Abstract
In Tijuana, Mexico, HIV is concentrated in sub-epidemics of key populations: persons who inject drugs (PWID), sex workers (SW), and men who have sex with men (MSM). To date, data on engagement in the HIV care continuum among these key populations, particularly in resource-constrained settings, are sparse. We pooled available epidemiological data from six studies (N=3,368) to examine HIV testing and treatment uptake in these key populations; finding an overall HIV prevalence of 5.7%. Of the 191 identified HIV-positive persons, only 11.5% knew their HIV-positive status and 3.7% were on ART. Observed differences between these HIV-positive key populations suggest PWID (vs. non-PWID) were least likely to have previously tested or initiate HIV care. MSM (vs. non-MSM) were more likely to have previously tested but not more likely to know their HIV-positive status. Of persons aware of their HIV-positive status, SW (vs. non-SW) were more likely to initiate HIV care. Findings suggest engagement of key populations in HIV treatment is far below estimates observed for similarly resource-constrained generalized epidemics in sub-Saharan Africa. These data provide one of the first empirical-snapshots highlighting the extent of HIV treatment disparities in key populations.
Keywords: HIV Continuum of Care, Key Populations, Secondary HIV prevention, HIV testing
INTRODUCTION
Tijuana, Mexico is a major Mexico-US border city, where contextual factors such as migration, deportation, drug use, and sex tourism facilitate a complex HIV risk environment.(1,2) Previous community-level HIV surveillance in Tijuana has detected dynamic HIV sub-epidemics where prevalence is primarily concentrated among key populations disproportionately affected by HIV in similar resourced-constrained settings,(3–5) namely men who have sex with men (MSM, 20%),(6) persons who inject drugs (PWID, 4%), female sex workers (FSW, 6%), and FSWs who inject drugs (FSW-PWID, 12%).(1) Comparatively, the state and national HIV prevalence are 0.80% and 0.24% respectively.(7) Viral genetic analyses of samples drawn from studies with these key populations in Tijuana demonstrated that two-thirds of incident HIV infections are phylogenetically unlinked, suggesting multiple new introductions of HIV and high transmission permeability.(8) Understanding the state of engagement in HIV care among key populations with such diverse transmission networks is critical for reducing future HIV incidence via targeted HIV treatment as prevention (TasP) efforts.
Universal access to healthcare is a constitutional right in Mexico; obtained via one of three mechanisms: 1) private healthcare sector, 2) social security-based health care sector, for individuals who are formally employed, and 3) healthcare services covered by the Ministries of Health, for individuals who are primarily unemployed.(9) Yet, 48.5% of the Mexican population may lack effective access to health services.(10) These estimates are likely higher for key populations who face additional stigma and discrimination.(11–13)
In 2001, Mexico began providing universal access to HIV care, including antiretroviral treatment (ART) based on the federal treatment guidelines (CD4 < 350 cells/mm3) through federally operated HIV clinics called, Centros Ambulatorios de Prevencion y Atención en SIDA e Infecciones de Transmision Sexual (CAPASITS).(14) Deportees and migrants in Mexico, who navigate high-risk HIV environments, may face additional challenges accessing care if they lack documentation of their Mexican citizenship (e.g., birth certificate).(15–17)
Tijuana’s HIV epicenter is located in the Zona Norte, adjacent to the Mexico-US border crossing, which is ~25km away from the local CAPASITS clinic. In 2012, CAPASITS partnered with a bi-national student-run free clinic, Health Frontiers in Tijuana (HFiT), to improve HIV treatment access via telemedicine consults with CAPASITS providers.(18) The HFiT Clinic, located in the Zona Norte, predominantly serves indigent/low resourced persons, including those affected by deportation and migration, by providing free basic primary care services three days a week.(19) These clinics, in addition to the Tijuana General Hospital, are the only public sources of HIV care in Tijuana for uninsured persons.
Relatively little is known about the state of engagement in HIV care among Tijuana’s key populations. We utilized established epidemiological data from key populations taking part in community-based studies or accessing services at the HFiT clinic to estimate levels of engagement in the HIV care continuum in Tijuana among highly vulnerable communities. Use of epidemiologic data may overcome some noted limitations of relying on clinic records to characterize rates of engagement across the continuum by allowing us to capture HIV-positive persons who may have transitioned between Mexican and US systems of care via migration or deportation,(11,16,17,20,21) as well as those who have never linked to HIV care or are lost-to-follow-up.(22–24) Such limitations may be particularly true for MSM, PWID and FSW disproportionately burdened with HIV, for whom stigma and related sociostructural factors (e.g., discrimination, violence, criminalization) often presents additional barriers to accessing HIV testing and treatment services.(3–5)
METHODS
Participants and Procedures
Estimates of engagement in the HIV care continuum were drawn from six epidemiologic studies conducted among key populations disproportionately affected by HIV in Tijuana (see Table I). Participant recruitment and baseline HIV testing protocols were complete for all six studies at time of analysis. Follow-up data from one study (El Cuete IV) is ongoing; this analysis includes data collected through December 11, 2013. Each study was selected because it used: 1) sampling methods to estimate HIV prevalence in its respective target population, 2) conducted HIV testing as part of study participation, and 3) had obtained survey data from HIV-positive participants documenting any previous HIV testing and treatment histories. This resulted in a pooled sample of 3,368 participants who were tested for HIV and surveyed on HIV-related risk factors. While most of these studies excluded participants if they were enrolled in another HIV study; it may be possible that a few individuals were sampled across more than one study.
