Abstract
The advent of more potent immunosuppressants led to the first successful human upper extremity transplantation in 1998. At this time, > than 100 upper extremity transplants, 30 face transplants and a variety of other vascularized composite allotransplantation (VCA) procedures have been performed around the world. VCA recipients present unique challenges for transplantation. The incidence of acute rejection exceeds 80% in hand and face transplantation and is well documented, whereas reports about antibody-mediated rejection and chronic rejection remain scarce. Immunosuppression protocols commonly used at US centers are derived from solid organ transplantation protocols. Novel approaches to minimize rejections in VCA may include improved HLA matching and considerations towards cytomegalovirus infection status. New graft preservation techniques may decrease immunogenicity prior to transplant. Novel monitoring methods such as valid biomarkers, ultrasound biomicroscopy and sentinel flaps may enable earlier diagnosis of rejection. Cell-based therapies are being explored in order to achieve immunosuppressive regimen minimization or even tolerance induction. The efficacy of local immunosuppression in clinical VCA remains controversial. In conclusion, although immunosuppressive strategies adapted from SOT have demonstrated good mid-term results, focusing on the unique features of VCA grafts may enable additional, more specific treatment strategies in the future and improved long-term graft outcomes.
Keywords: Vascularized Composite Allotransplantation, Composite Tissue Allotransplantation, Acute Rejection, Chronic Rejection, Antibody-Mediated Rejection, Immunosuppression
Introduction
Clinical vascularized composite allotransplantation (VCA) had been attempted as early as 1964. Although technically successful and despite the use of chemical immunosuppressants, the first allograft failed [4] due to irreversible acute rejection (AR), [5]. After all, early clinical results in addition to aggregated experimental experience led investigators to the belief that the skin’s potent immunogenicity would prevent the success of VCAs [6], resulting in a hiatus of three decades without major advances in VCA [7].
In the 1990s, the advent of more potent immunosuppressants rekindled the interest and successful experimental trials in rodents and pre-clinical large animal VCA models were performed [8]. The first successful human (unilateral) upper extremity transplantation was performed in 1998 in France [9]. At this time, > than 100 upper extremity transplants [20] and 30 face transplants [12] have been performed around the world.
Recently, chronic rejections have been reported in face and hand transplant recipients [21]. At the same time, we and others have reported on antibody mediated rejections in face and hand transplant patients [22, 23] supporting the concept that novel immunosuppressive approaches are urgently needed to prevent acute, antibody-mediated and chronic VCA rejection.
Assessment of pre-existing Immunological conditions prior to VCA
Several aspects require consideration during the pre-transplant screening of VCA candidates: Pre-sensitization is common in patients awaiting VCA. The transfusion of blood in addition to skin allografting in extensively burned patients often leads to HLA sensitization prior to transplantation. In a cohort of severely burned patients of which 50% had received skin allografts in addition to an average of > than 35 packed blood cell units (PRBC), the vast majority (28/29 patients) presented with anti-HLA antibodies and 18 out of 29 had been considered highly sensitized (calculated panel reactive antibodies (cPRA) ≥ 85%) [24]. In vitro and animal studies suggest a weaker immune response to glycerol-preserved skin allografts compared to cryopreserved skin allografts [25, 26]. Clinical studies with a larger sample size will need to further elucidate this suggestion. The treatment of highly sensitized VCA patients is currently debated controversially. Novel desensitization approaches including the utilization of the entire medical armamentarium treating humoral immune responses may make the transplantation against positive flow or positive B-cell CDC crossmatches possible. The decision to do so will be largely based on an individualized decision based on titers, patient selection and needs.
Cytomegalovirus (CMV) has been reported to decrease patient and graft survival in SOT [27]. Moreover, CMV increases opportunistic infections, cardiovascular risk, the risk of new-onset diabetes as well as severe acute rejection episodes in SOT [28]. There is only sparse information on the effects of CMV infections in VCA. However, there are reports associating active CMV infections with increased rates of acute rejections in VCA [29, 30]. Standard prophylaxis against CMV infection is recommended based on the donor/recipient serology. While discussed controversially in the community at this time, we feel that high risk constellations do not support an absolute contraindication for VCA transplants.
HLA-matching has not been a primary focus of VCA allocation with a limited pool of donors presenting with compatible skin color, sex and age [31]. A study reviewing 68 VCA rejection episodes suggests a link between the number of acute rejection episodes and the number of HLA mismatches, albeit differences have not been significant [29].
An additional restriction in VCA allocation has been the necessity to maintain brief ischemic times. At our institution, we accept currently a maximum ischemia time of four hours in order to minimize ischemia-reperfusion injury.
Acute Rejections in VCA
The incidence of acute rejection exceeds 80% in hand and face transplantation [32]. At this time, it remains unclear why the incidence of acute rejections in VCA surpasses that of SOT. Contributing aspects may include a potentially less compromised immune system in VCA recipients compared to SOT recipients, VCA specific immune responses and immunogenicity, and an overall limited experience with immunosuppression in a fairly young field [31]. It is assumed that skin remains the major target of alloimmune responses in VCA [33–35]. Basic immunological aspects of skin allograft rejection presume that recipient T-cells are the primary effectors behind epidermal and dermal microvascular target cell injury [36, 37]. A sequential study of acute rejections in five face transplants at our own institution revealed lymphocyte-mediated injuries of microvessels, stem cell-rich epidermal and follicular microcompartments, and associated target cell apoptosis in anagen hair follicles that persist after therapy-induced remission [38]. Of note, donor T cells residing in the facial allograft had been characterized as major constituents of rejection [38].
