Abstract
In order to investigate the effects of SKF96365 (SKF), which is a non-selective cationic channel blocker, on K+ channel currents, we recorded currents through ATP sensitive K+ (IKATP), voltage-gated K+ (IKv) and Ca2+ activated K+ channels (IBK) in the absence and presence of SKF in single small intestinal myocytes of mice with patch-clamp techniques. SKF (10 µM) reversibly abolished IKATP that was induced by cromakalim (10 µM), which is a selective ATP sensitive K+ channel opener. These inhibitory effects were induced in a concentration-dependent and voltage-independent manner. The 50% inhibitory concentration (IC50) was 0.85 µM, which was obviously lower than that reported for the muscarinic cationic current. In addition, SKF (1 µM ≈ the IC50 value in IKATP suppression) reversibly inhibited the IKv that was induced by repetitive depolarizing pulses from −80 to 20 mV. However, the extent of the inhibitory effects was only ~30%. In contrast, SKF (1 µM) had no significant effects on spontaneous transient IBK and caffeine-induced IBK. These results indicated that SKF inhibited ATP sensitive K+ channels and voltage-gated K+ channels, with the ATP sensitive K+ channels being more sensitive than the voltage-gated K+ channels. These inhibitory effects on K+ channels should be considered when SKF is used as a cationic channel blocker.
Keywords: ATP sensitive K+ channel, Ca2+ activated K+ channel, intestinal smooth muscle, SKF96365, voltage-gated K+ channel
In gastrointestinal smooth muscles, the stimulation of muscarinic receptors by cholinergic agonists, including acetylcholine, increases the intracellular concentration of Ca2+ ([Ca2+]i), which results in a contractile response [45]. The increase in [Ca2+]i is induced by the Ca2+ release from internal stores and Ca2+ entry into the cell through L-type voltage-gated Ca2+ channels (CaV1.2). The activation of CaV1.2 can be achieved by depolarization due to muscarinic stimulation of non-selective cationic channels [18, 23, 50]. Cationic channels are thought to consist of transient potential canonical channels 4 and 6 (TRPC4 and 6) [43], which are also permeable to Ca2+ and involved in Ca2+ entry [47]. In addition, muscarinic receptors directly or indirectly regulate the activities of K+ channels that are expressed in gastrointestinal smooth muscle cells, including ATP sensitive K+ (KATP), voltage-gated K+ (KV) and large conductance Ca2+ activated K+ (BK) channels, through which the electrical activities of the muscles are modulated [5, 6, 12, 14, 20].
SKF96365 (SKF) is an imidazole compound that inhibits receptor-mediated Ca2+ entry [27]. SKF has been reported to inhibit the opening of TRPC channels in guinea-pig [52] and mouse ileal cells [9]. Although other inhibitors of TRPC channels, such as quinine, quinidine, La3+ and flufenamic acid, are well known [9, 15, 24], these chemicals are not specific for the TRPC channels. Therefore, in investigations of the physiological and pharmacological properties of muscarinic receptor-gated TRPC channels, SKF has been widely used as a channel inhibitor in gastrointestinal smooth muscle cells [9, 26, 52]. However, it has been reported that application of SKF evoked membrane depolarization in gastrointestinal smooth muscles [15, 51], indicating additional effects of SKF on ion channels other than TRPC channels. The depolarizing action can make it more difficult to analyze the physiological roles of TRPC channels. Schwarz et al. [36] have suggested that SKF blocks inwardly and outwardly rectifying K+ currents in endothelial cells (IC50; ≥40 µM). Thus, it is possible that SKF can block K+ channels that are expressed in gastrointestinal smooth muscles together with TRPC channels, thus resulting in membrane depolarization. However, the effects of SKF on the activities of the K+ channels that are expressed in gastrointestinal smooth muscle cells remain to be elucidated. In the present study, we investigated the effects of SKF on the currents through the KATP (IKATP), KV (IKv) and BK (IBK) channels in longitudinal smooth muscle cells that were isolated from mouse small intestine, which have often been used to investigate the activation mechanisms of TRPC channels.
MATERIALS AND METHODS
The nomenclature of the ion channels was in accordance with Alexander et al. [2]. All of the animal care and experimental procedures described below complied with the guidelines of the local animal ethics committee of the Faculty of Life Sciences, Kyoto Sangyo University.
Cell preparation: Mice (hybrid of 129S4 or 129S6 and CF1) of either sex who were above 2-month-old and who weighed (mean ± standard deviation) 25.1 ± 3.0 g were sacrificed by cervical dislocation. A 15-cm-long gut segment was then removed from the region over the jejunum and ileum, except for the 2.0-cm terminal portion, and placed in physiological salt solution (PSS; for composition, see below). The gut segment was cut into 1.0- to 1.5-cm pieces, and the longitudinal muscle layer was carefully peeled off from each of the pieces. Single smooth muscle cells were isolated enzymatically from the longitudinal muscle layers as described previously [40]. The cells were suspended in PSS containing 0.5 mM CaCl2. A small aliquot was placed on coverglass and stored at 4°C for 2–7 hr until its use on the same day.
Whole-cell current recording: Whole-cell membrane current recordings were performed at room temperature (21–25°C) with standard patch-clamp techniques [11] with 4–7 MΩ patch pipettes. The current signals were amplified with a current amplifier (CEZ-2300 or 5100, Nihon Kohden Corp., Tokyo, Japan) filtered at 1 KHz and captured at a sampling rate of 4 KHz with an analog-digital converter (Digidata 1440A, Molecular Devices, LLC, Sunnyvale, CA, U.S.A.) that was interfaced with a computer (Think Centre A58 Small, Lenovo, Morrisville, NC, U.S.A.) that was running the pCLAMP program (version 10, Molecular Devices, LLC).
For the IKATP recordings, the cells were bathed in a 60-mM K+ solution and intracellularly dialyzed with a K+-rich high BAPTA pipette solution (for compositions, see below) at a holding potential of −80 mV in order to increase the driving force of K+ and minimize the activities of the KV and BK channels [12, 20, 31]. Under these conditions, the K+ equilibrium potential (EK) was estimated at −21 mV, and the K+ currents were inwardly activated. IKv was recorded in cells that was bathed in Ca2+-free PSS (for composition, see below) and dialyzed with the K+-rich high-BAPTA pipette solution in order to minimize the currents through the BK and CaV1.2 channels. The whole-cell voltage clamp mode was held at −80 mV, and 2 sec step pulses to 20 mV that were generated by the pCLAMP program were repeatedly applied to the cell every 20 sec to elicit IKv. When currents were recorded through IBK, a K+-rich low EGTA pipette solution (for composition, see below) was used. The cells were bathed in PSS and held at a holding potential of 0 mV. Recordings of the muscarinic cationic channel currents (mITRPC) were made from cells that were bathed in Cs+-rich external solution and intracellularly dialyzed with Cs+-rich pipette solution (for compositions, see below) at a holding potential of −60 mV in order to eliminate any K+ currents.
