Abstract
Microsporidia are obligate intracellular mitochrondria-lacking pathogens that rely on host cells to grow and multiply. Microsporidia, currently classified as fungi, are ubiquitous in nature and are found worldwide. They infect a large number of mammals and are recognized as opportunistic infection agents in HIV-AIDS patients. Its importance for veterinary medicine has been unveiled in recent years through the description of clinical and subclinical forms of infection in domestic and wild animals. Domestic and wild birds may be infected by the same human microsporidia, reinforcing their zoonotic potential. Microsporidiosis in fish is prevalent and causes significant economic losses for fish farming. Some species of microsporidia have been propagated in cell cultures, which may provide conditions for the development of diagnostic techniques, understanding of pathogenesis and immune responses and for the discovery of potential therapies. Unfortunately, the cultivation of these parasites is not fully standardized in most research laboratories, especially in the veterinary field. The aim of this review is to relate the most important microsporidia of veterinary interest and demonstrate how these pathogens can be grown and propagated in cell culture for diagnostic purposes or for pathogenesis studies. Cultivation of microsporidia allowed the study of its life cycle, metabolism, pathogenesis and diagnosis, and may also serve as a repository for these pathogens for molecular, biochemical, antigenic and epidemiological studies.
Keywords: animal, cell culture, diagnosis, microsporidia, zoonosis
Microsporidia are obligate intracellular parasites that are found in both invertebrate and vertebrate hosts [1]. These organisms were described as Protozoa, however, it is now recognized that they are atypical fungi without mitochondria [7]. Of over 1,200 species of organisms classified in the phylum Microsporidia, 17 species of microsporidia are known to infect animals and humans [6]. The species of microsporidia described simultaneously in humans and other animals have great genotypic diversity, and therefore, epidemiological studies are conducted to understand the dynamics of infection and the zoonotic potential from animals to humans (Table 1) [1, 5, 43]. Early knowledge of human and animal microsporidiosis is limited, because of difficulties in identifying the microscopic spores of microsporidia, which are small even at the highest magnification (1,000×) by light microscopy (3–5 µm) [6]. Therefore, the cultivation of microsporidia cells is very important for the improvement of diagnostic techniques or for studies about the pathogenesis of diseases.
Table 1. Microsporidia identified in animals and in human.
| Species | Animals and other hosts | Clinical Presentation in humans |
|---|---|---|
| Encephalitozoon cuniculi | Mammals (rabbits, rodents, dogs, blue fox, cats, cows, horses, pigs, primates) | Eye, respiratory, gastrointestinal, genitourinary and disseminated infection |
| Birds | ||
| Encephalitozoon hellem | Birds (psittacine birds, ostrich, finches, pigeons) | Eye, gastrointestinal and disseminated infection |
| Encephalitozoon intestinalis | Mammals (dogs, pigs, donkeys, cows, goats, primates) | GI infection, ocular, genitourinary and respiratory tracts |
| Birds | ||
| Enterocytozoon bieneusi | Mammals (dogs, pigs, donkeys, cows, goats, primates) | GI and respiratory infection |
| Birds | ||
| Anncalia algerae | Mosquitos | Eye, muscular and skin infection |
| Pleistophora sp. | Fish, amphibians and reptiles | Muscular infection |
| Tubulinosema acridophagus | Insects (grasshoppers) | Disseminated and muscular infection |
| Endoreticulatus sp. | Lepidopteran insects | Muscle infection |
Microsporidia infection has been described in a wide variety of domestic and wild mammals, birds, amphibians, reptiles and fish [5]. The first descriptions in mammals identified Encephalitozoon cuniculi in commercial breeding rabbits and laboratories rodents. E. cuniculi is the most microsporidian species identified in non-human vertebrates [17]. More recently, E. hellem has been identified in wild birds and pigeons [16], with or without clinical disease [9, 13], and Enterocytozoon bieneusi has been identified in cattle [34].