VCAs have the advantage to allow for visual monitoring, earlier detection and subsequent treatment of acute rejections [35]. Sentinel vascularized composite tissue flaps coined sentinel flaps have been used as secondary monitoring sites for rejection in VCA and have shown to correlate with findings in primary VCAs (i.e. the facial allograft), at least during severe acute rejections. Sentinel flaps, when used in face transplant recipients, may also help to distinguish rejection from dermatological conditions that are not related to rejection [35]. Thus far, all acute T-cell mediated rejections in VCAs have been reversible with the utilization of established rescue protocols [39]. Steroid bolus application has been sufficient to resolve > 80% of acute rejections in face and hand transplant recipients. In cases of steroid-resistant rejections, increasing maintenance immunosuppression, ATG, basiliximab or alemtuzumab treatment, clobetasol and tacrolimus ointments as well as dexamethasone rinses have been successful [32, 40, 41].
Antibody-Mediated Rejection
Allograft rejection can be mediated at a cellular level via cytotoxic cells (mainly T cells) and also at a humoral level through donor specific antibodies (DSA) by B lymphocytes. Reports on antibody-mediated rejection (AMR) in VCA are limited [39]. A rat limb transplant model inducing multiple acute rejection episodes did not show conclusive evidence of antibody-mediated alloresponses [42]. A previous report on two bilateral hand transplant recipients demonstrated focal and diffuse C4d deposition in the absence of DSAs [43]. An additional, more recent case reported on a B-cell driven rejection episode with presence of DSA and C4d positivity in a patient 9 years after forearm transplantation [22]. While those cases had shown some signs of antibody involvement, it remains unclear if rejections were ‘truly’ antibody mediated or if antibodies were bystanders of the rejection process. Our group recently reported on a highly sensitized patient who upon receiving a full face transplant, developed a fulminating AMR with strongly positive capillary staining for C4d (4+/4+) [23] and highly elevated donor specific antibodies (DSA) titers. This has been the first case demonstrating all characteristic signs of AMR including C4d deposition, histomorphological changes and DSA positivity in a face transplant recipient [44, 45].
Chronic allograft deterioration
Chronic allograft deterioration remains to be defined in VCA. The condition is not yet included in the 2007 BANFF classification system for diagnosis of rejection in skin-containing composite tissues [39]. The overall incidence of chronic allograft deterioration in VCA recipients is low, but has to be put in context with the short follow-up periods for most VCA [12, 20]. Of clinical relevance, skin components of VCAs display signs of acute rejection early and therefore allow early diagnosis and treatment that may potentially preempt chronic changes [35]. Furthermore, specific markers may point out possible chronic changes [46] such as myointimal proliferation of arteries and arterioles, loss of adnexa, skin and muscular atrophy, and fibrosis of deep tissues. In addition, late nail lesions have also been described as well [47]. The University of Louisville Hand Transplant Program reported chronic changes in five of six hand transplant patients with three patients demonstrating minimal-to-mild and two patients showing severe intimal hyperplasia [44]. Interestingly, advanced changes occurred within the first nine months after transplantation, whereas the minimal-to-mild changes occurred in patients that had been followed for 2–12 years [44]. In this context, intravascular ultrasound examinations after cardiac allograft transplantation indicated that most coronary artery intimal thickening occurs during the first 12 months [48].
In a more recent VCA study, chronic degradation requiring amputation of a unilateral hand allograft has been reported 13 years after transplantation. The patient had developed four prior acute rejection episodes, at least in part related to non-compliance and refused the treatment of a fifth rejection episode [49]. Comparable changes in a non-human primate face transplants have been reported with all five grafts developing vasculopathy with intimal proliferation and progressive luminal occlusion subsequent to weaning of immunosuppression [50]. The impact of multiple acute rejections accounting for chronic rejection has been characterized in a rodent study as well [51]. Several sub-optimally treated acute rejection episodes led to significant intimal proliferation with luminal occlusion, muscle and skin atrophy as well as upregulation of profibrotic genes. A recent report has been suggestive of chronic rejection in a face transplant recipient and describes progressively sclerotic skin changes with pigmented macules on a background of hypopigmentation and teleangiectasias [21]. Interestingly, only dermal capillaries showed intimal proliferation and luminal narrowing, whereas the large vessels looked. Skin biopsies demonstrated epidermal atrophy with basal cell vacuolization and diffuse dermal sclerosis in the absence of significant dermal cell infiltration. Neither DSA elevation nor vascular C4d deposit had been detected, suggesting an absence of antibody involvement. Of note, all reports suggestive of chronic rejection thus far have involved periods of suboptimal immunosuppression. Overall, the significance and mechanisms of chronic changes in VCA are poorly understood and need further exploration.