Solutions: The PSS that was used in the experiments had the following composition (mM): NaCl, 126; KCl, 6; CaCl2, 2; MgCl2, 1.2; glucose, 14; and HEPES, 10.5; and the pH was adjusted to 7.2 with NaOH. The 60-mM K+ solution was made by changing the concentrations of NaCl and KCl in the PSS to 72 mM and 60 mM, respectively. If necessary, the KCl in the 60-mM K+ solution was replaced with CsCl. The Ca2+-free PSS was prepared by omitting CaCl2 from the PSS and adding 0.5 mM EGTA. The Cs+-rich external solution had the following composition (mM): CsCl, 120; glucose, 12; and HEPES, 10; and the pH was adjusted to pH 7.4 with CsOH. The compositions of each patch-pipette solution (mM) were as follows. The K+-rich low-EGTA pipette solution contained KCl, 134; Na2GTP, 1.0; MgCl2, 1.2; MgATP, 1.0; glucose, 14; EGTA, 0.05; and HEPES, 10.5; and the pH was adjusted to 7.2 with KOH. For the K+-rich high-BAPTA pipette solution, CaCl2 was removed, and 20 mM BAPTA was added. In this case, in order to adjust the total concentration of K+ to 134 mM and the pH to 7.2, the concentration of KCl was reduced to ~67 mM, and KOH was added. The Cs+-rich pipette solution contained CsCl, 80; Na2GTP, 1.0; creatine, 5; MgATP, 1.0; glucose, 20; HEPES, 10; BAPTA, 10; and CaCl2, 4.6 (calculated free calcium=100 nM), with the pH adjusted to 7.4 with CsOH.
Drugs: The following drugs were used: 1-[2-(4-Methoxyphenyl)-2-[3-(4-methoxyphenyl)propoxy]ethyl-1H-imidazole hydrochloride (SKF), cromakalim (Tocris Bioscience, Bristol, U.K.), carbamylcholine chloride (CCh), glibenclamide (Sigma-Aldrich Co. LLC, St. Louis, MO, U.S.A.), tetraethylammonium Chloride (TEA; Tokyo Chemical Industry Co., Ltd., Tokyo, Japan) and caffeine (Wako Pure Chemical Industries Ltd., Osaka, Japan). Cromakalim and glibenclamide were dissolved in dimethyl sulfoxide to form a stock solution and was stored at −20°C. The drug concentrations herein are expressed as the final concentrations that were applied to the cells.
Data analysis: The amplitude of IKATP that was induced by cromakalim was measured, and the difference in the current level after the application of glibenclamide (10 µM) was calculated. In order to determine the current-voltage (I-V) relationships of IKATP, a 350-msec ramp pulse that ranged from −100 mV to 60 mV was applied to the cells before and after SKF application, and the I-V curves were constructed by subtracting the current after the application of glibenclamide from the current in the presence of cromakalim alone or with SKF [31]. The amplitude of IKv was measured by subtracting the leakage component from the outward current that was induced by the depolarizing pulses [44]. The leakage component was estimated in each experiment as follows. A 10-mV hyperpolarizing pulse was applied to the cells at the holding potential (−40 mV), and the amplitude of the current was considered the leakage current for the 10-mV step. The leakage current that corresponded to the 100-mV depolarizing step pulse was subtracted from each of the outward currents that were evoked by the depolarizing pulse. The amplitudes of the spontaneous transient outward IBK (STOC) and caffeine-induced IBK were calculated as the difference from the holding current. The amplitude of mITRPC was calculated as the difference from the holding current before the application of CCh. The extent of the SKF-induced inhibition of each current was expressed as the percentage reduction from the control current level immediately before its application.
The values are presented herein as mean ± S.E.M. The statistical significance of the difference between two groups was tested with a Student’s unpaired or paired t-test. When multiple groups were compared, a one-way analysis of variance (ANOVA) that was followed by a posthoc Bonferroni-type multiple comparison test was used. The differences were considered significant when the P values were less than 0.05. The averaged curves that represented the relationship between the concentration of SKF and its inhibitory effects were constructed by curve fitting with GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, U.S.A.), and these provided the SKF concentration that was required to produce half maximal inhibition (IC50). The cells that were obtained from male and female mice showed similar amplitudes for IKATP, IKv, STOC and mITRPC.
RESULTS
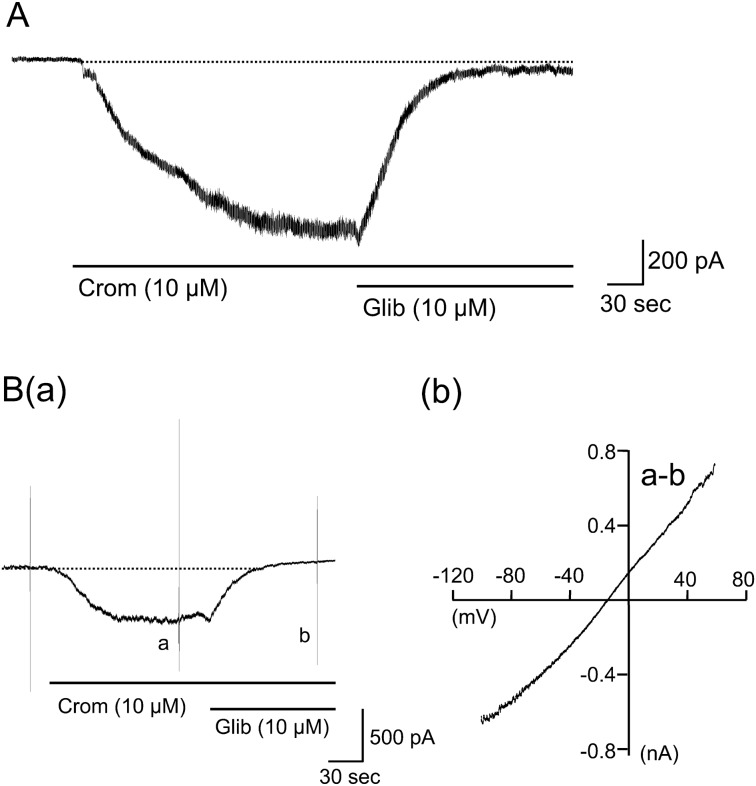
Current through the KATP channels in mouse small intestinal smooth muscles: The cells were dialyzed with the K+-rich high-BAPTA pipette solution and bathed in the 60-mM K+ external solution at a holding potential of −80 mV. Under these conditions, application of the KATP channel opener cromakalim (10 µM) induced an inward current, as has been reported by Nuttle & Farley [31]. The current developed progressively with time and reached a plateau about 1–3 min later (Fig. 1A). Further application of the KATP channel inhibitor, glibenclamide (10 µM), almost completely abolished the current. The mean amplitude of the cromakalim-induced current was −895.2 ± 66.5 pA (n=39). When the extracellular K+ in the bath solution was completely replaced with Cs+, the cromakalim-induced inward current was immediately abolished at −80 mV (n=5; data not shown). The I-V relationships of the cromakalim-induced current were determined by applying a voltage ramp pulse that ranged from −100 mV up to +60 mV over 350 msec (Fig. 1Ba). As shown in Fig 1Bb, the I-V curve was linear across the voltages tested. The polarity of the current was reversed at −18.8 ± 2.0 mV (n=7), which corresponded to the estimated EK of −21 mV, thus indicating that this current was carried through K+ channels. These results indicated that the cromakalim-induced current was carried through KATP channels.
