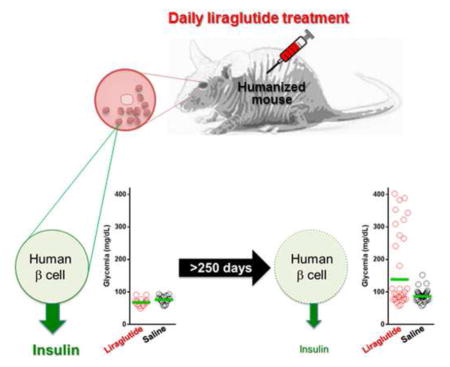
Abstract
Incretin mimetics are frequently used in the treatment of type 2 diabetes because they potentiate beta-cell response to glucose. Clinical evidence showing short-term benefits of such therapeutics (e.g., liraglutide) is abundant, however, there have been several recent reports of unexpected complications in association with incretin mimetic therapy. Importantly, clinical evidence on the potential effects of such agents on the beta cell and islet function during long-term multi-year use remains lacking. We now show that prolonged daily liraglutide treatment of >200 days in humanized mice, transplanted with human pancreatic islets in the anterior chamber of the eye, is associated with compromised release of human insulin and deranged overall glucose homeostasis. These findings raise concern about the chronic potentiation of beta-cell function through incretin mimetic therapy in diabetes.
Graphical Abstract
INTRODUCTION
Incretin mimetics or glucagon-like peptide-1 (GLP-1) analogs are a relatively new family of antidiabetic agents. Several GLP-1 analogs are currently used and more are being developed for the treatment of type 2 diabetes (T2D) (Saulsberry et al., 2015; Tella and Rendell, 2015). GLP-1 is an incretin, a polypeptide secreted by intestinal L-cells in response to food ingestion. Incretins contribute to glucose homeostasis by stimulating insulin secretion from pancreatic beta cells, suppressing prandial glucagon secretion from alpha-cells, reducing gastric emptying and intestinal absorption, and promoting satiety. GLP-1 is also rapidly inactivated by dipeptidyl peptidase-4 (DPP-4). While the GLP-1 analog liraglutide shares 97% homology to human GLP-1, it is however less susceptible to degradation by DPP-4 and, hence, its effects last longer. This has led to the use of GLP-1 analogs, such as liraglutide, as long-acting incretin mimetics to improve glycemic control in T2D patients. Although liraglutide has longer activity compared to GLP-1, its half-life is still limited thus requiring daily injections (Buse et al., 2009; Buse et al., 2010). While extended-release formulations of GLP-1 analogs (e.g., dulaglutide) have been developed to allow once-weekly administration, these and other antidiabetic agents (e.g., sitagliptin and metformin) have demonstrated less impressive reductions in glycated hemoglobin (A1C) and body mass index (BMI) for T2D patients than liraglutide (Chitnis et al., 2014; Dungan et al., 2014; Lee et al., 2014; Trujillo and Nuffer, 2014).
Available clinical data show the short-term benefits of therapy with incretin mimetics (e.g., liraglutide) in diabetes and other conditions typically during the first few years of use (Davies et al., 2015; Inoue et al., 2014; Katout et al., 2014; Mateos and Wajchenberg, 2012). However, pre-clinical and clinical data on the potential impact of such therapeutics on the human beta cell after continuous multi-year use are currently lacking (Consoli and Di Biagio, 2011; Devaraj and Maitra, 2014; Inoue et al., 2014; Wajchenberg, 2007). Notably, reports on undesired side effects and potentially life-threatening complications in association with the use of GLP-1 analogs have been emerging, and concerns about these effects after long-term use of such agents are increasingly being expressed (No authors listed, 2015). Gastrointestinal adverse effects are frequently reported with incretin mimetic therapy, and there have been several reports on increased risk of pancreatitis and pancreatic or neuroendocrine tumors with these therapies (Butler et al., 2013; Chalmer et al., 2014; Consoli and Formoso, 2015; Devaraj and Maitra, 2014).
Although incretin mimetics have been used in the clinic for more than a decade, a conclusive review of their potential effects on the human islet during long-term continuous use has been difficult due to inconsistencies in treatment duration and reporting biases in clinical trials (Butler et al., 2013; Consoli and Formoso, 2015; Devaraj and Maitra, 2014). Additionally, it is neither feasible to exert varied yet well-controlled experimental conditions nor ethical to manipulate treatment conditions in human subjects to investigate this outstanding question in the meantime. Liraglutide’s beneficial effects on pancreatic beta cells were initially demonstrated by pioneering studies using mice (Bock et al., 2003; Larsen et al., 2001; Sturis et al., 2003). Liraglutide was reported to reduce hyperglycemia in T2D mouse models by increasing pancreatic beta cell mass through enhanced proliferation (Rolin et al., 2002). While rodent islets have been and are likely to remain the workhorses of research in islet biology, increasing evidence showing distinct structural and functional features of the rodent and human islets underscores the need for conducting studies with primary human islets (Cabrera et al., 2006; Rodriguez-Diaz et al., 2011). This may also help in mitigating the common inconsistencies between pre-clinical findings and clinical outcomes. We therefore employed a “humanized” mouse model generated by transplanting human islets into the anterior chamber of the eye of diabetic nude mice.
