Abstract
Dronedarone is a class III antiarrhythmic that has been used for management of atrial fibrillation in humans, but limited information was found in dogs. The objective of this study was to determine the acute effects of escalating concentrations of dronedarone on electrocardiograms (ECG), hemodynamics and cardiac mechanics in healthy dogs. A total of 7 beagle dogs were anesthetized with isoflurane and instrumented to obtain lead II ECG, pressures at ascending aorta, right atrium, pulmonary artery and left ventricle, and left ventricular pressure-volume relationship. Five dogs were given vehicle and followed by escalating doses of dronedarone (0.5, 1.0 and 2.5 mg/kg, 15 min for each dose), and two dogs were used as a vehicle-treated control. All parameters were measured at 15 min after the end of each dose. The results showed that all parameters in vehicle-treated dogs were unaltered. Dronedarone at 2.5 mg/kg significantly lengthened PQ interval (P<0.01), reduced cardiac output (P<0.01) and increased systemic vascular resistance (P<0.01). Dronedarone produced negative inotropy assessed by significantly lowered end-systolic pressure-volume relationship, preload recruitable stroke work, contractility index and dP/dtmax. It also impaired diastolic function by significantly increased end-diastolic pressure-volume relationship, tau and dP/dtmin. These results suggested that acute effects of dronedarone produced negative dromotropy, inotropy and lusitropy in anesthetized dogs. Care should be taken when given dronedarone to dogs, especially when the patients have impaired cardiac function.
Keywords: canine, dronedarone, hemodynamics, inotropy, lusitropy
Dronedarone is a class III antiarrhythmic drug and possesses multiple cardiac ion channels blocking effects including voltage-gated L-type calcium channel (ICa,L), adrenergic receptors, voltage-gated sodium channel (INa) and rapid component of delayed rectifier potassium channel (IKr) [9]. It has a similar pharmacological profile with amiodarone, while minimizing adverse effects due to lack of iodine molecules in its structure [25]. In addition, dronedarone contains methylsulfonamide group resulted in a reduction of lipophilicity and toxicity [27, 40, 42]. Dronedarone has proven to be useful in humans for management of atrial fibrillation [31]. Several large clinical trials in patients with nonpermanent atrial fibrillation (AF) demonstrated that dronedarone reduced mortality and hospitalization rates, whereas others had found increased mortality and morbidity in patients with AF and heart failure (HF) [7, 30, 34]. Many clinical trials in patients also suggested that dronedarone would rather have better safety profile than amiodarone [16, 18, 27, 30, 35].
Based on the successful clinical trials of dronedarone in humans, dronedarone would be beneficial for management of AF in dogs. However, there is sparse information in the literature on hemodynamics and cardiac functions assessed simultaneously with cardiac electrophysiology in dogs. In canine cardiac papillary muscle preparation, acute effect of dronedarone (0.1–10 µmol/l) shortened action potential duration (APD), whereas the sustained effect did not alter APD [37]. In canine left ventricular Purkinje fibers and tissue slices, dronedarone lowered the incidence of triggered activities [22, 37]. Only a few studies have reported the dronedarone effects on QT and QTc intervals in healthy and complete AV block dogs [26, 37, 38]. It has been suggested that in vivo effects of dronedarone depend on duration of administration and species [26]. In dogs with complete atrioventricular block, intravenous administration of dronedarone produced shortening of APD, while the sustained oral administration (20 mg/kg, twice per day) lengthened the QTc interval [38]. In contrast, chronic dronedarone administration (25 mg/kg, twice per day) in normal dogs did not prolong the QTc interval [37]. In α-chloralose anesthetized dog, intravenous effects of dronedarone produced significant increases in sinus cycle length, effective refractory period of atrioventricular node and Wenckebach cycle length [20]. All of those studies did not investigate effects of dronedarone on hemodynamics and cardiac mechanics. The objective of the following study was to elucidate the acute effects of escalating concentration of intravenous dronedarone on electrocardiograms (ECG), hemodynamics and cardiac mechanics in intact dogs anesthetized with isoflurane.
MATERIALS AND METHODS
Approvals: This study was approved by the Institutional Animal Care and Use Committee of QTest Labs, LLC, Columbus, OH, U.S.A. (Protocol number SPD13-032). All experimental animal procedures were performed at QTest Labs and in compliance with QTest IACUC regulation, and followed the guidelines outlined in the Guide for the Care and Use of Laboratory Animals [24].
Animals: Seven healthy mature Beagles of either gender were purchased from Marshall BioResources (North Rose, NY, U.S.A.). The animal was housed individually from the time of arrival to the end of study in a dog run maintained at a temperature of 21 ± 2°C, a relative humidity of 50 ± 20% and a 12 hr:12 hr light:dark cycle. All animals were received commercial chow twice daily, and water was provided ad libitum in stainless steel containers.
Physical examination, routine lead II ECG recording, complete blood count and blood chemistry analysis were performed to evaluate healthy status in all dogs before beginning of the experiment. Experimental procedures were started after at least 6 hr period of fasting.
Drug preparation: Dronedarone (Multaq® 400 mg tablet, Sanofi-Aventis U.S. LLC, Bridge water, NJ, U.S.A.) 400 mg was dissolved with polyethylene glycol (PEG 400) (Sigma-Aldrich, St. Louis, MO, U.S.A.) and distilled water (2:1), and the mixture was heated on hot plate (<90°C) until all drugs are dissolved. The solution was cooled down at room temperature and filtered with 0.8 µm sterile-syringe filter before given intravenously to the animal. The drug preparation and dose selection were based on our pilot study and previous publication [20].
Experimental procedures: All dogs were given butorphanol (0.1 mg/kg, intravenously) 10 min before receiving propofol (4–6 mg/kg, intravenously, to effect). Orotracheal intubation was performed and ventilated mechanically with ascending-bellows, volume-cycled, pressure-regulated ventilator. The ventilator was set to deliver a tidal volume of 12–15 ml/kg (maximum allowed pressure, 20 cmH2O) at a rate of 8 to 12 breaths per min, sustaining the end-tidal partial pressure of CO2 between 35 and 45 mmHg and that of O2 greater than 80 mmHg. The endotracheal tube was connected to a circle anesthetic rebreathing circuit, and anesthesia was maintained with isoflurane in oxygen delivered by a use of vaporizer. The end-tidal inhalant concentration was maintained between 1.4–1.6%. Body temperature was maintained at 36.5–37°C by a warm water heating pump.
