Abstract
Preservation of indigenous gastrointestinal microbiota is deemed to be critical for successful captive breeding of endangered wild animals, yet its biology is poorly understood. Here, we investigated cecal bacterial communities in wild Japanese rock ptarmigans (Lagopus muta japonica) and compared them with those in Svalbard rock ptarmigans (L. m. hyperborea) in captivity. Ultra-deep sequencing of 16S rRNA gene indicated that the community structure of cecal microbiota in wild rock ptarmigans was remarkably different from that in captive Svalbard rock ptarmigans. Fundamental differences between bacterial communities in the two groups of birds were detected at the phylum level. Firmicutes, Actinobacteria, Bacteroidetes and Synergistetes were the major phyla detected in wild Japanese rock ptarmigans, whereas Firmicutes alone occupied more than 80% of abundance in captive Svalbard rock ptarmigans. Furthermore, unclassified genera of Coriobacteriaceae, Synergistaceae, Bacteroidaceae, Actinomycetaceae, Veillonellaceae and Clostridiales were the major taxa detected in wild individuals, whereas in zoo-reared birds, major genera were Ruminococcus, Blautia, Faecalibacterium and Akkermansia. Zoo-reared birds seemed to lack almost all rock ptarmigan-specific bacteria in their intestine, which may explain the relatively high rate of pathogenic infections affecting them. We show evidence that preservation and reconstitution of indigenous cecal microflora are critical for successful ex situ conservation and future re-introduction plan for the Japanese rock ptarmigan.
Keywords: captive rock ptarmigan, cecal bacteriome, wild rock ptarmigan
The Japanese rock ptarmigan (Lagopus muta japonica) is a typical endangered species, and recently, a national program for its conservation was approved [19]. Apart from the in situ conservation program, a number of ex situ conservation approaches are currently being implemented at zoos. However, several health problems, such as diarrhea and kidney failure, due to lithiasis are frequently detected in captive bred and reared Japanese rock ptarmigans ([20, 23] and N. Miyano, personal communication), and thus, treatment with antibiotics has been obligatory. Unbalanced intestinal microbiota in captive birds may cause these symptoms, because, in absence of the barrier function of normal gastro-intestinal (GIT) microbiota, diarrhea is usually caused by opportunistic enteropathogens [10], and kidney calculus is caused by accumulation of oxalic acid which, under normal circumstances, is degraded by indigenous intestinal bacteria [1, 15].
Identification of indigenous, health-promoting GIT microorganisms in rock ptarmigans is important for the success of captive breeding of these birds. Therefore, in the present study, we employed next-generation sequencing, also known as ultra-deep sequencing, in order to reveal the distinctiveness and key components of GIT microbiota in healthy wild rock ptarmigans in the Tateyama Mountains, Toyama Prefecture, Japan, by comparing the results with those from captive bred Svalbard rock ptarmigans (L. m. hyperborea) in a captive facility. Hatching in artificial settings for several generations may have caused Svalbard rock ptarmigans to lose rock ptarmigan-specific indigenous GIT microbiota, because these microorganisms are likely to be transferred from hens to nestlings during nesting. Although vertical transmission has been demonstrated only for enteropathogens due to their economical importance [6, 9], other indigenous bacteria in hens are likely to be transmitted in the same way. Once identified, indigenous and potentially health-promoting bacteria in wild birds could be re-inoculated into captive bred ptarmigan nestlings as a kind of probiotic-based vaccine to prevent disorders, such as those mentioned above.
Moreover, reconstitution of cecal microbiota in captive birds was found to be essential for future re-introduction efforts according to data from a successful ex situ conservation program investigating toxin degradation. Indeed, this work showed that the food foraged by wild Japanese rock ptarmigans [16] were Rhododendron aureum, Vaccinium vitis and Empetrum nigrum, which belong to the family Ericaceae. Ericaceous plants contain a wide range of plant toxins and anti-nutritional compounds, such as grayanotoxin and rhodojaponin [25], which indicates that toxin-degrading GIT bacteria may be involved in the protection of wild ptarmigans from toxicity, as evidenced in other animals, such as the Hawaiian native goat [2]. If GIT microbiota communities essential for survival are actually lacking in captive rock ptarmigans, it is imperative to re-establish them before these birds can be re-introduced into the wild.