Table I.
Pooled Sample and Individual Study Characteristics
| Pooled Sample | Proyecto H | El Cuete III | El Cuete IV | Mujer Segura | Mujer Mas Segura | Salud Fronteriza | |
|---|---|---|---|---|---|---|---|
| Total Sample (N) | 3,368 | 201 | 1056 | 737 | 474* | 302* | 598 |
| Study Design | Cross-sectional | Cohort† | Cohort† | RCT† | RCT† | Cross-sectional | |
| Sampling Method | RDS | RDS and Community Outreach | RDS and Community Outreach | Community Outreach | Community Outreach | Clinic patients | |
| Study Recruitment | 08/2012–05/2014 | 04/2006–04/2007 | 03/2011–05/2013 | 01/2004–01/2006 | 10/2008–10/2009 | 01/2013–05/2013 | |
| Target Population | MSM | PWID | PWID | FSW | FSW-PWID | All patients accessing care | |
| HIV Testing Protocol | Double Rapid IFA confirmed | Single Rapid EIA & IFA confirmed | Single Rapid EIA & IFA confirmed | Single Rapid EIA & Western-blot confirmed | Single Rapid EIA & IFA confirmed | Double Rapid | |
|
| |||||||
| HIV+ Sample (N) | 191 | 35 | 47 | 36 | 36 | 19 | 18 |
| HIV Prevalence (%) | 5.7% | 17.4% | 4.5% | 4.9% | 7.6% | 6.3% | 3.0% |
| Gender | |||||||
| Male | 90 | 30 | 31 | 20 | -- | -- | 9 |
| Female | 95 | -- | 16 | 16 | 36 | 19 | 8 |
| Transgender | 6 | 5 | 0 | 0 | 0 | 0 | 1 |
| HIV Transmission Risk§ | |||||||
| MSM | 38 | 35 | 0 | 2 | -- | -- | 1 |
| PWID | 116 | 1 | 47 | 36 | 9 | 19 | 4 |
| Sex Work (SW) | 81 | 6 | 11 | 4 | 36 | 19 | 5 |
| SW-PWID | 44 | 0 | 11 | 1 | 9 | 19 | 1 |
RCT (Randomized Controlled Trial), RDS (Respondent-driven Sampling), Confirmatory HIV testing protocols: IFA (immunofluorescence assay), EIA (HIV-1 enzyme immunoassay).
Study protocols with longitudinal follow-up.
Total sample and participant data was restricted to participants from the Tijuana study sites only.
Participants may have reported more than one HIV transmission risk behavior.
Study methods for these individual studies have either previously been published or are currently under review.(6,25–28) In brief, across all studies, eligible participants were required to be ≥18 years of age and meet the studies’ target population requirements: Proyecto H enrolled biologically male participants who had engaged in sex with another man in the past 2 months.(6) El Cuete III and El Cuete IV enrolled PWID who reported having injected illicit drugs in the past month, confirmed by injection stigmata (i.e., track marks).(25,26) Mujer Segura enrolled biologically female participants who had engaged in condom-unprotected sex work (SW) with ≥1 male client in the past 2 months.(27) Mujer Mas Segura enrolled biologically female participants who reported both condom-unprotected sex work with ≥1 male client and sharing drug injection equipment in the past month.(28) In Salud Fronteriza, all patients accessing free primary care services at the HFiT clinic were sequentially asked to participate in their survey and HIV testing protocol; participants generally reflect the Zona Norte community, a primarily indigent and low-resourced population affected by migration and deportation.
For each study, data collection was interviewer-administered in English or Spanish based on participants’ preference using computer-assisted personal interviewing (CAPI) or similar tablet-based surveys; except Proyecto H which was administered via paper and pencil. HIV and confirmatory testing protocols varied, but were adherent to the most current national, US (CDC) and Mexico (CENSIDA), or international, WHO, testing recommendations during their respective study periods. All participants provided informed consent and were compensated for their time. Participants who refused HIV testing as part of study participation were excluded from the current analysis. Each study obtained human subjects approval from the University of California, San Diego and respective institutions in Mexico.
Measures
For each of the six studies, we assessed the total number of participants enrolled who completed HIV testing. Study staff abstracted survey data for all HIV-positive participants regardless of whether or not they knew their HIV-positive status prior to testing. Survey data was used to dichotomously characterize (Yes=1, No=0) the pooled sample based on behaviors associated with increased HIV-transmission (i.e., self-reported sharing of needles/syringes, unprotected sex with clients or other sex partners). These data were also used to dichotomously characterize (Yes=1, No=0) their HIV testing history prior to study participation, and level of engagement in the HIV care continuum.