RESULTS AND DISCUSSION
Although direct extrapolation from rodents to humans is not straightforward, the technical and ethical limitations associated with research in human subjects have historically motivated the use of animal models, and mice have been at the forefront of this effort (Budhu et al., 2014; Peters et al., 2007; Rosenthal and Brown, 2007). Importantly, mice engrafted with human tissue, also known as humanized mice, hold great promise in improving our understanding of human diseases (Ito et al., 2012; Rahmig et al., 2015). Mice have also been used to study natural processes such as aging (Demetrius, 2006; Vanhooren and Libert, 2013). Our recent findings have shown that aged mice have pancreatic islets with hallmarks consistent with human islets from aged individuals (Almaca et al., 2014). This further supported the notion that the human islets in our mouse model are likely to be exposed to a systemic aging process during a prolonged liraglutide treatment of ≥250 days as in situ islets in the human pancreas during long-term multi-year use of incretin mimetic therapy (Flurkey et al., 2007; Jucker et al., 1994).
In the current study, we transplanted a marginal mass of human islets to accelerate human beta cell exhaustion and overall changes in glucose homeostasis. We adapted our intraocular islet transplantation model (Abdulreda et al., 2013; Speier et al., 2008a), and transplanted five hundred human islet equivalents into each eye (Caicedo et al., 2009; Rodriguez et al., 2009) (Figs. 1a and 1b). This is significantly less than what is typically transplanted into the kidney subcapsular space of mice to restore and maintain normoglycemia (Figs. S1a and S1b) (Ichii et al., 2005). Therefore, we were able to properly randomize the animals in our studies by matching the liraglutide- and saline-treated recipients to the same human islet preparation, and further circumventing the experimental variability associated with different human islet preparations from different donors. Although mice transplanted with human pancreatic islets under the kidney subcapsular space or other ectopic sites have been used more widely than the anterior chamber of the eye, it has also been shown in our personal experience that diabetic nude mice transplanted with up to 2000 IEQs human islets under the kidney capsule tend to lose function within 2 – 3 months after transplantation) (Ichii et al., 2005). Consequently, our studies requiring extended follow up well beyond 3 months would not have been possible in mice transplanted under the kidney capsule. By contrast, the intraocular islet transplantation model allowed us to gain insight about the potential effects of prolonged use of liraglutide on the human islet function during the ≥250 days of continuous treatment. Moreover, intraocular transplantation allowed longitudinal and noninvasive monitoring of the human islets, as previously described for mouse and monkey islets (Abdulreda and Berggren, 2013; Abdulreda et al., 2011; Perez et al., 2011; Speier et al., 2008a).
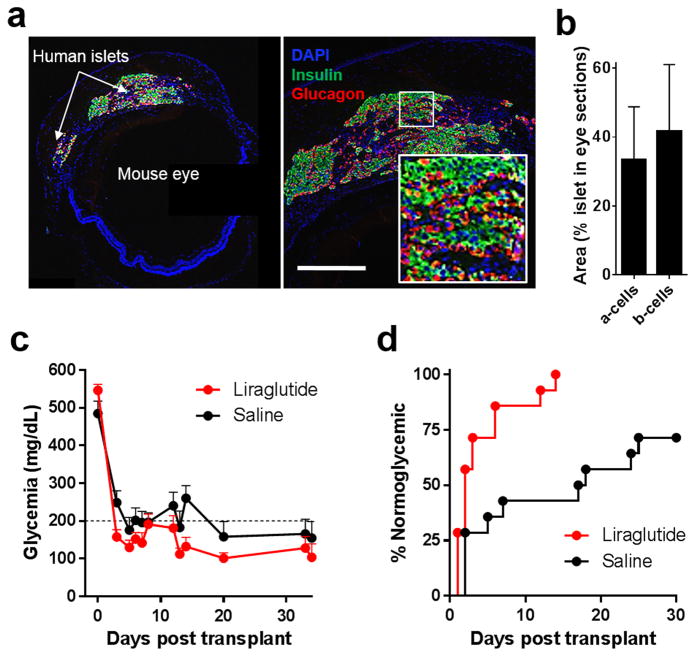
Fig. 1. Humanized mouse model to study pancreatic islet function in vivo.
Human islets transplanted into the anterior chamber of the eye of streptozotocin (STZ)-induced diabetic nude mice engraft on top of the iris and restore normoglycemia. (a) Representative fluorescence micrograph of a cross-section of a recipient nude mouse eye showing (left) the transplanted human islets on top of the iris and (right) a typical distribution of alpha (glucagon; red) and beta (insulin; green) cells (inset) in the intraocular human islet grafts (blue: DAPI nuclear counterstain; scale bar = 200μm). (b) Ratio of alpha and beta cells in immunostained cross-sections of the intraocular human islets. Ratios (shown as means ± SD) were acquired by dividing the total glucagon or insulin-positive area (including nuclei) by the total area of the corresponding islet(s) in immunostained transplanted-eye sections obtained at the conclusion of the studies ≥250 days after transplantation/treatment initiation. Data based on 19 and 29 analyzed eye sections derived from ≥three liraglutide-treated and saline-treated recipients, respectively. (c) Nonfasting glycemia before and ~35 days after human islet intraocular transplantation in diabetic nude mice treated with liraglutide (300 μg/kg/day s.c.) (Merani et al., 2008) or saline (control). Daily liraglutide or saline treatments were started two days prior to transplantation (n = 14 mice/treatment; data shown as means ± SEM). (d) Kaplan-Meyer curves showing % of normoglycemic animals following human islet transplantation in recipients treated with liraglutide or saline (n = 14 mice/treatment). Median diabetes reversal time was 2 days and 17.5 days in liraglutide vs. saline-treated animals, respectively (p<0.05).