The cultivation of microsporidia started with the inoculation of Nosema bombycis, an important parasite to silkworms, causing pebrine disease and is cultivated in silkworm ovarian tube lining cells [11, 38]. Interest in microsporidia cultivation increased greatly with continuous cultivation of E. cuniculi in rabbit kidney cell cultures (RK cell) [35], but it was in the 90s that cultivation of microsporidia really improved due to increased reports of human disease [40]. In vitro cultivation continues to provide confirmatory diagnosis of microsporidiosis in both humans and animals, as well as providing spores of microsporidia for experimental infections, which have provided relevant data regarding immune response against these pathogens [23]. In vitro culture has also been used to determine the efficacy of antimicrobial agents against several microsporidia, including E. cuniculi, E. hellem and E. intestinalis [44]. Although microsporidia cannot be grown axenically without the host cell, Encephalitozoon sp., Trachipleistophora hominis, Vittaforma corneae and Brachiola algerae have been successfully grown in in vitro cell cultivation. Cultivation of microsporidia has allowed the study of the life cycle, metabolism, pathogenesis and diagnosis, and also serves as a repository for these pathogens for molecular, biochemical, antigenic and epidemiological studies [24]. The study of microsporidiosis in veterinary medicine is still incipient, and therefore, this paper aims to (i) describe the most important microsporidia in the veterinary area and (ii) describe in vitro cultivation of these pathogens.
Microsporidia in animals: Encephalitozoonosis caused by E. cuniculi is a naturally occurring disease in domestic rabbits and laboratory rodents. Many rabbits have a latent infection of E. cuniculi, but only part of them, develops clinical disease. Three clinical syndromes are recognized and include encephalitis and renal and ocular lesions (uveitis and cataracts). The animals infected with neurological involvement are inactive, have torticollis, ataxia, circling and paresis and progress to death. When the pathogen affects the kidney, it causes chronic interstitial nephritis with kidney failure and death [17].
Among domestic animals, the dog is the most affected by Encephalitozoon infection. Moreover, encephalitozoonosis due to E. cuniculi genotype/strain I has been described as the cause of fatal encephalitis and nephritis in domestic dogs [5, 36]. Moreover, E. cuniculi was detected in a cat with cerebral hypoplasia, causing granulomatous encephalitis, hepatitis and mild interstitial nephritis, myocarditis and enteritis [30].
The first descriptions of microsporidia in cattle were mostly in Europe and North America over the past decade [32]. Enterocytozoon bieneusi was the microsporidia identified in 23% of 571 cattle examined from 14 farms [34]. Moreover, in South Africa, the presence of E. bieneusi in domestic cattle in rural communities was confirmed by PCR, with a genotype pathogenic for humans [33]. E. bieneusi was detected in pigs [4], and a prevalence of 35% was determined in Switzerland [3], affecting especially piglets. The pathogen was detected in pigs of all ages with varying prevalence, but the data suggest that E. bieneusi is not pathogenic to swine, and the same was observed in cattle.
Several reports described microsporidian infections in wild carnivores (red fox, arctic foxes and wild dogs) maintained as captive or in Zoos [10, 28, 39]. Disseminated natural infections resulting in high morbidity and marked encephalitis caused by Encephalitozoon like organism have been reported in stillborn and young squirrel monkeys (Saimiri sciureus) in the United States [45]. Recently, strain III (“dog strain”) of E. cuniculi was identified in tamarin colonies (Saguinus imperator, Oedipomidas oedipus and Leontopithecus rosalia rosalia) in two Zoos in Europe, causing marked disseminated infection with high mortality in infants [8]. In monkeys, experimental or even naturally acquired infection by transplacental transmission resulted in fatal multifocal granulomatous encephalitis [45].
The most common human microsporidian species, E. bieneusi, E. intestinalis, E. hellem and E. cuniculi, have been reported in various species of birds. The first case of microsporidiosis in birds was reported in masked lovebirds (Agaponis personta) in 1975 [13]. E. bieneusi was detected in chickens (Gallus gallus) in a poultry abattoir in Germany [31], in captive falcons in the Arab Emirates [27] and in pigeons [9]. In Brazil, we found 24.5% of positivity among 196 fecal samples analyzed by PCR- and Gram-Chromotrope staining technique; the prevalence was higher in pigeons (31.1%) than the free-ranging birds (18.8%) [12].
Microsporidia are a major cause of disease in fish and may have an economically important impact on fish stocks [19, 20]. Many fish diseases of considerable economic importance have been attributed to microsporidia, including diseases that impede aquaculture of fish and have been a factor in the weakening or collapse of several fisheries [2]. Fish are hosts of 156 species of microsporidia allocated to 14 genera, as well known: Glugea, Heterosporis, Ichthyosporidium, Kabatana, Loma, Microfilum, Microgemma, Neonosemoides, Nosemoides, Nucleospora, Ovipleistophora, Pleistophora, Spraguea, Tetramicra and the collective group Microsporidium, which is not considered a genus [19]. Microsporidiosis is highly destructive to the infected tissue, resulting in high mortality rates in fish [14]. Microsporidia are widely distributed in sea water, estuaries and freshwater. Ambient temperature affects the development of microsporidia, (e.g. infections by Glugea stephani and Loma salmonae), which increases during summer, when water temperatures rise [29]. Prevalence is especially high when the density increases, raising both morbidity and mortality of young fish [29].