Fig. 1.
Characterization of cromakalim-induced KATP channel currents (IKATP) in single longitudinal smooth muscle cells isolated from mouse small intestine. Cells were dialyzed with the K+-rich high-BAPTA pipette solution and bathed in the 60-mM K+ external solution at a holding potential of −80 mV. Cromakalim (10 µM) was extracellularly applied to the cells. A: a typical example of the cromakalim-induced sustained inward IKATP. B shows the current-voltage (I-V) relationship of cromakalim-induced IKATP. (a): a typical current trace, where a voltage ramp pulse from −100 mV up to +60 mV over 350 msec was applied in the presence of cromakalim alone (current a) and in combination with glibenclamide (10 µM) (current b). (b): the I-V curve constructed by subtraction of the current b from the current a.
SKF reversibly suppressed the KATP channel current (IKATP): After the cromakalim-induced IKATP reached a steady level, SKF (10 µM) was applied in the presence of cromakalim. As shown in Fig. 2A, SKF rapidly decreased the amplitude of IKATP and abolished the current within about 1 min. The time constant (τ) of the suppression (7.3 ± 1.6 sec, n=7) was significantly shorter than the value (35.6 ± 5.8 sec, n=10) in the glibenclamide-induced abolition of IKATP. The removal of SKF from the bath solution allowed the recovery of IKATP with a slower time course. Figure 2Ba shows a typical example of IKATP suppression that was induced by cumulative applications of SKF with a series of ascending concentrations (0.03–10 µM). The inhibitory effects increased with increasing SKF concentrations, and the maximal inhibition was attained at 10 µM. The mean IC50 value that was estimated by the curve fitting of the data from each myocyte was 0.85 ± 0.12 µM (n=6) (Fig. 2Bb).
Fig. 2.
Effects of SKF96365 (SKF) on cromakalim-induced IKATP. IKATP was induced by cromakalim (10 µM) in the cells under the same condition as those mentioned in Fig. 1. A shows a typical current trace when SKF (10 µM) was extracellularly applied to the cell in the presence of cromakalim (10 µM). B shows a relationship between concentration of SKF and its inhibitory effect on IKATP. (a): a typical example of the IKATP suppression induced by cumulative application of SKF (0.03-10 µM). (b): the averaged concentration-inhibition curve of IKATP. Each point indicates the mean ± S.E.M. of measurements in 6 cells. C shows a voltage sensitivity of the SKF-induced IKATP suppression. (a): a typical example of IKATP suppression induced by SKF (1 µM), where a voltage ramp pulse from −100 mV up to +60 mV over 350 msec was applied in the presence of cromakalim (10 µM) alone (current a), cromakalim + SKF (1 µM) (current b) and those agents together with glibenclamide (10 µM) (current c). (b): the I-V curves constructed by subtraction of the current c from the current a or b. (c): the mean percentage of IKATP suppression at each holding potential. Each column indicates mean + 1 S.E.M. The numbers of cells used are presented in parenthesis.
In order to investigate the voltage dependency of the SKF-induced IKATP suppression, the ramp pulses were applied in the absence and presence of SKF (1 µM), and the I-V curves were obtained, as shown in Fig. 2Ca. The I-V curves of IKATP remained linear, and the reversal potential was almost unchanged after the application of SKF (cromakalim alone, −18.5 ± 0.82 mV and cromakalim with SKF, −16.4 ± 0.97 mV; n=6) (Fig. 2Cb). IKATP suppression at −80, −40 and 40 mV was comparable (Fig. 2Cc). These results indicated that the SKF-induced IKATP suppression was voltage-independent.
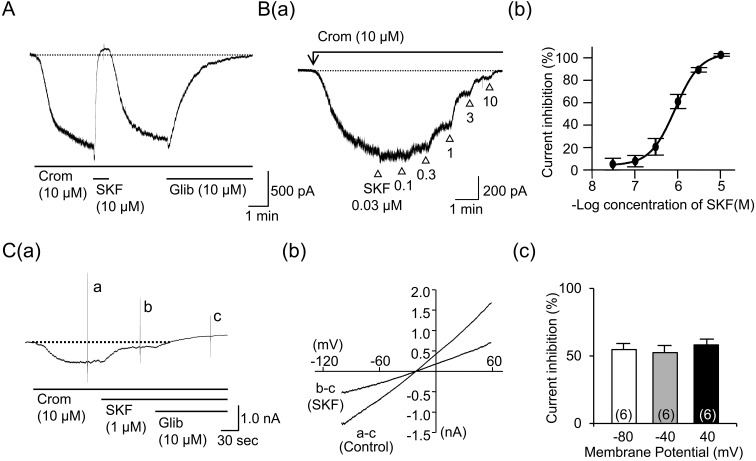
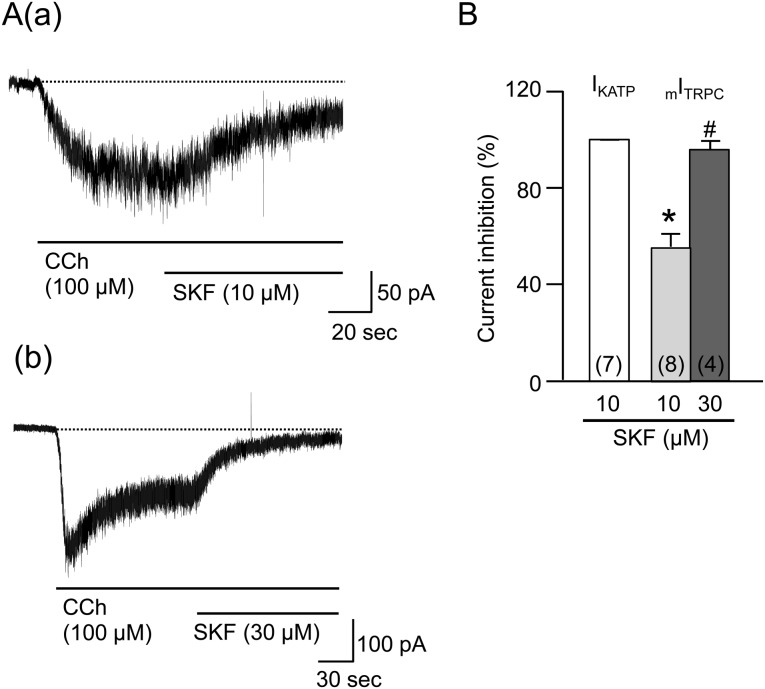
Comparison of the SKF-induced mITRPC suppression with the IKATP suppression: The cells that were bathed in the Cs+-rich external solution were clamped at a voltage of −60 mV with patch pipettes that were filled with the Cs+-rich pipette solution in order to block any K+ currents ([Ca2+]i buffered to 100 nM). Under these conditions, the application of CCh (100 µM) induced an inward mITRPC, as previously reported by Sakamoto et al. [34]. SKF (10 µM), which completely abolished IKATP, was applied to the bath solution in the presence of CCh when the mITRPC reached a steady level. The amplitude of the mITRPC gradually decreased after the application of SKF and reached a plateau about 1 min later (Fig. 3Aa). However, the mean suppression of mITRPC was only 55.5 ± 5.6% (n=8) (Fig. 3B). The time constant (τ) of the inhibitory effect was 38.7 ± 8.3 sec (n=7), which was significantly longer than the value in the IKATP suppression. When a higher concentration of SKF (30 µM) was applied, it almost completely abolished the mITRPC (n=4, Fig. 3Ab and 3B). The abolition had a longer time constant (τ), 12.6 ± 5.3 sec (n=3), than the value in IKATP suppression.