In our humanized mouse model, intraocular human islet grafts restored normoglycemia in the recipient diabetic nude mice (Fig. 1). The human islets maintained typical cytoarchitecture and cellular composition ≥250 days post-transplant (Figs. 1a – 1c) (Cabrera et al., 2006). Consistent with previous findings in rodent islets (Toyoda et al., 2008), the results showed improved initial function of the transplanted human islets in liraglutide-treated (300 μg/kg/day s.c.) mice compared to matched saline-treated controls (Figs. 1c and 1d) (Merani et al., 2008). The mice tolerated well the liraglutide treatment and exhibited no adverse systemic side-effects. However, long-term follow up in the same mice for ≥200 days after initiating the liraglutide treatment showed no additional improvement in function of the human islets. Instead, the results showed unexpected progressive deterioration in glycemic control in the mice following long-term continuous treatment with liraglutide (Figs. 2a and 2b; also see Fig. S2). This effect was also observed during high glucose challenges performed throughout the long-term liraglutide treatment (Figs. 2c–2f). Human C-peptide plasma levels were lower, albeit not significantly, in fed mice after 168 days of liraglutide treatment compared to saline (Fig. 2f; p=0.073). Importantly, insulin plasma levels during high glucose challenges performed after ≥175 days of treatment indicated slower kinetics of insulin release from the human islets in the liraglutide-treated recipients (Fig. 2e). Insulin levels peaked at 3 min after glucose bolus in saline-treated mice whereas more gradual increase was observed in those treated with liraglutide.
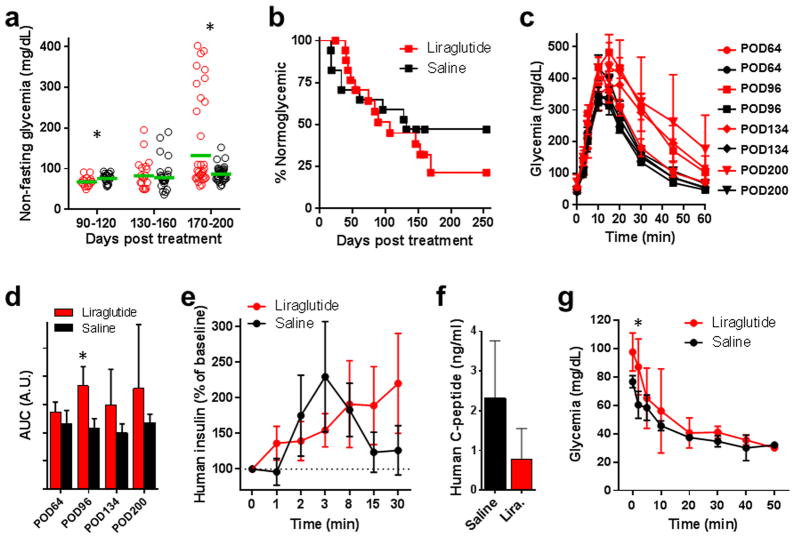
Fig. 2. Effects of long-term daily liraglutide treatment on glucose homeostasis.
Longitudinal in vivo follow up in “humanized” mice revealed compromised glycemic control in association with long-term daily treatment with liraglutide. (a) Nonfasting glycemia in originally diabetic nude mice that were transplanted with human islets and treated daily with liraglutide (300 μg/kg/day s.c.) or saline (n = 6 mice/treatment) (red: liraglutide; black: saline). Either treatment was initiated two days prior to transplantation and continued for ≥250 days. The data were binned for each treatment group for indicated time points on the X axis. Green lines represent the geometric mean (Asterisks indicate significance; p=0.045 for [90–120], p=0.724 for [130 – 160], p=0.00012 for [170 – 200]). (b) Kaplan-Meyer curves showing a comprehensive record of % normoglycemic animals during the extended follow up of ≥250 days after treatment initiation (n = 17 – 18 mice/treatment). Liraglutide-driven improvement in human islet function was initially evident based on the higher number of normoglycemic mice during the first ~80 days after treatment initiation (day 0). However, islet function started to deteriorate in the liraglutide-treated mice at ~150 days of treatment as evidenced by the lower % of normoglycemic mice. The overall median “survival” (i.e., normoglycemia) time did not differ significantly between the liraglutide vs. saline-treated mice (107 vs. 132, receptively; p=0.4861). (c) Blood glucose excursion curves during high glucose challenges (intraperitoneal glucose challenge test) performed 64 ± 2, 96 ± 4, 134 ± 7, and 200 days post-treatment initiation (red: liraglutide; black: saline). The challenges were performed only in mice with normoglycemia at the time of the test. These data showed the progressive deterioration of glycemic control by the human islets in the challenged mice (n = 6 – 7 mice/treatment; data shown as means ± SEM). (d) Area under the curve (AUC; shown as means ± SD) values of the corresponding glucose challenge tests shown in 2c (Asterisks indicate significance; p=0.132 for POD64, p=0.00071 for POD96, p=0.0764 for POD134, p=0.326 for POD200). (e) Plasma levels of human insulin measured in blood samples collected from liraglutide- and saline-treated mice during two glucose challenges performed 176 ± 15 days after treatment initiation (shown as means ± SEM). (f) Plasma levels of human C-peptide measured on POD168 under fed conditions (shown as means ± SD; p=0.073). (g) Blood glucose values (shown as means ± SD) during an insulin tolerance test performed on day 243 post-treatment initiation (asterisks indicate significance; p=0.092 for T0, p=0.0347 for T2, p=0.578 for T5, p=0.391 for T10, p=0.791 for T20, p=0.597 for T30, p=0.675 for T40, p=0.9 for T50).