Organism and life cycle: Microsporidia are nucleated, single-celled, obligate intracellular pathogens that were considered to be early-branching eukaryotic organisms, based on the presence of prokaryote-like ribosome and the apparent absence of true Golgi, peroxisomes and mitochondria [5, 37, 41]. The life cycle of microsporidia is characterized by three phases: the infective or environmental phase (spores); the proliferative phase, identified by merogony; and the sporogonic or spore-forming phase [12].
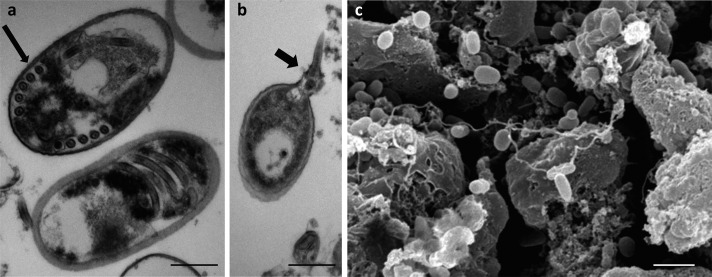
Spores of microsporidia are generally small, oval or pyriform-shaped with 1 to 12 µm in length (Figs. 1 and 2). Microsporidian spores contain a long and convoluted tubule extrusion apparatus (polar tubule), which distinguishes them from other organisms (Fig. 1). The polar tube has a crucial role in invasion of host cell (Fig. 1) [18, 37]. Spore approaches the host cell, and its polar tubular is everted to enter the cell and inject its sporoplasm into the cell cytoplasm (Fig. 1). Phagocytosis of spores by host cells or cell culture by Encephalitozoon involves binding of the spores to the host cell surface glycosaminoglycans (GAGs) [12].
Fig. 1.
Encephalitozoon sp. spores from Vero cell. a) Transmission electron microscopy (TEM) of young E. intestinalis spore with five filament (arrow) coils of polar filament (scale bar=0.4 µm); b) Extruded (arrow) polar filament of E. cuniculi spore (TEM, scale bar=0.6 µm); c) Scanning electron microscopy of E. cuniculi (scale bar=3 µm).
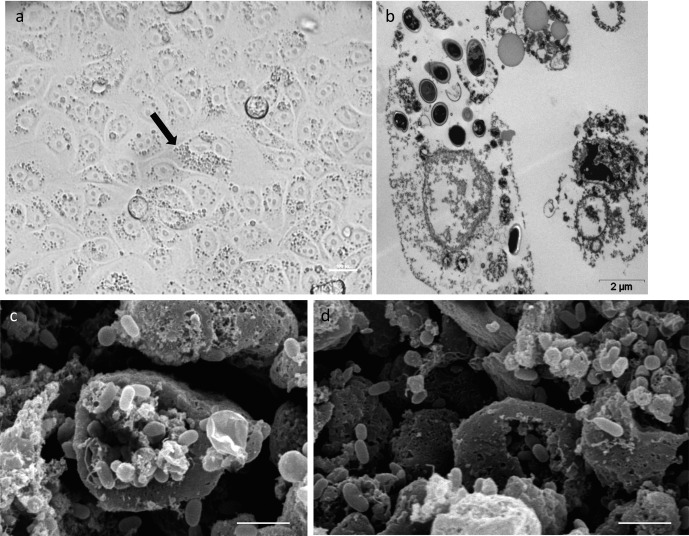
Fig. 2.
a) Parasitophorous vacuole (arrow) in a distended RK-13 cell. b) Fully formed parasitophorous vacuole with sporogonial stages and spores in Vero cell (TEM, Scale bar=2 µm). c) and d) Scanning electron microscopy of Vero cell culture infected with E. cuniculi (scale bar=5 µm).
The proliferative phase (schizogony and merogony) includes all cell growth and division from sporoplasm until spore formation. The sporoplasm injected into the host cell becomes meronts and proliferates by repeated binary or multiple fission or by plasmotomy [7]. The sporogony includes sporonts (cells produce two to more sporoblasts), sporoblasts (cells undergo metamorphosis into spores) and spores. Meronts develop into sporonts, which is the stage that divides into sporoblasts. The beginning of sporogony to microsporidia which have diplokaryotic nuclei takes place after meiosis, whereas for other species, sporogony occurs in the presence of plasmalemmal thicking. Sporonts are usually characterized by the development of a thick, electron-dense surface coat that will later become the exospore layer of the spore wall (Fig. 1). Sporonts can multiply by binary fission or multiple, acquire specialized organelles and become spores. Subsequently, spores spread through the tissues of the host by infecting new cells and continuing the cycle [12].