Fig. 3.
Effects of SKF96365 (SKF) on carbachol (CCh)-induced mITRPC. Cells bathed in the Cs+-rich external solution were held under voltage clamp at −60 mV using patch pipettes filled with the Cs+-rich pipette solution to block any K+ currents. Aa and Ab show a typical current trace when SKF (10 and 30 µM) was extracellularly applied to the cell in the presence of CCh (100 µM), respectively. B shows the mean percentages of IKATP and mITRPC suppressions. Each column indicates mean + 1 S.E.M. The numbers of cells used are presented in parenthesis. * in (B) represents significantly smaller inhibition of mITRPC induced by SKF (10 µM) relative to the IKATP one (P<0.05). # in (B) represents significantly greater inhibition of mITRPC induced by SKF (30 µM) relative to the corresponding one induced by SKF (10 µM) (P<0.05).
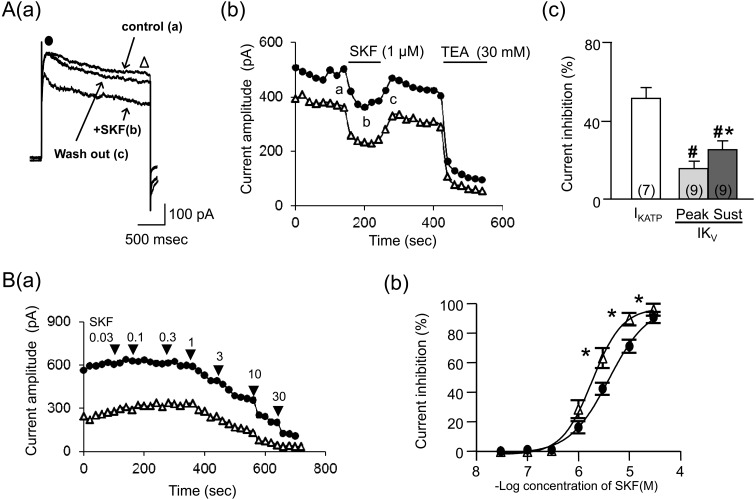
SKF reversibly suppressed IKv: The cells that were bathed in the Ca2+-free PSS were clamped at a voltage of −80 mV with patch pipettes that were filled with the K+-rich high-BAPTA pipette solution. Under these conditions, a 2 sec-step pulse to 20 mV was repeatedly applied every 20 sec in order to elicit IKv. Applications of the depolarizing pulse evoked an initial transient outward current (IKVpeak) with an additional sustained component (IKVsustained), as was previously described in mouse intestinal smooth muscles [25]. As shown in Fig. 4Aa, the amplitudes of both IKVpeak and IKVsustained reduced after the application of SKF (1 µM). The IKv suppressions developed progressively with time and reached a plateau about 1–2 min later (Fig. 4Ab). The suppression of the IKVpeak had a longer time constant (τ) (66.8 ± 23.1 sec, n=8) than the IKVsustained suppression did (21.1 ± 5.4 sec, n=8), although the difference was not statistically significant. The mean suppressions of IKVpeak and IKVsustained by SKF (1 µM) were significantly smaller than IKATP suppression (Fig. 4Ac). The removal of SKF allowed for a partial recovery of IKVpeak and IKVsustained with a slow time course (Fig. 4Ab). The restored IKv was strongly inhibited by the application of the K+ channel blocker TEA (30 mM).
Fig. 4.
Effects of SKF on voltage-gated K+ channel currents (IKv). Cells bathed in the Ca2+-free PSS were held under voltage clamp at −80 mV using patch pipettes filled with the K+-rich high-BAPTA pipette solution. A 2 sec step pulse to 20 mV was repeatedly applied every 20 sec in order to elicit IKv. Aa: IKVpeak (●) and IKVsustained (Δ) recorded in a cell when SKF (1 µM) was extracellularly applied. Ab: time courses of the change in IKVpeak (●) and IKVsustained (Δ) in the cell in a plotted against time (the beginning of depolarizing pulse applications was taken as zero) when SKF (1 µM) was extracellularly applied. Points a-c in the graph correspond with actual IKv records (a–c) in Aa. Ac shows the mean percentages of IKATP and IKv suppressions induced by SKF (1 µM). Each column indicates mean + 1 S.E.M. The numbers of cells used are presented in parenthesis. B (a): time courses of the change in IKVpeak (●) and IKVsustained (Δ) when SKF (0.03-30 µM) was cumulatively applied. Bb: the averaged concentration-inhibition curves of IKVpeak (●) and IKVsustained (Δ). Each point in Bb indicates the mean ± S.E.M. of measurements in 3–5 cells. # in (Ac) represents significantly smaller inhibition of IKv induced by SKF (1 µM) relative to the IKATP one (P<0.05). Asterisks in (Ac) and (Bb) represent significantly greater inhibition of the IKVsustained relative to the IKVpeak (P<0.05).
The cumulative application of SKF (0.03–30 µM) at increasing concentrations inhibited both IKVpeak and IKVsustained in a concentration-dependent manner. The inhibitory effects reached a maximum at 30 µM (Fig. 4Ba). However, the IKVsustained suppressions were significantly larger than the IKVpeak suppressions at 3, 10 and 30 µM (Fig. 4Bb). The mean IC50 value that was estimated by the curve fitting of the data from each myocyte was 3.3 ± 0.24 µM in the IKVpeak (n=5), which was significantly higher than the value in the IKVsustained (2.0 ± 0.35 µM, n=5). These IC50 values were significantly higher than the value in the IKATP suppression.