Additional results from an insulin tolerance test performed >240 days of liraglutide treatment did not show decreased peripheral insulin sensitivity in the liraglutide-treated recipients compared to saline-treated counterparts (Fig. 2g). Both liraglutide and saline-treated mice maintained similar body weights during the extended follow up (Fig. S1b). These results support the notion that long-term treatment with liraglutide associated with compromised hormone release from the human beta cell in our model. It has been reported that secretory dysfunction and beta cell apoptosis in the pancreas may occur under oxidative stress and inflammatory conditions (Kim and Yoon, 2011; Nguyen et al., 2015; Talukdar et al., 2015). Apoptosis may have also been increased by direct toxicity of liraglutide to beta cells because the dose in our studies (300 μg/kg/day) was approximately 7 times that of what is currently recommended in diabetic patients. However, this is unlikely because our immunostaining data showed relatively intact human islets at the conclusion of the studies (Fig. 1b). Therefore, we conclude that, rather than apoptosis or compromised insulin biogenesis, continuous liraglutide treatment for ≥250 days primarily induced deranged insulin release in the human islets in our mouse model.
Although the results did not show increased peripheral insulin resistance after exposure to liraglutide for ≥250 days, we cannot rule out this possibility early in the treatment in our model. Liraglutide-treated mice may have initially experienced decreased peripheral insulin sensitivity due to increased human beta cell output and hyperinsulinemia driven by liraglutide and/or augmented priming effects by the increased combined alpha cell mass in the transplanted islets (Fig. 1b) and in the pancreas (Fig. S1c) (Rodriguez-Diaz et al., 2011). Nonetheless, the current results indicate that the observed derangement in glucose homeostasis in our model cannot be solely explained by changes in peripheral insulin sensitivity or body mass. Future studies are needed to fully characterize the mechanism(s) underlying the potential effects of long-term liraglutide treatment on the human beta cell.
In summary, we show that chronic activation of human islets by prolonged daily liraglutide treatment in a humanized mouse model is associated with initial improvement in function that is followed by progressive deterioration over time. These findings are consistent with the notion that “excessive” activation of an already overworked beta cell under diabetic conditions during long-term treatment with incretin mimetics may lead to metabolic exhaustion of the beta cell (Araki et al., 2003; Larque et al., 2011) and, ultimately, compromised glucose homeostasis. This is conceptually important to consider prior to prescribing long-term multi-year continuous usage of GLP-1 analogs for the treatment of T2D.
MATERIAL AND METHODS
Animals and drugs
Animal procedures were performed under protocols reviewed and approved by the University of Miami IACUC. Athymic nude mice (Nu/Nu; JAX stock # 002019) were purchased from Jackson Laboratory (JAX; Bar Harbor, Maine). Animals were housed in Virus Antibody Free (VAF) rooms and kept in micro-isolated cages with free access to autoclaved food and water. Liraglutide was purchased as synthetic trifluoroacetate salt from Bachem, Switzerland. A 1000x stock solution was prepared by dissolving 18 mg in 1 ml of sterile normal saline solution (0.9% sodium chloride), divided in single-use aliquots, and stored at −20° C.
Diabetes induction
Acute diabetes induction in the mice was achieved via single intravenous or intraperitoneal injection of Streptozotocin (STZ; 150–220 mg/kg). The mice were fasted overnight (~ 17h) before STZ administration in order to measure and compare fasting glycemia before and after development of STZ-induced diabetes. When needed, up to two additional doses of STZ were administered at least three days apart to confirm frank diabetes (three consecutive readings of nonfasting glycemia ≥300 mg/dL).
Human islets and islet transplantation into the eye
Human pancreatic islets were obtained through the City of Hope integrated islet distribution program (IIDP). Islets were incubated at 22° C overnight in serum-free Miami Media supplemented with glutathione (1 mg/100ml) (Bottino et al., 1997). Islets destined for transplantation into liraglutide-treated diabetic recipients were cultured for 48h in Miami Media supplemented with liraglutide (0.1 nM) (Bohman et al., 2007). Recipient treatment with either liraglutide (300 μg/kg/day s.c.) (Merani et al., 2008) or saline was also started two days prior to transplantation. The rationale for pretreatment was to establish baseline drug levels in the recipient mice before transplantation. Islet transplantation into the anterior chamber of the eye of diabetic nude mice was performed as previously described (Abdulreda et al., 2013; Speier et al., 2008a; Speier et al., 2008b). A total of 1000 human islet equivalents (IEQs) (500 IEQs in each eye) were transplanted into confirmed hyperglycemic nude mouse recipients.