In vitro culture of microsporidia of mammals:
Samples for culture. For diagnosis, reports indicate the use of urine, sputum, bronchoalveolar lavage, feces, duodenal aspirates, conjunctival scraping, corneal biopsies, cerebrospinal fluids, muscle biopsies and brain tissue as possible tissues to harvest. The addition of antibiotics (gentamicin, penicillin and streptomycin) and amphotericin B prevents bacterial and fungal growth without compromising the development of microsporidia [24]. In the case of samples with mucus (e.g. respiratory secretions), a mucolytic should be used, then the sample will be centrifuged, and its pellet will be washed with distilled water before inoculating into cell culture [40]. Tissue from biopsies should be macerated mechanically or by enzymatic action before being used to inoculate into cell monolayers. In the case of urine use for initiating a culture, it must be centrifuged at 1,500 × g for 30 min, and the pellet should be suspended in Dulbecco’s Modified Eagle’s Medium - DEMEM containing penicillin, streptomycin and amphotericin B and subsequently be incubated for 4 hr. Afterwards, a new centrifugation is carried out, and the pellet with spores should be inoculated in monolayers of Madin-Darby Canine Kidney cell line (MDCK cells) with Modified Eagle’s Medium - MEM and 10% fetal bovine serum [24, 40].
Maintenance and preservation of microsporidian cultures. Maintaining microsporidia cell cultures is relatively simple. Cultures should be microscopically examined every week before the change of medium (Figs. 2 and 3). An inverted microscope equipped with differential interference contrast optics or phase is needed, where cells filled with spores or free spores can be observed [22]. The culture medium should preferably be exchanged twice a week, and this favors the development of microsporidia and host cells, however, in our experience, a medium exchange weekly is sufficient to culture flasks, and spores can survive for several months to a year or even more.
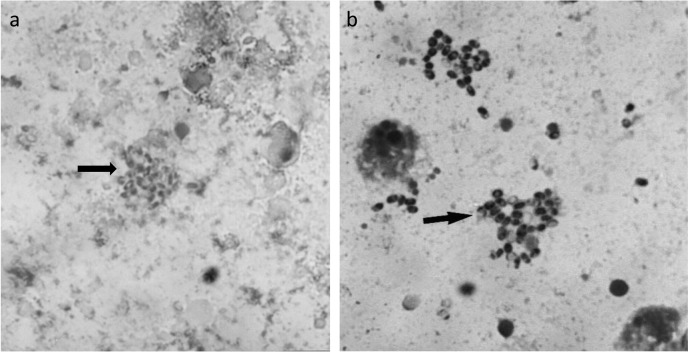
Fig. 3.
Culture smears of Encephalitozoon cuniculi spores stained with Chromotrope (a) and Gram-Chromotrope technique (b).
In case of need to rapidly expand the cultures to obtain a large number of spores, new cultures inoculated with spores can be obtained from the supernatant of infected cultures. Cultures that have more than 80% of infected cells may be scraped or trypsinized, and the cells are transferred to new flasks for expansion cultures [24, 40]. The culture medium collected from the inoculated flasks should be centrifuged (1,500 × g for 20 min at 4°C), and the pellet containing spores should be suspended in culture medium and stored at 4°C or used to inoculate new cell cultures [12, 42].
A number of cell lines have been used (Table 2), including monkey and rabbit kidney cell lines (Vero and RK-13), a human fetal lung fibroblasts cell line (MRC-5) and the MDCK cell line. Unfortunately, one of the most common human microsporidial pathogens, E. bieneusi, has been propagated only in short-term cultures [17].