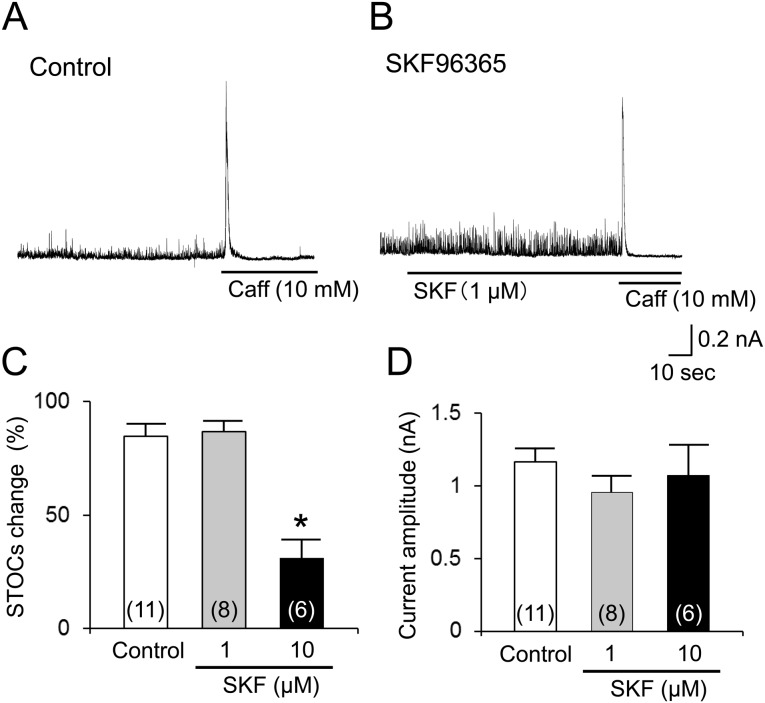
IBK was less sensitive to SKF: It is well known that Ca2+ releases from Ca2+ stores can induce IBK responses [34, 40]. Thus, under the appropriate conditions (see methods), IBK that was evoked by the potent Ca2+ releaser caffeine was recorded in the presence of SKF. As shown in Fig. 5A, the STOCs that were evoked by Ca2+ sparks [7] occurred with various frequencies and amplitudes, as previously reported by Sakamoto et al. [33]. When [Ca2+]i was strongly chelated, the STOCs were not observed at all (see Figs. 1 and 2). The K+ channel blocker TEA (5 mM) almost abolished the STOCs. In the presence of TEA, the application of CCh and the subsequent application of caffeine failed to evoke the IBK (data not shown, n=3). These findings strongly indicated that the STOCs and caffeine-induced IBK were conducted through BK channels. SKF (1 µM) had no obvious effects on the STOCs (Fig. 5A and 5B). The change rate of the STOC amplitude was similar to the time-dependent rundown that was observed in the cells without SKF (Fig. 5C). Caffeine (10 mM) consistently evoked a brief IBK, even in the presence of SKF (1 µM) (Fig. 5B). The mean amplitude of the IBK that was induced by caffeine did not differ significantly from the value without SKF (Fig. 5D). The higher concentration of SKF (10 µM) reduced the STOCs, but not the caffeine-induced IBK (Fig. 5C and 5D).
Fig. 5.
Less sensitivity of SKF to Ca2+ activated K+ channel current (IBK). Cells were bathed in PSS and held at a holding potential of 0 mV using the K+-rich low EGTA pipette solution. Spontaneous transient or caffeine (10 mM)-induced Ca2+ release events were detected as IBK. A and B: typical current traces in the absence (A) and presence (B) of SKF (1 µM), respectively. C shows the mean change rates of spontaneous transient outward IBK (STOCs) in the presence or absence of SKF. D shows summary of caffeine-induced IBK in the presence or absence of SKF. Each column indicates mean + 1 S.E.M. The numbers of cells used are presented in parentheses. * in (C) represents significantly larger reduction in STOCs in the presence of SKF (10 µM) relative to the time-dependent rundown of STOCs in the absence of SKF (P<0.05).
DISCUSSION
Activation of the muscarinic receptors induces the opening of cationic channels comprising TRPC4 and 6 [43]. SKF has been used as an inhibitor of the TRPC channels [9, 26, 52]. However, it has been reported to inhibit other types of ion channels, including CaV1, CaV2, CaV3 [27, 37], NaV1 [13] and inwardly or outwardly rectifying K+ channels [36]. In this study, we investigated the effects of SKF on the activities of the KATP, KV and BK channels that are expressed in gastrointestinal smooth muscle cells.
KATP channels have been suggested to regulate electrical excitability in gastrointestinal smooth muscles [19,20,21]. Cromakalim induced a sustained inward current that was abolished by glibenclamide in small intestinal smooth muscles of the mouse, as has been reported by Nuttle & Farley [31]. The I-V curve of the cromakalim-induced current was almost linear, which was consistent with the results obtained for rabbit esophageal [12] and swine tracheal [31] smooth muscle cells. The reversal potential of the current was similar to the estimated EK value, and the replacement of extracellular K+ with Cs+ abolished the current, which indicated that the charge carrier of this current was K+. These results indicated that the cromakalim-induced current was carried through the KATP channels. The extracellular application of SKF reversibly inhibited the cromakalim-induced IKATP in a concentration-dependent manner. The IKATP was abolished at a SKF concentration of 10 µM. Although SKF (10 µM) also inhibited the CCh-induced mITRPC, the mean percentage of suppression was only about 50% in the present study, which was consistent with the IC50 (6.5–14.5 µM) that has been reported for SKF-induced mITRPC suppression [52]. The mean IC50 value (0.85 µM) for the SKF-induced IKATP suppression in the present study was obviously lower than the reported IC50 value for the mITRPC suppression, which suggested that SKF suppressed the KATP channels with a higher affinity than that required for inhibition of TRPC channels.
The KATP channels are composed of Kir6 and SUR subunits. Two Kir6 genes (Kir6.1 and Kir6.2) and two SUR genes (SUR1 and SUR2) have been identified [1]. The pharmacological properties of the KATP channels, including the sensitivity to its channel opener and blocker, depend on the channel subunits [16, 17, 49]. KATP channels, which have been reported in pancreatic β cells, cardiac muscles and vascular smooth muscles, consist of the Kir6.2/SUR1 [10, 28], Kir6.2/SUR2A [4] and Kir6.1/SUR2B [29, 39, 49] subunits, respectively. Nakayama et al. [30] have reported the expression of Kir6.1 and SUR2B in mouse ileal smooth muscles. Quayle et al. [32] have reported that the IC50 values of glibenclamide, tolbutamide, which is a selective KATP channel inhibitor, and TEA, which is a nonselective K+ channel blocker, were 101 nM, 351 µM and 6.2 mM, respectively, in the smooth muscles from the rabbit mesenteric artery. Taken together with our results, the order of affinity to the KATP channels appears to be glibenclamide >SKF >> tolbutamide >> TEA. Future investigations are needed on the sensitivity of SKF to other types of KATP channels
Because the I-V curve of the IKATP that was obtained from the myocytes in the presence of SKF remained linear, the inhibitory effects of SKF on IKATP seemed to be voltage-independent. Glibenclamide inhibits the KATP by occupying sulfonylurea receptors [8]. The IKATP abolition that was induced by glibenclamide developed more slowly than that induced by SKF, as shown by the time constants of the abolitions. Thus, SKF may inhibit the KATP in a different manner from that of glibenclamide. The short time course of the SKF-induced IKATP suppression implies the involvement of a simple mechanism, such as direct blockade of the channel pore, which is located in Kir6, as was suggested in caffeine-induced IKATP suppression [41]. If so, the binding site might not be a voltage sensor of the channels, and its binding would be reversible. Future investigations with the single channel patch clamp technique are needed to elucidate the blockade mechanisms of SKF.