Immunosuppression
Induction therapy
Reperfusion after transplantation triggers mechanisms leading to strong activation of the recipient’s immune system targeting donor cells and tissues [52, 53]. Subsequently, donor antigen-presenting cells can drain into the lymphatic tissue and activate the recipient’s adaptive alloimmune response [54, 55]. Antithymocyte globulin (ATG) is currently the most commonly used T cell depleting induction agent in the United States. Other alternative approaches include the use of Alemtuzumab and Basiliximab.
Maintenance therapy
Protocols commonly used at US centers are derived from SOT protocols. The most common regimen in VCA is a triple therapy with tacrolimus, MMF and steroids [32]. In our own experience, we were able to maintain VCA recipients on a dual maintenance immunosuppression regimen subsequent to ATG induction. Long-term outcomes with this approach are pending [40].
Tacrolimus is the most commonly used CNI [59]. For the initial period of 1–5 months after transplantation, protocols aim for trough levels of 10–15 ng/mL and 5–10 ng/mL thereafter [60, 61]. A potential benefit of tacrolimus in VCA is the side effect of enhancing axonal regeneration through targeting of the PI3K/Akt and Ras/ERK signaling cascades [62]. Nephrotoxicity mandates a switch to the mTOR inhibitor sirolimus in some patients [63].
Most hand transplantation centers taper steroids rapidly in the early post-transplant period with a subsequent maintenance of 5 to 15 mg/d for 6 to 12 months in most patients [60]. Initial experiences with 4 face transplant and 1 bilateral hand transplant patients that had early steroid withdrawal between 2 and 12 months post-transplant were recently reported [40]. Tacrolimus trough levels < 5ng/mL appeared to be associated with a higher risk for acute rejection. Although common protocols in VCA have demonstrated effective prevention of graft loss, acute rejections continue to occur more frequently than in SOT.
Rescue therapy
Pulsed steroid therapy and increasing maintenance immunosuppression have successfully treated early and late acute rejections in VCA with slightly better results when compared to SOT (81–90% in VCA vs. 60–75% in SOT [32, 64]. In the case of steroid resistant acute rejections, ATG and Alemtuzumab reversed all reported episodes successfully. Some centers have also used topical treatment with skin ointments and mouth flushes to treat rejection [65]. However, these applications have not yet been proven to provide a benefit over systemic treatment alone.
AMR therapy
Recently, we reported the first VCA in a highly presensitized recipient with positive donor-recipient crossmatch and conclusive evidence of AMR [23]. In addition to the initial induction regimen, the patient received total plasma exchange (TPE) every other day starting on postoperative day (POD) 1 and subsequent 10g intravenous immunoglobulin (IVIG, 150mg/kg) to prevent a rebound antibody secretion. Due to facial erythema and elevated blood DSA levels, TPE had been switched to complement blockade with eculizumab once per week and a second steroid pulse and taper had been necessary. With a persistent erythema, continuously rising DSA levels and emerging positive staining for C4d, 6 more runs of TPE and IVIG were administered over the course of 8 days. Additional doses of eculizumab and bortezomib had become necessary. With resolving symptoms by 1 month, reflected by decreasing DSA levels and C4d deposition, the above regimen was continued an additional two weeks and the patient continues to do well currently on a triple immunosuppression 780 days after transplantation. In an additional case suggestive of AMR in a forearm recipient treatment with rituximab had been successful [22].
Novel approaches
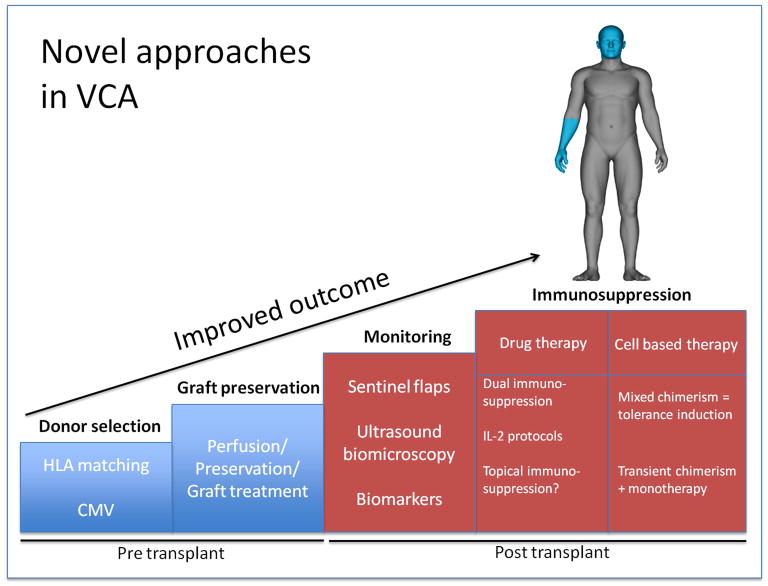
Minimization of graft immunogenicity and prevention of graft injury are critical for improved outcomes in VCA (Fig. 1). Expanding the donor pool may enable improved HLA matching that will be particularly relevant when transplanting sensitized patients.
Figure 1.
Approaches that may improve outcomes and decrease complications after VCA.