Table 2. Examples of cell line culture used in the cultivation of microsporidia from mammals, birds and fish.
| Microsporidian species | Cell culture |
|---|---|
| Mammals and birds | |
| Encephalitozoon cuniculi | Monkey kidney (E6); Madin-Darby canine kidney (MDCK); Rabbit kidney (RK-13); Human lung fibroblast (HLF); Human lung fibroblast (MRC-5) |
| Encephalitozoon hellem | |
| Encephalitozoon intestinalis | Monkey kidney (E6); Madin-Darby canine kidney (MDCK); Rabbit kidney (RK-13); Human lung fibroblast (HLF); Human colorectal adenocarcinoma (HT-29); Human colorectal adenocarcinoma (CACO-2) |
| Anncaliia algerae | Monkey kidney (E6); Human lung fibroblast (HLF) |
| Trachipleistophora hominis | Madin-Darby canine kidney (MDCK); Rabbit kidney (RK-13); Monkey kidney (COS-1) |
| Vittaforma corneae | Monkey kidney (E6); Rabbit cornea (SIRC); Madin-Darby canine kidney (MDCK); Human lung fibroblast (HLF); Human lung fibroblast (MRC-5) |
| Fish | |
| Nucleospora salmonis | Primary culture of leukocytes from peripheral blood of chinook salmon |
| Nucleospora salmonis | Primary culture of epithelial-like cell from kidney of rainbow trout |
| Glugea sp. | Cell line CHSE-214 from salmon embryo |
| Pseudoloma neurophilia | Cell line CCO- fibroblast from ovary of channel catfish; Cell line SJD.1- fibroblast from fin of zebrafish; Cell line EPC- epithelial –like from skin of carp; Cell line EPC-epithelial–like from connective tissue and muscle of fathead minnow |
| Heteroporis anguillarum | Cell line EP-1–epithelial like from infected tissues of elves of Japanese eel |
Culture of cells infected with microsporidia can be scraped or trypsinized and mixed with an equal volume of culture medium containing 20% DMSO. This mixture should be aliquoted in a 1.0 ml volume into plastic cryovials. The samples at room temperature should be placed in a freezing unit that cools −1°C/min to reach −40°C, and only then, they can be transferred to liquid nitrogen. Another possibility is to place the sample in Nalgene 1°C freezing chamber. The flasks should be placed at −80°C for 2 hr to overnight period, and then, they should be transferred to liquid nitrogen [24, 40].
Cell culture of fish microsporidia. Compared to microsporidia of mammals, in vitro culture of fish microsporidia has a limited success (Table 2). Although primary cultures and cell lines are available for culturing various tissues and organs of fish, microsporidia cultures typically are of short duration (about 48 hr or less). Therefore, culture of fish microsporidia has been employed to study their interaction with cells of the innate immune system, macrophages and neutrophils [15].
Primary cultures of leukocytes from salmonids can give relatively greater longevity to the development of microsporidia in fish. In vitro cultures of fish microsporidia have been successfully demonstrated by using epithelial cell cultures of Aedes albopictus at 28°C [21]. Culture of Glugea sp. was obtained from specimens of the fish Hyperoplus lanceolatus that was captured by commercial boats in the coast of Andalusia, Spain. The same microsporidium was not able to develop more than 48 hr in epithelial cells from salmon embryo at 21°C. It is worth mentioning that the cell lines of fish promote the growth of Anncaliia (Brachiola/Nosema) algereae, a microsporidium that was first described in insects, but is currently found in many vertebrate hosts, including humans [25]. Epithelial-like cells (PE-1) derived from tissues of eel (Anguilla japonica) are considered immortal lineages and have allowed the development of Heterosporis (Pleistophora) anguillarum. In this cultivation, microsporidian spores are not formed, however, inoculation of infected culture in eel causes muscular disease and spore formation [26].
CONCLUSION
Several species of microsporidia have been identified in domestic and wild animals, and many are zoonotic, and lethal pathogens. However, aspects related to the pathogenesis of infections in animals remain unknown, and therefore, microsporidia should be further studied in animals. In conclusion, cell culture is an essential tool for the study of microsporidia important for veterinary medicine and can be implanted in research and diagnostic laboratories using already established cell lines culture. Therefore, the establishment of cultures is essential for acting an implementation of knowledge of these emerging and opportunistic pathogens in different areas of scientific knowledge.
Acknowledgments
We thank Magna Aparecida Mautauro Soares (Butantan Institute) for making the material for light microscopy and Michelle Sanchez Freitas Correia (Paulista University) for performing the scanning electron microscopy. We thank the Butantan Institute for allowing us to use their electron microscope obtained by FAPESP project number 18070806074.