In this report, we show the marked differences in composition between the GIT bacteriome in wild and captive rock ptarmigans. The results of our study suggest the presence of rock ptarmigan-specific GIT bacteria and their health-conferring benefits.
MATERIALS AND METHODS
Sample collections: Using a pair of sterilized, stainless tweezers, we collected immediately after defecation fresh cecal feces of two male (WL4 and WL5) and two female (WL7 and WL8) wild Japanese rock ptarmigans off the snow surface in the Murodo area (36° 34’N, 137° 36’E, 2,450 m, above sea level) of the Tateyama Mountains in Toyama Prefecture, Japan, on May 2014. The ages of the birds were unknown. At the time of sampling, research field was mostly covered by snow, and birds apparently fed leaves of crowberry (Empetrum nigrum var. japonicum), Rhododendron (Rhododendron brachycarpum) and Japanese stone pine (or hai-matsu) (Pinus pumila). Similarly, we collected cecal feces of two male (YL2 and YL4) and three female (YL1, YL3 and YL5) captive Svalbard rock ptarmigans artificially reared off the floor of individual pens in the Preservation and Research Center, The City of Yokohama, Japan. These birds were all one year old and maintained on pelleted food for rabbit (RM-4, Funabashi Farm, Funabashi, Japan) supplemented with fresh Japanese mustard spinach (Brassica rapa var. perviridis) and Blueberries (Vaccinium spp.) purchased from the local market. All birds were in normal condition, but YL5 experienced chronic diarrhea of unknown causes and treated with a probiotic (Miyarisan, MIYARISAN Pharmaceutical Co., Ltd., Tokyo, Japan) 10 days before the sample collection.
In both cases, special care was taken not to contaminate the feces with snow or dirt. Samples were immersed in DNA conservation solution [11], and bacterial DNA was extracted within 24 hr.
DNA extractions and high-throughput, ultra-deep sequencing of 16S rRNA gene: To obtain bacterial pellets, samples were centrifuged at 20,000 × g for 10 min with a TOMY MX-307 centrifuge (TOMY SEIKO, Tokyo, Japan), and the pellets were washed with Tris-EDTA several times to remove residual plant metabolites, such as phenolic compounds.
Genomic DNA was extracted from the pellets using a Quick Gene DNA Tissue kit (Kurabo, Tokyo, Japan) as per the manufacturer’s instructions (n=3). DNA concentration was determined with a Quant-iT™ dsDNA HS assay kit using a Qubit® fluorometer (Invitrogen, Carlsbad, CA, U.S.A.).
The microbial community structure was analyzed by 16S rRNA gene amplicon sequencing carried out with a MiSeq Desktop Sequencer (Illumina, Hayward, CA, U.S.A.) as per the manufacturer’s instructions (https://support.illumina.com/content/dam/illumina-support/documents/documentation/chemistry_documentation/16s/16s-metagenomic-library-prep-guide-15044223-b.pdf). Partial 16S rRNA gene sequences including V3 and V4 regions were amplified using primers Bakt_341F and Bakt_805R [12] with Illumina overhang adaptor sequences attached to their 5′ ends. The reaction mixture (25 µl) contained 1 × KAPA HiFi HotStart ReadyMix (Kapa Biosystems, Wilmington, MA, U.S.A.), 0.2 µM of each primer and 2 µl of template DNA. PCR was performed with Takara’s Thermal Cycler Dice (TAKARA BIO Inc., Kyoto, Japan) under the following cycling conditions: initial annealing at 95°C for 3 min, followed by 25 cycles at 95°C for 30 sec, 55°C for 30 sec and 72°C for 30 sec and a final extension at 72°C for 5 min. The Illumina sequencing adaptor and index tag sequences were added to the amplicons using a Nextera XT Index kit (Illumina) as per the manufacturer’s instructions. Resulting amplicons were purified using a AMPure XP kit (Beckman Coulter Inc., Brea, CA, U.S.A.) and determined by a Quant-iT™ dsDNA HS assay kit using the Qubit® fluorometer (Invitrogen). Amplicons from triplicate samples were pooled in equal quantity. Resulting DNA was mixed with Phi X control DNA in a ratio of 80:20 and used as a template for paired-end sequencing using a MiSeq Reagent Kit v3 (600 cycles) and the MiSeq desktop sequencer (Illumina).