Several steps appear of critical importance to increase donation rates in VCA: i) An evolving acceptance of VCA procedures within the general public has already been recognized [66]; ii) Face and hand allografts were recently defined as organs by the United Network for Organ Sharing (UNOS; http://optn.transplant.hrsa.gov/news/board-approves-initial-policies-regarding-limb-and-face-transplantation-new-policies-for-pediatric-heart-allocation/) iii) the development of perfusion devices that preserve isolated solid organs for extended periods of time are currently investigated. Step ii) may not only increase donation rates, but also help facilitate the exchange of organs across regions; while the development of step iii) is supported by promising data emerging from clinical trials for kidney, heart and lung transplantation [67].
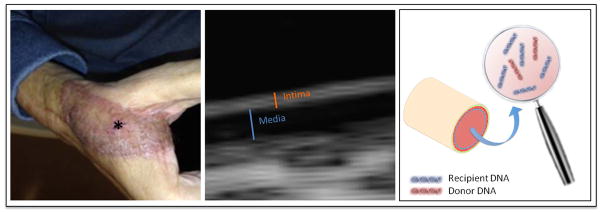
Moreover, the identification of valid biomarkers may not only help the diagnosis of rejections but may also help increase the efficiency of immunosuppressive strategies. For instance, data from SOT demonstrate interesting innovations including the feasibility of non-invasive heart transplant monitoring by measuring circulating cell-free donor DNA in a prospective cohort study [68]. Ultrasound biomicroscopy (UBM) has recently been investigated to assess intima changes subsequent to hand transplants [44] and the approach is currently tested in face transplant recipients [69] (Fig 2).
Figure 2. Enhancing the diagnosis of acute and chronic rejection.
Left: acute Banff Grade III rejection in a face transplant recipient displayed on his sentinel flap (*); middle: vessel wall of a radial artery assessed with a high frequency UBM ultrasound technique; right: free donor DNA measured in VCA recipient’s blood may display acute rejection
Of note, neither AMR nor chronic graft deterioration have been implemented into the BANFF 2007 working classification for skin-containing composite tissue allograft pathology [46]. The recent BANFF 2013 Meeting reported: “Current observations are that chronic VCA rejection is more similar than different from that in other organ transplants.” It was agreed to review and collect data for potential changes to the VCA-Banff system at future meetings [70].
Novel immunosuppressive therapies aiming to minimize immunosuppressive regimens and tolerance protocols are being explored. For example, a cell-based therapy has been tested in 5 hand transplant recipients [71]. In this trial, patients received an induction treatment with alemtuzumab and steroids with a subsequent tacrolimus monotherapy as maintenance immunosuppression; donor bone marrow was transferred on POD 14. Importantly, peripheral blood chimerism had not been observed and low-dose tacrolimus proved to be sufficient as monotherapy, at least short-term with trough levels of 4 to 12 ng/ml. Kidney tolerance protocols have been successfully implemented with both transient and stable chimerism [72]. On an experimental level, durable mixed chimerism following nonmyeloablative conditioning and hematopoetic cell transplantation resulted in tolerance of all VCA components in a miniature pig model of VCA [73]. This protocol is based on donor pretreatment with a 7-day course of IL3 and stem cell factors, as well as apheresis over 3 days in addition to 100cGy total body irradiation 2 days prior to transplantation.
A combination of long-lasting human IL2 fusion protein (hIL-2/Fc) with antilymphocyte serum (ALS) and short-term cyclosporine A (CsA) achieved tolerance in a rat hind limb transplant [74]. ALS was given intraperitoneally (for 5 days, starting on day −4; the regimens of CsA and hIL-2/Fc continued for 3 weeks after transplantation. The authors showed that treatment with hIL-2/Fc increased regulatory T cell proliferation while suppressing effector T cells. Six of 11 limb transplant recipients (55%) achieved long-term allograft survival (>150 days, p<0.05).
The efficacy of local immunosuppression in clinical VCA remains controversial. Topical treatments with tacrolimus and clobetasol ointments have achieved sufficient resolution of low-grade (Banff Grade 1 to 1–2) rejections [65]. Pre-clinical studies of topical tacrolimus and clobetasol demonstrated a benefit for allograft survival in rodent hind limb and hemi-face VCA models [75]. Topical application of tacrolimus increases skin concentrations of the drug substantially, however, not resulting in measurable systemic changes [75]. Side effects were minor when applying topical tacrolimus; steroid application, however, had been linked to skin atrophy in some cases. Site specific release approaches may gain interest. A system that utilizes an enzyme-responsive hydrogel releasing tacrolimus in response to the presence of proteolytic enzymes overexpressed during inflammation has been reported [76]. A one-time local injection after hindlimb transplantation in rats significantly prolonged graft survival in this system.
Moreover, as in SOT, controlling the activation of innate immunity may also be a promising strategy in VCA. Strategies may include anti-ischemic interventions with free radical scavengers, targeting toll-like receptor(s) the transcription factor NF-κB (in consequence down-regulating pro-inflammatory production or release) or adhesion molecules, complement inhibition and targeted small interfering RNA (siRNA) delivery [77]. RNA interference through targeted siRNA delivery represents a novel and potentially powerful approach with the ability to knock down genes by targeting and cleaving complementary mRNA. siRNA carriers such as multifunctional inorganic nanoparticles may encapsulate and escort siRNA into the cytosol. Indeed, VCA is a unique setting for a successful siRNA application not only as an addition to the organ preservation solution but also through direct application onto the recipient’s skin.
