Abstract
The role of IL-17A in host defense against Legionella pneumophila remains elusive. To address this issue, we used Il17a−/−, Il17f−/−, and Il17a/Il17f−/− mice on a C57Bl/6 (non-permissive) background and IL-17 neutralizing Abs in mice on an A/J (permissive) background. Higher bacterial (L.pneumophila) counts in the lung and blood along with reduced neutrophil recruitment were detected in Il17a−/−, but not Il17f−/−, mice. We found that neutrophils produce IL-17A homodimer (IL-17A) during L. pneumophila infection, and hematopoietic cell-derived IL-17A is known to be important for bacterial clearance. Thus, intratracheal (i.t) administration of WT neutrophils or recombinant IL-17A restored bacterial clearance and neutrophil recruitment in Il17a−/− mice. Furthermore, neutrophil-depleted Rag2−/− and Rag2/Il-2rγ−/− mice exhibited increased bacterial burden, reduced neutrophil influx and IL-17A production in the lung. Recombinant IFN-γ administration in Il17a−/− mice augmented bacterial elimination whereas IL-17A administration in Ifnγ−/− mice did not augment bacterial clearance. IFN-γ is produced by T cells, but not neutrophils or macrophages, suggesting that neutrophil-derived IL-17A induces IFN-γ in a paracrine fashion. Human pneumonic lungs and human neutrophils challenged with L. pneumophila exhibited increased numbers of IL-17A producing cells. These findings display a novel function of neutrophil-derived IL-17A in antibacterial defense via the induction of IFN-γ in a paracrine manner.
INTRODUCTION
Bacterial pneumonia is a leading cause of mortality, morbidity, and health care costs worldwide 1. The intracellular pathogen Legionella pneumophila causes both nosocomial and community pneumonia termed Legionnaires’ disease 2, 3. In addition to the mouse model of L. pneumophila 4 infection, which mimics Legionnaires' disease in immunocompetent individuals, 5, 6 macrophages from the inbred A/J mouse strain are often used in studies as they are permissive for growth of L. pneumophila 6, 7 whereas macrophages from other inbred mice are not 4.
Recruitment of neutrophils is a hallmark of bacterial pneumonia 8, 9. Neutrophil influx into the lung is critical for host defense against L. pneumophila 10, as the selective depletion of neutrophils leads to impaired bacterial clearance. Th17 (IL-17-producing) T cell subsets are known to be involved in neutrophil migration 11. IL-17A and IL-17F are homodimeric cytokines produced by Th17 cells that bind to the same receptors (IL-17RA or IL-17RC) 12, 13. Murine IL-17A is the well characterized 21-kDa glycoprotein with 147 amino acids that shares 63% amino acid homology with human IL-17A (155 aa) 14.
In a recent investigation, Il17a/Il17f−/− mice on a Balb/c background were utilized to show that IL-17A/F is important for controlling bacterial burden, inducing neutrophil influx into the lung, and for survival in response to L. pneumophila 15. However, to further expand upon this initial study, we used: 1) single and double knockout mice on C57Bl/6 background and antibody blocking in A/J mice; 2) the role of IL-17A or IL-17F individually or together are important for host defense; 3) bone marrow chimeras and adoptive transfer experiments to determine which cell types are responsible for IL-17A and IL-17F production; 4) IL-17 homodimer (IL-17A or IL-17F) or heterodimer (IL-17A/F) to determine which is more effective at inducing host resistance; and 5) IL-17-mediates IFN-γ production via paracrine manner to induce host defense.
RESULTS
Bacterial infection induces production of IL-17A and IL-17F in lungs
To determine whether bacterial infection regulates the production of IL-17A and IL-17F, we performed time course experiments (24, 48, and 72 h) in a well-established mouse model of L. pneumophila infection. As shown in Figure S1A, IL-17A and IL-17F along with TNF-α, IL-12/IL-23 (p40) and IL-23 (p19) were upregulated at the mRNA level and peaked at 6 h postinfection. Since IL-17A and IL-17F can exist as either homo- or heterodimers 16, 17, we specifically determined the levels of IL-17A, IL-17A/F, and IL-17F in the lungs following infection and found them all to be induced at the protein level in lung tissue, with concentrations peaking at 24 h postinfection (Supplemental Fig. 1B). Using specific ELISA kits, IL-17A/F expression was detected at much higher levels than either IL-17A or IL-17F at all time points (Supplemental Fig. 1B).
Bacterial clearance is dependent on IL-17A, but not IL-17F
While, it was recently reported that deletion of both IL-17A and IL-17F genes (Il17a/Il17f−/− ; double knockout (DKO) mice) results in impaired L. pneumophila clearance in the lungs, the individual roles of IL-17A and IL-17F with regard to L. pneumophila infection remained unclear 15. To this end, we compared wild-type (C57Bl/6), Il17a−/−, Il17f−/−, and Il17a/Il17f−/− (DKO) mice. Similar to the findings reported previously 15, we found that Il17a/Il17f−/− mice exhibited a higher bacterial burden in the lung and increased dissemination at 24, 48, and 72 h postinfection as compared with their WT counterparts. Intriguingly, compared to Il17f−/− mice, Il17a−/− exhibited a higher bacterial burden in the lung at 48 and 72 h postinfection and enhanced bacterial dissemination (liver CFU) at 24, 48, and 72 h postinfection (Fig. 1A-B).
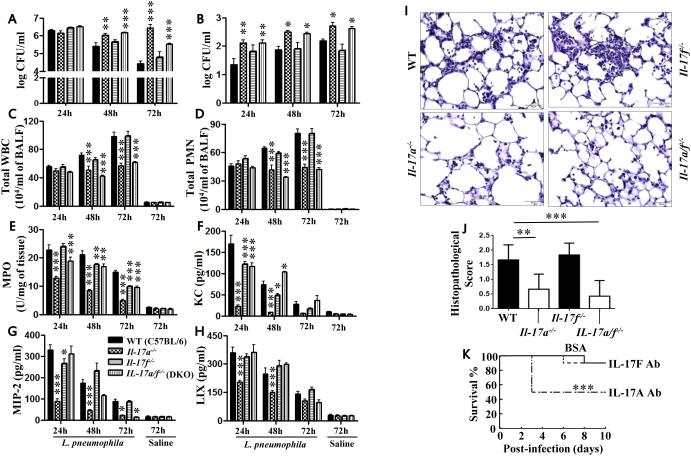
Figure 1. IL-17A mediates bacterial clearance, cellular recruitment, and cytokine/chemokine production following L. pneumophila infection.
Whole lung (A) and liver (B) CFU of WT, Il17a−/−, Il17f−/−, and Il17a/Il17f−/− mice following L. pneumophila infection (107 CFU/mouse). Lung or liver homogenates at 24, 48, and 72 h were used to enumerate the bacterial colony forming units (CFU). C-D. BALF was collected at 24, 48, and 72 h post-L. pneumophila inoculation from WT, Il17a−/−, Il17f−/−, and Il17a/Il17f−/− mice and cell enumeration was performed to determine total WBC (C) and neutrophil (PMN) recruitment (D) to the airspaces. E. Neutrophil recruitment to the lung parenchyma was determined by myeloperoxidase (MPO) activity in the lungs of L. pneumophila-infected mice. F-H. Chemokine levels in BALF at 24, 48, and 72 h postinfection were measured by sandwich ELISA. I-J. Lung sections were prepared at 72 h post bacterial infection and were stained with Haemotoxylin and Eosin. These are representative sections of 6 mice with identical results at a magnification of 40X (I). Scores of 6 histolopathological sections from WT, Il17a−/−, Il17f−/−, and Il17a/Il17f−/− mice were performed in a blinded fashion (J). For A-J, n=6-9 mice/group). *, p<0.05; **, p<0.01;***, p<0.001; (significance as compared to L. pneumophila-infected C57Bl/6 mice). K. IL-17A or IL-17F blocking antibody (10 mg/mouse) administered A/J (control) mice were inoculated i.t. with 108 CFUs of K. pneumoniae 1 h post-antibody challenge and mortality was monitored up to 10 days (***, p<0.001 by log rank test) (n=10 mice/group).