Available measures collected across these six studies allowed the following variables to be operationalized across the HIV care continuum. HIV-positive participants were those who tested HIV-positive via study participation. Previous HIV Test represents participants who self-reported having had an HIV test prior to study participation, regardless of test results. Knew HIV+ Status represents participants who self-reported “knowing he or she was HIV-positive” prior to testing HIV-positive as part of study participation. Ever Linked to HIV Care represents participants who self-reported “ever discussing their HIV-positive status with a provider” or that “a provider had ever offered them medications to treat their HIV”. These measures do not allow us to establish a time frame post-diagnosis during which the participant engaged in HIV care (i.e., timely vs. delayed linkage to care), length of time in care, or that these discussions occurred with an ART-prescribing provider (i.e., HIV care vs. general medical care); however, they serve as a general approximation that interactions with the medical care system regarding one’s HIV serostatus did occur at some point post-diagnosis. Receiving ART represents participants’ self-report that they were currently taking HIV medications.
For study protocols with longitudinal follow-up, measures of previous testing history and knowledge of HIV-positive status were taken from assessment periods prior to first testing HIV-positive, while measures of having ever linked to HIV care or being on ART were assessed across each available follow up visit. If a participant had initiated ART but their last available follow up data indicated they had stopped taking ART, they were classified as not receiving ART. Measures for retention in HIV care, timely ART initiation, ART adherence, or viral suppression were not available across all six epidemiological studies, and therefore are not included in the current analysis.
Statistical Analysis
HIV-positive participants’ HIV transmission, testing, and treatment histories were first characterized for each of the six studies independently, and used to evaluate HIV prevalence. Prevalence estimates were not weighted for the pooled sample. Data from all HIV-positive participants was then pooled to characterize the state of engagement in HIV treatment among key populations. Following protocols established by Gardner and colleagues, (29,30) we sought to identify the proportion of all HIV-positive participants who were retained at each step across the HIV care continuum from the previous step. For example, among all HIV-positive participants, what proportion had previously tested for HIV? Of those who had previously tested for HIV, what proportion knew their HIV-positive status? Once these proportions had been established for key populations in Tijuana across the HIV care continuum, Chi Square tests were used to explore potential differences in engagement in HIV care by self-reported HIV transmission-related behaviors (e.g., MSM, PWID, sex work regardless of gender (SW), both SW and PWID), gender, and sample type (i.e., recruited via community or clinic). Due to limited statistical representation, six participants identifying as transgender male-to-female were excluded from the analyses exploring differences in HIV testing and treatment behaviors by gender.
RESULTS
HIV Prevalence and Participant Characteristics
A total of 191 HIV-positive participants were identified across the entire pooled sample of 3,368 participants, for an overall HIV prevalence of 5.7% (see Table I). Prevalence was highest among studies of MSM (17.4%), followed by FSW (7.6%) and FSW-PWID (6.3%). Prevalence in studies of PWID were marginally lower (4.5–4.9%) as was prevalence among the sample of indigent/resource-limited patients accessing free clinical care in the Zona Norte (3.0%).
Females (49.7%) and males (47.1%) were equally represented across the pooled sample. Six participants identified as male-to-female transgender (3.1%). Regarding engagement in specific types of HIV transmission-related behaviors across the pooled sample, almost one fifth (19.9%) were MSM, 60.7% were PWID, and 42% engaged in SW. A total of four SW were male and four were male-to-female transgender (data not shown). Less than one fourth of the sample (23.0%) engaged in both SW and injection drug use (SW-PWID).
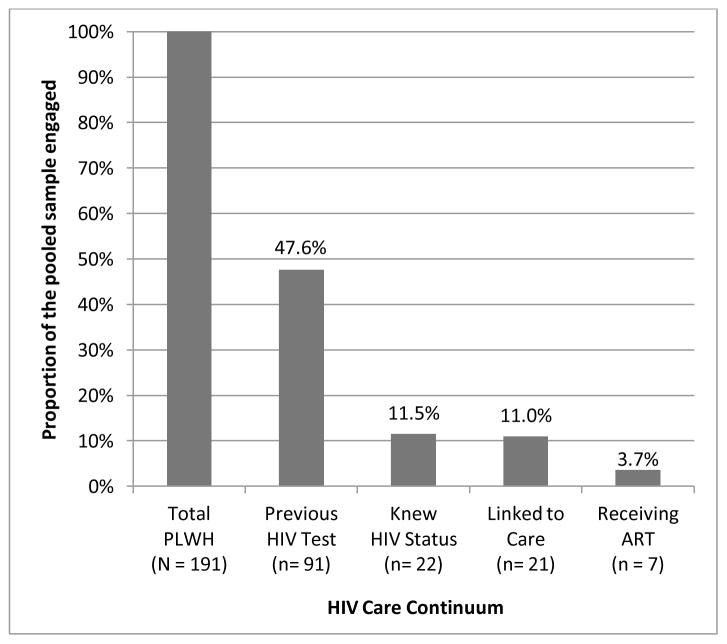
Proportion of People Living with HIV (PLWH) Engaged across the HIV Care Continuum
As depicted in Figure 1, almost half of all identified HIV-positive participants in the pooled sample had previously tested for HIV (47.6%). Of those who had ever been tested (N=91), 24.2% knew their HIV-positive status reflecting 11.5% of the pooled sample. Among those who knew their HIV-positive serostatus (N=22), almost all (95.5%) reported initiating HIV care at some point following their HIV diagnosis (11.0% of the pooled sample). Of those who had ever linked to HIV care (N=21), 33.3% reported they were currently receiving ART (N=7), indicating that only 3.7% of all identified PLWH were on active treatment across all six studies.
