was calculated by taking mean of these times. Throughout the 30-min experimental time period, blood was adsorbed on Kim-wipes at every 20 sec. Hemoglobin was extracted by soaking the wipes in 20 ml of solution of ABX Lysebio (France) for 2 h or more. Hemoglobin was also extracted from known volumes of freshly collected mouse blood to generate a standard curve for calculating the volume of blood loss. Total blood loss (in the entire 30-min experimental period) was divided by the number of hemostasis events achieved in this period to determine blood loss between each hemostasis. Mice were killed at the end of 30 min bleeding period.
Statistics
Statistical significance of differences between two groups was calculated using nonparametric Mann-Whitney test. ATH and blood loss in LTF and HTF hemophilia mice treated with the same concentration of rFVIIa were compared to evaluate the role of TF in rFVIIa-induced hemostatic effect in hemophilia.
Results and discussion
In preliminary studies, we analyzed a panel of five hFVIII mAb (Green Mountain Antibodies, VT), sheep anti-hFVIII and rabbit anti-hFVIII polyclonal antibodies for their ability to inhibit mFVIII activity in vitro. Among them, we found that GMA 8015 mAb was the most suitable for inhibiting mFVIII activity. It had a titer of ~2500 mouse Bethesda Units/mg and the inhibition reached about 95% at optimal concentrations of the anitbody. Administration of this antibody (1 mg/kg) intravenously to mice inhibited 92 to 95% of mFVIII activity. Initial trial studies with this antibody on prolonging the bleeding time in the saphenous vein bleeding model indicated that administration of the antibody for 120 min prior to the injury induced maximal bleeding.
Administration of FVIII mAb to wild-type mice markedly prolonged the bleeding time and increased blood loss (Fig. 1A and 1B). Administration of 1 or 4 mg/kg of rFVIIa fully restored hemostasis in Ab-induced hemophilia mice. Administration of 0.25 mg/Kg rFVIIa partially corrected the bleeding, but the correction was not statistically significant. Overall, these data indicate that Ab-induced hemophilia model system is a suitable system to assess the role of TF in rFVIIa-induced hemostasis in hemophilia with inhibitors. It may be pertinent to point out here that the Ab-induced bleeding phenotype was not as severe as that observed in FVIII−/− mice. FVIII−/− mice bled more profusely, and no hemostasis was achieved in the 30-min experimental period. Our earlier studies showed that administration of 1 mg/kg of rFVIIa was not sufficient to restore hemostasis in these mice and required 4 mg/kg rFVIIa to fully restore hemostasis [15].
Fig. 1.
Antibody-induced hemophilia in mice and effect of rFVIIa in restoring hemostasis following the saphenous vein incision. Wild-type mice (C57BL) were administered with saline or FVIII mAb (1 mg/kg) intravenously via the tail vein. After 2 h, mice administered with FVIII mAb were treated with varying concentrations of rFVIIa intravenously. Five minutes after rFVIIa administration, mice were subjected to saphenous vein incision. Average time to achieve hemostasis (A) and blood loss (B) was determined as described in methods (n = 5). For comparison, data obtained with hemophilia A (FVIII−/−) was also shown. All p values were calculated using the nonparametric Mann-Whitney test comparing the group receiving rFVIIa with the group receiving saline. In the group receiving saline, one of the mice had an 1800 sec hemostasis time and 4 mice with an average of about 450 seconds. Exclusion of 1800 sec data point from the statistical analysis did not alter the overall outcome of statistical significance analysis between the groups but the significance values are slightly altered (recalculated p values for Fig. 1A: the group receiving saline vs. the group receiving 0.25 mg/kg rFVIIa, 0.19; : the group receiving saline vs. the group receiving 1 mg/kg rFVIIa, 0.015; : the group receiving saline vs. the group receiving 4 mg/kg rFVIIa, 0.015; recalculated p values for Fig. 1B: the group receiving saline vs. the group receiving 0.25 mg/kg rFVIIa, 0.73; the group receiving saline vs. the group receiving 1 mg/kg rFVIIa, 0.015; : the group receiving saline vs. the group receiving 4 mg/kg rFVIIa, 0.015).