Phylogenetic analyses and community comparisons: Read files (fastq.gz) were generated using MiSeq Reporter software version 2.3.32 (Illumina). Forward and reverse sequence reads were aligned using PANDAseq assembler [18]. The assembled sequencing data were analyzed using QIIME open-source bioinformatics pipeline version 1.8.0 [5]. Quality-based filtering was carried out using the following settings: Maximum number of ambiguous bases=1, minimum sequence length=380 bp, maximum sequence length=600 bp, no ambiguous bases allowed and maximum number of homopolymers=8. To check for chimeric sequences, USEARCH was used [8]. The sequences were clustered into operational taxonomic units (OTUs) with a criterion of 98% identity by USEARCH. Taxonomic assignment was conducted using a BLAST search against the Greengenes database (version 13_8; ftp://greengenes.microbio.me/greengenes_release/gg_13_5/gg_13_8_otus.tar.gz). Sequences affiliated with eukaryotes, chloroplasts and unknown organisms were eliminated. Good’s coverage was calculated using the formula (1–n/N), where n is the number of singletons and N is the total number of sequences. Inverse Simpson and Shannon were also calculated. Weighted UniFrac analyses were also performed to calculate the pairwise distances between the bacterial communities [17], and principal coordinates analysis (PCA) was applied to visualize the results.
To estimate the possible source of these bacterial OTUs, we identified the 16S rRNA gene sequences of top 20 OTUs in each sample by conducting a BlastN search [3] against the NCBI non-redundant database with identity >98% and E-value <1e−6 in the top 5,000 hits. We categorized constructed OTUs into human-origin, other mammalian-origin, bird-origin or environmental origin with a sub-classification of body part, such as the gut or skin, according to the reference information from the database.
Ethics: Sampling was conducted under the supervision of an officer of Toyama Research Association of rock ptarmigan (Toyama Raicho Kenkyukai), which holds a permission from The Japanese Ministry of Environment and the local forestry office for the access to wild Japanese ptarmigans in Tateyama. Sampling from Svalbard rock ptarmigans in the Yokohama city zoo was permitted by the zoo administration.
Nucleotide sequence accession numbers: Sequencing datasets have been submitted to the DDBJ Sequence Read Archive (DRA) under accession number PRJDB3819. 16S rRNA gene sequences of top 20 OTUs were also deposited to DDBJ with Accession numbers LC055731 to LC055757.
RESULTS AND DISCUSSION
We performed 16S rRNA gene sequencing analyses with an Illumina Miseq sequencer to probe and retrieve bacterial community structures in the samples. After quality filtering, the Miseq sequnece dataset consisted of 886,713 reads with an average of 98,524 ± 76,499 reads (mean ± SD) per sample and an average sequence length of 407 bp. Good’s coverage values ranged between 0.74 and 0.77 for wild rock ptarmigans and between 0.77 and 0.84 for rock ptarmigans in captivity (Supplementary Table 1). In total, 107,612 and 67,895 operational taxonomic units (OTUs) defined with 98% sequence similarity were found in samples from wild and captive rock ptarmigans, respectively. Although the rarefaction curves (Supplementary Fig. 1) indicated that our sequencing effort was not sufficiently large to comprehend the diversity of bacteria in wild and captive rock ptarmigans, we detected about 10,000–20,000 OTUs with the Good’s coverage as high as 74 to 84%. GIT microbiota of birds have not been well explored by deep sequencing approach comparing to mammals including human [24]. Particularly, information about wild birds is still very limited; from 560 to 2,000 OTUs were identified from the feces of various wild penguins [7] and from 150 to 500 OTUs were identified in cecal contents of Emu in captivity [4]. Present detection level is apparently bigger than those previously determined OTUs in bird GIT.
The bacterial community structure of cecal microbiota in wild rock ptarmigans was remarkably different from that in captured rock ptarmigans (Tables 1, 2 and Fig. 1). Cecal microbiota in artificially reared rock ptarmigans were predominantly Firmicutes (84.1%) followed by Actinobacteria (6.2%) and Bacteroidetes (5.6%). Proteobacteria and Verrucomicrobia had minor contributions (ca. 1.5%), and other phyla, such as Tenericutes, Cyanobacteria, Synergistetes and TM7 [13], had only smaller contributions (<1.0%). In contrast, Firmicutes were less abundant (28.6%) in cecal microbiota in wild individuals. Actinobacteria (32.1%), Bacteroidetes (17.6%) and Synergistetes (11.5%) were the most contributing communities after Firmicutes (Table 1).