Figure 1.
Estimated levels of engagement in the HIV care continuum among key populations affected by HIV/AIDS in Tijuana, Mexico.
Differences in HIV Test and Treat Behaviors
Differences in HIV testing, knowledge of HIV-positive status and linkage to HIV care are reported in Table II. HIV-positive participants identified through clinic-derived (N=18) compared to community-derived sampling were more likely to report having previously tested for HIV (χ2= 4.813, p=.028) and more likely to know their HIV-positive status (χ2= 10.497, p=.001). Further, all clinic-recruited participants (100.0%) reported ever linking to HIV care vs. 67.7% of community-recruited participants; however, this difference was only marginally significant (χ2= 3.471, p=.062).
Table II.
Differences by Sample Characteristics in HIV Testing and Treatment Behaviors among Key Populations in Tijuana, Mexico (N=191)
| Measured Behaviors | Observed Differences | |||||
|---|---|---|---|---|---|---|
| Sample Method | Gender† | MSM | PWID | SW | SW-PWID | |
|
|
|
|||||
| Previously tested for HIV | χ2=4.813 ** | χ2=0.979 | χ2= 8.141 * | χ2= 12.105 ** | χ2= 2.253 | χ2= 0.051 |
| p=.028 | p=.323 | p=.004 | p=.001 | p=.133 | p=.822 | |
| n=191 | n=185 | n=184 | n=189 | n=179 | n=179 | |
| Community (78/173), 45.1% |
Female (47/95), 49.5% |
MSM (26/38), 68.4% |
PWID (43/116), 37.1% |
SW (43/81), 53.1% |
SW-PWID (20/44), 45.5% |
|
| Clinic (13/18), 72.2% |
Male (38/90), 42.2% |
non-MSM (62/146), 42.5% |
non-PWID (46/73), 63.0% |
non-SW (41/98), 41.8% |
non-SW-PWID (64/135), 47.4% |
|
| Knew HIV+ status | χ2=10.497 ** | χ2=5.152 ** | χ2= 0.080 | χ2= 1.305 | χ2= 0.138 | χ2= 0.022 |
| p=.001 | p=.023 | p=.777 | p=.253 | p=.711 | p=.881 | |
| n=95 | n=89 | n=91 | n=93 | n=88 | n=88 | |
| Community (16/82), 19.5% |
Female (8/49), 16.3% |
MSM (6/27), 22.2% |
PWID (14/47), 29.8% |
SW (9/45), 20.0% |
SW-PWID (5/22), 22.7% |
|
| Clinic (8/13), 61.5% |
Male (15/40), 37.5% |
non-MSM (16/64), 25.0% |
non-PWID (9/46), 19.6% |
non-SW (10/43), 23.3% |
non-SW-PWID (14/66), 21.2% |
|
| Ever linked to HIV care | χ2=3.471 | χ2=0.038 | χ2= 2.141 | χ2= 4.212 ** | χ2= 4.163 * | χ2= 1.145 |
| p=.062 | p=.846 | p=.143 | p=.040 | p=.041 | p=.228 | |
| n=39 | n=38 | n=37 | n=38 | n=34 | n=34 | |
| Community (21/31), 67.7% |
Female (15/20), 75.0% |
MSM (5/5), 100.0% |
PWID (19/29), 65.5% |
SW (14/16), 87.5% |
SW-PWID (10/12), 83.3% |
|
| Clinic (8/8), 100.0% |
Male (13/18), 72.2% |
non-MSM (22/32), 68.8% |
non-PWID (9/9), 100.0% |
non-SW (10/18), 55.6% |
non-SW-PWID (14/22), 63.6% |
|
Based on available behavioral HIV data, participants across the pooled sample could be classified as follows: Community-derived (n=173; referent) vs. clinic-derived (n=18) sampling methods; Gender represented as female (n=95; referent) vs. male (n=90); MSM (n=38) vs. non-MSM (n=146), missing data (n=7); PWID (n=116) vs. non-PWID (n=73), missing data (n=2); sex workers regardless of gender (SW; n=81) vs. non-SW (n=98), missing data (n=12); SW-PWID (n=44) vs. non-SW-PWID (n=135), missing data (n=12). Proportions of each sub-group engaged in the target behavior are presented as: [(n1/Ntotal), %].
indicates a significantly smaller proportion of the referent group engaged in the behavior.
indicates a significantly larger proportion of the referent group engaged in the behavior.