Discussion
VCA recipients present unique challenges for transplantation. Currently, centers utilize immunosuppressive protocols derived from the experience in SOT with excellent survival rates thus far. However, acute rejection rates remain high and unique features of VCAs have not been implemented in the design of immunosuppressive protocols. VCA specific aspects appear of relevance and are reflected, for example, by split rejections demonstrating that skin can be rejected while muscle and bone compartments appear intact [33]. Data on topical treatment are not conclusive thus far while the concept of a delivery to the target site appears intriguing.
Sensitization in VCA recipients is expected to gain relevance and more detailed approaches in analyzing mechanisms of rejection in VCA may help to better design immunosuppression.
The clinical significance of chronic graft deterioration and its mechanisms in VCA will require more intense investigations. Meanwhile, sensitive monitoring strategies including UBM may assist in an early detection.
Interesting approaches in achieving tolerance, mainly coming from animal models have been reported. Novel agents such as IL-2/Fc still need to demonstrate efficacy in pre-clinical large animal models.
As centers offering VCA are rapidly expanding, the donor pool may increase providing the potential to implement HLA-matching and a more careful selection of high viral donor/recipient risk constellations. Moreover, novel perfusion and preservation methods may not only improve the quality but also allow the allocation of VCAs across broader geographic distances and an early treatment of recipients. Targeted approaches using siRNA application and nano-particle carriers may be useful future approaches to reduce graft inflammation.
In conclusion, although immunosuppressive strategies adapted from SOT have demonstrated good mid-term results, focusing on the unique features of VCA grafts may enable additional, more specific treatment strategies in the future and improved long-term graft outcomes.
Acknowledgments
We wish to acknowledge the Hand Registry (http://www.handregistry.com/) for providing valuable data about VCA in transplant recipients worldwide.
Furthermore we would like to acknowledge funding support from the U.S. Department of Defense (to B.P., E.M.B., M.K., S.F. and S.G.T. receive partial support from Department of Defense Biomedical Translational Initiative research contract #W911QY-09-C-0216) and the National Institutes of Health (to S.G.T., RO1 AG039449).
Footnotes
Conflict of interest statement: All authors have no conflict of interest.
References
- 1.Harrison JH, Merrill JP, Murray JE. Renal homotransplantation in identical twins. Surg Forum. 1956;6:432–6. [PubMed] [Google Scholar]
- 2.Murray JE, et al. Study on transplantation immunity after total body irradiation: clinical and experimental investigation. Surgery. 1960;48:272–84. [PubMed] [Google Scholar]
- 3.Merrill JP, et al. Successful transplantation of kidney from a human cadaver. JAMA. 1963;185:347–53. doi: 10.1001/jama.1963.03060050025015. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert R. Transplant is successful with a cadaver forearm. Med Trib Med News. 1964;5:20–22. [Google Scholar]
- 5.Gilbert R. Hand transplanted from cadaver is reamputated. Med Trib Med News. 1964;5:23–25. [Google Scholar]
- 6.Murray JE. Organ transplantation (skin, kidney, heart) and the plastic surgeon. Plast Reconstr Surg. 1971;47(5):425–31. doi: 10.1097/00006534-197105000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Tobin GR, et al. The history of human composite tissue allotransplantation. Transplant Proc. 2009;41(2):466–71. doi: 10.1016/j.transproceed.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 8.Diaz-Siso JR, et al. Vascularized composite tissue allotransplantation--state of the art. Clin Transplant. 2013;27(3):330–7. doi: 10.1111/ctr.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubernard JM, et al. Human hand allograft: report on first 6 months. Lancet. 1999;353(9161):1315–20. doi: 10.1016/S0140-6736(99)02062-0. [DOI] [PubMed] [Google Scholar]
- 10.Hofmann GO, et al. Vascularized knee joint transplantation in man: a report on the first cases. Transpl Int. 1998;11(Suppl 1):S487–90. doi: 10.1007/s001470050525. [DOI] [PubMed] [Google Scholar]
- 11.Monaco AP. Transplantation of the larynx--a case report that speaks for itself. N Engl J Med. 2001;344(22):1712–4. doi: 10.1056/NEJM200105313442211. [DOI] [PubMed] [Google Scholar]
- 12.Fischer S, et al. Functional outcomes of face transplantation. Am J Transplant. 2015;15(1):220–33. doi: 10.1111/ajt.12956. [DOI] [PubMed] [Google Scholar]