Neutrophils are critical to the augmentation of host defense against L. pneumophila 15. Attenuated recruitment of total WBCs or neutrophils in airspaces of Il17a−/− and Il17a/Il17f−/− mice was observed at both 48 and 72 h post-L. pneumophila infection (Fig. 1C-D). Furthermore, neutrophil recruitment to the lung parenchyma, as measured by myeloperoxidase (MPO) activity in lung homogenates of Il17a−/− mice, was reduced at 24, 48, and 72 h following L. pneumophila infection (Fig. 1E). Conversely, total WBC and neutrophil counts in airspaces and lung parenchyma of Il17f−/− mice were not attenuated Il17f−/− mice (Fig. 1C-E) whereas neutrophil recruitment to the lung parenchyma was modestly reduced at 48 and 72 h after infection in Il17f−/− mice (Fig. 1E).
Consistent with reduced neutrophil accumulation in the lungs, attenuated levels of chemokines (KC/CXCL1, MIP-2/CXCL2, LIX/CXCL5) were observed in Il17a−/− mice following L. pneumophila infection (Fig. 1F-H). As compared with control mice, Il17f−/− mice exhibited modest or no reduction in either chemokines or cytokines at any of the time points examined (Fig. 1F-H). Moreover, lungs obtained from WT (control) mice demonstrated moderate suppurative bronchopneumonia whereas Il17a−/− and Il17a/Il17f−/− mice displayed only mild suppurative bronchopneumonia at 72 h following L. pneumophila infection (Fig. 1I-J). Furthermore, Il17f−/− mouse lungs demonstrated no significant difference in histopathological changes as compared to control mice (Fig. 1I-J). These results suggest that IL-17A is an importanrt mediator for host defense and that IL-17A deficiency plays a dominant role in Il17a/Il17f−/− mice.
Blocking IL-17A attenuates bacterial clearance in permissive (A/J) mice
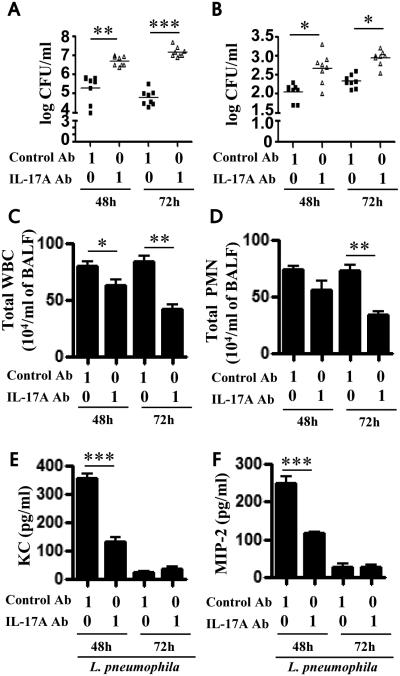
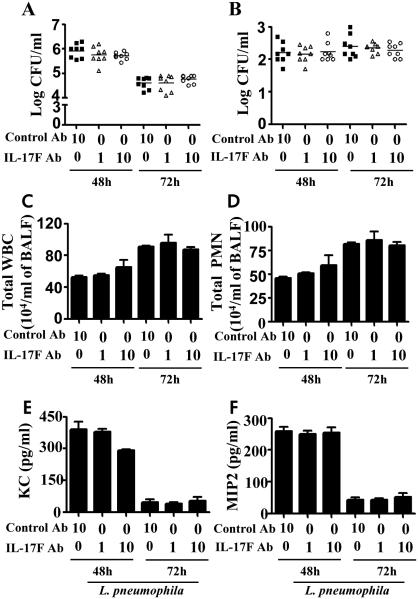
In order to validate the findings obtained in an L. pneumophila non-permissive host (C57Bl/6 strain), we used a permissive host (A/J mice) in which blocking antibodies to IL-17A (1 μg/mouse) or IL-17F (1 or 10 μg/mouse) were administered 1 h prior to L. pneumophila challenge. Survival experiments in A/J mice using IL-17A Ab (10 μg/mouse) , but not IL-17F Ab, following L. pneumophila infection (108 CFU/50 μl/mouse) resulted in reduction in survival (Fig. 1K). Intratracheal (i.t.) administration of an IL-17A neutralizing antibody increased bacterial burden in the lungs (Fig. 2A) and liver (Fig. 2B) as well as decreased recruitment of WBCs and neutrophils to the lungs (Fig. 2C-D). Furthermore, blocking IL-17A also reduced chemokine (KC, MIP2) levels (Fig. 2E-F). On the other hand, blocking of IL-17F had no significant effect on bacterial clearance, neutrophil influx, or chemokine production following infection (Fig. 3A-F).
Figure 2. Blockade of IL-17A in A/J mice attenuates the host immune response during pulmonary L. pneumophila infection.
A-B. A/J mice were treated intratracheally with IL-17A blocking antibody (1 μg/mouse) or control IgG 1 h before L. pneumophila (107 CFU/mouse) infection. Unlavaged lung (A) and liver (B) were isolated and homogenized and bacterial CFUs enumerated. C-D. BALF was obtained at 48 and 72 h post-L. pneumophila inoculation from blocking Ab or control Ab-treated mice, and cell enumeration was performed to determine total WBC (C) and neutrophil infiltration (D). E-F. Chemokine levels in BALF were measured by sandwich ELISA following infection with L. pneumophila and Ab blockade. (n=6-8 mice/group). *, p<0.05; **, p<0.01;***, p<0.001; (significance as compared to L. pneumophila-infected C57Bl/6 mice).
Figure 3. Blockade of IL-17F in A/J mice attenuates the host immune response during pulmonary L. pneumophila infection.
A-F. A/J mice were treated intratracheally with IL-17F blocking antibody (1 or 10 μg/mouse) or control IgG 1 h after L. pneumophila (107 CFU/mouse) inoculation. Unlavaged lung (A) and liver (B) were isolated and tissues were homogenized. The lung or liver homogenates were used to enumerate the bacterial CFU. C2-D2. BALF was obtained post-L. pneumophila inoculation from Ab treated mice, and cell enumeration was performed to determine total WBC (C) and neutrophil recruitment (D). E-F. Chemokine levels in BALF were measured by sandwich ELISA following antibody blockade and infection with L. pneumophila. For experiments A2-F2, (n=6-8 mice/group). *, p<0.05; **, p<0.01;***, p<0.001; (significance as compared to L. pneumophila-infected C57Bl/6 mice). Cont-Ab: Isotype-matched control antibody.
IL-17A homodimer rescues the impaired bacterial clearance in Il17a−/− mice
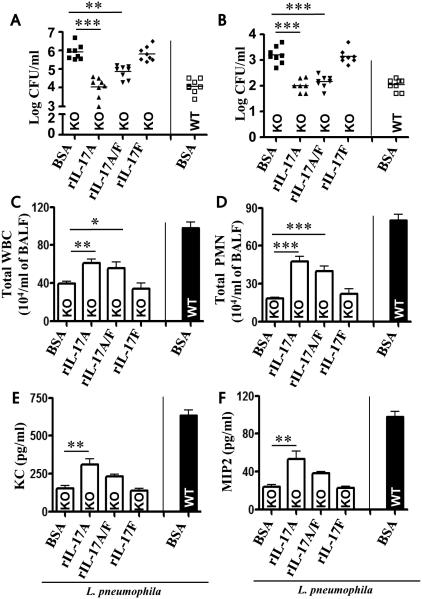
Because IL-17A and IL-17F form not only homodimers, but also a heterodimer (IL-17A/F) 11, 18, we next asked which is effective in rescuing neutrophil-dependent host defense in Il17a−/− mice following L. pneumoniae infection. Each homodimer, the heterodimer, or vehicle control (BSA) was administered 1 h post-L. pneumophila infection. Administration of a single dose of IL-17A and IL-17A/F, but not IL-17F, augmented bacterial clearance in the lungs (Fig. 4A), attenuated bacterial dissemination to liver (Fig. 4B), and enhanced neutrophil influx (Fig. 4C-D) and cytokine/chemokine expression in Il17a−/− mice (Fig. 4E-F). Intriguingly, exogenous IL-17A is much more potent than IL-17A/F in rescuing host defense function in Il17a−/− mice following L. pneumophila infection (Fig. 4A-F).