Due to limited statistical representation, analysis of observed differences by gender excludes persons who identify as transgender (n=6 male-to-female).
No differences were observed between females (N=95) and males in terms of previous HIV testing history (χ2= 0.979, p=.323) or linkage to HIV care (χ2= 0.038, p=.846). HIV-positive males were significantly more likely to know their HIV-positive status than females (χ2= 5.152, p=.023).
HIV-positive MSM (N=38) were more likely to have previously tested for HIV compared to non-MSM in the pooled sample (χ2= 8.141, p=.004), but were no more likely to know their HIV-positive status (χ2= 0.080, p=.777) or link to HIV care (χ2= 2.141, p=.143) than non-MSM. MSM were still more likely to have previously tested for HIV (χ2= 8.827, p=.003), but were marginally less likely to know their HIV-positive status (χ2= 3.306, p=.069) when this comparison was restricted to men who were non-MSM (data not shown). MSM appeared more likely to link to HIV care compared to non-MSM men, although this difference was not statistically significant (100% vs. 58.3%; χ2= 2.424, p=.119).
HIV-positive PWID (N=116) were less likely to have previously tested for HIV (χ2= 12.105, p=.001) and were less likely to have ever linked to HIV care (χ2= 4.212, p=.040) compared to PLWH who did not inject in the pooled sample. No differences were observed for knowing one’s HIV-positive status based on injection drug use status (χ2= 1.305, p=.253).
No differences in HIV testing history were observed between HIV-positive SW (N=81) and PLWH who did not report engaging in sex work, regardless of gender (χ2= 2.253, p=.133), nor were differences observed with respect to knowledge of HIV-positive status (χ2= 0.138, p=.711). However, SW were more likely to report having ever linked to HIV care than the remainder of the pooled sample (χ2= 4.163, p=.041). When the referent group was restricted to FSW (N=73) only (data not shown), no significant differences were observed for HIV testing and linkage behaviors (Previously tested for HIV: χ2= 0.282, p= .595; Ever linked to HIV care: χ2= 1.377, p= .241). However, observed trends suggested fewer FSW (15.8%) knew their HIV-positive status compared to the pooled sample (32.0%; χ2=3.026, p=.082), while a higher proportion of FSW (84.6%) reported having ever linked to HIV care when the comparison group was restricted to non-FSW women only (40.0%; χ2=3.583, p=.058).
For HIV-positive SW who also reported injection drug use (SW-PWID; N=44), no differences were observed in previous HIV testing history (χ2= 0.051, p=.822), knowing one’s HIV-positive status (χ2=0.022p=.881), or having ever linked to HIV care (χ2= 1.145, p=.228) compared to the pooled sample. This same pattern of results was observed when the comparison was restricted to SW who did not inject (data not shown; Previously tested for HIV: χ2= 2.253, p= .133; Knew HIV+ Status: χ2= 0.200, p= .655; Ever linked to HIV care: χ2= 0.762, p= .383). Similarly, no differences were observed when the referent category was restricted to FSW-PWID (data not shown; Previously tested for HIV: χ2= 0.171, p= .679; Knew HIV+ Status: χ2= 0.521, p= .470; Ever linked to HIV care: χ2= 0.621, p= .431).
DISCUSSION
This study provides some of the first epidemiologic estimates characterizing the state of engagement in the HIV care continuum across key populations affected by HIV in resourced-constrained countries. Most striking is that among HIV-positive MSM, PWID, SW, and indigent/low resourced persons affected by migration and deportation in Tijuana, only 11.5% knew their HIV-positive status and 3.7% were receiving ART. These estimates are sobering when compared to those obtained from the United States, where 80% of all HIV-positive persons know their serostatus and 36% are estimated to be receiving ART when clinically indicated.(30) Comparing estimates with data available from other resourced-constrained countries in sub-Saharan Africa (e.g., Uganda, South Africa, Zambia), an estimated 51% of all HIV-positive persons know their HIV-positive status and 32% are on ART.(31) In the context of TasP, our estimates across six epidemiological studies in Tijuana are far below any meaningful threshold for HIV treatment to affect community-level reduction in future HIV transmission, morbidity, or mortality.
Like many other resourced-constrained settings, Tijuana’s HIV epidemic is concentrated among key, stigmatized, populations who are least likely to access HIV prevention and treatment services.(1,3–5) Under the backdrop of Mexico’s universal treatment policies,(9,15) these estimates highlight the particular challenges TasP efforts face in sufficiently reaching key populations, among whom specific gaps in the HIV care continuum may differ.(3–5,32,33) For example, our data suggests that PWID were least likely to have previous HIV testing histories or be linked to care. MSM may have had more exposure to testing, but were no more likely to know their HIV-positive status or link to care. Once aware of their status, SW were more likely to link to HIV care, which may reflect public health efforts tied to permits that enable sex work to be quazi-legal in Tijuana. Regardless, lack of ART utilization among key populations suggests substantial problems retaining these patients long enough to initiate ART once linked to HIV care.