Table 1. Plylum level prokaryotic microbiome in cecal feces from rock ptarmigans (% in total reads).
| Wild Japanese rock ptarmigans (n=4) | Captive bred Svalbard rock ptarmigans (n=5) | ||||||
|---|---|---|---|---|---|---|---|
| Actinobacteria | 32.08 | ± | 6.58 | Firmicutes | 84.05 | ± | 9.58 |
| Firmicutes | 28.57 | ± | 1.88 | Actinobacteria | 6.21 | ± | 4.33 |
| Bacteroidetes | 17.61 | ± | 2.54 | Bacteroidetes | 5.57 | ± | 7.64 |
| Synergistetes | 11.50 | ± | 2.27 | Proteobacteria | 1.56 | ± | 1.56 |
| Proteobacteria | 4.53 | ± | 0.89 | Verrucomicrobia | 1.42 | ± | 2.82 |
| Spirochaetes | 1.54 | ± | 0.66 | Tenericutes | 0.43 | ± | 0.32 |
| Euryarchaeota | 0.64 | ± | 0.32 | Cyanobacteria | 0.14 | ± | 0.19 |
| Cyanobacteria | 0.04 | ± | 0.03 | TM7a) | 0.12 | ± | 0.16 |
| Tenericutes | 0.03 | ± | 0.02 | Synergistetes | 0.02 | ± | 0.02 |
| Acidobacteria | 0.02 | ± | 0.01 | Chloroflexi | 0.01 | ± | 0.00 |
Data are shown with mean ± standard deviations, a) Hugenholtz et al. [13].
Table 2. Genus level prokaryote microbiome in cecal feces from rock ptarmigans (% in total reads).
| Taxon | Wild Japanese rock ptarmigans (n=4) | Taxon | Captive bred Svalbard rock ptarmigans (n=5) | ||||
|---|---|---|---|---|---|---|---|
| Unidentified genus in Coriobacteriaceae | 17.26 | ± | 5.17 | Unidentified genus in Ruminococcaceae | 24.65 | ± | 6.30 |
| Unidentified genus in Synergistaceae | 11.07 | ± | 2.52 | Unidentified genus in Clostridiales | 18.43 | ± | 4.82 |
| Bacteroides | 8.05 | ± | 0.72 | Ruminococcus | 10.52 | ± | 1.67 |
| Actinomyces | 5.96 | ± | 1.92 | Faecalibacterium | 8.47 | ± | 3.79 |
| Megasphaera | 5.44 | ± | 1.12 | Unidentified genus in Lachnospiraceae | 5.17 | ± | 1.45 |
| Unidenitified genus in Clostridiales | 5.23 | ± | 0.50 | Bifidobacterium | 4.86 | ± | 4.30 |
| Bifidobacterium | 4.99 | ± | 1.15 | Oscillospira | 4.06 | ± | 1.12 |
| Unidentified genus in Ruminococcaceae | 3.94 | ± | 0.84 | Blautia | 3.00 | ± | 0.52 |
| Dialister | 3.36 | ± | 1.88 | Bacteroides | 2.37 | ± | 2.99 |
| Asteroleplasma | 3.32 | ± | 0.76 | Coprobacillus | 2.08 | ± | 0.78 |
| Slackia | 3.12 | ± | 2.12 | Eubacterium | 1.72 | ± | 1.41 |
| Genus YRC22 in Paraprevotellaceae | 2.51 | ± | 0.48 | Akkermansia | 1.47 | ± | 0.67 |