A lead II electrocardiogram was obtained (Ponemah 12 lead ECG amplifier, DSI, St. Paul, MN, U.S.A.). All catheterization procedures were performed under fluoroscopic guidance. A 5 French thermodilution catheter (Edwards Lifesciences, Irvine, CA, U.S.A.) was inserted into the jugular vein and advanced into the pulmonary artery to permit simultaneously continuous monitoring of central venous (RAP) and pulmonary arterial (PAP) pressures and intermittent determination of cardiac output by a use of thermodilution technique. A 5 French pressure-volume (PV) loop admittance catheter (Scisense Inc., London, Canada) was inserted into the internal carotid artery, and the tip was advanced into the left ventricle (LV). This catheter was used to record pressure-volume relationship. A 12 French venous occlusion catheter was inserted into the right femoral vein and positioned at the caudal vena cava for periodic occlusion of the vein to generate a family of pressure-volume loop. A 6 French fluid-filled catheter was inserted into the left femoral artery, and the tip advanced into the thoracic aorta to record arterial blood pressure (AoP). Each dog was in a stabilized anesthetic state for approximately 30 min before recording baseline data.
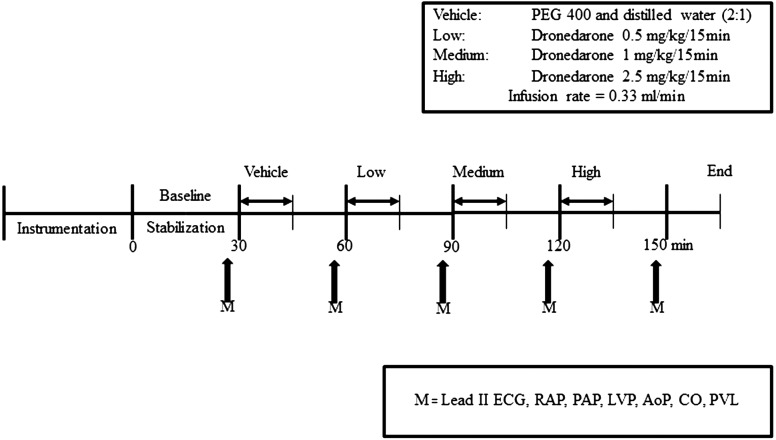
After stabilization, vehicle (PEG 400 and distilled water (2:1)) was infused for 15 min, at a rate of 0.33 ml/min. All hemodynamics and left ventricular function were observed for 15 min after the end of vehicle. Then, escalating concentrations of dronedarone (0.5, 1 and 2.5 mg/kg) were given at the same rate as vehicle for 15 min per each concentration with an observation period of 15 min between each dose. The initial dose was selected, because it was found to be a no-effect dose in our preliminary studies. While the ECG and blood pressure were recorded throughout the experiment, parameters were analyzed at 30 min after the beginning of each concentration (Fig. 1). Pressure-volume loops were obtained at 30 min after the beginning of infusion as previously described [17]. Briefly, at a given time point after dosing, left ventricular preload was acutely reduced by means of brief (~8–10 beats) caudal vena caval occlusions in order to generate a family of pressure-volume curves; approximately three occlusions were performed at each time point, allowing for hemodynamic recovery between occlusions. The cardiac output (CO) was determined by a modified Stewart-Hamilton indicator dilution equation [1]. Basically, the COM-2 machine integrates area under the curve at the instant of saline injection (5 ml) and terminates integration when the exponential decay reaches a value of about 30%. The computer then extrapolates the exponential decay to baseline. The CO measurement was performed 3 times for each time-point, and the mean value was calculated. The CO and pressure-volume loop relationship were measured at 30 min after injection of each concentration.
Fig. 1.
Experimental procedure to study acute effects of escalating doses of dronedarone on electrocardiograms (ECG), hemodynamics and left ventricular functions in anesthetized dogs. RAP=right atrial pressure, PAP=pulmonary arterial pressure, LVP=left ventricular pressure, AoP=aortic pressure, CO=cardiac output, PVL=pressure-volume loop
Before commencing the main experiments, vehicle-treated dogs (n=2) were performed to establish that there was no difference in ECG, hemodynamic and cardiac function parameters at each time point for 150 min after stabilization period. The average data of each measurement in vehicle-treated dogs were presented in the same figure as of the dronedarone-treated dogs, but none of the standard deviation and standard error of mean was calculated.
Due to a severe cardiac suppression by dronedarone, all animals were euthanized at the end of the experiment, while they were under general anesthesia with sodium pentobarbital (200 mg/kg; Somnasol, Butler Animal Health Supply, Dublin, OH, U.S.A.) in accordance with American Veterinary Medical Association guidelines [2].
Data analysis: Electrocardiographic data were analyzed for rhythm and rate, including P wave, QRS complex, QT and QTc. Value of each parameter was averaged from cardiac cycles over 30 sec of each time point. Corrected QT interval (QTc interval) was calculated by using Fridericia equation [10].
Arterial blood pressure (BP) was collected at a specific time point from AoP and calculated for mean blood pressure (MBP). The average of three cardiac output determinations was calculated to obtain cardiac output value at each time point. Systemic vascular resistance (SVR) was calculated by using the following equation: SVR=(AoP–RAP)/CO. Pulmonary vascular resistance (PVR) was calculated as the following equation: PVR=(PAP–RAP)/CO. The maximal rate of rise of the left ventricular pressure during isovolumetric contraction (dP/dtmax) was obtained from the LV pressure. Contractility index (CI), the ratio of maximal rate of rise of the left ventricular pressure over the left ventricular pressure at that point, was calculated from the following equation: CI=(dP/dtmax)/P. The maximal rate of fall of the left ventricular pressure during isovolumetric relaxation (dP/dtmin) was obtained from the LV pressure. Isovolumic relaxation time constant (τ, tau), the exponential decline of ventricular pressure during isovolumic relaxation, was calculated from Glantz method [29].
The resulting left ventricular pressure and volume data were analyzed both on- and off-line in order to generate relationships representing the contractile and energetic state of the myocardium to each dose of vehicle and dronedarone. End systolic pressure-volume relationship (ESPVR), the maximal ventricular development pressure at any given left ventricular volume, was obtained from the family of PV loops, and the slope of linear relation of ESPVR was obtained. In addition, PRSW, the slope of the relations between stroke work (SW) and end-diastolic volume (linear), was derived by graphing LV pressure versus LV volume generated during brief periods of venous occlusion. End-diastolic pressure-volume relationship (EDPVR), the ventricular passive filling curve, was also obtained from the PV loops and used as an index of LV relaxation.
Statistical analysis: Statistical analyses were performed with commercially available software. Data are presented as mean ± standard error of the mean. Comparisons were made for each parameter in dronedarone-treated dogs versus baseline of the same group, because none of the parameters in the vehicle-treated dogs changed with time. Differences among time points were determined using one-way ANOVA with repeated measures design. When indicated by a significant F-statistic, specific means were compared by Dunnett’s test multiple comparison. Values of P<0.05 were considered significance for all analyses.