Figure 4. Administration of recombinant IL-17A and IL-17A/F, but not IL-17F, rescues bacterial clearance, neutrophil recruitment and cytokine production in the lungs of Il-17a−/− mice following L. pneumophila infection.
Intratracheal administration of BSA (as control; 1 μg/mouse) or 1 μg/50μl/mouse of rm-IL-17A, rmIL-17A/F, or rm-IL-17F to L. pneumophila infected IL-17A−/− mice 1 h postinfection. BALF and lung tissues were harvested at 72 h after L. pneumophila infection. CFU in the lung (A) and liver (B), total leukocytes (WBC) and neutrophils (PMN) in BALF (C-D), cytokine/chemokine levels in BALF and lung homogenates (E-F) were quantified at 72 h postinfection. *Indicates difference between control (BSA) and cytokine administered mice. n = 6-10 mice in each group at each time-point. *p < 0.05, **p < 0.01,*** p < 0.001.
PMNs are the major source of IL-17A and IL-17F in lungs
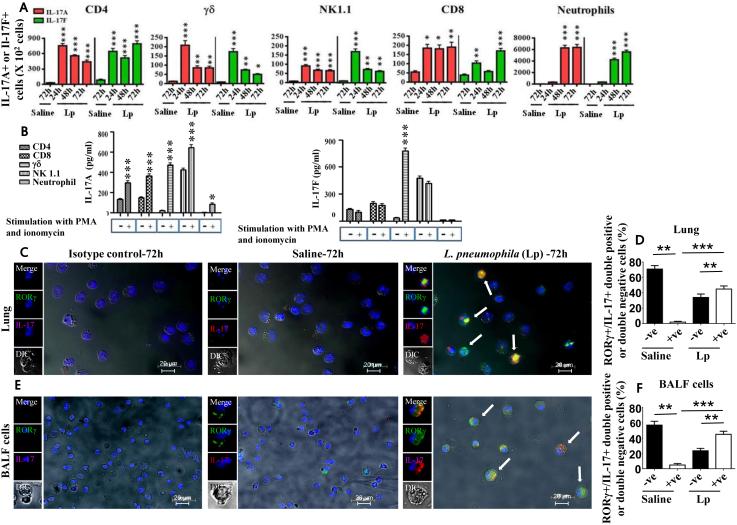
A critical event in bacterial pneumonia is the migration of circulating neutrophils to the inflammed tissues 8, 9, 19. To determine the cell types within the infected lung lesions that produce IL-17A and/or IL-17F, we utlized flow cytometry. Our results show increased accumulation of IL-17A+ and IL-17F+ T cell subsets (CD4, CD8, γδ and NK1.1+ cells) compared to neutrophils in infected lungs at 24 h postinfection, whereas numbers of IL-17A+ and IL-17F+ neutrophils infiltrating into the lungs were much higher than T cells at 48 and 72 h post-infection (Fig. 5A). Moreover, the numbers of IL-17F+ neutrophils were slightly less than IL-17A+ neutrophils at 48 and 72 h post-infection (Fig. 5A). Furthermore, macrophages isolated from the infected lungs did not produce either IL-17A or IL-17F (data not shown). We then explored if IL-17A or IL-17F is produced by T cell subsets and neutrophils on an individual cell basis upon L. pneumophila stimulation and observed that T cell subsets (CD4, γδ, NK1.1, CD8, neutrophils) and neutrophils produce IL-17A at 24 h post-infection. Remarkably, T cell subsets produce more IL-17A than neutrophils on an individual cell basis (Fig. 5B). However, only γδ cells produce IL-17F following L. pneumophila infection (Fig. 5B). Immunocytochemistry was performed on neutrophils isolated from L. pneumophila-infected lung homogenates and BALF to assess IL-17A expression and ROR-γt, constitutive expression of the transcription factor for IL-17. In neutrophils from uninfected lungs (parenchyma or airspace) minimal ROR-γt+ IL-17A producing neutrophils were detected whereas neutrophils from infected lungs and BALF exhibited substantial ROR-γt+ IL-17A production at 72 h postinfection (Fig. 5C-F). To further evaluate the ROR-γt+ IL-17A producing neutrophils we obtained purified neurophils from uninfected and infected lungs at 72 h postinfection and using flow cytometry found that neutrophils both from infected lung paranchma (Supplemental Fig. 2A) and airspaces (Supplemental Fig. 2B) produce IL-17A. Moreover, we found greater expression of IL-17 receptor A (IL-17RA) than IL-17RC on neutrophils from infected airspaces at 72 h postinfection (Supplemental Fig. 2C).
Figure 5. L. pneumophila infection regulates IL-17A and IL-17F production by neutrophils.
A. Flow cytometric analysis was performed on cells obtained from whole lung digests at 24, 48, or 72 h postinfection with L. pneumophila (Lp) (107 CFU/mouse) as described in Materials and Methods. Total numbers of IL-17A and IL-17F positive cells from each lung following saline challenge or infection. For experiments A-B, a total of 5-8 mice/group were used. *, p<0.05; **, p<0.01; ***, p<0.001 (compared with 24 h L. pneumophila infected mice). B. CD4, γδ, NK1.1, and CD8 cells were purified from the spleen whereas neutrophils were isolated from digested lungs of L. pneumophila-infected mice at 24 h using magnetic selection (positive selection for γδ cells; negative selection for CD4, CD8, NK1.1 cells and neutrophils). Production of IL-17A and IL-17F was determined by ELISA of the culture supernatants following stimulation of 1 × 104 cells with PMA and ionomycin for 5 h. For experiment B, a total of 6-8 mice were used to isolate cells. C-F. Neutrophils express IL-17A and RORγt following L. pneumophila infection. Neutrophils were purified from the lungs (C-D) or BALF (E-F) and stained with antibodies against IL-17A and RORγt. Shown is a representative image of 6 slides with similar results and 500 cells were counted in lung sections or BALF cytospins and plotted. *, p<0.05; **, p<0.01; ***, p<0.001 (compared with samples with saline challenge).
IL-17+ neutrophils rescue impaired host defense in Il17a−/− mice
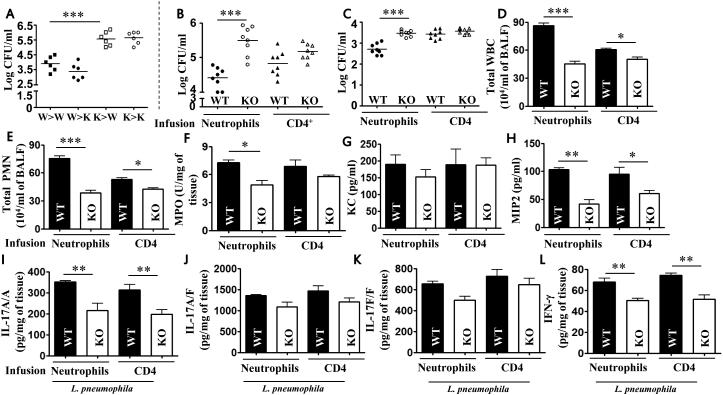
We generated bone marrow chimeras to assess the contribution of bone marrow-derived IL-17A versus stromal cell-derived IL-17A in bacterial clearance, neutrophil accumulation, and cytokine/chemokine expression. WT (W) → WT (W) and WT (W) → Il17a−/− (K) mice showed efficient bacterial clearance whereas Il17a−/− (K) → WT (W) and Il17a−/− (K) → Il17a−/− (K) mice showed impaired bacterial clearance (Fig. 6A). Moreover, in WT → Il17a−/− mice the rescued host defense effects were blocked by addition of 1 μg IL-17A blocking Ab (data not shown). These studies suggest that IL-17A-producing, bone marrow-derived cells predominantly contribute to host resistance. Since IL-17+ neutrophils get recruited to the lungs at 48 and 72 h following L. pneumophila infection, we wanted to determine the functional significance of these cells during L. pneumophila infection. To accomplish this, we adoptively transferred WT or Il17a−/− neutrophils (106 cells/mouse) or CD4 T cells (106 cells /mouse) to recipient Il17a−/− mice 1 h postinfection. Bacterial clearance, neutrophil accumulation, and cytokines/chemokines including Il-17A were reconstituted in WT neutrophils → Il17a−/− mice but not in Il17a−/− neutrophils → Il17a−/− mice (Fig. 6B-L). As compared to WT mice, WT CD4+ T cell infusion into Il17a−/− mice did not result in substantial differences either in bacterial clearance in the lungs or bacterial dissemination to liver (Fig. 6B-C), but increases in neutrophil accumulation (Fig. 6D-F) and cytokine/chemokine production (Fig. 6G-L) were observed. However, influsion of neutrophils or CD4 T cells did not induce lung injury as determined by histopathology (data not shown).