| Prevotella | 2.14 | ± | 1.06 | Odoribacter | 1.41 | ± | 3.15 |
| Unidentified genus in Bacteroidales | 1.84 | ± | 0.62 | Coprococcus | 1.39 | ± | 2.95 |
| Unidentified genus in S24-7 [Bacteroidales] | 1.63 | ± | 0.21 | Unidentified genus in Rikenellaceae | 1.38 | ± | 0.53 |
| Oscillospira | 1.58 | ± | 0.42 | Unidentified genus in Coriobacteriaceae | 1.17 | ± | 1.86 |
| Treponema | 1.46 | ± | 0.93 | Unidentified genus in Enterobacteriaceae | 1.08 | ± | 0.54 |
| Desulfovibrio | 1.40 | ± | 0.68 | Streptococcus | 0.98 | ± | 1.18 |
| Unidentified genus in Lachnospiraceae | 1.36 | ± | 0.40 | Clostridium | 0.94 | ± | 1.30 |
| Genus p-75-a5 in Erysipelotrichaceae | 1.32 | ± | 0.89 | Unidentified genus in Erysipelotrichaceae | 0.71 | ± | 0.76 |
| Parabacteroides | 1.14 | ± | 0.15 | Dorea | 0.56 | ± | 0.28 |
| Genus RFN20 in Erysipelotrichaceae | 0.96 | ± | 0.26 | Unidentified genus in Family RF39 | 0.43 | ± | 0.10 |
| Sutterella | 0.83 | ± | 0.76 | Turicibacter | 0.40 | ± | 0.37 |
| Megamonas | 0.74 | ± | 0.32 | Unidentified genus in Clostridiaceae | 0.37 | ± | 0.65 |
| Genus vadinCA11in Methanomassiliicoccaceae | 0.72 | ± | 0.50 | Lactobacillus | 0.33 | ± | 0.25 |
| Unidentified genus of Enterobacteriaceae | 0.62 | ± | 0.37 | Sutterella | 0.32 | ± | 0.70 |
| Unidentified genus of Bifidobacteriaceae | 0.61 | ± | 0.65 | Rikenella | 0.32 | ± | 0.25 |
| Eubacterium | 0.58 | ± | 0.12 | Unidentified genus in Barnesiellaceae | 0.26 | ± | 0.55 |
Data are shown with mean ± standard deviations.
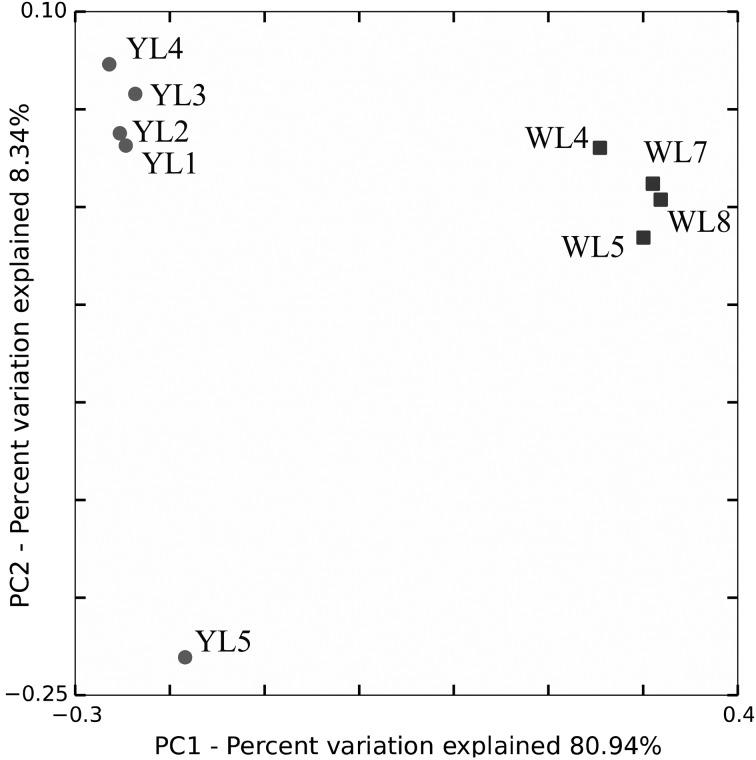
Fig. 1.
The principal components analysis (PCA) plot of all 16S rRNA gene sequences found in the cecal bacteriome samples in wild (WL) and artificially (YL) reared rock ptarmigans. Cecal feces of two male (WL4 and WL5) and two female (WL7 and WL8) wild Japanese rock ptarmigans were subjected to the study. The ages of the birds were unknown. One-year old two male (YL2 and YL4) and three female (YL1, YL3 and YL5) captive Svalbard rock ptarmigans in the Preservation and Research Center, The City of Yokohama, Japan. All birds were in normal condition, but YL5 experienced chronic diarrhea of unknown causes and treated with a probiotic.