A major strength of this study is the use of community-derived sampling methods to identify MSM, PWID and FSW in the Zona Norte, thus capturing hard-to-reach PLWH typically missed (e.g., untested, never in care, lost-to-follow-up) when clinic records are used to characterize the state of engagement in HIV care for a given population.(22–24) Similar community-based recruitment and intervention approaches may be needed to facilitate sustained engagement in HIV care and viral suppression in these key populations.(18)
In the context of universal access to HIV care, future work is needed to ensure that barriers to HIV testing and treatment are identified and ameliorated for these key populations. An encouraging sign is that HIV testing efforts in Tijuana(1) and previous host countries, such as the US,(34) have successfully reached almost half (47.6%) of all persons identified as HIV-positive. HIV prevention and testing efforts in these key populations in Tijuana has been enhanced by the use of mobile HIV testing units (Prevenmovihl).(1) However, the recent loss of resources from the Global Fund currently limits the capacity for increasing staff outreach and initiating programs within these key populations to target repeat (i.e., annual) HIV testing, and for sustaining access to free sterile syringes and condoms. In collaboration with Tijuana’s HIV care sites, CAPASITS and HFiT, enhanced capacities could allow the Prevenmovihl to adopt a combination prevention approach integrating basic HIV care services and routine ART distribution to HIV-positive patients in the Zona Norte. Such integrated efforts, alongside the current decentralization efforts to provide HIV care via telemedicine in the Zona Norte, could help maintain high rates of linkage to HIV care (95.5%) observed among those who knew their HIV-positive status while supporting improvements in retention in HIV care, timely ART initiation, and ART adherence behaviors requisite to attain viral suppression. In addition, recent work found that patients who co-accessed HIV and non-HIV services (e.g., appointments with the psychologist) at Tijuana’s CAPASITS clinic were better retained in HIV care;(35) suggesting additional benefit to expanding access to psychosocial services in this region for key populations once HIV care is initiated.
Our ability to understand barriers to repeat HIV testing, retention in HIV care, and ART adherence was limited by available epidemiologic data. Systematically assessing the full continuum of HIV care behaviors for persons testing HIV-positive in future epidemiological studies in Tijuana and other resourced-constrained regions with concentrated epidemics are warranted. We are further limited in our ability to follow patterns of engagement/disengagement in HIV care over time across all studies. While HIV serostatus was confirmed by established testing protocols, all other data on HIV-related transmission, testing, and treatment behaviors were limited to self-report. Our datasets included some of the most marginalized individuals living with HIV, resulting in a possible overestimation of poor engagement in HIV treatment among all people living with HIV in the region. This inclusion of highly-vulnerable populations; however, provides much needed information about engagement in HIV care that is often lacking in the research literature. The use of de-identified data further restricted the ability to validate our findings with data from Mexico’s national HIV treatment database, as well as our ability to confirm that cases were unduplicated across studies. Linking clinical HIV treatment data to prospective studies evaluating structural, social, and individual-level determinants of engagement in the HIV care continuum is needed to better inform targeted intervention development with these key populations.
Despite these limitations, public health officials at the national, state, and local level are committed to taking action. These data are being used to inform programmatic development that will enable promotores (peer outreach workers) to identify poorly engaged PLWH within the Zona Norte and promote stronger relationships with available HIV services to facilitate ART uptake and adherence. This development will capitalize on the benefits of telemedicine-delivered HIV care at the HFiT clinic; increasing local access to HIV treatment for vulnerable populations, such as those in this study, by reducing travel and economic barriers to HIV care.(18)
Acknowledgments
This work was supported by the National Institutes of Health training grants [T32 DA023356 and K01 DA039767 funding LRS, K01 MH095680 funding JLB, K01 DA025504 funding VDO], Fogarty AITRP training grant [D43 TW008633]; research awards [R01 MH065849 to TLP; R01 DA023877, R01 DA019829, and R37 DA019829 to SAS]; the UC GloCal Fellowship funded by the Fogarty International and the National Institutes of Health [R25 TW009343 to VDO], and the UCSD Nash Williams Foundation Fund. We sincerely thank the participants and field staff of these six epidemiologic studies, as well as our non-profit partner Prevencasa, A.C., especially Luis Alberto Segovia and Liliana Pacheco for their contributions to supporting this work within the city of Tijuana, Mexico. We wish to recognize David Goodman-Meza, MD, for his conceptualization and contributions to obtaining current HIV prevalence data for MSM in Tijuana, as well as the doctors, students, and volunteers that support the Health Frontiers in Tijuana (HFiT) Clinic’s endeavors to provide care to the region’s most vulnerable populations. All ideas expressed in this manuscript are those of the authors and should not be attributed to the funding agencies.