Figure 6. Neutrophil-derived IL-17A rescues bacterial clearance, cellular recruitment, and cytokine expression following L. pneumophila infection.
A. Bone marrow chimeras were generated by lethal irradiation of Il17a−/− (knockout mice; K) and WT mice (W) and reconstitution with bone marrow cells from K or W mice via tail injection. Bacterial CFU in the lungs were enumerated at 72 h post-L. pneumophila infection. (n=6 mice/group). B-I. Adoptive transfer of neutrophils or CD4 T cells. Bacterial clearance in the lungs (B) and liver (C), leukocyte/neutrophil influx (D-F) and cytokine expression (G-L) in BALF or lung homogenates of IL-17A−/− mice 72 h after L. pneumophila infection followed by adoptive transfer of cells at 1 h postinfection (106 cells/mouse). (n=6-9 mice/group). *, p<0.05; **, p<0.01;***, p<0.001.
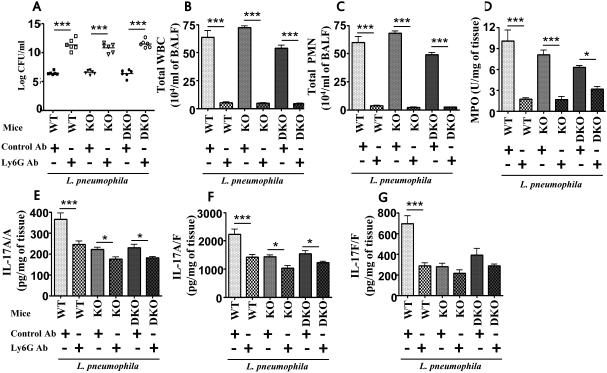
Neutrophil depletion increases CFU and reduces IL-17A, IL-17A/F, and IL-17F production in Rag2−/− and Rag2−/−/Il2rg−/− mice
To further explore the role of neutrophils in bacterial burden and IL-17A, IL-17A/F, and IL-17F production, we depleted neutrophils from Rag2−/− (lack T and B cells; knockout mice - KO) and Rag2−/−/Il2rg−/− mice (lack T cells, B cells, and NK/NKT cells; Double knockout mice - DKO). Neutrophil depletion in Rag2−/− and Rag2−/−/Il2rg−/− mice resulted in higher bacterial burden in the lungs (Fig. 7A) and reduced IL-17A, IL-17A/F, and IL-17F following L. pneumophila infection (Fig. 7E-G), confirming that neutrophils are important for bacterial clearance and indeed a major source of IL-17 production. We confirmed neutrophil depletion in the airspaces and lung parenchyma by the Ly6G Ab (Fig. 7B-D).
Figure 7. Neutrophil depletion in Rag2−/− and Rag2/Ilrg−/− mice enhances bacterial burden and reduces IL-17A, IL-17A/F, and IL-17F production following L. pneumophila infection.
WT, T cell- and B cell–deficient Rag2−/− (KO) mice or T cell-, B cell-, and NK/NKT cell-deficient Rag2/Il2rg−/− (DKO) mice were pretreated with neutrophil (Ly6G) depleting mAb (IA8) at 12 h and 2 h prior to L. pneumophila infection. Bacterial burdern in the lungs, leukocyte/neutrophil numbers and cytokine (IL-17A, IL-17A/F, and IL-17F) levels were determined at 72 h postinfection. For A-F, n=6-8 mice/group; *, p<0.05; **, p<0.01;***, p<0.001. Cont-Ab: Isotype-matched control antibody.
IL-17A is upstream of IFN-γ in bacterial clearance
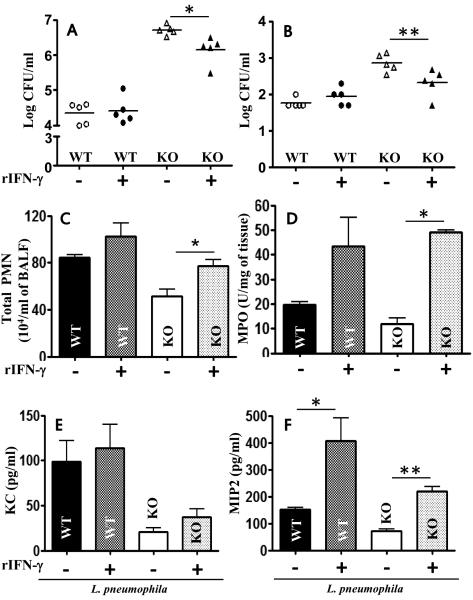
Il17a−/− mice reconstituted with WT PMNs exhibited enhanced bacterial elimination from lungs and liver (Fig. 6B-C) and IFN-γ production in the lungs following bacterial challenge (Fig. 6L). Furthermore, IFN-γ is known to be critical for control of L. pneumophila multiplication in macrophages and the lungs 20-23. In a mouse kidney ischemia-referfusion injury model, IL-17A has been shown to regulate IFN-γ 24. To determine the relationship between the IL-17A production and IFN-γ levels in the lung, exogenous recombinant mouse IFN-γ (50 ng) was administered i.t. to Il17a−/− mice 1 hour after infection. As compared with controls, IFN-γ reduced bacterial burden in the lung (Fig. 8A), dissemination (Fig. 8B), enhanced PMN recruitment (Figs. 8C-D), and chemokine expression (Fig. 8E-F). Next, we determined the cellular origin of IFN-γ in L. pneumophila-infected lungs and found T cells, but not neutrophils or macrophages, to be the major source of IFN-γ in infected lungs (Supplemental Fig. 3). On the other hand, administration of exogenous IL-17A (1 μg/mouse) in Ifnγ−/− mice 1 h prior to L. pneumophila challenge did not decrease the bacterial burden in the lungs or spleen at 72 h post-infection (Supplemental Fig. 4). These results suggest that neutrophil-deived IL-17A stimulates IFN-γ production via a paracrine manner and IFN-γ is critical to control L. pneumophila infection in the lungs.
Figure 8. Recombinant IFN-γ rescues bacterial clearance, neutrophil recruitment, and chemokine expression following L. pneumophila infection in IL-17A KO mice.
Mice were i.t. treated with IFN-γ treatment 1 h postinfection with L. pneumophila. Bacterial CFU in lungs (A) and spleen (B), leukocyte/neutrophil influx (C-D) and chemokine expression (E-F) were determined at 72 h postinfection in control (WT) and IL-17A KO mice. n = 5-8/group; *, p<0.05; **, p<0.01;***, p<0.001.
Human neutrophils produce IL-17A
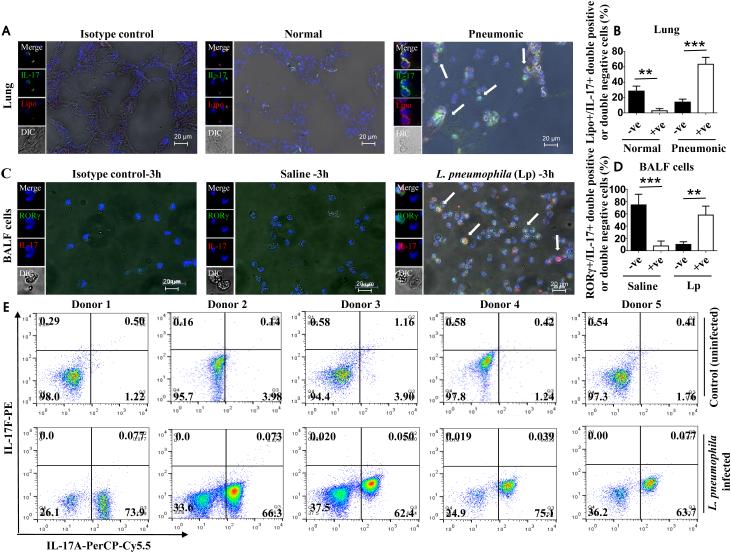
To assess whether IL-17A is expressed in lung tissues during human bacterial pneumonia, we performed immunofluorescence staining for IL-17A on lung sections of patients who succumbed to bacterial (Gram-negative) pneumonia and from patients who had died from a non-pulmonary infection. Although control lung sections indicated a small number of lipocalin-2 (Lipo; a neutrophil marker) expressing IL-17A+ cells, lung sections from patients who succumbed to bacterial pneumonia showed higher numbers of Lipo+/IL-17A+ (double positive) neutrophils (Fig. 9A-B). To confirm that IL-17A producing neutrophils are RORγt-positive in response to L. pneumophila infection in humans, we purified neutrophils from peripheral blood by negative selection and incubated these cells with L. pneumophila for 3 h at an MOI of 1.0. Using immunofluorescence staining, we detected an increase in RORγt+IL-17A+ (double positive) neutrophils at 3 h postinfection (Fig. 9C-D). Moreover, using flow cytometry, we confirmed that human neutrophils from five healthy donors indeed produce IL-17A, but not IL-17F, following L. pneumophila infection (Fig. 9E).