Glycemia, body weight monitoring, blood sampling, and enucleation
Animals were weighed 2 − 3 times per week and blood glucose was measured using portable glucometers (OneTouchUltra2; LifeScan, CA). Blood samples (~100 μL) for hormone measurements during glucose challenge were collected from the tail vein into tubes containing K2 EDTA, and immediately supplemented with 5 μL aprotinin (10000 KIU/ml). Given the time constraints of bleeding mice through the tail vein, glycemia was not measured during these challenges which were conducted specifically for the purpose of hormone measurements. Plasma insulin levels were measured with Human insulin ELISA (Mercodia, Uppsala, Sweden; 10-1113-01, normal range). C-peptide levels were measured with Human C-Peptide ELISA (Mercodia, 10-1136-01). Enucleation (i.e., removal of eyes bearing the islet graft) was approved by the University of Miami IACUC and was performed under general anesthesia. The eyes were carefully resected and the orbit was packed with sterile gauze saturated with neomycin ointment to prevent bleeding. Glycemia during the first 20–30 mins was measured while the mouse is still under anesthesia and without thereafter. The mice were euthanized immediately after the last glycemia measurement.
IPGTT and ITT
Intraperitoneal glucose tolerance tests (IPGTTs) were performed after overnight fasting. Mice were injected with 200–300 ml glucose solution (4 g/kg B.W.) and blood glucose (IPGTT) or insulin (ITT) was monitored at predetermined time points after the injection. The higher concentration glucose bolus was used to elicit glucose excursion during challenges with glucose or insulin, as the typical 2 g/kg B.W. bolus did not induce measurable change in glycemia in nude mice transplanted with human islets. In our hands, mice transplanted with human islets typically maintained fed glycemia around ≤100 mg/dl and returned to fasting glycemia levels within 60 min during IPGTTs. Given the tight glucose regulation by the human islet grafts, transplanted mice were not fasted prior to ITT to avoid severe hypoglycemia.
Immunofluorescence staining
Frozen/fixed mouse eyes bearing human islet grafts and recipient mouse pancreata were thawed at 4ºC, transferred into Sakura cassettes and fixation process was repeated with 10% formalin buffered solution for six hours at RT. Dehydration of eye tissue was performed in a VIP3000 after which eyes were embedded into paraffin and sectioned (4 μm) for immunofluorescence staining. Used antibodies included: guinea pig anti-insulin (Dako, diluted 1:35) and rabbit anti-glucagon (Dako, diluted 1:50), rabbit anti-Ki67 (Ventana).
Statistical Analysis
Statistical analysis was performed using Graphpad Prism (La Jolla, CA USA). All data were expressed as means ± SD (unless otherwise stated). Parametric (Paired/Unpaired t- test) and non-parametric tests (Mann Whitney test and Wilcoxon test) were used to perform pairwise comparisons. Multiple comparisons were by ordinary one-way ANOVA followed by either the parametric Holm-Sidak's multiple comparisons test, or the non-parametric Dunn's or Kruskal-Wallis multiple comparisons tests. Comparison of median diabetes reversal or survival times in Kaplan-Meier curves was done using the Log-rank (Mantel-Cox) test. Asterisks denote significance with p<0.05.
Supplementary Material
Acknowledgments
This work was supported by funds from the Diabetes Research Institute Foundation (DRIF), grants from the NIH/NIDDK (F32DK083226/DK097194/R01DK084321); the Swedish Diabetes Association Funds; the Swedish Research Council, Novo Nordisk Foundation, the Family Erling-Persson Foundation, Strategic Research Program in Diabetes at Karolinska Institutet, the ERC-2013-AdG 338936-BetaImage, the Family Knut and Alice Wallenberg Foundation, Skandia Insurance Company Ltd, Diabetes and Wellness Foundation, the Bert von Kantzow Foundation, and the Stichting af Jochnick Foundation. P-O.B. is cofounder and CEO of Biocrine, an unlisted biotech company that is using the anterior chamber of the eye technique as a research tool. M.H.A. is consultant for the same company. We thank pRED at F. Hoffmann - La Roche, Basel, Switzerland for bioanalytic and islet morphology analyses and support of this study. We also thank Dr. April Mann from the University of Miami’s Writing Center for feedback on the manuscript. We acknowledge the organ donors and their families for enabling our research with human pancreatic islets.
Footnotes
AUTHOR CONTRIBUTION
M.H.A. designed and conducted experiments, analyzed and interpreted data, and wrote the manuscript.
R.R-D. designed and conducted experiments, analyzed and interpreted data, and edited the manuscript.
A.C. designed and conducted experiments, interpreted data, and edited the manuscript.