RESULTS
In general, all parameters at each time-point were measurable from all dogs anesthetized with isoflurane. There was no substantial change among parameters of ECG, hemodynamics and cardiac functions in vehicle-treated dogs at each time-point for at least 150 min after stabilization period. From beginning to the end of experiment in vehicle-treated dogs, the heart rate was maintained between 102–114 bpm, while mean aortic pressure was maintained between 83–91 mmHg.
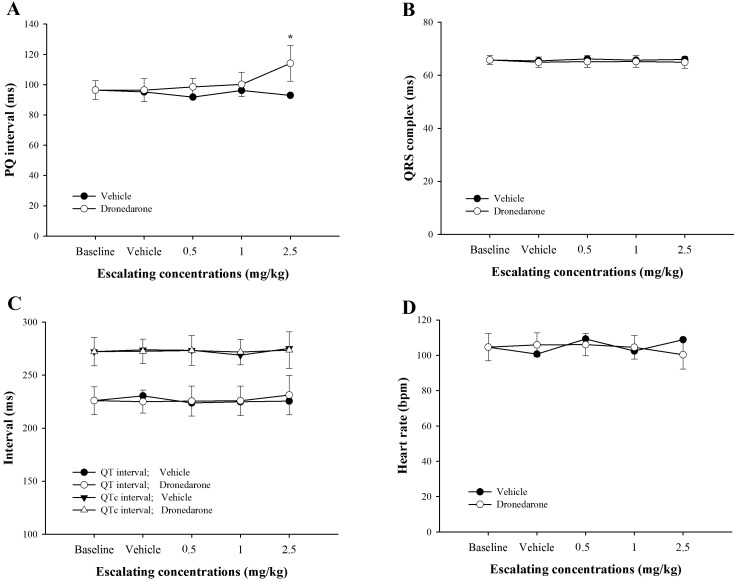
Acute effects of dronedarone on electrocardiograms: At the highest dose of dronedarone (2.5 mg/kg), PQ interval was significantly prolonged by 18.3% when compared with baseline (P<0.01; Fig. 2A). In both vehicle group and dronedarone group, all 3 cumulative doses did not alter QRS complex, QT and QTc intervals and heart rate (Fig. 2B–2D). It can be noticed that all ECG parameters of dogs receiving only vehicle were unaltered.
Fig. 2.
Effects of escalating doses of dronedarone (0.5, 1.0 and 2.5 mg/kg) versus vehicle-treated dogs measured at the same time-point on baseline adjusted PQ interval (A), QRS complex (B), QT and QTc intervals (C) and heart rate (D). Values were presented as mean ± standard error of means (SEM) in dronedarone-treated dogs (n=5), while those values in the vehicle-treated dogs were presented as an average of 2 dogs. * indicates P<0.05.
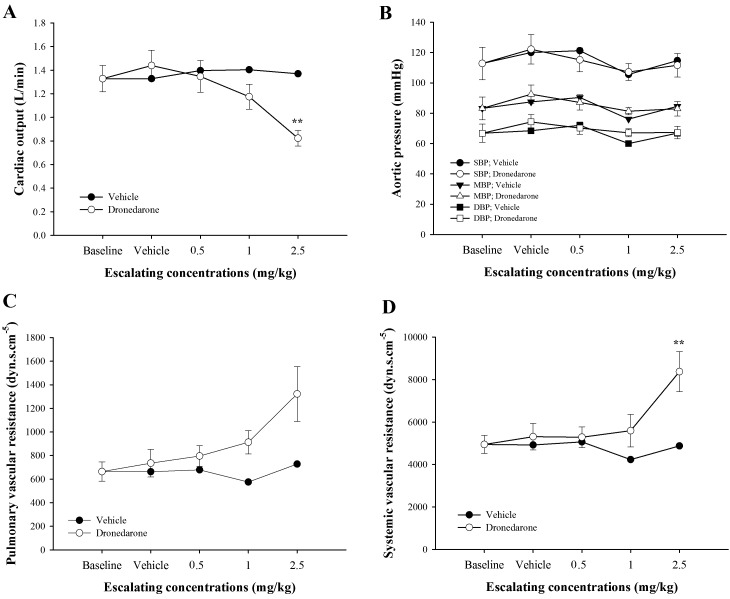
Acute effects of dronedarone on hemodynamics: Figure 3 reveals plots of baseline adjusted cardiac output (A), mean aortic pressure (B), pulmonary vascular resistance (C) and systemic vascular resistance (D) versus cumulative doses obtained during incremental dosing in dronedarone-treated dogs and in vehicle-treated dogs. When dogs were given dronedarone, cardiac output tended to decrease at 1.0 mg/kg and significantly decreased at 2.5 mg/kg when compared with baseline (P<0.01), whereas mean AoP did not change. In response to graded doses of dronedarone, systemic vascular resistance was sharply increased at 2.5 mg/kg when compared with baseline (P<0.01), while PVR did not change. Mean pulmonary arterial pressure (mPAP) and mean RAP at baseline were 14.2 ± 0.82 mmHg and 4.79 ± 0.8 mmHg, respectively. While the mPAP was remained unchanged in dogs receiving either escalating dronedarone or vehicle from baseline to the end of experiment, the mRAP was significantly increased in dogs receiving dronedarone at 2.5 mg/kg (6.65 ± 1.28, P<0.05) when compared with baseline.
Fig. 3.
Effects of escalating doses of dronedarone (0.5, 1.0 and 2.5 mg/kg) versus vehicle-treated dogs measured at the same time-point on baseline adjusted cardiac output (A), mean aortic pressure (B), pulmonary vascular resistance (PVR, C) and systemic vascular resistance (SVR, D). Values were presented as mean ± standard error of means (SEM) in dronedarone-treated dogs (n=5), while those values in the vehicle-treated dogs were presented as an average of 2 dogs. **indicates P<0.01.
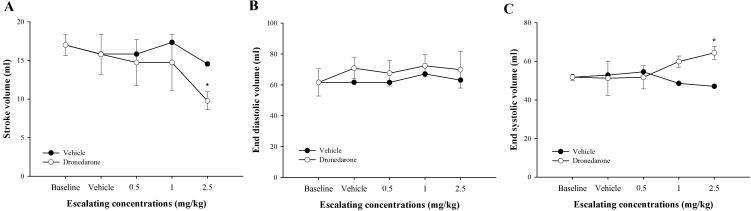
Figure 4 reveals plots of baseline adjusted stroke volume, end-diastolic volume and end-systolic volume versus escalating doses of dronedarone or vehicle. In response to incremental doses of dronedarone, SV and ESV were trivially changed at 0.5 mg/kg and 1.0 mg/kg. Then, the SV was significantly decreased at 2.5 mg/kg (P<0.05, −42.48%) when compared to baseline, whereas the ESV was increased at 1.0 mg/kg and significantly increased at 2.5 mg/kg (P<0.05) when compared to baseline (15.57% and 24.40%, respectively). The EDV did not change in response to either dronedarone or vehicle. The SV, EDV and ESV were unchanged in dogs receiving vehicle.