Figure 9. Human neutrophils produce IL-17A following L. pneumophila infection.
Bacterial (Gram-negative) pneumonic or non-pneumonic lung sections were stained with lipocalin-2 (lipo; neutrophil-specific) Ab, IL-17A Ab, or control Ab and 500 cells in each section were counted. Shown is a representative section of five lungs from 5 pneumonic or normal in each group (A) and the mean ± SE of 500 cells from 5 slides (B). Blood was obtained from healthy volunteers and neutrophils were purified using negative selection with magnetic beads. Neutrophils were stimulated with L. pneumophila at a MOI of 1.0. At 3 h postinfection, neutrophils were stained with Abs against RORγt and IL-17A and immunofluorescence performed. Shown is a representative image of neutrophils from 5 healthy individuals (C) and the mean ± SE of 500 cells from 5 slides (D). Production of IL-17A and IL-17F by neutrophils following L. pneumophila infection. For A-D, p<0.05; **, p<0.01;***, p<0.001. E. Human neutrophils were purified and stimulated with L. pneumophila at an MOI of 1.0. At 3 h postinfection, neutrophils were stained with IL-17A antibody or IL-17F antibody. The upper panel indicates control or uninfected cells whereas the lower panel indicates stimulated cells. Dot plot shows IL-17A and/or IL-17F producing cells upon stimulation.
DISCUSSION
The present study was conducted to determine the role of IL-17A versus IL-17F in lung inflammation and host defense against L. pneumophila pneumonia. Using two completely different approaches, gene-deficient mice on a non-permissive background for L. pneumophila growth and IL-17 neutralization on a permissive background for L. pneumophila growth, we established a critical role for the IL-17A homodimer and showed it is produced primarily by neutrophils during host protection following bacterial challenge. Furthermore, we demonstrated that IL-17A is essential for the production of IFN-γ, which regulates neutrophil-mediated host defense. Moreover, IL-17F is not only dispensable for the induction of host responses but does not show additive, synergistic, or compensatory effects with IL-17A in these responses. These findings of interest since both IL-17A and IL-17F are produced by neutrophils and T cells in the lungs following infection.
IL-17A has been shown to contribute to host responses to a variety of bacterial and fungal agents, such as Klebsiella pneumoniae, Streptococcus pneumoniae, Bordetella pertussis, Yersinia pestis, Mycobacterium tuberculosis, and Candida albicans 25. IL-17 is secreted by a variety of cells including T helper type 17 (Th17) cells, CD8, and natural killer (NK) cells, and as a result induces Th17-type host responses characterized by neutrophil recruitment and production of proinflammatory cytokines and chemokines 18, 26. However, in most of these studies the role of IL-17F in host defense has not been examined. Previously, a study using IL-17A/F-deficient mice infected with L. pneumophila found reduced neutrophil migration, survival, and production of proinflammatory cytokines (IL-6 and TNF-α) and chemokines (KC, LIX, and MIP-2); however, it is not possible to determine the individual contributions of IL-17A and IL-17F from this study. In the current investigation, we unequivocally demonstrate the critical role of IL-17A, but not IL-17F in regulating neutrophil-mediated host defense against pulmonary L. pneumophila infection.
Bone marrow-derived cells in the lung produce numerous neutrophil chemoattractants, such as KC and MIP-2 27, 28, whereas stromal cells, including alveolar epithelial type II cells, produce other neutrophil chemoattractants including LIX 29. Our findings are consistent with prior reports of the role of hematopoietic and resident cells during lung inflammation. MyD88 expression in hematopoietic cells is more important for LPS-induced expression of TNF-α and IL-12p40 30 than is its expression in stromal cells, despite the fact that both hematopoietic and resident cell-derived MyD88 signaling are essential for LPS-induced neutrophil accumulation 31, 32. On the other hand, MD-2 signaling in both hematopoietic and resident cells is essential for neutrophil-mediated inflammation and the expression of MIP-2, TNF-α, and IL-6 is mediated by both cell types in the lungs after LPS challenge 33. In a similar manner, KC produced by both hematopoietic and stromal cells is important for bacterial clearance and neutrophil recruitment to the lung in response to K. pneumonia infection 9. In this study, using bone marrow chimeras and adoptive cell transfers we specifically identified one hematopoietic cell, the neutrophil, as a key mediator for bacterial clearance in the lungs following L. pneumophila infection.
The hallmark of Th17 cells is expression of the cytokine IL-17A 18, 34. Interestingly, Th17 cells also express IL-17F which shares strong homology to IL-17A 11, 35 and is expressed form the same choromosomal region 36. In previous studies, we demonstrated that γδ and CD4+ T cells are the key producers of IL-17A in the lungs in response to Gram-negative extracellular bacteria, such as E. coli 19. Regarding the source of IL-17A and IL-17F, the findings from the current study indicated both T cells (CD4, CD8, NK 1.1 and γδ) and neutrophils produce both IL-17A and IL-17F following L. pneumophila infection, while we did not observe IL-17A or IL-17F production by macrophages following infection (data not shown). Although multiple reports show the production of IL-17A and IL-17F by T cell subsets during infection 19, 37, recent studies also demonstrated that IL-17A production by neutrophils in the lung during Aspergillus fumigates and Yersinia pestis infection contributes to host resistance 38. Additionally, during Y. pestis infection IL-17A produced by neutrophils (Ly6G+), and not by γδT or NKT cells, was shown to be important for host protection 39.
Although prior studies have conclusively demonstrated the effects of mouse IL-17A both in vitro and in vivo non-infectious models, the comparisons between IL-17A and IL-17F were performed using human proteins. The primary conclusion from these studies is that IL-17A is more potent to IL-17A/F in the production of inflammatory mediators from epithelial cells 40. In a study using eukaryotic-derived IL-17A and IL-17F homodimers and the heterodimer showed IL-17A was more potent than IL-17A/F, and IL-17A/F more potent than IL-17F in vivo 40. Although our observations confirm this in regard to neutrophil recruitment 41, our results also show that IL-17A is more potent to IL-17A/F in rescuing bacterial CFU in the lung and liver, neutrophil recruitment and chemokine (KC and MIP-2) production in Il-17a−/− mice following L. pneumophila infection. While the mechanistic differences between human IL-17 and mouse IL-17 signaling remain unclear, this may be mediated via different receptor usage. In this regard, human IL-17A and IL-17F induces signaling through two receptors, IL-17RA and IL-17RC 12, whereas mouse IL-17A, IL-17A/F, and IL-17F-induced intracellular signaling requires IL-17RA 42. As a consequence, receptor binding affinity or avidity may contribute to the differential effect. However, several reports suggest that these proteins may not share the same receptor or may have different functions mediated via distinct signaling cascades. For instance, Starnes et al 43 demonstrated that IL-17F can induce TGF-β expression in endothelial cells. Furthermore, the same report indicated that IL-17A can enhance angiogenesis in tumor cells whereas IL-17F can reduce angiogenesis 43. From our studies, however, IL-17RA is induced at a greater level in bacteria-infected neutrophils than IL-17RC, suggesting IL-17RA may be the major receptor for IL-17A binding (Supplemental Fig. 2C).