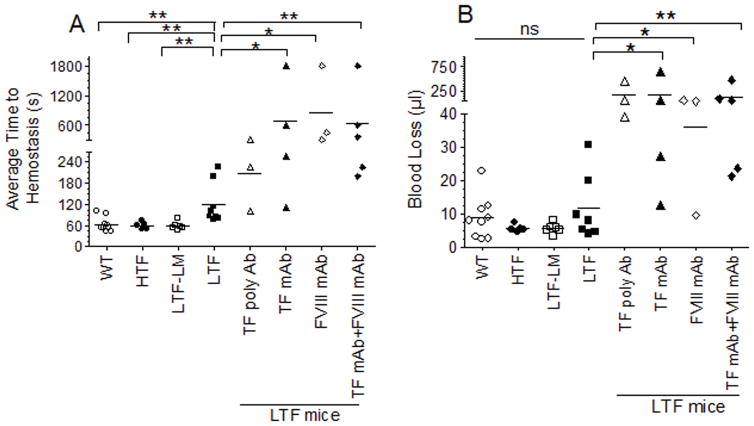
The importance of TF in hemostasis was clearly evident as the average time to achieve hemostasis in LTF mice (121 ± 20.4 s) following the injury was significantly prolonged compared to that observed in the littermate heterozygote controls (58.9 ± 4.9 s), WT (63.3 ± 6.9 s) or HTF (60 ± 4.8 s) mice (mean ± SEM, n = 5 to 10 mice/group). This bleeding time was prolonged further by administration of neutralizing antibodies against hTF, either polyclonal or monoclonal antibodies (Fig. 2A). These data indicate that very low levels of TF present in LTF mice may be sufficient to afford the partial hemostatic effect in these mice, or LTF mice may have more TF than previously reported (~1%) [12]. As expected, administration of FVIII mAb significantly increased the bleeding time in LTF mice. Administration of both TF mAb and FVIII mAb together did not further increase the bleeding time. Although blood loss was also slightly higher in LTF mice compared to the littermate controls and HTF mice, the difference between them was not statistically significant (Fig. 2B). Administration of TF mAb and/or FVIII mAb significantly increased the blood loss in LTF mice (Fig. 2B).
Fig. 2.
Comparison of bleeding times and blood loss following the saphenous vein incision in wild-type, HTF, LTF, LTF heterozygote littermate controls, and LTF mice administered with TF Ab, FVIII mAb or both. Wild-type, HTF, LTF and LTF littermate controls were subjected to saphenous vein incision and an average time to achieve hemostasis (A) and blood loss (B) were measured as described in methods. In additional groups, LTF mice were injected with rabbit polyclonal antibodies against human TF, TF mAb (5G9), FVIII mAb (GMA 8015) or both TF mAb and FVIII mAb (1 mg/kg, each) intravenously 2 h before the saphenous vein incision (n = 3 to 9). *, p < 0.05; **, p <0.01; ns, not statistically significant (p > 0.05).
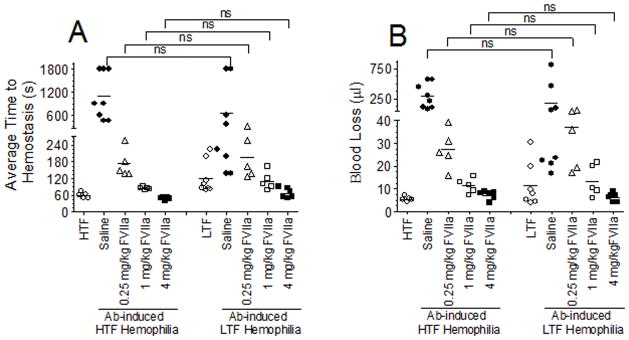
Next, we investigated the effect of rFVIIa administration in correcting the bleeding in FVIII Ab-induced LTF and HTF hemophilia mice (Fig. 3). To inhibit the remaining TF in LTF mice, these mice were also administered with TF mAb along with FVIII mAb. The bleeding phenotype of Ab-induced LTF hemophilia mice was effectively corrected with both 4 and 1 mg/kg rFVIIa administration. Administration of 0.25 mg/Kg rFVIIa restored the hemostasis partially in both the groups. At all doses tested, there was no statistical significant difference on the effectiveness of rFVIIa in restoring hemostasis in LTF and HTF hemophilia mice. The present data provide strong evidence that pharmacological doses of rFVIIa restore hemostasis in hemophilia independent of TF. This conclusion was consistent with the conclusion reached in a recent study using a murine chimeric FIX gla-FVIIa that does not bind to TF in correcting bleeding in hemophilia B mice [9].
Fig. 3.
Factor VIIa restores hemostasis in Ab-induced hemophilia independent of TF. LTF and HTF mice were administered intravenously with FVIII mAb (1 mg/kg) to induce hemophilia. LTF mice were also administered with TF mAb (1 mg/kg) along with FVIII mAb. After 2 h, 0.25, 1 or 4 mg/kg rFVIIa was given to mice intravenously and the bleeding was initiated by saphenous vein incision. Average time to achieve hemostasis (A) and blood loss (B) were determined (n = 5 to 9). ns, not statistically significant difference.
Essentials.
The role of tissue factor (TF) in recombinant factor VIIa (rFVIIa) therapy in hemophilia is unclear.
Acquired mouse hemophilia model having very low or normal levels of human TF was used in the study.
rFVIIa is equally effective in correcting the bleeding in mice expressing low or normal levels of TF.