Fig. 4.
Effects of cumulative doses of dronedarone (0.5, 1.5 and 4 mg/kg) versus vehicle-treated dogs measured at the same time-point on baseline adjusted stroke volume (SV, A), end-diastolic volume (EDV, B) and end-systolic volume (ESV, C). Values were presented as mean ± standard error of means (SEM) in dronedarone-treated dogs (n=5), while those values in the vehicle-treated dogs were presented as an average of 2 dogs. It can be noticed that acute dronedarone administration significantly reduced stroke volume, whereas the end-systolic volume was significantly increased. There is no significant change in end-diastolic volume. *indicates P<0.05.
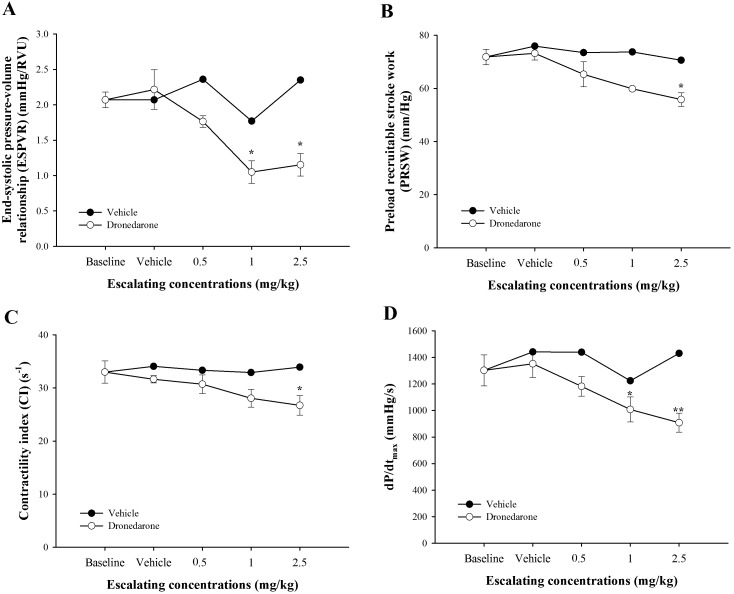
Acute effects of dronedarone on left ventricular functions: Inotropic and lusitropic properties of left ventricle were assessed by parameters obtained from both pressure-volume loop and left ventricular pressure (Figs. 5–7). In response to escalating doses of dronedarone, ESPVR, PRSW, CI and dP/dtmax were decreased in a dose-dependent manner. The end-systolic pressure volume relationship (Fig. 5A) was significantly decreased at 1.0 and 2.5 mg/kg when compared to baseline (P<0.05; −49.30% and −44.37%, respectively). The representative family of pressure-volume loops in 1 dog receiving vehicle and escalating doses of dronedarone (0.5 and 1.0 mg/kg) was also plot in Fig. 6. The PRSW (Fig. 5B) and CI (Fig. 5C) were decreased continuously from baseline and became significantly decreased at 2.5 mg/kg when compared with baseline (P<0.05; −22.29% and −19.02%, respectively). The dP/dtmax (Fig. 5D) was continuously decreased from a dose of 0.5 mg/kg, and the change became significant at 1.0 and 2.5 mg/kg when compared to baseline (P<0.05, −22.62% and P<0.01, −30.26%, respectively). All parameters of contractility were unchanged in dogs receiving a vehicle.
Fig. 5.
Effects of escalating doses of dronedarone (0.5, 1.0 and 2.5 mg/kg) versus vehicle-treated dogs measured at the same time-point on baseline adjusted introtropic indices, end-systolic pressure-volume relationship (ESPVR, A), preload recruitable stroke work (PRSW, B), contractility index (CI, C) and dP/dtmax (D). Values were presented as mean ± standard error of means (SEM) in dronedarone-treated dogs (n=5), while those values in the vehicle-treated dogs were presented as an average of 2 dogs. *indicates P<0.05, and **indicates P<0.01.
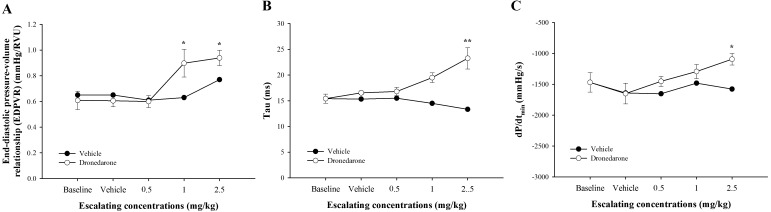
Fig. 7.
Effects of escalating doses of dronedarone (0.5, 1.0 and 2.5 mg/kg) versus vehicle-treated dogs measured at the same time-point on baseline adjusted lusitropic indices, end-diastolic pressure-volume relationship (EDPVR, A), tau (B) and dP/dtmin (C). Values were presented as mean ± standard error of means (SEM) in dronedarone-treated dogs (n=5), while those values in the vehicle-treated dogs were presented as an average of 2 dogs. *indicates P<0.05, and **indicates P<0.01.
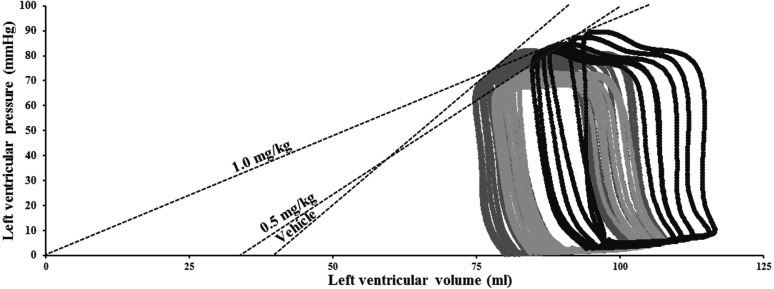
Fig. 6.
Representative left ventricular pressure-volume relationship in a single dog receiving vehicle and after administration of dronedarone (0.5 and 1.0 mg/kg) in isoflurane anesthetized dog. The slopes of the end-systolic pressure-volume relationship were fitted by linear.