- 13.Pomahac B, et al. Three patients with full facial transplantation. N Engl J Med. 2012;366(8):715–22. doi: 10.1056/NEJMoa1111432. [DOI] [PubMed] [Google Scholar]
- 14.Selvaggi G, et al. Abdominal wall transplantation: surgical and immunologic aspects. Transplant Proc. 2009;41(2):521–2. doi: 10.1016/j.transproceed.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 15.Sun Q, Zheng J, Zhao C. Nerve transplantation and accompanying peripheral vessels for repair of long nerve defect. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2012;26(7):832–6. [PubMed] [Google Scholar]
- 16.Birchall M. Tongue transplantation. Lancet. 2004;363(9422):1663. doi: 10.1016/S0140-6736(04)16287-9. [DOI] [PubMed] [Google Scholar]
- 17.Jones TR, Humphrey PA, Brennan DC. Transplantation of vascularized allogeneic skeletal muscle for scalp reconstruction in renal transplant patient. Transplant Proc. 1998;30(6):2746–53. doi: 10.1016/s0041-1345(98)00802-1. [DOI] [PubMed] [Google Scholar]
- 18.Johannesson L, et al. Uterus transplantation trial: 1-year outcome. Fertil Steril. 2015;103(1):199–204. doi: 10.1016/j.fertnstert.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 19.Zhang LC, Zhao YB, Hu WL. Ethical issues in penile transplantation. Asian J Androl. 2010;12(6):795–800. doi: 10.1038/aja.2010.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shores JT, Brandacher G, Lee WA. Hand and Upper Extremity Transplantation: An Update of Outcomes in the Worldwide Experience. Plast Reconstr Surg. 2014 doi: 10.1097/PRS.0000000000000892. [DOI] [PubMed] [Google Scholar]
- 21.Petruzzo P, et al. Clinicopathological Findings of Chronic Rejection in a Face Grafted Patient. Transplantation. 2015 doi: 10.1097/TP.0000000000000765. [DOI] [PubMed] [Google Scholar]
- 22.Weissenbacher A, et al. Antibody-mediated rejection in hand transplantation. Transpl Int. 2014;27(2):e13–7. doi: 10.1111/tri.12233. [DOI] [PubMed] [Google Scholar]
- 23.Chandraker A, et al. The management of antibody-mediated rejection in the first presensitized recipient of a full-face allotransplant. Am J Transplant. 2014;14(6):1446–52. doi: 10.1111/ajt.12715. [DOI] [PubMed] [Google Scholar]
- 24.Duhamel P, et al. Anti-HLA sensitization in extensively burned patients: extent, associated factors, and reduction in potential access to vascularized composite allotransplantation. Transpl Int. 2015 doi: 10.1111/tri.12540. [DOI] [PubMed] [Google Scholar]
- 25.Cinamon U, et al. A simplified testing system to evaluate performance after transplantation of human skin preserved in glycerol or in liquid nitrogen. J Burn Care Rehabil. 1993;14(4):435–9. doi: 10.1097/00004630-199307000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Richters CD, et al. Immunogenicity of glycerol-preserved human cadaver skin in vitro. J Burn Care Rehabil. 1997;18(3):228–33. doi: 10.1097/00004630-199705000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Kotton CN. CMV: Prevention, Diagnosis and Therapy. Am J Transplant. 2013;13(Suppl 3):24–40. doi: 10.1111/ajt.12006. quiz 40. [DOI] [PubMed] [Google Scholar]
- 28.Couzi L, et al. Direct and Indirect Effects of Cytomegalovirus-Induced gammadelta T Cells after Kidney Transplantation. Front Immunol. 2015;6:3. doi: 10.3389/fimmu.2015.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonastre J, et al. Factors influencing acute rejection of human hand allografts: a systematic review. Ann Plast Surg. 2012;68(6):624–9. doi: 10.1097/SAP.0b013e318255a411. [DOI] [PubMed] [Google Scholar]
- 30.Schneeberger S, et al. Cytomegalovirus-related complications in human hand transplantation. Transplantation. 2005;80(4):441–7. doi: 10.1097/01.tp.0000168454.68139.0a. [DOI] [PubMed] [Google Scholar]
- 31.Weissenbacher A, et al. Vascularized composite allografts and solid organ transplants: similarities and differences. Curr Opin Organ Transplant. 2013;18(6):640–4. doi: 10.1097/MOT.0000000000000019. [DOI] [PubMed] [Google Scholar]
- 32.Fischer S, et al. Acute rejection in vascularized composite allotransplantation. Curr Opin Organ Transplant. 2014;19(6):531–44. doi: 10.1097/MOT.0000000000000140. [DOI] [PubMed] [Google Scholar]
- 33.Sinha I, Pomahac B. Split rejection in vascularized composite allotransplantation. Eplasty. 2013;13:e53. [PMC free article] [PubMed] [Google Scholar]
- 34.Mathes DW, et al. Split tolerance to a composite tissue allograft in a swine model. Transplantation. 2003;75(1):25–31. doi: 10.1097/00007890-200301150-00005. [DOI] [PubMed] [Google Scholar]
- 35.Kueckelhaus M, et al. Utility of sentinel flaps in assessing facial allograft rejection. Plast Reconstr Surg. 2015;135(1):250–8. doi: 10.1097/PRS.0000000000000797. [DOI] [PubMed] [Google Scholar]