P-O.B initiated the study, designed experiments, interpreted data, and edited the manuscript
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Glucose-lowering treatment of type 2 diabetes. Part II--Glucose-lowering drugs after metformin: a choice based largely on adverse effects. Prescrire Int. 2015;24:130–135. No authors listed. [PubMed] [Google Scholar]
- Abdulreda MH, Berggren PO. Islet inflammation in plain sight. Diabetes Obes Metab. 2013;15(Suppl 3):105–116. doi: 10.1111/dom.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdulreda MH, Caicedo A, Berggren P-O. Transplantation into the Anterior Chamber of the Eye for Longitudinal, Non-invasive In vivo Imaging with Single-cell Resolution in Real-time. J Vis Exp. 2013:e50466. doi: 10.3791/50466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdulreda MH, Faleo G, Molano RD, Lopez-Cabezas M, Molina J, Tan Y, Echeverria OA, Zahr-Akrawi E, Rodriguez-Diaz R, Edlund PK, et al. High-resolution, noninvasive longitudinal live imaging of immune responses. Proc Natl Acad Sci U S A. 2011;108:12863–12868. doi: 10.1073/pnas.1105002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almaca J, Molina J, Arrojo EDR, Abdulreda MH, Jeon WB, Berggren PO, Caicedo A, Nam HG. Young capillary vessels rejuvenate aged pancreatic islets. Proc Natl Acad Sci U S A. 2014 doi: 10.1073/pnas.1414053111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki E, Oyadomari S, Mori M. Impact of endoplasmic reticulum stress pathway on pancreatic beta-cells and diabetes mellitus. Exp Biol Med (Maywood) 2003;228:1213–1217. doi: 10.1177/153537020322801018. [DOI] [PubMed] [Google Scholar]
- Bock T, Pakkenberg B, Buschard K. The endocrine pancreas in non-diabetic rats after short-term and long-term treatment with the long-acting GLP-1 derivative NN2211. APMIS. 2003;111:1117–1124. doi: 10.1111/j.1600-0463.2003.apm1111207.x. [DOI] [PubMed] [Google Scholar]
- Bottino R, Inverardi L, Valente U, Ricordi C. Serum-free medium and pyruvate improve survival and glucose responsiveness of islet beta cells in culture. Transplant Proc. 1997;29:1978. doi: 10.1016/s0041-1345(97)00191-7. [DOI] [PubMed] [Google Scholar]
- Budhu S, Wolchok J, Merghoub T. The importance of animal models in tumor immunity and immunotherapy. Curr Opin Genet Dev. 2014;24:46–51. doi: 10.1016/j.gde.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, Zychma M, Blonde L, Group LS. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6) Lancet. 2009;374:39–47. doi: 10.1016/S0140-6736(09)60659-0. [DOI] [PubMed] [Google Scholar]
- Buse JB, Sesti G, Schmidt WE, Montanya E, Chang CT, Xu Y, Blonde L, Rosenstock J Group, L.E.A.i.D.-S. Switching to once-daily liraglutide from twice-daily exenatide further improves glycemic control in patients with type 2 diabetes using oral agents. Diabetes Care. 2010;33:1300–1303. doi: 10.2337/dc09-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AE, Campbell-Thompson M, Gurlo T, Dawson DW, Atkinson M, Butler PC. Marked expansion of exocrine and endocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon-producing neuroendocrine tumors. Diabetes. 2013;62:2595–2604. doi: 10.2337/db12-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A. 2006;103:2334–2339. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caicedo A, Rodriguez-Diaz R, Molano RD, Ricordi C, Beggren PO, Pileggi A. A Now Experimental Platform To Study Human Islet Cell Biology In Vivo. Diabetes. 2009;58:A57–A57. [Google Scholar]
- Chalmer T, Almdal TP, Vilsbøll T, Knop FK. Adverse drug reactions associated with the use of liraglutide in patients with type 2 diabetes - focus on pancreatitis and pancreas cancer. Expert Opin Drug Saf. 2014:1–10. doi: 10.1517/14740338.2015.975205. [DOI] [PubMed] [Google Scholar]
- Chitnis AS, Ganz ML, Benjamin N, Langer J, Hammer M. Clinical effectiveness of liraglutide across body mass index in patients with type 2 diabetes in the United States: a retrospective cohort study. Adv Ther. 2014;31:986–999. doi: 10.1007/s12325-014-0153-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consoli A, Di Biagio R. Protective effects of glucagon-like peptide-1 on beta-cells: preclinical and clinical data. G Ital Cardiol (Rome) 2011;12:5–9. doi: 10.1714/985.10684. [DOI] [PubMed] [Google Scholar]
- Consoli A, Formoso G. Potential side effects to GLP-1 agonists: understanding their safety and tolerability. Expert Opin Drug Saf. 2015;14:207–218. doi: 10.1517/14740338.2015.987122. [DOI] [PubMed] [Google Scholar]