In response to escalating doses of dronedarone, end-diastolic pressure volume relationship (EDPVR), tau and dP/dtmin were increased in a dose-dependent manner (Fig. 7). The EDPVR (Fig. 7A) was unchanged at 0.5 mg/kg, but it significantly increased at 1.0 and 2.5 mg/kg when compared with baseline (P<0.05; 47.55% and 54.33%, respectively). The tau (Fig. 7B) and dP/dtmin (Fig. 7C) were significantly increased only at 2.5 mg/kg when compared with baseline (P<0.01, 50.85%; P<0.05, 25.61%, respectively). All parameters of relaxation were unchanged in dogs receiving vehicle.
DISCUSSION
Dronedarone has been used widely for management of AF in humans. While the acute effects of dronedarone on ECG parameters and His bundle electrograms were studied previously [20], there are no references to hemodynamics and cardiac mechanics in dogs. This study was conducted to determine acute effects of dronedarone on electrocardiograms, hemodynamics and cardiovascular functions, especially the left ventricular mechanics in intact dogs anesthetized with isoflurane.
In this study, an escalating dose of dronedarone 2.5 mg/kg caused PQ interval to lengthen to values greater than obtained during baseline and vehicle. This prolongation of PQ interval can be explained by dronedarone binding to its binding site in cardiac calcium channel, which reduces calcium conductance through voltage-gated L-type calcium channel (ICa,L). A previous in vitro whole cell patch clamp study showed that dronedarone blocks ICa,L with IC50 of 0.18 µM at a stimulation frequency of 0.033 Hz in a use- and frequency-dependent manner [12]. Similarly, acute dronedarone application in dog’s papillary muscle demonstrated strong ICa,L inhibitory effect [37]. In α–chloralose anesthetized dogs, intravenous administration of dronedarone (5 mg/kg) has been demonstrated to markedly lengthen PQ interval [20]. The lengthening of PQ interval may be attributed to blocking of the ICa,L or the fast sodium channel (INa) by dronedarone. However, it has been shown previously that dronedarone did not affect the HV interval together with a lack of any effect on QRS interval in this study, suggesting that acute effect of dronedarone at an escalating dose of 2.5 mg/kg did not significantly alter sodium channel. Thus, our study confirmed the findings of previous studies in that acute effect of intravenous dronedarone caused PQ interval prolongation [20, 39]. It has been suggested that the effect of dronedarone on APD and QT/QTc intervals is depending on species and the duration of drug administration partly due to its protein-binding property that may interfere with its electrical properties [26]. In this study, the QT and QTc intervals were unchanged. The result of QTc interval was agreed with previous studies [20, 37]. In α–chloralose anesthetized dogs, a cumulative dose of dronedarone at 4.5 mg/kg did not affect QTc interval. In conscious normal dogs, chronic dronedarone administration orally (25 mg/kg, twice per day, 4 weeks) did not show any effect on QT interval. In contrast to our results, intravenous dronedarone in dogs with complete atrioventricular block produced a QTc shortening effect and suppressed EAD-induced torsades de pointes [38]. Varro and colleagues [37] suggested that it might be because dronedarone has multiple sites of action involving ICa,L, INa and rapid component of delayed rectifier potassium channel (IKr). These multiple blocking effects may balance depolarizing and repolarizing currents resulted in unaltered QTc intervals. Therefore, the possible explanation for unchanged QT/QTc intervals in our study would be the duration of drug administration (i.e. acute vs sustained administration) together with the multichannel blocking effects. It has been known that dronedarone reduces heart rate by blockade of both ICa,L and β-adrenergic receptors. In addition, recent studies have shown that dronedarone also inhibits funny channel (If) in pacemaker cells [3, 32, 39]. The heart rate measured in this study was minimally changed in response to escalating doses of dronedarone. Similar results were observed in the study of Hodeige and colleagues in which intravenous dronedarone administration at either 1 mg/kg or 5 mg/kg failed to attenuate isoprenaline-induced increases in HR in anesthetized dog, but oral dose of dronedarone (12.5 mg/kg) significantly reduced the elevation of HR induced by isoprenaline in conscious dogs [15]. In contrast to our result, a cumulative dose of 4.5 mg/kg demonstrated a reduction of heart rate 10–20% in dogs anesthetized with α–chloralose, an anesthetic known to produce minimal cardiac and respiratory depression. The differences between previous studies and our study are the anesthetic regimens and the experimental conditions which may be responsible for the different outcome [20].
A previous study in anesthetized pigs showed that cumulative doses of dronedarone (5 mg/kg, intravenously) had no effect on mean arterial pressure and contractility as evaluated by left ventricular dP/dtmax [32]. In our study, left ventricular contractility was decreased significantly at an escalating dose of 2.5 mg/kg as assessed by end-systolic pressure-volume relationship (ESPVR), preload recruitable stroke work (PRSW) and dP/dtmax. The first two parameters were derived from a family of pressure-volume loops, a gold standard for measurements of cardiac contractility, since they are load-independent indices [33]. On the other hand, dP/dtmax, a measure of baroinometry, is determined by loading conditions (i.e. preload, afterload), heart rate (Bowditch effect) and myocardial contractility [14]. Since the mean arterial blood pressure, end-diastolic volume and heart rate remained unchanged in this experiment, the decrease in dP/dtmax could be a consequence of the negative inotropy of the dronedarone. Therefore, the reduction in LV contractility in our study could be explained by multichannel blocking properties of dronedarone mainly ICa,L and non-competitive binding to β-adrenergic receptors [6]. The poor LV contraction resulted in increased end-systolic volume. Since the end-diastolic volume did not change, the stroke volume was markedly reduced. As a result, a cardiac output (CO) in this study was markedly reduced (−38.02%) from baseline.
Interestingly, while cardiac output markedly decreased, the blood pressure remained unchanged. Since blood pressure is a product of CO and total peripheral resistance (TPR), the reduction in CO must be counteracted by an elevation of TPR. In our study, systemic vascular resistance (SVR) was noticeably increased at an escalating dose of 2.5 mg/kg, while the pulmonary vascular resistance (PVR) was trivially changed. The SVR was mainly determined by the diameter of the blood vessels. However, dronedarone has been known to possess both α- and β-adrenergic blocking effects which may dilate the resistant vessels. This discrepancy could be explained by the fact that when test article with β-blockade property was acutely administered to the dog, the compensatory rise in SVR was usually observed because a fall in CO activates baroreceptor reflex [19, 36]. However, the compensatory increase in heart rate was not detected, since the blocking effects of dronedarone on adrenergic receptors and If channels prevent the compensatory reflex.