Zholos et al. [52] have reported that the inhibition of mITRPC by SKF was greater at positive membrane potentials than that at negative ones, which indicated the voltage-dependency of its inhibitory effects. Thus, the mechanisms of SKF-induced suppression of the mITRPC may be somewhat different from those for the IKATP. This idea is supported by the fact that the time constant of the SKF-induced mITRPC suppression was much longer than the corresponding value of IKATP suppression. The different characteristics of these suppressions can provide useful information when SKF is used as a blocker of TRPC or KATP.
KV channels, which are widely expressed in gastrointestinal smooth muscles, are involved in the setting of the resting membrane potential and the determination of amplitude and rhythmicity of action potentials [22, 48]. Application of the depolarizing pulse evoked a biphasic outward current that consisted of IKVpeak and IKVsustained and that was strongly inhibited by TEA. SKF (1 µM), which was a concentration that corresponded to the IC50 in the IKATP suppression, reversibly inhibited the IKv. However, the mean percentages of the SKF-induced IKv suppressions were only ~30%, and the IC50 values (>2 µM), which were significantly higher than the value in the IKATP suppression, were similar to the value reported for mITRPC suppression. A great contribution of the KV4 and KV2 family to the IKVpeak and IKVsustained, respectively, has been suggested [3, 25, 35]. The heterogeneity of IKv can make it difficult to determine the sensitivity of the KV family to SKF due to the lack of commercial blockers with high selectivity. However, the IKVsustained suppression was greater than the IKVpeak suppression with a lower IC50 value (IKVsustained: 2.2 µM vs. IKVpeak: 3.3 µM). Furthermore, the time constant of the IKVsustained suppression was shorter than the value for the IKVpeak suppression. These findings may reflect decreased sensitivity of the KV4 family for SKF compared with KV2. The short time course of the SKF-induced IKVsustained suppression may imply a direct bind of SKF to the channel, as indicated in TEA or 4-Aminopyridine-induced KV channel suppressions [38, 42] as well as the IKATP suppression.
In gastrointestinal smooth muscles, BK channels are expressed and supposed to involve in a negative feedback mechanism to prevent cytosolic Ca2+ overload induced by the muscarinic Ca2+ mobilization [46]. SKF (1 µM) had no significant effects on STOCs and caffeine-induced IBK which reflected the Ca2+ release from the internal stores in the myocytes. These results indicated that BK channel is less sensitive to SKF. Taken together, SKF can inhibit KATP, KV and BK channels in addition to TRPC channel, and the order of affinity of SKF to those channels appears to be KATP >KV ≈ TRPCs >BK. SKF (3–50 µM) has been reported to evoke membrane depolarization in gastrointestinal muscles (15, 51). The depolarization can be explained by the inhibitory effects of SKF on those K+ channels. The depolarization can cause to underestimate a contribution of TRPC channel to mechanical and electrical responses. Thus, the inhibitory effects of those K+ channels should be considered when SKF is used as a TRPC channel blocker in gastrointestinal smooth muscles. Characterizing the SKF-induced suppression, such as IC50 and voltage-dependency, and additional tests with other TRPC blockers may be necessary to assess an involvement of TRPC channel in a response.
In conclusion, SKF can suppress KATP, KV and BK channels. The order of affinity of SKF to those channels is KATP > KV > BK.
Acknowledgments
This work was supported by JSPS KAKENHI Grant Numbers 25870891 and 26450402. All the authors were responsible for the content and editorial decisions for this manuscript. The authors have no competing interests to declare.
REFERENCES
- 1.Aguilar-Bryan L., Clement J. P., 4th, Gonzalez G., Kunjilwar K., Babenko A., Bryan J.1998. Toward understanding the assembly and structure of KATP channels. Physiol. Rev. 78: 227–245. [DOI] [PubMed] [Google Scholar]
- 2.Alexander S. P., Benson H. E., Faccenda E., Pawson A. J., Sharman J. L., Catterall W. A., Spedding M., Peters J. A., Harmar A. J., CGTP Collaborators2013. The Concise Guide to PHARMACOLOGY 2013/14: ion channels. Br. J. Pharmacol. 170: 1607–1651. doi: 10.1111/bph.12447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amberg G. C., Koh S. D., Hatton W. J., Murray K. J., Monaghan K., Horowitz B., Sanders K. M.2002. Contribution of Kv4 channels toward the A-type potassium current in murine colonic myocytes. J. Physiol. 544: 403–415. doi: 10.1113/jphysiol.2002.025163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashcroft F. M., Gribble F. M.1998. Correlating structure and function in ATP-sensitive K+ channels. Trends Neurosci. 21: 288–294. doi: 10.1016/S0166-2236(98)01225-9 [DOI] [PubMed] [Google Scholar]
- 5.Benham C. D., Bolton T. B.1986. Spontaneous transient outward currents in single visceral and vascular smooth muscle cells of the rabbit. J. Physiol. 381: 385–406. doi: 10.1113/jphysiol.1986.sp016333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolton T. B., Lim S. P.1989. Properties of calcium stores and transient outward currents in single smooth muscle cells of rabbit intestine. J. Physiol. 409: 385–401. doi: 10.1113/jphysiol.1989.sp017504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolton T. B., Gordienko D. V.1998. Confocal imaging of calcium release events in single smooth muscle cells. Acta Physiol. Scand. 164: 567–575. doi: 10.1046/j.1365-201X.1998.00464.x [DOI] [PubMed] [Google Scholar]
- 8.Dörschner H., Brekardin E., Uhde I., Schwanstecher C., Schwanstecher M.1999. Stoichiometry of sulfonylurea-induced ATP-sensitive potassium channel closure. Mol. Pharmacol. 55: 1060–1066. [DOI] [PubMed] [Google Scholar]