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Strathdee SA, Magis-Rodriguez C, Mays VM, Jimenez R, Patterson TL. The emerging HIV epidemic on the Mexico-US border: an international case study characterizing the role of epidemiology in surveillance and response. Ann Epidemiol. 2012;22(6):426–438. doi: 10.1016/j.annepidem.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strathdee SA, Magis-Rodriguez C. Mexico's evolving HIV epidemic. JAMA. 2008;300(5):571–573. doi: 10.1001/jama.300.5.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karim QA. The Global HIV Epidemic: Current Status and Challenges. Curr HIV/AIDS Rep. 2013;10(2):111–112. doi: 10.1007/s11904-013-0160-1. [DOI] [PubMed] [Google Scholar]
- 4.Vermund SH. Global HIV Epidemiology: A Guide for Strategies in Prevention and Care. Current HIV/AIDS Reports. 2014;11(2):93–98. doi: 10.1007/s11904-014-0208-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shannon K, Strathdee SA, Goldenberg SM, Duff P, Mwangi P, Rusakova M, et al. Global epidemiology of HIV among female sex workers: influence of structural determinants. The Lancet. 2014 doi: 10.1016/S0140-6736(14)60931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pitpitan EV, Goodman-Meza D, Burgos JL, Abramovitz D, Chavarin CV, Torres K, et al. Prevalence and correlates of HIV among men who have sex with men in Tijuana, Mexico. J Int AIDS Soc. 2015 Feb 9;18(1):19304. doi: 10.7448/IAS.18.1.19304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CENSIDA. Vigilancia Epidemiológica de casos de VIH/SIDA en México Registro Nacional de Casos de SIDA Actualización al cierre de 2013. 2014 [Google Scholar]
- 8.Mehta S, Brouwer K, Strathdee S, et al. HIV Transmission Dynamics in Tijuana, Mexico: Isolated clusters suggest multiple new sources of viral introductions. 20th International AIDS Conference; July 23, 2014; p. Abstract No. WEPE177. [Google Scholar]
- 9.Bautista-Arredondo S, Mane A, Bertozzi SM. Economic impact of antiretroviral therapy prescription decisions in the context of rapid scaling-up of access to treatment: lessons from Mexico. AIDS. 2006;20(1):101–109. doi: 10.1097/01.aids.0000198096.08444.53. [DOI] [PubMed] [Google Scholar]
- 10.Gutiérrez JP, García-Saisó S, Dolci GF, Ávila MH. Effective access to health care in Mexico. BMC health services research. 2014;14(1):186. doi: 10.1186/1472-6963-14-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldenberg SM, Strathdee SA, Perez-Rosales MD, Sued O. Mobility and HIV in Central America and Mexico: A critical review. Journal of Immigrant and Minority Health. 2012;14(1):48–64. doi: 10.1007/s10903-011-9505-2. [DOI] [PubMed] [Google Scholar]
- 12.Herrera C, Campero L, Caballero M, Kendall T. Relación entre médicos y pacientes con VIH: influencia en apego terapéutico y calidad de vida. Revista de Saúde Pública. 2008;42(2):249–255. doi: 10.1590/s0034-89102008000200009. [DOI] [PubMed] [Google Scholar]
- 13.Infante C, Zarco A, Cuadra SM, Morrison K, Caballero M, Bronfman M, et al. El estigma asociado al VIH/SIDA: el caso de los prestadores de servicios de salud en México. Salud Pública de México. 2006;48(2):141–150. doi: 10.1590/s0036-36342006000200007. [DOI] [PubMed] [Google Scholar]
- 14.Caro-Vega Y, Volkow P, Sierra-Madero J, Colchero MA, Crabtree-Ramírez B, Bautista-Arredondo S. Did Universal Access to ARVT in Mexico Impact Suboptimal Antiretroviral Prescriptions? AIDS research and treatment. 2013 doi: 10.1155/2013/170417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saavedra JA. HIV treatment continues to progress in Mexico. Ministry of Health director discusses advances. Aids Alert. 2005 May;20(5 suppl):2–4. [PubMed] [Google Scholar]
- 16.Brouwer K, Lozada R, Cornelius W, Cruz MF, Magis-Rodriguez C, de Nuncio MZ, et al. Deportation along the US–Mexico border: its relation to drug use patterns and accessing care. Journal of Immigrant and Minority Health. 2009;11(1):1–6. doi: 10.1007/s10903-008-9119-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magis-Rodríguez C, Gayet C, Negroni M, Leyva R, Bravo-García E, Uribe P, et al. Migration and AIDS in Mexico: an overview based on recent evidence. JAIDS J Acquired Immune Defic Syndromes. 2004;37:S215–S226. doi: 10.1097/01.qai.0000141252.16099.af. [DOI] [PubMed] [Google Scholar]
- 18.Burgos JL. Use of Technology to Improve Linkage in HIV Care Among the Underserved in Tijuana, Mexico. National Hispanic Scientific Network on Drug Abuse. 2014 Sep 5; [Google Scholar]