In this study, effect of dronedarone on cardiac relaxation was assessed by dP/dtmin and tau. These indices are known to occur during isovolumetric relaxation not after ventricle had filled completely [11]. The left ventricular dP/dtmin is determined by lusitrope, reduction in heart rate, diastolic systemic arterial pressure, structural properties of myocardium and constriction of the pericardium or pericardial effusion [21]. Tau is determined by both heart rate and β-blocking effect of dronedarone. In response to escalating doses of dronedarone, dP/dtmin and tau changed in a dose-dependent manner. Since the heart rate remained unchanged throughout the experiment, the observed change in tau (i.e. negative lusitrope) may result from β-blocking effect of dronedarone which prevents phosphorylation of phospholamban; therefore, less calcium is resequestrated through the SERCa2+ channel resulted in slow relaxation [4]. This result is also in accordance with our previous study in which dP/dtmin and tau were lengthened when metoprolol was given to anesthetized guinea pigs [17]. The end-diastolic pressure-volume relationship (EDPVR) has been used as an index of lusitrope, since it measures the relationship between pressure and volume at the end-diastole [5]. Similar to the results of tau and dP/dtmin, EDPVR was elevated at an escalating dose of 2.5 mg/kg compared to baseline, suggesting diastolic dysfunction due to acute effect of β-blockade. The similar result was observed when escalating doses of metoprolol were given intravenously in anesthetized guinea pigs [17].
It is well known that dronedarone and other amiodarone-like agents had a slow onset of action [23]. It is also known that these compounds had profound effects on hemodynamics when given intravenously [8]. Therefore, the parameters of ECG, hemodynamics and cardiac mechanics obtained in this study were measured 15 min after the end of infusion of each dose to allow the recovery of hemodynamics during infusion period and permit the drug to stabilize.
In humans, dronedarone is used for either maintaining sinus rhythm or reducing ventricular rate in atrial fibrillation [30]. Based on successful clinical trials in humans, dronedarone might be useful in veterinary medicine as well. This study was conducted on healthy beagle dogs anesthetized with isoflurane. The typical clinical patients with arrhythmias, especially AF, are large breed dogs or small breed dogs with heart diseases [13, 41]. Data from this study must be interpreted cautiously in clinical patients. Since dronedarone exerts its negative inotropy and lusitropy, caution should be exercised when using dronedarone in dogs with supra- or ventricular arrhythmias with ventricular compromise, especially when the arrhythmias comorbidity with unstable heart failure. Furthermore, it is difficult for veterinarian practitioners to extrapolate therapeutic dosage from the present study, since the cumulative doses were investigated intravenously. Therefore, a further study should be performed to explore therapeutic oral dose in conscious dogs or dogs with atrial fibrillation.
Study limitations: We did not measure plasma concentrations of dronedarone and its metabolite, N-debutyl metabolite. In humans, the steady state plasma concentration of oral dronedarone (a therapeutic dose of 400 mg, twice daily) is 84–167 ng/ml [26]. As far as we are aware, there is only one study measured the plasma and tissue levels of dronedarone in dogs after chronic oral administration (25 mg/kg, twice per day, 4 weeks) [37]. After 4 weeks, the plasma concentration of dronedarone was 1.01 ± 0.32 µg/ml, and the plasma concentration of N-debutyl metabolite was 0.09 ± 0.03 µg/ml. That plasma concentration does not produce any change on APD and QT/QTc intervals, except for a strong use-dependent Vmax depression. The pharmacokinetics of dronedarone have been reported in dogs previously [28]. After an oral dose, the time to maximum plasma concentration (tmax) is between 1–4 hr, and the steady state plasma concentrations were achieved between 7–14 days [26, 28]. The absolute bioavailability of dronedarone was between 14–22%. Dronedarone was greatly bound to dog’s plasma proteins (>99.5%) without concentration dependent. It is rapidly and extensively distributed to several organs (i.e. kidneys, spleen, lung and liver). After absorption, dronedarone was extensively metabolized to metabolites (i.e. N-debutyl metabolite). It is eliminated by metabolic clearance and excreted mainly by biliary excretion. The escalating doses of dronedarone used in our study are within the range of a previous study (1–17 mg/kg, intravenously) that have been demonstrated to produce physiological effects in anesthetized dogs [20]. Based on the pharmacokinetics of dronedarone in dogs, the lowest and highest cumulative doses of dronedarone in our study (0.5 and 4 mg/kg) would yield a plasma concentration approximately 10 and 80 times higher than the study of Varro and colleagues [37]. Since that study did not report the percentage of recovery of dronedarone’s extraction process, it is possible that the cumulative doses of dronedarone in our study are lower than those calculations.
Conclusions: This study demonstrated significant acute cardiovascular effects of drondarone in anesthetized dogs. The effects can be explained by calcium channel and adrenergic receptors blockade leading to prolong PQ interval, decrease contractility and worsen lusitropic properties of the left ventricle. To explain more fully effects of dronedarone observed in this study, future studies should be directed at measuring alterations in myocyte calcium homeostasis and chronic cardiovascular effects.
Acknowledgments
ACKNOWLEDGMENTS. This study was supported by Ratchadaphisek Sompot endowment fund from Chulalongkorn University (the 72th and 90th Anniversary of Chulalongkorn University Fund (GCUGR1125572059D); Special task Force for Activating Research (GSTAR 56-008-31-001)) and Qtest Labs, LLC. Dr. Nakkawee Saengklub was partly supported by Graduate School, Chulalongkorn University to performed research while she was at QTest labs. We also thank Drs. Carlos del Rio and Yukie Ueyama at QTest Labs for their help with equipment set up.