References
- 1.Anane S., Attouchi H.2010. Microsporidiosis: epidemiology, clinical data and therapy. Gastroenterol. Clin. Biol. 34: 450–464. doi: 10.1016/j.gcb.2010.07.003 [DOI] [PubMed] [Google Scholar]
- 2.Ashley P. J.2007. Fish welfare: current issues in aquaculture. Appl. Anim. Behav. Sci. 104: 199–235. doi: 10.1016/j.applanim.2006.09.001 [DOI] [Google Scholar]
- 3.Breitenmoser A. C., Mathis A., Bürgi E., Weber R., Deplazes P.1999. High prevalence of Enterocytozoon bieneusi in swine with four genotypes that differ from those identified in humans. Parasitology 118: 447–453. doi: 10.1017/S0031182099004229 [DOI] [PubMed] [Google Scholar]
- 4.Deplazes P., Mathis A., Müller C., Weber R.1996. Molecular epidemiology of Encephalitozoon cuniculi and first detection of Enterocytozoon bieneusi in faecal samples of pigs. J. Eukaryot. Microbiol. 43: 93S. doi: 10.1111/j.1550-7408.1996.tb05018.x [DOI] [PubMed] [Google Scholar]
- 5.Didier E. S.2005. Microsporidiosis: an emerging and opportunistic infection in humans and animals. Acta Trop. 94: 61–76. doi: 10.1016/j.actatropica.2005.01.010 [DOI] [PubMed] [Google Scholar]
- 6.Fayer R., Santin-Duran M.2014. Epidemiology of microsporidia in human infections. pp.135–164. In: Microsporidia −Pathogens of Opportunity (Weiss, L. M. and Becnel, J. J. eds.), Willey Blackwell, Oxford. [Google Scholar]
- 7.Franzen C.2008. Microsporidia: a review of 150 years of research. Open Parasitol. J. 2: 1–34. doi: 10.2174/1874421400802010001 [DOI] [Google Scholar]
- 8.Guscetti F., Mathis A., Hatt J. M., Deplazes P.2003. Overt fatal and chronic subclinical Encephalitozoon cuniculi microsporidiosis in a colony of captive emperor tamarins (Saguinus imperator). J. Med. Primatol. 32: 111–119. doi: 10.1034/j.1600-0684.2003.00016.x [DOI] [PubMed] [Google Scholar]
- 9.Haro M., Henriques-Gil N., Fenoy S., Izquierdo F., Alonso F., Del Aguila C.2006. Detection and genotyping of Enterocytozoon bieneusi in pigeons. J. Eukaryot. Microbiol. 53Suppl 1: S58–S60. doi: 10.1111/j.1550-7408.2006.00173.x [DOI] [PubMed] [Google Scholar]
- 10.Hersteinsson P., Gunnarsson E., Hjartardóttir S., Skírnisson K.1993. Prevalence of Encephalitozoon cuniculi antibodies in terrestrial mammals in Iceland, 1986 to 1989. J. Wildl. Dis. 29: 341–344. doi: 10.7589/0090-3558-29.2.341 [DOI] [PubMed] [Google Scholar]
- 11.Jaronski S. T.1984. Microsporidia in cell culture. pp.183–229. In: Advances in Cell Culture (Maramorosch, K. ed.). Academic Press, New York. [Google Scholar]
- 12.Keeling P. J., Fast N. M.2002. Microsporidia: biology and evolution of highly reduced intracellular parasites. Annu. Rev. Microbiol. 56: 93–116. doi: 10.1146/annurev.micro.56.012302.160854 [DOI] [PubMed] [Google Scholar]
- 13.Kemp R. L., Kluge J. P.1975. Encephalitozoon sp. in the blue-masked lovebird, Agapornis personata (Reichenow): first confirmed report of microsporidan infection in birds. J. Protozool. 22: 489–491. doi: 10.1111/j.1550-7408.1975.tb05214.x [DOI] [PubMed] [Google Scholar]
- 14.Kent M. L., Shaw R. W., Sanders J. L.2014. Microsporidia in fish. pp. 493–520. In: Microsporidia −Pathogens of Opportunity (Weiss, L. M. and Becnel, J. J. eds.), Willey Blackwell, Oxford. [Google Scholar]
- 15.Kim J. H., Ogawa K., Wakabayashi H.1999. Lectin-reactive components of the microsporidian Glugea plecoglossi and their relation to spore phagocytosis by head kidney macrophages of ayu Plecoglossus altivelis. Dis. Aquat. Organ. 39: 59–63. doi: 10.3354/dao039059 [DOI] [PubMed] [Google Scholar]