- 36.Dvorak HF, et al. Rejection of first-set skin allografts in man. the microvasculature is the critical target of the immune response. J Exp Med. 1979;150(2):322–37. doi: 10.1084/jem.150.2.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhan AK, Mihm MC, Jr, Dvorak HF. T cell subsets in allograft rejection. In situ characterization of T cell subsets in human skin allografts by the use of monoclonal antibodies. J Immunol. 1982;129(4):1578–83. [PubMed] [Google Scholar]
- 38.Lian CG, et al. Biomarker evaluation of face transplant rejection: association of donor T cells with target cell injury. Mod Pathol. 2014;27(6):788–99. doi: 10.1038/modpathol.2013.249. [DOI] [PubMed] [Google Scholar]
- 39.Petruzzo P, Dubernard JM. The International Registry on Hand and Composite Tissue allotransplantation. Clin Transpl. 2011:247–53. [PubMed] [Google Scholar]
- 40.Diaz-Siso JR, et al. Initial Experience of Dual Maintenance Immunosuppression With Steroid Withdrawal in Vascular Composite Tissue Allotransplantation. Am J Transplant. 2015 doi: 10.1111/ajt.13103. [DOI] [PubMed] [Google Scholar]
- 41.Schneeberger S, et al. First forearm transplantation: outcome at 3 years. Am J Transplant. 2007;7(7):1753–62. doi: 10.1111/j.1600-6143.2007.01837.x. [DOI] [PubMed] [Google Scholar]
- 42.Unadkat JV, et al. Investigation of antibody-mediated rejection in composite tissue allotransplantation in a rat limb transplant model. Transplant Proc. 2009;41(2):542–5. doi: 10.1016/j.transproceed.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 43.Landin L, et al. CD3+-mediated rejection and C4d deposition in two composite tissue (bilateral hand) allograft recipients after induction with alemtuzumab. Transplantation. 2009;87(5):776–81. doi: 10.1097/TP.0b013e318198dbc7. [DOI] [PubMed] [Google Scholar]
- 44.Kaufman CL, et al. Graft vasculopathy in clinical hand transplantation. Am J Transplant. 2012;12(4):1004–16. doi: 10.1111/j.1600-6143.2011.03915.x. [DOI] [PubMed] [Google Scholar]
- 45.Petruzzo P, et al. Long-term follow-up in composite tissue allotransplantation: in-depth study of five (hand and face) recipients. Am J Transplant. 2011;11(4):808–16. doi: 10.1111/j.1600-6143.2011.03469.x. [DOI] [PubMed] [Google Scholar]
- 46.Cendales LC, et al. The Banff 2007 working classification of skin-containing composite tissue allograft pathology. Am J Transplant. 2008;8(7):1396–400. doi: 10.1111/j.1600-6143.2008.02243.x. [DOI] [PubMed] [Google Scholar]
- 47.Thione A, et al. Late nail lesions rejection in a stable bilateral forearm allograft at 60 months posttransplantation. Ann Plast Surg. 2014;73(5):612–4. doi: 10.1097/SAP.0000000000000349. [DOI] [PubMed] [Google Scholar]
- 48.Tsutsui H, et al. Lumen loss in transplant coronary artery disease is a biphasic process involving early intimal thickening and late constrictive remodeling: results from a 5-year serial intravascular ultrasound study. Circulation. 2001;104(6):653–7. doi: 10.1161/hc3101.093867. [DOI] [PubMed] [Google Scholar]
- 49.Kanitakis J, et al. Graft vasculopathy in the skin of a human hand allograft: implications for diagnosis of rejection of vascularized composite allografts. Transpl Int. 2014;27(11):e118–23. doi: 10.1111/tri.12399. [DOI] [PubMed] [Google Scholar]
- 50.Mundinger GS, et al. Histopathology of chronic rejection in a nonhuman primate model of vascularized composite allotransplantation. Transplantation. 2013;95(10):1204–10. doi: 10.1097/TP.0b013e31828d1528. [DOI] [PubMed] [Google Scholar]
- 51.Unadkat JV, et al. Composite tissue vasculopathy and degeneration following multiple episodes of acute rejection in reconstructive transplantation. Am J Transplant. 2010;10(2):251–61. doi: 10.1111/j.1600-6143.2009.02941.x. [DOI] [PubMed] [Google Scholar]
- 52.Menke J, et al. The effect of ischemia/reperfusion on the kidney graft. Curr Opin Organ Transplant. 2014;19(4):395–400. doi: 10.1097/MOT.0000000000000090. [DOI] [PubMed] [Google Scholar]
- 53.Rao J, Lu L, Zhai Y. T cells in organ ischemia reperfusion injury. Curr Opin Organ Transplant. 2014;19(2):115–20. doi: 10.1097/MOT.0000000000000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lunsford KE, Barbas AS, Brennan TV. Recent advances in immunosuppressive therapy for prevention of renal allograft rejection. Curr Opin Organ Transplant. 2011;16(4):390–7. doi: 10.1097/MOT.0b013e328348b420. [DOI] [PubMed] [Google Scholar]
- 55.Slegtenhorst BR, et al. Ischemia/reperfusion Injury and its Consequences on Immunity and Inflammation. Curr Transplant Rep. 2014;1(3):147–154. doi: 10.1007/s40472-014-0017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thiyagarajan UM, Ponnuswamy A, Bagul A. Thymoglobulin and its use in renal transplantation: a review. Am J Nephrol. 2013;37(6):586–601. doi: 10.1159/000351643. [DOI] [PubMed] [Google Scholar]