REFERENCES
- 1.Armengol J., Man G. C., Balsys A. J., Wells A. L.1981. Effects of the respiratory cycle on cardiac output measurements: reproducibility of data enhanced by timing the thermodilution injections in dogs. Crit. Care Med. 9: 852–854. doi: 10.1097/00003246-198112000-00010 [DOI] [PubMed] [Google Scholar]
- 2.Artwhol J., Carbone L., Flecknell P., Friedman D. P., Pritchett-Corning K.2013. AVMA Guidelines for the Euthanasia of Animals: 2013 Edition. the American Veterinary Medical Association, Schaumburg. [Google Scholar]
- 3.Bogdan R., Goegelein H., Ruetten H.2011. Effect of dronedarone on Na+, Ca2+ and HCN channels. Naunyn Schmiedebergs Arch. Pharmacol. 383: 347–356. doi: 10.1007/s00210-011-0599-9 [DOI] [PubMed] [Google Scholar]
- 4.Bristow M. R.2011. Treatment of chronic heart failure with β-adrenergic receptor antagonists: a convergence of receptor pharmacology and clinical cardiology. Circ. Res. 109: 1176–1194. doi: 10.1161/CIRCRESAHA.111.245092 [DOI] [PubMed] [Google Scholar]
- 5.Burkhoff D.2013. Pressure-volume loops in clinical research: a contemporary view. J. Am. Coll. Cardiol. 62: 1173–1176. doi: 10.1016/j.jacc.2013.05.049 [DOI] [PubMed] [Google Scholar]
- 6.Chatelain P., Meysmans L., Mattéazzi J. R., Beaufort P., Clinet M.1995. Interaction of the antiarrhythmic agents SR 33589 and amiodarone with the beta-adrenoceptor and adenylate cyclase in rat heart. Br. J. Pharmacol. 116: 1949–1956. doi: 10.1111/j.1476-5381.1995.tb16397.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christiansen C. B., Torp-Pedersen C., Køber L.2010. Efficacy and safety of dronedarone: a review of randomized trials. Expert Opin. Drug Saf. 9: 189–199. doi: 10.1517/14740330903514105 [DOI] [PubMed] [Google Scholar]
- 8.Cushing D. J., Cooper W. D., Gralinski M. R., Lipicky R. J.2010. The hypotensive effect of intravenous amiodarone is sustained throughout the maintenance infusion period. Clin. Exp. Pharmacol. Physiol. 37: 358–361. doi: 10.1111/j.1440-1681.2009.05303.x [DOI] [PubMed] [Google Scholar]
- 9.Ehrlich J. R., Nattel S.2009. Novel approaches for pharmacological management of atrial fibrillation. Drugs 69: 757–774. doi: 10.2165/00003495-200969070-00001 [DOI] [PubMed] [Google Scholar]
- 10.Fridericia L. S.1920. Die Systolendauer im Elektrokardiogramm bei normalen Menschen und bei Herzkranken. Acta Med. Scand. 53: 469–486. doi: 10.1111/j.0954-6820.1920.tb18266.x [DOI] [Google Scholar]
- 11.Garcia M. J., Smedira N. G., Greenberg N. L., Main M., Firstenberg M. S., Odabashian J., Thomas J. D.2000. Color M-mode Doppler flow propagation velocity is a preload insensitive index of left ventricular relaxation: animal and human validation. J. Am. Coll. Cardiol. 35: 201–208. doi: 10.1016/S0735-1097(99)00503-3 [DOI] [PubMed] [Google Scholar]
- 12.Gautier P., Guillemare E., Marion A., Bertrand J. P., Tourneur Y., Nisato D.2003. Electrophysiologic characterization of dronedarone in guinea pig ventricular cells. J. Cardiovasc. Pharmacol. 41: 191–202. doi: 10.1097/00005344-200302000-00007 [DOI] [PubMed] [Google Scholar]
- 13.Guglielmini C., Chetboul V., Pietra M., Pouchelon J. L., Capucci A., Cipone M.2000. Influence of left atrial enlargement and body weight on the development of atrial fibrillation: retrospective study on 205 dogs. Vet. J. 160: 235–241. doi: 10.1053/tvjl.2000.0506 [DOI] [PubMed] [Google Scholar]
- 14.Hamlin R. L., del Rio C.2012. dP/dtmax—a measure of ‘baroinometry’. J. Pharmacol. Toxicol. Methods 66: 63–65. doi: 10.1016/j.vascn.2012.01.001 [DOI] [PubMed] [Google Scholar]
- 15.Hodeige D., Heyndrickx J. P., Chatelain P., Manning A.1995. SR 33589, a new amiodarone-like antiarrhythmic agent: anti-adrenoceptor activity in anaesthetized and conscious dogs. Eur. J. Pharmacol. 279: 25–32. doi: 10.1016/0014-2999(95)00130-D [DOI] [PubMed] [Google Scholar]
- 16.Hohnloser S. H., Crijns H. J., van Eickels M., Gaudin C., Page R. L., Torp-Pedersen C., Connolly S. J., Investigators A., ATHENA Investigators2009. Effect of dronedarone on cardiovascular events in atrial fibrillation. N. Engl. J. Med. 360: 668–678. doi: 10.1056/NEJMoa0803778 [DOI] [PubMed] [Google Scholar]
- 17.Kijtawornrat A., Ueyama Y., del Rio C., Sawangkoon S., Buranakarl C., Chaiyabutr N., Hamlin R. L.2014. Test of the usefulness of a paradigm to identify potential cardiovascular liabilities of four test articles with varying pharmacological properties in anesthetized guinea pigs. Toxicol. Sci. 137: 458–468. doi: 10.1093/toxsci/kft244 [DOI] [PubMed] [Google Scholar]
- 18.Køber L., Torp-Pedersen C., McMurray J. J., Gøtzsche O., Lévy S., Crijns H., Amlie J., Carlsen J., Dronedarone Study Group2008. Increased mortality after dronedarone therapy for severe heart failure. N. Engl. J. Med. 358: 2678–2687. doi: 10.1056/NEJMoa0800456 [DOI] [PubMed] [Google Scholar]
- 19.Lund-Johansen P., Omvik P.1991. Acute and chronic hemodynamic effects of drugs with different actions on adrenergic receptors: a comparison between alpha blockers and different types of beta blockers with and without vasodilating effect. Cardiovasc. Drugs Ther. 5: 605–615. doi: 10.1007/BF03029729 [DOI] [PubMed] [Google Scholar]
- 20.Manning A., Thisse V., Hodeige D., Richard J., Heyndrickx J. P., Chatelain P.1995. SR 33589, a new amiodarone-like antiarrhythmic agent: electrophysiological effects in anesthetized dogs. J. Cardiovasc. Pharmacol. 25: 252–261. doi: 10.1097/00005344-199502000-00010 [DOI] [PubMed] [Google Scholar]
- 21.McConnell B. K., Popovic Z., Mal N., Lee K., Bautista J., Forudi F., Schwartzman R., Jin J. P., Penn M., Bond M.2009. Disruption of protein kinase A interaction with A-kinase-anchoring proteins in the heart in vivo: effects on cardiac contractility, protein kinase A phosphorylation, and troponin I proteolysis. J. Biol. Chem. 284: 1583–1592. doi: 10.1074/jbc.M806321200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moro S., Ferreiro M., Celestino D., Medei E., Elizari M. V., Sicouri S.2007. In vitro effects of acute amiodarone and dronedarone on epicardial, endocardial, and M cells of the canine ventricle. J. Cardiovasc. Pharmacol. Ther. 12: 314–321. doi: 10.1177/1074248407306906 [DOI] [PubMed] [Google Scholar]
- 23.Naccarelli G. V., Jalal S.1995. Intravenous amiodarone. Another option in the acute management of sustained ventricular tachyarrhythmias. Circulation 92: 3154–3155. doi: 10.1161/01.CIR.92.11.3154 [DOI] [PubMed] [Google Scholar]
- 24.National Research Council (U.S.)2011. Guide for the Care and Use of Laboratory Animals, 8th ed., National Academies Press, Washington D.C. [Google Scholar]
- 25.Nattel S., Khairy P., Roy D., Thibault B., Guerra P., Talajic M., Dubuc M.2002. New approaches to atrial fibrillation management: a critical review of a rapidly evolving field. Drugs 62: 2377–2397. doi: 10.2165/00003495-200262160-00005 [DOI] [PubMed] [Google Scholar]
- 26.Patel C., Yan G. X., Kowey P. R.2009. Dronedarone. Circulation 120: 636–644. doi: 10.1161/CIRCULATIONAHA.109.858027 [DOI] [PubMed] [Google Scholar]
- 27.Piccini J. P., Hasselblad V., Peterson E. D., Washam J. B., Califf R. M., Kong D. F.2009. Comparative efficacy of dronedarone and amiodarone for the maintenance of sinus rhythm in patients with atrial fibrillation. J. Am. Coll. Cardiol. 54: 1089–1095. doi: 10.1016/j.jacc.2009.04.085 [DOI] [PubMed] [Google Scholar]
- 28.Product Monograph2014. “Subject: PrMULTAQ® Dronedarone Tablets 400 mg dronedarone (as dronedarone hydrochloride) Antiarrhythmic Agent” (online). Available: http://products.sanofi.ca/en/multaq.pdf.