- 16.Lallo M. A., Calábria P., Milanelo L.2012. Encephalitozoon and Enterocytozoon (Microsporidia) spores in stool from pigeons and exotic birds: microsporidia spores in birds. Vet. Parasitol. 190: 418–422. doi: 10.1016/j.vetpar.2012.06.030 [DOI] [PubMed] [Google Scholar]
- 17.Leipig M., Matiasek K., Rinder H., Janik D., Emrich D., Baiker K., Hermanns W.2013. Value of histopathology, immunohistochemistry, and real-time polymerase chain reaction in the confirmatory diagnosis of Encephalitozoon cuniculi infection in rabbits. J. Vet. Diagn. Invest. 25: 16–26. doi: 10.1177/1040638712466394 [DOI] [PubMed] [Google Scholar]
- 18.Leitch G. J., He Q., Wallace S., Visvesvara G. S.1993. Inhibition of the spore polar filament extrusion of the microsporidium, Encephalitozoon hellem, isolated from an AIDS patient. J. Eukaryot. Microbiol. 40: 711–717. doi: 10.1111/j.1550-7408.1993.tb04463.x [DOI] [PubMed] [Google Scholar]
- 19.Lom J.2002. A catalogue of described genera and species of microsporidians parasitic in fish. Syst. Parasitol. 53: 81–99. doi: 10.1023/A:1020422209539 [DOI] [PubMed] [Google Scholar]
- 20.Lom J., Nilsen F.2003. Fish microsporidia: fine structural diversity and phylogeny. Int. J. Parasitol. 33: 107–127. doi: 10.1016/S0020-7519(02)00252-7 [DOI] [PubMed] [Google Scholar]
- 21.Lores B., Rosales M. J., Mascaró C., Osuna A.2003. In vitro culture of Glugea sp. Vet. Parasitol. 112: 185–196. doi: 10.1016/S0304-4017(02)00411-9 [DOI] [PubMed] [Google Scholar]
- 22.Lowman P. M., Takvorian P. M., Cali A.2000. The effects of elevated temperatures and various time-temperature combinations on the development of Brachiola (Nosema) algerae N. Comb. in mammalian cell culture. J. Eukaryot. Microbiol. 47: 221–234. doi: 10.1111/j.1550-7408.2000.tb00041.x [DOI] [PubMed] [Google Scholar]
- 23.Meng X., Zheng J., He X., Jia H., Zhang Y.2014. First characterization in China of Encephalitozoon cuniculi in the blue fox (Alopex lagopus). J. Eukaryot. Microbiol. 61: 580–585. doi: 10.1111/jeu.12135 [DOI] [PubMed] [Google Scholar]
- 24.Molestina R., Becnel J. J., Weiss L. M.2014. Culture and propagation of microsporidia. pp. 457–467. In: Microsporidia–Pathogens of Opportunity (Weiss, L. M. and Becnel, J. J. eds.), Willey Blackwell, Oxford. [Google Scholar]
- 25.Monaghan S. R., Rumney R. L., Vo N. T., Bols N. C., Lee L. E.2011. In vitro growth of microsporidia Anncaliia algerae in cell lines from warm water fish. In Vitro Cell. Dev. Biol. Anim. 47: 104–113. doi: 10.1007/s11626-010-9366-3 [DOI] [PubMed] [Google Scholar]
- 26.Monaghan S. R., Kent M. L., Watral V. G., Kaufman R. J., Lee L. E., Bols N. C.2009. Animal cell cultures in microsporidial research: their general roles and their specific use for fish microsporidia. In Vitro Cell. Dev. Biol. Anim. 45: 135–147. doi: 10.1007/s11626-008-9172-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Müller M. G., Kinne J., Schuster R. K., Walochnik J.2008. Outbreak of microsporidiosis caused by Enterocytozoon bieneusi in falcons. Vet. Parasitol. 152: 67–78. doi: 10.1016/j.vetpar.2007.11.019 [DOI] [PubMed] [Google Scholar]
- 28.Murphy T. M., Walochnik J., Hassl A., Moriarty J., Mooney J., Toolan D., Sanchez-Miguel C., O’Loughlin A., McAuliffe A.2007. Study on the prevalence of Toxoplasma gondii and Neospora caninum and molecular evidence of Encephalitozoon cuniculi and Encephalitozoon (Septata) intestinalis infections in red foxes (Vulpes vulpes) in rural Ireland. Vet. Parasitol. 146: 227–234. doi: 10.1016/j.vetpar.2007.02.017 [DOI] [PubMed] [Google Scholar]
- 29.Noga E. J.2010. Fish Disease Diagnosis and Treatment. pp.247–252. 2nd ed., Wiley-Blackwell, Ames. [Google Scholar]