- Davies MJ, Bergenstal R, Bode B, Kushner RF, Lewin A, Skjoth TV, Andreasen AH, Jensen CB, DeFronzo RA. Efficacy of Liraglutide for Weight Loss Among Patients With Type 2 Diabetes: The SCALE Diabetes Randomized Clinical Trial. Jama. 2015;314:687–699. doi: 10.1001/jama.2015.9676. [DOI] [PubMed] [Google Scholar]
- Demetrius L. Aging in mouse and human systems: a comparative study. Ann N Y Acad Sci. 2006;1067:66–82. doi: 10.1196/annals.1354.010. [DOI] [PubMed] [Google Scholar]
- Devaraj S, Maitra A. Pancreatic safety of newer incretin-based therapies: are the "-tides" finally turning? Diabetes. 2014;63:2219–2221. doi: 10.2337/db14-0545. [DOI] [PubMed] [Google Scholar]
- Dungan KM, Povedano ST, Forst T, González JG, Atisso C, Sealls W, Fahrbach JL. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomised, open-label, phase 3, non-inferiority trial. Lancet. 2014;384:1349–1357. doi: 10.1016/S0140-6736(14)60976-4. [DOI] [PubMed] [Google Scholar]
- Flurkey K, Currer J, Harrison D. The mouse in aging research. Burlington, MA: American College of Laboratory Animal Medicine (Elsevier); 2007. [Google Scholar]
- Ichii H, Inverardi L, Pileggi A, Molano RD, Cabrera O, Caicedo A, Messinger S, Kuroda Y, Berggren PO, Ricordi C. A novel method for the assessment of cellular composition and beta-cell viability in human islet preparations. Am J Transplant. 2005;5:1635–1645. doi: 10.1111/j.1600-6143.2005.00913.x. [DOI] [PubMed] [Google Scholar]
- Inoue K, Maeda N, Fujishima Y, Fukuda S, Nagao H, Yamaoka M, Hirata A, Nishizawa H, Funahashi T, Shimomura I. Long-term impact of liraglutide, a glucagon-like peptide-1 (GLP-1) analogue, on body weight and glycemic control in Japanese type 2 diabetes: an observational study. Diabetol Metab Syndr. 2014;6:95. doi: 10.1186/1758-5996-6-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Takahashi T, Katano I, Ito M. Current advances in humanized mouse models. Cell Mol Immunol. 2012;9:208–214. doi: 10.1038/cmi.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jucker M, Walker LC, Kuo H, Tian M, Ingram DK. Age-related fibrillar deposits in brains of C57BL/6 mice. A review of localization, staining characteristics, and strain specificity. Mol Neurobiol. 1994;9:125–133. doi: 10.1007/BF02816112. [DOI] [PubMed] [Google Scholar]
- Katout M, Zhu H, Rutsky J, Shah P, Brook RD, Zhong J, Rajagopalan S. Effect of GLP-1 mimetics on blood pressure and relationship to weight loss and glycemia lowering: results of a systematic meta-analysis and meta-regression. Am J Hypertens. 2014;27:130–139. doi: 10.1093/ajh/hpt196. [DOI] [PubMed] [Google Scholar]
- Kim JW, Yoon KH. Glucolipotoxicity in Pancreatic beta-Cells. Diabetes Metab J. 2011;35:444–450. doi: 10.4093/dmj.2011.35.5.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larque C, Velasco M, Navarro-Tableros V, Duhne M, Aguirre J, Gutierrez-Reyes G, Moreno J, Robles-Diaz G, Hong E, Hiriart M. Early endocrine and molecular changes in metabolic syndrome models. IUBMB Life. 2011;63:831–839. doi: 10.1002/iub.544. [DOI] [PubMed] [Google Scholar]
- Larsen PJ, Fledelius C, Knudsen LB, Tang-Christensen M. Systemic administration of the long-acting GLP-1 derivative NN2211 induces lasting and reversible weight loss in both normal and obese rats. Diabetes. 2001;50:2530–2539. doi: 10.2337/diabetes.50.11.2530. [DOI] [PubMed] [Google Scholar]
- Lee WC, Dekoven M, Bouchard J, Massoudi M, Langer J. Improved real-world glycaemic outcomes with liraglutide versus other incretin-based therapies in type 2 diabetes. Diabetes Obes Metab. 2014;16:819–826. doi: 10.1111/dom.12285. [DOI] [PubMed] [Google Scholar]
- Mateos JL, Wajchenberg BL. Liraglutide: new results in the treatment of type 2 diabetes mellitus. Drugs Today (Barc) 2012;48(Suppl B):1–17. doi: 10.1358/dot.2012.48(Suppl.B).1876681. [DOI] [PubMed] [Google Scholar]
- Merani S, Truong W, Emamaullee JA, Toso C, Knudsen LB, Shapiro AM. Liraglutide, a long-acting human glucagon-like peptide 1 analog, improves glucose homeostasis in marginal mass islet transplantation in mice. Endocrinology. 2008;149:4322–4328. doi: 10.1210/en.2008-0501. [DOI] [PubMed] [Google Scholar]
- Nguyen TT, Quan X, Hwang KH, Xu S, Das R, Choi SK, Wiederkehr A, Wollheim CB, Cha SK, Park KS. Mitochondrial oxidative stress mediates high-phosphate-induced secretory defects and apoptosis in insulin-secreting cells. Am J Physiol Endocrinol Metab. 2015;308:E933–941. doi: 10.1152/ajpendo.00009.2015. [DOI] [PubMed] [Google Scholar]