Neutrophils are known to play a critical role in host defense during pulmonary L. pneumophila infection via immunomodulation, but not via direct bacterial killing 44, 45. In this context, it has been demonstrated that neutrophils produce IL-12 in the lungs to drive a Th1-type host response leading to IFN-γ production 15, 46. In addition, it is well established that IFN-γ is critical for control of L. pneumophila infection in the lungs 21. In this study, we found that neutrophil-derived IL-17 induced IFN-γ drives bacterial clearance. However, we did not observe IFN-γ production by neutrophils following L. pneumophila infection (Supplemental Fig. 3). These results suggest that IL-17A may induce IFN-γ through paracrine manner. The observations from this study somethat differ from those of a prior investigation using L. pneumophila in which no differences in IFN-γ expression were seen between WT and IL-17A/F deficient mice because individual contribution of IL-17A or IL-17F was not examined by the previous study 15. However, in another study using gene-deficient mice or macrophage-depletion it was shown that IFN-γ is important for macrophage-activated protection (M1 phenotype) against Y. pestis although the role of neutrophils has not been examined 39. The current study indicated that the IL-17A-IFN-γ signaling axis contributes to innate immune responses associated with L. pneumophila lung infection in paracrine manner (Supplemental Figure 5). In a previous study, it has been shown that infiltrating NKT cells produce IFN-γ within short time (3 hours) of ischemia-reperfusion injury (IRI) 47. In the same investigation, IFN-γ was primarily produced by CD1d-restricted NKT cells and GR-1+ neutrophils in kidney following IRI 47. Therefore, in the previous study, CD4+ NKT cells provides an explanation for how T cells, the traditional players of the adaptive immune ersponses, play an essential role in innate immunity. However, future studies are needed to determine the role of this cell type in producing IFN-γ in response to IL-17 during L. pneumophila infection.
Hyper IgE Syndrome (HIES) patients have low levels of IL-17 and have recurrent bacterial infections in skin and the lung due to decreased neutrophil recruitment to tissues 48. A recent study reported cavitation of Legionella pneumonia in an HIES patient 49, showing the critical role of IL-17 in human pulmonary Legionella infections. Further, we found IL-17A+ neutrophils in bacterial pneumonic lungs and induction of IL-17A in human neutrophils by L. pneumophila infection, validating the results obtained from the preclinical (mouse) model.
Overall, the novel observations in this study unveil a previously unrecognized function of neutrophils to augment host defense via the IL-17A-IFN-γ axis and thus, establish a paradigm shift in the way clinicians and researchers think about the role of neutrophils in host defense during bacterial pneumonia followed by sepsis. Moreover, these findings are fundamental to the design of improved treatment and/or prevention strategies to control bacterial pneumonias. Additionally, these mechanisms might contribute to inflammation and host defense in response to infections that are caused or complicated by other bacterial pathogens.
METHODS
Bacterial burden, neutrophil influx, and flow cytometry
Philadelphia 1 strain (ATCC 33152) of L. pneumophila was used 50. The bacterium was grown on buffered charcoal-yeast extract (BCYE) agar for 2 days at 37 °C before use. Experimental L. pneumophila infection and creation of bone marrow chimeras were conducted as described 9, 51. Bacterial numbers in tissues were enumerated by serial dilutions whereas neutrophil recruitment to in bronchoalveolar lavage fluid (BALF) and lung homogenates were measured by total/differential counts and myeloperoxidase assays, respectively 8, 9. BALF and tissue homogenates were used to measure cytokines using sandwich ELISA kits for KC (R&D Systems, MN 55413), MIP-2 (R&D Systems), LIX (R&D Systems), IL-17A/A (eBioscience, Inc., CA 92121), IL-17A/F (eBioscience, Inc.), IL-17F/F (eBioscience, Inc.) and IFN-γ (R&D Systems) based on manufacturer’s protocols. CD4, CD8, NK1.1, and total T cells were purified from the spleens of L. pneumophila-infected C57Bl/6 mice whereas neutrophils were obtained from collagenase-digested lungs by negative magnetic selection (StemCell Technologies, Vancouver, Canada) 52. Neutrophils were purified from the digested lungs of L. pneumophila-infected mice using an antibody cocktail (CD5, CD4, CD45R/B220, TER119, F4/80, CD11c, c-KIT) for negative selection whereas γδ cells were isolated by positive selection using PE-conjugated anti-γδ TCR (BD Biosciences) as previously described 8.
Animals, in vivo methods, and tissue isolation
Female Il17a−/− (Iwakura), Il17f−/− (Iwakura), Il17a/f−/− (Iwakura), Rag2−/− (Taconic), and Rag2/Il-2rg−/− (Taconic), and control female mice on a C57BL/6 background (Taconic and Jackson) were used in compliance with institutional animal use regulations. Neutrophil depletion in mice was induced by intraperitoneal (i.p.) injection of 50 μg of anti-Ly6G mAb (clone IA8; BD Biosciences, CA 95131) or isotype control Ab (clone R35-95; BD Biosciences) at 12 h and 2 h prior to pathogen challenge as described earlier 8, 9, 51. IL-17A or IL-17F activity was blocked by i.p. administration of 1 μg of anti-IL-17A (monoclonal – rat IgG2a, Cat # MAB421/CF; R&D Systems), anti-IL-17F (polyclonal, Cat # AF2057/CF; R&D Systems), control Ab for anti-IL-17A (rat IgG2a, cat # MAB006/CF, R&D Systems) or control Ab for anti-IL-17F (goat IgG, cat # AB-108-C, R&D Systems) in A/J mice (Jackson). Recombinant IL-17A (Cat # 421-ML/CF, R&D Systems), IL-17A/F (Cat # 5390-IL/CF, R&D Systems), IL-17F (Cat # 2057-IL/CF, R&D Systems), or IFN-γ (Cat # 485-MI/CF, R&D Systems) was administered intratrachealy (i.t.) at a concentration of 1 μg/mouse whereas the control mice were administered with PBS supplemented with BSA (R&D Sysystems, MN). For adoptive transfer, naïve bone marrow neutrophils or splenic CD4+ T cells (106 cells/mouse) were injected via the tail vein. Methods for BALF collection, organ harvest, cell isolation (T cells and neutrophils) and histology were described in earlier publications 8, 9.
Mouse lung preparation for histopathology
Lung tissue was prepared by intratracheal infusion of pressurized, low-melting agarose containing formalin. Isolated tissue was placed in 4% formalin overnight. Five μM sections were cut and H&E staining was performed. Slides were scored for inflammation/injury by a Veterinary Pathologist in a blinded fashion (0-4 scale) using a scoring system described by Matute Bello et al 53. Further details regarding this method are provided in the online supplement.
Immunohistochemistry
In brief, lung sections were deparaffinised, fixed and permeabilized with the buffer containing 0.1% Triton X-100 and then blocked with serum. For mouse lungs, sections were incubated with anti-IL-17 (rabbit polyclonal, cat # ab91649, Abcam, MA 02139) or ROR-γ (rabbit polyclonal, cat # ab78077, Abcam). For human lungs, pneumonic and normal lung sections were incubated with anti-IL-17 (rabbit polyclonal, cat # ab136668, Abcam) or Liopcalin-2 (rabbit polyclonal, cat # ab41105, Abcam). Rabbit IgG, polyclonal (cat # ab27478, Abcam) was used as an isotype control. As a control, Lung sections were washed and incubated with Alexa-conjugated secondary antibodies (Invitrogen, CA). Lung sections were dehydrated and mounted using Vectashield mounting medium (Vector Laboratories, CA). The slides were viewed using a Zeiss Axioskop 2 Plus microscope.
Immunocytochemistry
The method is the same as described in immunohistochemistry, including antibodies used to demonstate proteins in lung sections, except we used neutrophils in these experiments.
Bone marrow transplantation
Donor and recipient C57Bl/6 mice between 6-8 wk old were used. Recipient mice were gamma-irradiated with two 525-rad doses 3 h apart. Bone marrow cells (8 × 106/mouse) were injected into the tail vein of the irradiated recipients, and mice were maintained on 0.2% neomycin sulfate for the first 3 wks. The reconstituted mice were used for experiments 6 wks after transplantation. We found that greater than 78-86% of blood leukocytes were derived from donor mice at the time the mice were used for experiments. Irradiated mice that were not transplanted with donor cells died between days 18 and 20 after irradiation (data not shown).