- 30.Rebel-Bauder B., Leschnik M., Maderner A., Url A.2011. Generalized encephalitozoonosis in a young kitten with cerebellar hypoplasia. J. Comp. Pathol. 145: 126–131. doi: 10.1016/j.jcpa.2010.12.009 [DOI] [PubMed] [Google Scholar]
- 31.Reetz J., Rinder H., Thomschke A., Manke H., Schwebs M., Bruderek A.2002. First detection of the microsporidium Enterocytozoon bieneusi in non-mammalian hosts (chickens). Int. J. Parasitol. 32: 785–787. doi: 10.1016/S0020-7519(02)00045-0 [DOI] [PubMed] [Google Scholar]
- 32.Rinder H., Thomschke A., Dengjel B., Gothe R., Löscher T., Zahler M.2000. Close genotypic relationship between Enterocytozoon bieneusi from humans and pigs and first detection in cattle. J. Parasitol. 86: 185–188. doi: 10.1645/0022-3395(2000)086[0185:CGRBEB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 33.Abu Samra N., Thompson P. N., Jori F., Zhang H., Xiao L.2012. Enterocytozoon bieneusi at the wildlife/livestock interface of the Kruger National Park, South Africa. Vet. Parasitol. 190: 587–590. doi: 10.1016/j.vetpar.2012.06.031 [DOI] [PubMed] [Google Scholar]
- 34.Santín M., Trout J. M., Fayer R.2005. Enterocytozoon bieneusi genotypes in dairy cattle in the eastern United States. Parasitol. Res. 97: 535–538. doi: 10.1007/s00436-005-1482-8 [DOI] [PubMed] [Google Scholar]
- 35.Shadduck J. A.1969. Nosema cuiculi: in vitro isolation. Science 166: 516–517. doi: 10.1126/science.166.3904.516 [DOI] [PubMed] [Google Scholar]
- 36.Snowden K. F., Lewis B. C., Hoffman J., Mansell J.2009. Encephalitozoon cuniculi infections in dogs: a case series. J. Am. Anim. Hosp. Assoc. 45: 225–231. doi: 10.5326/0450225 [DOI] [PubMed] [Google Scholar]
- 37.Texier C., Vidau C., Viguès B., El Alaoui H., Delbac F.2010. Microsporidia: a model for minimal parasite-host interactions. Curr. Opin. Microbiol. 13: 443–449. doi: 10.1016/j.mib.2010.05.005 [DOI] [PubMed] [Google Scholar]
- 38.Trager W.1937. The hatching of spores of Nosema bombycis Nägeli and the partial development of the organism in tissue cultures. J. Parasitol. 23: 226–227. doi: 10.2307/3272075 [DOI] [Google Scholar]
- 39.Van Heerden J., Bainbridge N., Burroughs R. E. J., Kriek N. P. J.1989. Distemper-like disease and encephalitozoonosis in wild dogs (Lycaon pictus). J. Wildl. Dis. 25: 70–75. doi: 10.7589/0090-3558-25.1.70 [DOI] [PubMed] [Google Scholar]
- 40.Visvesvara G. S.2002. In vitro cultivation of microsporidia of clinical importance. Clin. Microbiol. Rev. 15: 401–413. doi: 10.1128/CMR.15.3.401-413.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vossbrinck C. R., Maddox J. V., Friedman S., Debrunner-Vossbrinck B. A., Woese C. R.1987. Ribosomal RNA sequence suggests microsporidia are extremely ancient eukaryotes. Nature 326: 411–414. doi: 10.1038/326411a0 [DOI] [PubMed] [Google Scholar]
- 42.Waller T.1975. Growth of Nosema cuniculi in established cell lines. Lab. Anim. 9: 61–68. doi: 10.1258/002367775780994835 [DOI] [PubMed] [Google Scholar]
- 43.Weber R., Bryan R. T., Schwartz D. A., Owen R. L.1994. Human microsporidial infections. Clin. Microbiol. Rev. 7: 426–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolk D. M., Johnson C. H., Rice E. W., Marshall M. M., Grahn K. F., Plummer C. B., Sterling C. R.2000. A spore counting method and cell culture model for chlorine disinfection studies of Encephalitozoon syn. Septata intestinalis. Appl. Environ. Microbiol. 66: 1266–1273. doi: 10.1128/AEM.66.4.1266-1273.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeman D. H., Baskin G. B.1985. Encephalitozoonosis in squirrel monkeys (Saimiri sciureus). Vet. Pathol. 22: 24–31. [DOI] [PubMed] [Google Scholar]