- 57.Weissenbacher A, et al. Alemtuzumab in solid organ transplantation and in composite tissue allotransplantation. Immunotherapy. 2010;2(6):783–90. doi: 10.2217/imt.10.68. [DOI] [PubMed] [Google Scholar]
- 58.Ramirez CB, I, Marino R. The role of basiliximab induction therapy in organ transplantation. Expert Opin Biol Ther. 2007;7(1):137–48. doi: 10.1517/14712598.7.1.137. [DOI] [PubMed] [Google Scholar]
- 59.Thomson AW, Bonham CA, Zeevi A. Mode of action of tacrolimus (FK506): molecular and cellular mechanisms. Ther Drug Monit. 1995;17(6):584–91. doi: 10.1097/00007691-199512000-00007. [DOI] [PubMed] [Google Scholar]
- 60.Schneeberger S, Khalifian S, Brandacher G. Immunosuppression and monitoring of rejection in hand transplantation. Tech Hand Up Extrem Surg. 2013;17(4):208–14. doi: 10.1097/BTH.0000000000000019. [DOI] [PubMed] [Google Scholar]
- 61.Khalifian S, et al. Facial transplantation: the first 9 years. Lancet. 2014;384(9960):2153–63. doi: 10.1016/S0140-6736(13)62632-X. [DOI] [PubMed] [Google Scholar]
- 62.Chan KM, et al. Improving peripheral nerve regeneration: from molecular mechanisms to potential therapeutic targets. Exp Neurol. 2014;261:826–35. doi: 10.1016/j.expneurol.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 63.Sehgal SN. Rapamune (RAPA, rapamycin, sirolimus): mechanism of action immunosuppressive effect results from blockade of signal transduction and inhibition of cell cycle progression. Clin Biochem. 1998;31(5):335–40. doi: 10.1016/s0009-9120(98)00045-9. [DOI] [PubMed] [Google Scholar]
- 64.McAlister VC, et al. Cyclosporin versus tacrolimus as primary immunosuppressant after liver transplantation: a meta-analysis. Am J Transplant. 2006;6(7):1578–85. doi: 10.1111/j.1600-6143.2006.01360.x. [DOI] [PubMed] [Google Scholar]
- 65.Schnider JT, et al. Site-specific immunosuppression in vascularized composite allotransplantation: prospects and potential. Clin Dev Immunol. 2013;2013:495212. doi: 10.1155/2013/495212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kiwanuka H, et al. Evolution of ethical debate on face transplantation. Plast Reconstr Surg. 2013;132(6):1558–68. doi: 10.1097/PRS.0b013e3182a97e2b. [DOI] [PubMed] [Google Scholar]
- 67.Ali F, Dua A, Cronin DC. Changing paradigms in organ preservation and resuscitation. Curr Opin Organ Transplant. 2015 doi: 10.1097/MOT.0000000000000180. [DOI] [PubMed] [Google Scholar]
- 68.De Vlaminck I, et al. Circulating cell-free DNA enables noninvasive diagnosis of heart transplant rejection. Sci Transl Med. 2014;6(241):241ra77. doi: 10.1126/scitranslmed.3007803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kueckelhaus MIA, Fischer S, Kumamaru K, Alhefzi M, Bueno EM, Wake N, Gerhard-Herman MD, Rybicki FJ, Pomahac B. Non-invasive monitoring of immune rejection in face transplant recipients. Plast Reconstr Surg. 2015 doi: 10.1097/PRS.0000000000001703. in press. [DOI] [PubMed] [Google Scholar]
- 70.Haas M, et al. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014;14(2):272–83. doi: 10.1111/ajt.12590. [DOI] [PubMed] [Google Scholar]
- 71.Schneeberger S, et al. Upper-extremity transplantation using a cell-based protocol to minimize immunosuppression. Ann Surg. 2013;257(2):345–51. doi: 10.1097/SLA.0b013e31826d90bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leonard DA, Kurtz JM, Cetrulo CL., Jr Achieving immune tolerance in hand and face transplantation: a realistic prospect? Immunotherapy. 2014;6(5):499–502. doi: 10.2217/imt.14.29. [DOI] [PubMed] [Google Scholar]
- 73.Leonard DA, et al. Vascularized composite allograft tolerance across MHC barriers in a large animal model. Am J Transplant. 2014;14(2):343–55. doi: 10.1111/ajt.12560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jindal R, et al. Spontaneous Resolution of Acute Rejection and Tolerance Induction With IL-2 Fusion Protein in Vascularized Composite Allotransplantation. Am J Transplant. 2015 doi: 10.1111/ajt.13118. [DOI] [PubMed] [Google Scholar]
- 75.Gharb BB, et al. Effectiveness of topical immunosuppressants in prevention and treatment of rejection in face allotransplantation. Transplantation. 2013;95(10):1197–203. doi: 10.1097/TP.0b013e31828bca61. [DOI] [PubMed] [Google Scholar]
- 76.Gajanayake T, et al. A single localized dose of enzyme-responsive hydrogel improves long-term survival of a vascularized composite allograft. Sci Transl Med. 2014;6(249):249ra110. doi: 10.1126/scitranslmed.3008778. [DOI] [PubMed] [Google Scholar]
- 77.Solhjou Z, et al. Emerging therapies targeting intra-organ inflammation in transplantation. Am J Transplant. 2015;15(2):305–11. doi: 10.1111/ajt.13073. [DOI] [PubMed] [Google Scholar]