- 9.Dresviannikov A. V., Bolton T. B., Zholos A. V.2006. Muscarinic receptor-activated cationic channels in murine ileal myocytes. Br. J. Pharmacol. 149: 179–187. doi: 10.1038/sj.bjp.0706852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gloyn A. L., Siddiqui J., Ellard S.2006. Mutations in the genes encoding the pancreatic beta-cell KATP channel subunits Kir6.2 (KCNJ11) and SUR1 (ABCC8) in diabetes mellitus and hyperinsulinism. Hum. Mutat. 27: 220–231. doi: 10.1002/humu.20292 [DOI] [PubMed] [Google Scholar]
- 11.Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J.1981. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 391: 85–100. doi: 10.1007/BF00656997 [DOI] [PubMed] [Google Scholar]
- 12.Hatakeyama N., Wang Q., Goyal R. K., Akbarali H. I.1995. Muscarinic suppression of ATP-sensitive K+ channel in rabbit esophageal smooth muscle. Am. J. Physiol. 268: C877–C885. [DOI] [PubMed] [Google Scholar]
- 13.Hong S. J., Lin W. W., Chang C. C.1994. Inhibition of the Sodium Channel by SK&F 96365, an Inhibitor of the Receptor-Operated Calcium Channel, in Mouse Diaphragm. J. Biomed. Sci. 1: 172–178. doi: 10.1007/BF02253347 [DOI] [PubMed] [Google Scholar]
- 14.Horowitz B., Ward S. M., Sanders K. M.1999. Cellular and molecular basis for electrical rhythmicity in gastrointestinal muscles. Annu. Rev. Physiol. 61: 19–43. doi: 10.1146/annurev.physiol.61.1.19 [DOI] [PubMed] [Google Scholar]
- 15.Hotta A., Kim Y. C., Nakamura E., Kito Y., Yamamoto Y., Suzuki H.2005. Effects of inhibitors of nonselective cation channels on the acetylcholine-induced depolarization of circular smooth muscle from the guinea-pig stomach antrum. J. Smooth Muscle Res. 41: 313–327. doi: 10.1540/jsmr.41.313 [DOI] [PubMed] [Google Scholar]
- 16.Inagaki N., Gonoi T., Clement J. P., 4th, Namba N., Inazawa J., Gonzalez G., Aguilar-Bryan L., Seino S., Bryan J.1995. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science 270: 1166–1170. doi: 10.1126/science.270.5239.1166 [DOI] [PubMed] [Google Scholar]
- 17.Inagaki N., Gonoi T., Clement J. P., Wang C. Z., Aguilar-Bryan L., Bryan J., Seino S.1996. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron 16: 1011–1017. doi: 10.1016/S0896-6273(00)80124-5 [DOI] [PubMed] [Google Scholar]
- 18.Inoue R., Isenberg G.1990. Effect of membrane potential on acetylcholine-induced inward current in guinea-pig ileum. J. Physiol. 424: 57–71. doi: 10.1113/jphysiol.1990.sp018055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jun J. Y., Yeum C. H., Yoon P. J., Chang I. Y., Kim S. J., Kim K. W.1998. ATP-sensitive K+ current and its modulation by substance P in gastric myocytes isolated from guinea pig. Eur. J. Pharmacol. 358: 77–83. doi: 10.1016/S0014-2999(98)00577-9 [DOI] [PubMed] [Google Scholar]
- 20.Jun J. Y., Kong I. D., Koh S. D., Wang X. Y., Perrino B. A., Ward S. M., Sanders K. M.2001. Regulation of ATP-sensitive K+ channels by protein kinase C in murine colonic myocytes. Am. J. Physiol. Cell Physiol. 281: C857–C864. [DOI] [PubMed] [Google Scholar]
- 21.Koh S. D., Bradley K. K., Rae M. G., Keef K. D., Horowitz B., Sanders K. M.1998. Basal activation of ATP-sensitive potassium channels in murine colonic smooth muscle cell. Biophys. J. 75: 1793–1800. doi: 10.1016/S0006-3495(98)77621-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koh S. D., Ward S. M., Dick G. M., Epperson A., Bonner H. P., Sanders K. M., Horowitz B., Kenyon J. L.1999. Contribution of delayed rectifier potassium currents to the electrical activity of murine colonic smooth muscle. J. Physiol. 515: 475–487. doi: 10.1111/j.1469-7793.1999.475ac.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Komori S., Kawai M., Takewaki T., Ohashi H.1992. GTP-binding protein involvement in membrane currents evoked by carbachol and histamine in guinea-pig ileal muscle. J. Physiol. 450: 105–126. doi: 10.1113/jphysiol.1992.sp019118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuriyama H., Kitamura K., Itoh T., Inoue R.1998. Physiological features of visceral smooth muscle cells, with special reference to receptors and ion channels. Physiol. Rev. 78: 811–920. [DOI] [PubMed] [Google Scholar]
- 25.Liu D. H., Huang X., Guo X., Meng X. M., Wu Y. S., Lu H. L., Zhang C. M., Kim Y. C., Xu W. X.2014. Voltage dependent potassium channel remodeling in murine intestinal smooth muscle hypertrophy induced by partial obstruction. PLoS ONE 9: e86109. doi: 10.1371/journal.pone.0086109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsuyama H., Tanahashi Y., Kitazawa T., Yamada M., Komori S., Unno T.2013. Evidence for M2 and M3 muscarinic receptor involvement in cholinergic excitatory junction potentials through synergistic activation of cation channels in the longitudinal muscle of mouse ileum. J. Pharmacol. Sci. 121: 227–236. doi: 10.1254/jphs.12231FP [DOI] [PubMed] [Google Scholar]
- 27.Merritt J. E., Armstrong W. P., Benham C. D., Hallam T. J., Jacob R., Jaxa-Chamiec A., Leigh B. K., McCarthy S. A., Moores K. E., Rink T. J.1990. SK&F 96365, a novel inhibitor of receptor-mediated calcium entry. Biochem. J. 271: 515–522. doi: 10.1042/bj2710515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miki T., Nagashima K., Tashiro F., Kotake K., Yoshitomi H., Tamamoto A., Gonoi T., Iwanaga T., Miyazaki J., Seino S.1998. Defective insulin secretion and enhanced insulin action in KATP channel-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 95: 10402–10406. doi: 10.1073/pnas.95.18.10402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miki T., Suzuki M., Shibasaki T., Uemura H., Sato T., Yamaguchi K., Koseki H., Iwanaga T., Nakaya H., Seino S.2002. Mouse model of Prinzmetal angina by disruption of the inward rectifier Kir6.1. Nat. Med. 8: 466–472. doi: 10.1038/nm0502-466 [DOI] [PubMed] [Google Scholar]
- 30.Nakayama S., Ohya S., Liu H. N., Watanabe T., Furuzono S., Wang J., Nishizawa Y., Aoyama M., Murase N., Matsubara T., Ito Y., Imaizumi Y., Kajioka S.2005. Sulphonylurea receptors differently modulate ICC pacemaker Ca2+ activity and smooth muscle contractility. J. Cell Sci. 118: 4163–4173. doi: 10.1242/jcs.02540 [DOI] [PubMed] [Google Scholar]
- 31.Nuttle L. C., Farley J. M.1997. Muscarinic receptors inhibit ATP-sensitive K+ channels in swine tracheal smooth muscle. Am. J. Physiol. 273: L478–L484. [DOI] [PubMed] [Google Scholar]