- Perez VL, Caicedo A, Berman DM, Arrieta E, Abdulreda MH, Rodriguez-Diaz R, Pileggi A, Hernandez E, Dubovy SR, Parel JM, et al. The anterior chamber of the eye as a clinical transplantation site for the treatment of diabetes: a study in a baboon model of diabetes. Diabetologia. 2011;54:1121–1126. doi: 10.1007/s00125-011-2091-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters LL, Robledo RF, Bult CJ, Churchill GA, Paigen BJ, Svenson KL. The mouse as a model for human biology: a resource guide for complex trait analysis. Nat Rev Genet. 2007;8:58–69. doi: 10.1038/nrg2025. [DOI] [PubMed] [Google Scholar]
- Rahmig S, Bornstein SR, Chavakis T, Jaeckel E, Waskow C. Humanized mouse models for type 1 diabetes including pancreatic islet transplantation. Horm Metab Res. 2015;47:43–47. doi: 10.1055/s-0034-1390446. [DOI] [PubMed] [Google Scholar]
- Rodriguez R, Molano RD, Ricordi C, Berggren PO, Pileggi A, Caicedo A. The Anterior Chamber of the Eye as a Platform To Study Human Islet Cell Biology In Vivo. American Journal of Transplantation. 2009;9:725–725. [Google Scholar]
- Rodriguez-Diaz R, Dando R, Jacques-Silva MC, Fachado A, Molina J, Abdulreda MH, Ricordi C, Roper SD, Berggren PO, Caicedo A. Alpha cells secrete acetylcholine as a non-neuronal paracrine signal priming beta cell function in humans. Nat Med. 2011;17:888–892. doi: 10.1038/nm.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolin B, Larsen MO, Gotfredsen CF, Deacon CF, Carr RD, Wilken M, Knudsen LB. The long-acting GLP-1 derivative NN2211 ameliorates glycemia and increases beta-cell mass in diabetic mice. Am J Physiol Endocrinol Metab. 2002;283:E745–752. doi: 10.1152/ajpendo.00030.2002. [DOI] [PubMed] [Google Scholar]
- Rosenthal N, Brown S. The mouse ascending: perspectives for human-disease models. Nat Cell Biol. 2007;9:993–999. doi: 10.1038/ncb437. [DOI] [PubMed] [Google Scholar]
- Saulsberry WJ, Coleman CI, Mearns ES, Zaccaro E, Doleh Y, Sobieraj DM. Comparative efficacy and safety of antidiabetic drug regimens added to stable and inadequate metformin and thiazolidinedione therapy in type 2 diabetes. Int J Clin Pract. 2015 doi: 10.1111/ijcp.12698. [DOI] [PubMed] [Google Scholar]
- Speier S, Nyqvist D, Cabrera O, Yu J, Molano RD, Pileggi A, Moede T, Köhler M, Wilbertz J, Leibiger B, et al. Noninvasive in vivo imaging of pancreatic islet cell biology. Nat Med. 2008a;14:574–578. doi: 10.1038/nm1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speier S, Nyqvist D, Kohler M, Caicedo A, Leibiger IB, Berggren PO. Noninvasive high-resolution in vivo imaging of cell biology in the anterior chamber of the mouse eye. Nat Protoc. 2008b;3:1278–1286. doi: 10.1038/nprot.2008.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturis J, Gotfredsen CF, Rømer J, Rolin B, Ribel U, Brand CL, Wilken M, Wassermann K, Deacon CF, Carr RD, et al. GLP-1 derivative liraglutide in rats with beta-cell deficiencies: influence of metabolic state on beta-cell mass dynamics. Br J Pharmacol. 2003;140:123–132. doi: 10.1038/sj.bjp.0705397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukdar R, Sasikala M, Kumar PP, Rao GV, Pradeep R, Reddy DN. T-Helper Cell-Mediated Islet Inflammation Contributes to beta-Cell Dysfunction in Chronic Pancreatitis. Pancreas. 2015 doi: 10.1097/MPA.0000000000000479. [DOI] [PubMed] [Google Scholar]
- Tella SH, Rendell MS. Glucagon-like polypeptide agonists in type 2 diabetes mellitus: efficacy and tolerability, a balance. Ther Adv Endocrinol Metab. 2015;6:109–134. doi: 10.1177/2042018815580257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda K, Okitsu T, Yamane S, Uonaga T, Liu X, Harada N, Uemoto S, Seino Y, Inagaki N. GLP-1 receptor signaling protects pancreatic beta cells in intraportal islet transplant by inhibiting apoptosis. Biochem Biophys Res Commun. 2008;367:793–798. doi: 10.1016/j.bbrc.2008.01.046. [DOI] [PubMed] [Google Scholar]
- Trujillo JM, Nuffer W. Albiglutide: a new GLP-1 receptor agonist for the treatment of type 2 diabetes. Ann Pharmacother. 2014;48:1494–1501. doi: 10.1177/1060028014545807. [DOI] [PubMed] [Google Scholar]
- Vanhooren V, Libert C. The mouse as a model organism in aging research: usefulness, pitfalls and possibilities. Ageing Res Rev. 2013;12:8–21. doi: 10.1016/j.arr.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Wajchenberg BL. beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev. 2007;28:187–218. doi: 10.1210/10.1210/er.2006-0038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.