Human ex vivo studies
Peripheral blood was isolated from healthy donors in compliance with institutional regulations and neutrophils were purified using negative magnetic selection (StemCell Technologies, Vancouver, Canada) 8. These cells were stimulated with 1 MOI of L. pneumophila. At 3 h post stimulation, cells were fixed and used for immunofluorescence microscopy and flow cytometry.
Statistics
Two-way ANOVA followed by Bonferroni's post hoc analysis was used for multiple comparisons. Data were analyzed with the Student’s t-test (between two groups). The results are from 2-3 independent experiments. Survival curves were compared by the Wilcoxon rank sign test. Unless otherwise stated, all data are expressed as mean and standard error of the mean (SE). Statistics were calculated with Prism version 4.0 (GraphPad). Differences were considered significant at *P<0.05, **P<0.01 and ***<0.001.
Supplementary Material
ACKNOWLEGMENTS
Supported by Scientist Awards from the Flight Attendant Medical Research Institute (CIA and YCSA to SJ; YCSA to SC and SB); and grants from the NIH (R01 HL-091958, R01 HL-091958S1 via ARRA and R01 AI113720 to SJ and R15 ES023151-02 to SB). We thank the Lung Biology members Mary Leissinger, Taylor Ferguson, Jonte Ellison, Liliang Jin, Ritwij Kulkarni, Pankaj Baral, and K. Jeyagowri for helpful discussions.
Footnotes
The authors have no conflicts of interest.
REFERENCES
- 1.Mizgerd JP. Lung infection--a public health priority. PLoS Med. 2006;3(2):e76. doi: 10.1371/journal.pmed.0030076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agullo-Ortuno MT, Garcia-Mancebo ML, Montes-Ares O, Noguera-Velasco JA. Biochemical and immunologic features of an outbreak of Legionnaires disease: comparative study between community-acquired pneumonias. Diagn Microbiol Infect Dis. 2006;56(1):7–11. doi: 10.1016/j.diagmicrobio.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Almirall J, Blanquer J, Bello S. Community-acquired pneumonia among smokers. Arch Bronconeumol. 2014;50(6):250–254. doi: 10.1016/j.arbres.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 4.Losick VP, Stephan K, Smirnova, Isberg RR, Poltorak A. A hemidominant Naip5 allele in mouse strain MOLF/Ei-derived macrophages restricts Legionella pneumophila intracellular growth. Infect Immun. 2009;77(1):196–204. doi: 10.1128/IAI.01011-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown AS, van Driel IR, Hartland EL. Mouse models of Legionnaires' disease. Curr Top Microbiol Immunol. 2013;376:271–291. doi: 10.1007/82_2013_349. [DOI] [PubMed] [Google Scholar]
- 6.Brieland J, Freeman P, Kunkel R, Chrisp C, Hurley M, Fantone J, et al. Replicative Legionella pneumophila lung infection in intratracheally inoculated A/J mice. A murine model of human Legionnaires' disease. Am J Pathol. 1994;145(6):1537–1546. [PMC free article] [PubMed] [Google Scholar]
- 7.Brieland JK, Loebenberg D, Menzel F, Hare RS. Efficacy of SCH27899 in an animal model of Legionnaires' disease using immunocompromised A/J mice. Antimicrob Agents Chemother. 2000;44(5):1333–1336. doi: 10.1128/aac.44.5.1333-1336.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Batra S, Cai S, Balamayooran G, Jeyaseelan S. Intrapulmonary administration of leukotriene B(4) augments neutrophil accumulation and responses in the lung to Klebsiella infection in CXCL1 knockout mice. J Immunol. 2012;188(7):3458–3468. doi: 10.4049/jimmunol.1101985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai S, Batra S, Lira SA, Kolls JK, Jeyaseelan S. CXCL1 regulates pulmonary host defense to Klebsiella Infection via CXCL2, CXCL5, NF-kappaB, and MAPKs. J Immunol. 2010;185(10):6214–6225. doi: 10.4049/jimmunol.0903843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tateda K, Moore TA, Deng JC, Newstead MW, Zeng X, Matsukawa A, et al. Early recruitment of neutrophils determines subsequent T1/T2 host responses in a murine model of Legionella pneumophila pneumonia. J Immunol. 2001;166(5):3355–3361. doi: 10.4049/jimmunol.166.5.3355. [DOI] [PubMed] [Google Scholar]
- 11.Dong C. Regulation and pro-inflammatory function of interleukin-17 family cytokines. Immunol Rev. 2008;226:80–86. doi: 10.1111/j.1600-065X.2008.00709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang SH, Dong C. Signaling of interleukin-17 family cytokines in immunity and inflammation. Cell Signal. 2011;23(7):1069–1075. doi: 10.1016/j.cellsig.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright JF, Bennett F, Li B, Brooks J, Luxenberg DP, Whitters MJ, et al. The human IL-17F/IL-17A heterodimeric cytokine signals through the IL-17RA/IL-17RC receptor complex. J Immunol. 2008;181(4):2799–2805. doi: 10.4049/jimmunol.181.4.2799. [DOI] [PubMed] [Google Scholar]
- 14.Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34(2):149–162. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 15.Kimizuka Y, Kimura S, Saga T, Ishii M, Hasegawa N, Betsuyaku T, et al. Roles of interleukin-17 in an experimental Legionella pneumophila pneumonia model. Infect Immun. 2012;80(3):1121–1127. doi: 10.1128/IAI.05544-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X, Angkasekwinai P, Dong C, Tang H. Structure and function of interleukin-17 family cytokines. Protein Cell. 2011;2(1):26–40. doi: 10.1007/s13238-011-1006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ely LK, Fischer S, Garcia KC. Structural basis of receptor sharing by interleukin 17 cytokines. Nat Immunol. 2009;10(12):1245–1251. doi: 10.1038/ni.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolls JK, Khader SA. The role of Th17 cytokines in primary mucosal immunity. Cytokine Growth Factor Rev. 2010;21(6):443–448. doi: 10.1016/j.cytogfr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balamayooran T, Batra S, Balamayooran G, Cai S, Kobayashi KS, Flavell RA, et al. RIP2 Controls Pulmonary Host Defense to E. coli Infection via the Regulation of IL-17A. Infect Immun. 2011 doi: 10.1128/IAI.05641-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schiavoni G, Mauri C, Carlei D, Belardelli F, Pastoris MC, Proietti E. Type I IFN protects permissive macrophages from Legionella pneumophila infection through an IFN-gamma-independent pathway. J Immunol. 2004;173(2):1266–1275. doi: 10.4049/jimmunol.173.2.1266. [DOI] [PubMed] [Google Scholar]
- 21.Deng JC, Tateda K, Zeng X, Standiford TJ. Transient transgenic expression of gamma interferon promotes Legionella pneumophila clearance in immunocompetent hosts. Infect Immun. 2001;69(10):6382–6390. doi: 10.1128/IAI.69.10.6382-6390.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harding CR, Stoneham CA, Schuelein R, Newton H, Oates CV, Hartland EL, et al. The Dot/Icm effector SdhA is necessary for virulence of Legionella pneumophila in Galleria mellonella and A/J mice. Infect Immun. 2013;81(7):2598–2605. doi: 10.1128/IAI.00296-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plumlee CR, Lee C, Beg AA, Decker T, Shuman HA, Schindler C. Interferons direct an effective innate response to Legionella pneumophila infection. J Biol Chem. 2009;284(44):30058–30066. doi: 10.1074/jbc.M109.018283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li L, Huang L, Vergis AL, Ye H, Bajwa A, Narayan V, et al. IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J Clin Invest. 2010;120(1):331–342. doi: 10.1172/JCI38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peck A, Mellins ED. Precarious balance: Th17 cells in host defense. Infect Immun. 2010;78(1):32–38. doi: 10.1128/IAI.00929-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guglani L, Khader SA. Th17 cytokines in mucosal immunity and inflammation. Curr Opin HIV AIDS. 2010;5(2):120–127. doi: 10.1097/COH.0b013e328335c2f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bozic CR, Kolakowski LF, Jr., Gerard NP, Garcia-Rodriguez C, von Uexkull-Guldenband C, Conklyn MJ, et al. Expression and biologic characterization of the murine chemokine KC. J Immunol. 1995;154(11):6048–6057. [PubMed] [Google Scholar]