- 32.Quayle J. M., Bonev A. D., Brayden J. E., Nelson M. T.1995. Pharmacology of ATP-sensitive K+ currents in smooth muscle cells from rabbit mesenteric artery. Am. J. Physiol. 269: C1112–C1118. [DOI] [PubMed] [Google Scholar]
- 33.Sakamoto T., Unno T., Matsuyama H., Uchiyama M., Hattori M., Nishimura M., Komori S.2006. Characterization of muscarinic receptor-mediated cationic currents in longitudinal smooth muscle cells of mouse small intestine. J. Pharmacol. Sci. 100: 215–226. doi: 10.1254/jphs.FP0050973 [DOI] [PubMed] [Google Scholar]
- 34.Sakamoto T., Unno T., Kitazawa T., Taneike T., Yamada M., Wess J., Nishimura M., Komori S.2007. Three distinct muscarinic signalling pathways for cationic channel activation in mouse gut smooth muscle cells. J. Physiol. 582: 41–61. doi: 10.1113/jphysiol.2007.133165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmalz F., Kinsella J., Koh S. D., Vogalis F., Schneider A., Flynn E. R., Kenyon J. L., Horowitz B.1998. Molecular identification of a component of delayed rectifier current in gastrointestinal smooth muscles. Am. J. Physiol. 274: G901–G911. [DOI] [PubMed] [Google Scholar]
- 36.Schwarz G., Droogmans G., Nilius B.1994. Multiple effects of SK&F 96365 on ionic currents and intracellular calcium in human endothelial cells. Cell Calcium 15: 45–54. doi: 10.1016/0143-4160(94)90103-1 [DOI] [PubMed] [Google Scholar]
- 37.Singh A., Hildebrand M. E., Garcia E., Snutch T. P.2010. The transient receptor potential channel antagonist SKF96365 is a potent blocker of low-voltage-activated T-type calcium channels. Br. J. Pharmacol. 160: 1464–1475. doi: 10.1111/j.1476-5381.2010.00786.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spruce A. E., Standen N. B., Stanfield P. R.1987. The action of external tetraethylammonium ions on unitary delayed rectifier potassium channels of frog skeletal muscle. J. Physiol. 393: 467–478. doi: 10.1113/jphysiol.1987.sp016833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki M., Li R. A., Miki T., Uemura H., Sakamoto N., Ohmoto-Sekine Y., Tamagawa M., Ogura T., Seino S., Marbán E., Nakaya H.2001. Functional roles of cardiac and vascular ATP-sensitive potassium channels clarified by Kir6.2-knockout mice. Circ. Res. 88: 570–577. doi: 10.1161/01.RES.88.6.570 [DOI] [PubMed] [Google Scholar]
- 40.Tanahashi Y., Unno T., Matsuyama H., Ishii T., Yamada M., Wess J., Komori S.2009. Multiple muscarinic pathways mediate the suppression of voltage-gated Ca2+ channels in mouse intestinal smooth muscle cells. Br. J. Pharmacol. 158: 1874–1883. doi: 10.1111/j.1476-5381.2009.00475.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teramoto N., Yunoki T., Tanaka K., Takano M., Masaki I., Yonemitsu Y., Sueishi K., Ito Y.2000. The effects of caffeine on ATP-sensitive K+ channels in smooth muscle cells from pig urethra. Br. J. Pharmacol. 131: 505–513. doi: 10.1038/sj.bjp.0703586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tseng G. N.1999. Different state dependencies of 4-aminopyridine binding to rKv1.4 and rKv4.2: role of the cytoplasmic halves of the fifth and sixth transmembrane segments. J. Pharmacol. Exp. Ther. 290: 569–577. [PubMed] [Google Scholar]
- 43.Tsvilovskyy V. V., Zholos A. V., Aberle T., Philipp S. E., Dietrich A., Zhu M. X., Birnbaumer L., Freichel M., Flockerzi V.2009. Deletion of TRPC4 and TRPC6 in mice impairs smooth muscle contraction and intestinal motility in vivo. Gastroenterology 137: 1415–1424. doi: 10.1053/j.gastro.2009.06.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Unno T., Komori S., Ohashi H.1996. Some evidence against the involvement of arachidonic acid in muscarinic suppression of voltage-gated calcium channel current in guinea-pig ileal smooth muscle cells. Br. J. Pharmacol. 119: 213–222. doi: 10.1111/j.1476-5381.1996.tb15973.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Unno T., Kwon S. C., Okamoto H., Irie Y., Kato Y., Matsuyama H., Komori S.2003. Receptor signaling mechanisms underlying muscarinic agonist-evoked contraction in guinea-pig ileal longitudinal smooth muscle. Br. J. Pharmacol. 139: 337–350. doi: 10.1038/sj.bjp.0705267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Unno T., Matsuyama H., Komori S.2003. Muscarinic signal transduction in gastrointestinal smooth muscle. Recent Res. Dev. Physiol 1: 577–597. [Google Scholar]
- 47.Unno T., Matsuyama H., Okamoto H., Sakamoto T., Yamamoto M., Tanahashi Y., Yan H. D., Komori S.2006. Muscarinic cationic current in gastrointestinal smooth muscles: signal transduction and role in contraction. Auton. Autacoid Pharmacol. 26: 203–217. doi: 10.1111/j.1474-8673.2006.00366.x [DOI] [PubMed] [Google Scholar]
- 48.Vogalis F.2000. Potassium channels in gastrointestinal smooth muscle. J. Auton. Pharmacol. 20: 207–219. doi: 10.1046/j.1365-2680.2000.00183.x [DOI] [PubMed] [Google Scholar]
- 49.Yamada M., Isomoto S., Matsumoto S., Kondo C., Shindo T., Horio Y., Kurachi Y.1997. Sulphonylurea receptor 2B and Kir6.1 form a sulphonylurea-sensitive but ATP-insensitive K+ channel. J. Physiol. 499: 715–720. doi: 10.1113/jphysiol.1997.sp021963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yan H. D., Okamoto H., Unno T., Tsytsyura Y. D., Prestwich S. A., Komori S., Zholos A. V., Bolton T. B.2003. Effects of G-protein-specific antibodies and G β γ subunits on the muscarinic receptor-operated cation current in guinea-pig ileal smooth muscle cells. Br. J. Pharmacol. 139: 605–615. doi: 10.1038/sj.bjp.0705289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zagorodnyuk V., Santicioli P., Maggi C. A.1998. Evidence for the involvement of multiple mechanisms in the excitatory action of bradykinin in the circular muscle of guinea-pig colon. Naunyn Schmiedebergs Arch. Pharmacol. 357: 197–204. doi: 10.1007/PL00005158 [DOI] [PubMed] [Google Scholar]
- 52.Zholos A. V., Tsytsyura Y. D., Philyppov I. B., Shuba M. F., Bolton T. B.2000. Voltage-dependent inhibition of the muscarinic cationic current in guinea-pig ileal cells by SK&F 96365. Br. J. Pharmacol. 129: 695–702. doi: 10.1038/sj.bjp.0703115 [DOI] [PMC free article] [PubMed] [Google Scholar]