- 19.Ojeda VD, Eppstein A, Lozada R, Vargas-Ojeda AC, Strathdee SA, Goodman D, et al. Establishing a Binational Student-Run Free-Clinic in Tijuana, Mexico: A Model for US–Mexico Border States. J Immigr Minor Health. 2013:1–3. doi: 10.1007/s10903-012-9769-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zúñiga ML, Brennan J, Scolari R, Strathdee SA. Barriers to HIV care in the context of cross-border health care utilization among HIV-positive persons living in the California/Baja California US-Mexico border region. Journal of Immigrant and Minority Health. 2008;10(3):219–227. doi: 10.1007/s10903-007-9073-7. [DOI] [PubMed] [Google Scholar]
- 21.Donohoe T, Reyes M, Armas L, Mandel N. Continuum of Care for HIV Patients Returning to Mexico. JANAC: Journal of the Association of Nurses in AIDS Care. 2008;19(5):335–337. doi: 10.1016/j.jana.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Smith MK, Powers KA, Muessig KE, Miller WC, Cohen MS. HIV treatment as prevention: the utility and limitations of ecological observation. PLoS Medicine. 2012;9(7):e1001260. doi: 10.1371/journal.pmed.1001260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geng EH, Nash D, Kambugu A, Zhang Y, Braitstein P, Christopoulos KA, et al. Retention in Care among HIV-infected Patients in Resource-limited Settings: Emerging insights and new directions. Curr HIV/AIDS Rep. 2010;7(4):234–244. doi: 10.1007/s11904-010-0061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jenness SM, Myers JE, Neaigus A, Lulek J, Navejas M, Raj-Singh S. Delayed entry into HIV medical care after HIV diagnosis: Risk factors and research methods. AIDS Care. 2012;24(10):1240–1248. doi: 10.1080/09540121.2012.656569. [DOI] [PubMed] [Google Scholar]
- 25.Strathdee SA, Lozada R, Pollini RA, Brouwer KC, Mantsios A, Abramovitz DA, et al. Individual, social, and environmental influences associated with HIV infection among injection drug users in Tijuana, Mexico. J Acquir Immune Defic Syndr. 2008 Mar 1;47(3):369–376. doi: 10.1097/QAI.0b013e318160d5ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robertson AM, Garfein RS, Wagner KD, Mehta SR, Magis-Rodriguez C, Cuevas-Mota J, et al. Evaluating the impact of Mexico's drug policy reforms on people who inject drugs in Tijuana, B.C., Mexico, and San Diego, CA, United States: a binational mixed methods research agenda. Harm Reduct J. 2014 Feb 12;11 doi: 10.1186/1477-7517-11-4. 4-7517-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patterson TL, Semple SJ, Staines H, Lozada R, Orozovich P, Bucardo J, et al. Prevalence and correlates of HIV infection among female sex workers in 2 Mexico-US border cities. J Infect Dis. 2008 Mar 1;197(5):728–732. doi: 10.1086/527379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strathdee SA, Lozada R, Martinez G, Vera A, Rusch M, Nguyen L, et al. Social and structural factors associated with HIV infection among female sex workers who inject drugs in the Mexico-US border region. PLoS One. 2011;6(4):e19048. doi: 10.1371/journal.pone.0019048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gardner EM, McLees MP, Steiner JF, del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clinical Infectious Diseases. 2011;52(6):793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen S, Van Handel M, Branson B, Blair J, Hall H, Hu X. Vital signs: HIV prevention through care and treatment-United States. MMWR Morb Mortal Wkly Rep. 2011;60(47):1618–1623. [PubMed] [Google Scholar]
- 31.UNAIDS. Access to Antiretroviral Therapy in Africa: Status report on progress towards the 2015 targets. 2013 [Google Scholar]
- 32.Qian H, Vermund SH. Are Low-and Middle-Income Countries Repeating Mistakes Made by High-Income Countries in the Control of HIV for Men who have Sex with Men? Journal of AIDS & clinical research. 2012:e001. doi: 10.4172/2155-6113.S4-e001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Degenhardt L, Mathers BM, Wirtz AL, Wolfe D, Kamarulzaman A, Carrieri MP, et al. What has been achieved in HIV prevention, treatment and care for people who inject drugs, 2010–2012? A review of the six highest burden countries. International Journal of Drug Policy. 2014;25(1):53–60. doi: 10.1016/j.drugpo.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Espinoza L, Hall HI, Hu X. Increases in HIV diagnoses at the US-Mexico border, 2003–2006. AIDS Education & Prevention. 2009;21(Supplement B):19–33. doi: 10.1521/aeap.2009.21.5_supp.19. [DOI] [PubMed] [Google Scholar]
- 35.Jaramillo AML, Zúñiga-Denuncio ML, Rangel-Gómez MG, Fuentes-Flores CM. Relación entre adherencia a citas médicas de pacientes VIH y la accesibilidad geográfica a servicios de salud entre quienes acuden al CAPASITS de Tijuana. Población y Salud en Mesoamérica. 2014;12(2) [Google Scholar]