- 28.Wolpe SD, Sherry B, Juers D, Davatelis G, Yurt RW, Cerami A. Identification and characterization of macrophage inflammatory protein 2. Proc Natl Acad Sci U S A. 1989;86(2):612–616. doi: 10.1073/pnas.86.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeyaseelan S, Manzer R, Young SK, Yamamoto M, Akira S, Mason RJ, et al. Induction of CXCL5 during inflammation in the rodent lung involves activation of alveolar epithelium. Am J Respir Cell Mol Biol. 2005;32(6):531–539. doi: 10.1165/rcmb.2005-0063OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noulin N, Quesniaux VF, Schnyder-Candrian S, Schnyder B, Maillet I, Robert T, et al. Both hemopoietic and resident cells are required for MyD88-dependent pulmonary inflammatory response to inhaled endotoxin. J Immunol. 2005;175(10):6861–6869. doi: 10.4049/jimmunol.175.10.6861. [DOI] [PubMed] [Google Scholar]
- 31.Skerrett SJ, Liggitt HD, Hajjar AM, Ernst RK, Miller SI, Wilson CB. Respiratory epithelial cells regulate lung inflammation in response to inhaled endotoxin. Am J Physiol Lung Cell Mol Physiol. 2004;287(1):L143–152. doi: 10.1152/ajplung.00030.2004. [DOI] [PubMed] [Google Scholar]
- 32.Quinton LJ, Jones MR, Robson BE, Simms BT, Whitsett JA, Mizgerd JP. Alveolar epithelial STAT3, IL-6 family cytokines, and host defense during Escherichia coli pneumonia. Am J Respir Cell Mol Biol. 2008;38(6):699–706. doi: 10.1165/rcmb.2007-0365OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cai S, Zemans RL, Young SK, Worthen GS, Jeyaseelan S. Myeloid differentiation protein-2-dependent and -independent neutrophil accumulation during Escherichia coli pneumonia. Am J Respir Cell Mol Biol. 2009;40(6):701–709. doi: 10.1165/rcmb.2008-0152OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khader SA. Th17 cytokines: the good, the bad, and the unknown. Cytokine Growth Factor Rev. 2010;21(6):403–404. doi: 10.1016/j.cytogfr.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 35.Reynolds JM, Angkasekwinai P, Dong C. IL-17 family member cytokines: regulation and function in innate immunity. Cytokine Growth Factor Rev. 2010;21(6):413–423. doi: 10.1016/j.cytogfr.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang XO, Chang SH, Park H, Nurieva R, Shah B, Acero L, et al. Regulation of inflammatory responses by IL-17F. J Exp Med. 2008;205(5):1063–1075. doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iwakura Y, Nakae S, Saijo S, Ishigame H. The roles of IL-17A in inflammatory immune responses and host defense against pathogens. Immunol Rev. 2008;226:57–79. doi: 10.1111/j.1600-065X.2008.00699.x. [DOI] [PubMed] [Google Scholar]
- 38.Werner JL, Gessner MA, Lilly LM, Nelson MP, Metz AE, Horn D, et al. Neutrophils produce interleukin 17A (IL-17A) in a dectin-1- and IL-23-dependent manner during invasive fungal infection. Infect Immun. 2011;79(10):3966–3977. doi: 10.1128/IAI.05493-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin JS, Kummer LW, Szaba FM, Smiley ST. IL-17 contributes to cell-mediated defense against pulmonary Yersinia pestis infection. J Immunol. 2011;186(3):1675–1684. doi: 10.4049/jimmunol.1003303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liang SC, Long AJ, Bennett F, Whitters MJ, Karim R, Collins M, et al. An IL-17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J Immunol. 2007;179(11):7791–7799. doi: 10.4049/jimmunol.179.11.7791. [DOI] [PubMed] [Google Scholar]
- 41.Linden A, Laan M, Anderson GP. Neutrophils, interleukin-17A and lung disease. Eur Respir J. 2005;25(1):159–172. doi: 10.1183/09031936.04.00032904. [DOI] [PubMed] [Google Scholar]
- 42.Hot A, Zrioual S, Toh ML, Lenief V, Miossec P. IL-17A- versus IL-17F-induced intracellular signal transduction pathways and modulation by IL-17RA and IL-17RC RNA interference in rheumatoid synoviocytes. Ann Rheum Dis. 2011;70(2):341–348. doi: 10.1136/ard.2010.132233. [DOI] [PubMed] [Google Scholar]
- 43.Starnes T, Robertson MJ, Sledge G, Kelich S, Nakshatri H, Broxmeyer HE, et al. Cutting edge: IL-17F, a novel cytokine selectively expressed in activated T cells and monocytes, regulates angiogenesis and endothelial cell cytokine production. J Immunol. 2001;167(8):4137–4140. doi: 10.4049/jimmunol.167.8.4137. [DOI] [PubMed] [Google Scholar]
- 44.Tateda K, Moore TA, Newstead MW, Tsai WC, Zeng X, Deng JC, et al. Chemokine-dependent neutrophil recruitment in a murine model of Legionella pneumonia: potential role of neutrophils as immunoregulatory cells. Infect Immun. 2001;69(4):2017–2024. doi: 10.1128/IAI.69.4.2017-2024.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horwitz MA, Silverstein SC. Interaction of the Legionnaires' disease bacterium (Legionella pneumophila) with human phagocytes. I. L. pneumophila resists killing by polymorphonuclear leukocytes, antibody, and complement. J Exp Med. 1981;153(2):386–397. doi: 10.1084/jem.153.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Friedman H, Yamamoto Y, Klein TW. Legionella pneumophila pathogenesis and immunity. Semin Pediatr Infect Dis. 2002;13(4):273–279. doi: 10.1053/spid.2002.127206. [DOI] [PubMed] [Google Scholar]
- 47.Li L, Huang L, Sung SS, Lobo PI, Brown MG, Gregg RK, et al. NKT cell activation mediates neutrophil IFN-gamma production and renal ischemia-reperfusion injury. J Immunol. 2007;178(9):5899–5911. doi: 10.4049/jimmunol.178.9.5899. [DOI] [PubMed] [Google Scholar]
- 48.Ma CS, Chew GY, Simpson N, Priyadarshi A, Wong M, Grimbacher B, et al. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med. 2008;205(7):1551–1557. doi: 10.1084/jem.20080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Di Stefano F, Verna N, Di Gioacchino M. Cavitary Legionella pneumonia in a patient with immunodeficiency due to Hyper-IgE syndrome. J Infect. 2007;54(3):e121–123. doi: 10.1016/j.jinf.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 50.Schmeck B, N'Guessan PD, Ollomang M, Lorenz J, Zahlten J, Opitz B, et al. Legionella pneumophila-induced NF-kappaB- and MAPK-dependent cytokine release by lung epithelial cells. Eur Respir J. 2007;29(1):25–33. doi: 10.1183/09031936.00141005. [DOI] [PubMed] [Google Scholar]
- 51.Cai S, Batra S, Wakamatsu N, Pacher P, Jeyaseelan S. NLRC4 Inflammasome-Mediated Production of IL-1beta Modulates Mucosal Immunity in the Lung against Gram-Negative Bacterial Infection. J Immunol. 2012 doi: 10.4049/jimmunol.1200195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Utting O, Sedgmen BJ, Watts TH, Shi X, Rottapel R, Iulianella A, et al. Immune functions in mice lacking Clnk, an SLP-76-related adaptor expressed in a subset of immune cells. Mol Cell Biol. 2004;24(13):6067–6075. doi: 10.1128/MCB.24.13.6067-6075.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matute-Bello G, Frevert CW, Kajikawa O, Skerrett SJ, Goodman RB, Park DR, et al. Septic shock and acute lung injury in rabbits with peritonitis: failure of the neutrophil response to localized infection. Am J Respir Crit Care Med. 2001;163(1):234–243. doi: 10.1164/ajrccm.163.1.9909034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.