- 29.Raff G. L., Glantz S. A.1981. Volume loading slows left ventricular isovolumic relaxation rate. Evidence of load-dependent relaxation in the intact dog heart. Circ. Res. 48: 813–824. doi: 10.1161/01.RES.48.6.813 [DOI] [PubMed] [Google Scholar]
- 30.Singh B. N., Connolly S. J., Crijns H. J., Roy D., Kowey P. R., Capucci A., Radzik D., Aliot E. M., Hohnloser S. H., EURIDIS and ADONIS Investigators2007. Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter. N. Engl. J. Med. 357: 987–999. doi: 10.1056/NEJMoa054686 [DOI] [PubMed] [Google Scholar]
- 31.Singh B. N., Cingolani E.2010. A new agent for atrial fibrillation: electrophysiological properties of dronedarone. J. Cardiovasc. Pharmacol. Ther. 15Suppl: 6S–14S. doi: 10.1177/1074248410377618 [DOI] [PubMed] [Google Scholar]
- 32.Sobrado L. F., Varone B. B., Machado A. D., Nearing B. D., Zeng D., Belardinelli L., Verrier R. L.2013. Dronedarone’s inhibition of If current is the primary mechanism responsible for its bradycardic effect. J. Cardiovasc. Electrophysiol. 24: 914–918. doi: 10.1111/jce.12155 [DOI] [PubMed] [Google Scholar]
- 33.Suga H., Sagawa K., Shoukas A. A.1973. Load independence of the instantaneous pressure-volume ratio of the canine left ventricle and effects of epinephrine and heart rate on the ratio. Circ. Res. 32: 314–322. doi: 10.1161/01.RES.32.3.314 [DOI] [PubMed] [Google Scholar]
- 34.Torp-Pedersen C., Crijns H. J., Gaudin C., Page R. L., Connolly S. J., Hohnloser S. H., Investigators A., ATHENA Investigators2011. Impact of dronedarone on hospitalization burden in patients with atrial fibrillation: results from the ATHENA study. Europace 13: 1118–1126. doi: 10.1093/europace/eur102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Touboul P., Brugada J., Capucci A., Crijns H. J., Edvardsson N., Hohnloser S. H.2003. Dronedarone for prevention of atrial fibrillation: a dose-ranging study. Eur. Heart J. 24: 1481–1487. doi: 10.1016/S0195-668X(03)00321-X [DOI] [PubMed] [Google Scholar]
- 36.van den Meiracker A. H., Man in ’t Veld A. J., van Eck H. J., Boomsma F., Schalekamp M. A.1988. Hemodynamic and hormonal adaptations to beta-adrenoceptor blockade. A 24-hour study of acebutolol, atenolol, pindolol, and propranolol in hypertensive patients. Circulation 78: 957–968. doi: 10.1161/01.CIR.78.4.957 [DOI] [PubMed] [Google Scholar]
- 37.Varró A., Takács J., Németh M., Hála O., Virág L., Iost N., Baláti B., Agoston M., Vereckei A., Pastor G., Delbruyère M., Gautier P., Nisato D., Papp J. G.2001. Electrophysiological effects of dronedarone (SR 33589), a noniodinated amiodarone derivative in the canine heart: comparison with amiodarone. Br. J. Pharmacol. 133: 625–634. doi: 10.1038/sj.bjp.0704106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verduyn S. C., Vos M. A., Leunissen H. D., van Opstal J. M., Wellens H. J.1999. Evaluation of the acute electrophysiologic effects of intravenous dronedarone, an amiodarone-like agent, with special emphasis on ventricular repolarization and acquired torsade de pointes arrhythmias. J. Cardiovasc. Pharmacol. 33: 212–222. doi: 10.1097/00005344-199902000-00006 [DOI] [PubMed] [Google Scholar]
- 39.Verrier R. L., Sobrado M. F., Pagotto V. P., Kanas A. F., Machado A. D., Varone B. B., Sobrado L. F., Nearing B. D., Zeng D., Belardinelli L.2013. Inhibition of I(f) in the atrioventricular node as a mechanism for dronedarone’s reduction in ventricular rate during atrial fibrillation. Heart Rhythm 10: 1692–1697. doi: 10.1016/j.hrthm.2013.08.007 [DOI] [PubMed] [Google Scholar]
- 40.Wegener F. T., Ehrlich J. R., Hohnloser S. H.2006. Dronedarone: an emerging agent with rhythm- and rate-controlling effects. J. Cardiovasc. Electrophysiol. 17Suppl 2: S17–S20. doi: 10.1111/j.1540-8167.2006.00583.x [DOI] [PubMed] [Google Scholar]
- 41.Westling J., Westling W., Pyle R. L.2008. Epidemiology of atrial fibrillation in the dog. Intern. J. Appl. Res. Vet. Med. 6: 151–154. [Google Scholar]
- 42.Zimetbaum P. J.2009. Dronedarone for atrial fibrillation—an odyssey. N. Engl. J. Med. 360: 1811–1813. doi: 10.1056/NEJMp0902248 [DOI] [PubMed] [Google Scholar]