Pharmacological doses of rFVIIa restore hemostasis in hemophilia independent of TF.
Acknowledgments
This work was supported by National Institutes of Health grants HL107483 (LVMR). The authors thank Nigel Mackman for providing LTF and HTF mice and James H. Morrissey for providing TF monoclonal antibodies.
Footnotes
Addendum
S. Keshava and J. Sundaram performed experiments. A. Rajulapati generated mice and assisted in performing experiments. S. Keshava, U. Pendurthi, and L. V. M. Rao analyzed and interpreted data. L. V. M. Rao supervised the study and wrote the manuscript. All authors approved the manuscript.
Disclosure of Conflicts of Interests
L. V. M. Rao reports grants from NHLBI during the conduct of the study.
Other authors declare no conflict of interest.
References
- 1.Hedner U. Factor VIIa and its potential therapeutic use in bleeding-associated pathologies. Thromb Haemost. 2008;100:557–62. [PubMed] [Google Scholar]
- 2.Abshire T, Kenet G. Recombinant factor VIIa: review of efficacy, dosing regimens and safety in patients with congenital and acquired factor VIII or IX inhibitors. J Thromb Haemost. 2004;2:899–909. doi: 10.1111/j.1538-7836.2004.00759.x. [DOI] [PubMed] [Google Scholar]
- 3.Rao LVM, Rapaport SI. Factor VIIa-catalyzed activation of factor X independent of tissue factor: Its possible significance for control of hemophilic bleeding by infused factor VIIa. Blood. 1990;75:1069–73. [PubMed] [Google Scholar]
- 4.Monroe DM, Hoffman M, Oliver JA, Roberts HR. Platelet activity of high-dose factor VIIa is independent of tissue factor. Br J Haematol. 1997;99:542–7. doi: 10.1046/j.1365-2141.1997.4463256.x. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman M, Monroe DM., III The action of high-dose factor VIIa (FVIIa) in a cell-based model of hemostasis. Semin Hematol. 2001;38:6–9. doi: 10.1016/s0037-1963(01)90140-4. [DOI] [PubMed] [Google Scholar]
- 6.van’t VC, Golden NJ, Mann KG. Inhibition of thrombin generation by the zymogen factor VII: implications for the treatment of hemophilia A by factor VIIa. Blood. 2000;95:1330–5. [PubMed] [Google Scholar]
- 7.Butenas S, Brummel KE, Branda RF, Paradis SG, Mann K. Mechanism of factor VIIa-dependent coagulation in hemophilia blood. Blood. 2002;99:923–30. doi: 10.1182/blood.v99.3.923. [DOI] [PubMed] [Google Scholar]
- 8.Shibeko AM, Woodle SA, Lee TK, Ovanesov MV. Unifying the mechanism of recombinant FVIIa action: dose dependence is regulated differently by tissue factor and phospholipids. Blood. 2012;120:891–9. doi: 10.1182/blood-2011-11-393371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng D, Whinna H, Monroe D, Stafford DW. FVIIa as used pharmacologically is not TF dependent in hemophilia B mice. Blood. 2014;123:1764–6. doi: 10.1182/blood-2013-08-522987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Augustsson C, Persson E. In vitro evidence of a tissue factor-independent mode of action of recombinant factor VIIa in hemophilia. Blood. 2014;124:3172–4. doi: 10.1182/blood-2014-05-576892. [DOI] [PubMed] [Google Scholar]
- 11.Rao LVM. Characterization of anti-tissue factor antibody and its use in immunoaffinity purification of human tissue factor. Thromb Res. 1988;51:373–84. doi: 10.1016/0049-3848(88)90373-8. [DOI] [PubMed] [Google Scholar]
- 12.Parry GC, Erlich JH, Carmeliet P, Luther T, Mackman N. Low levels of tissue factor are compatible with development and hemostasis in mice. J Clin Invest. 1998;101:560–9. doi: 10.1172/JCI814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pawlinski R, Tencati M, Holscher T, Pedersen B, Voet T, Tilley RE, Marynen P, Mackman N. Role of cardiac myocyte tissue factor in heart hemostasis. J Thromb Haemost. 2007;5:1693–700. doi: 10.1111/j.1538-7836.2007.02649.x. [DOI] [PubMed] [Google Scholar]
- 14.Buyue Y, Whinna HC, Sheehan JP. The heparin-binding exosite of factor IXa is a critical regulator of plasma thrombin generation and venous thrombosis. Blood. 2008;112:3234–41. doi: 10.1182/blood-2008-01-136820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sundaram J, Pendurthi UR, Esmon CT, Rao LV. Blockade of endothelial cell protein C receptor augments factor VIIa hemostatic effect in hemophilia treatment. Blood. 2014;124:3031–3. doi: 10.1182/blood-2014-09-600254. [DOI] [PMC free article] [PubMed] [Google Scholar]