The dominant genera in artificially reared individuals were Ruminococcus (10.5%) and unclassified Ruminococcaceae (24.7%) (Table 2). Higher abundance of Lachnospiraceae (i.e. the genus Blautia and unclassified Lachnospiraceae) and Faecalibacterium of Firmicutes, and Akkermansia of Verrucomicrobia were also distinctive in artificially reared birds, but not detected as major genera in wild individuals. Instead, the dominant genera in wild birds were the unidentified genera of Coriobacteriaceae (17.3%) and Synergistaceae (11.1%). In addition, genera Bacteroides, Actinomyces and Megasphaera, and an unidentified genus of Clostridiales, none of which were detected as major contributors in artificially reared individuals, significantly contributed to the cecal microbiome in wild individuals.
There were only 1,174 OTUs shared between the samples from wild and captive birds, whereas the other majorities of OTU, 106,438 OTUs and 66,721 OTUs, were specific to the wild and captivity environments, respectively. Obvious differences between samples in the PCA plot of weighted UniFrac can be seen in Fig. 1.
Supplementary Table 2 shows the phylogenetic identification of predominant genera by BlastN search. Although BlastN search does not directly prove the original source of bacteria, it may give some clues about the origin of the detected bacterial OTU. In this context, we found that the predominant bacterial OTU in the artificially reared birds suggested to have a origin from chicken (YL-OTU-2, 3, 8, 10, 12 and 19), human (YL-OTU-0, 7 and 16), pig (YL-OTU-4, 13) or rodent (YL-OTU-5 and 6), all of which showed high sequence identities (>99%) with known sequences. In contrast to that, predominant bacterial OTUs detected in wild rock ptarmigans showed lower sequence identities (<96%) with known sequences, except for the OTUs (WL-OTU-1, 4, 9, 12, 14 and 17) which showed high sequence identities (>99%) with those obtained from wild birds, such as Tetrao urogallus [26]. It is plausible that these 6 OTUs may be wild grouse -specific bacteria. Other possible sources, such as human, pig, cat and squirrels, were also suggested for some of the top 20 OTUs, but most of them showed low similarities as mentioned above, which suggested un-relatedness rather than relatedness to OTUs detected in wild rock ptarmigans instead (Supplementary Table 2).
The present results clearly show fundamental differences between cecal bacteriome in wild and artificially reared rock ptarmigans. Interesting but not surprising was the fact that artificially reared birds harbored poultry bacteria, most likely due to being fed on feed for poultry supplemented with fresh green vegetables. Moreover, human-hosted bacteria were also detected, which may be due to close contact with keepers or veternarians of the Center. Rearing without contact from mothers easily deprives rock ptarmigans from species-specific bacteria, which seems to be vertically transferred from progenitors, although several common bacterial OTUs (1,174 OTUs) were detected in both types of birds. Unlike most of all commonly shared OTUs, which were detected in a one-sided manner (Table 3), a limited number of OTUs, as listed in Table 4, were detected in both groups of birds at relatively similar abundance. However, as shown in Table 3, completely undetectable OTUs were rare even within the OTUs detected in the one-sided manner. This may indicate that bacteria in artificially reared birds would grow, if wild-like conditions, especially feeding, were established. However, it may prove difficult for wild condition-associated bacteria from such a small source to grow and rapidly replace bacteria associated with captivity conditions, and hence, artificial reconstitution of rock ptarmigan-specific cecal microbiota is most likely necessary.
Table 3. Species level comparison between wild and captive bred rock ptarmigans a).
| Seq_Code | Suggested species | DDBJ Accession | WL4 | WL5 | WL7 | WL8 | YL1 | YL2 | YL3 | YL4 | YL5 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ptarmigan-OTU0 | Unidentified sp. in Coriobacteriaceae (Olsenella sp.) | LC055731 | 29,747 | 2,181 | 7,776 | 5,203 | 1 | 135 | 96 | 52 | 43 |
| ptarmigan-OTU1 | Actinomyces sp.b) | LC055732 | 11,145 | 1,684 | 2,025 | 1,186 | 1 | 0 | 0 | 1 | 0 |
| ptarmigan-OTU2 | Unidentified species in Clostridiales | LC055733 | 5 | 1 | 2 | 0 | 2,027 | 2,853 | 10,997 | 2,447 | 4,583 |
| ptarmigan-OTU3 | Unidentified species in Synergistaceae (Cloacibacillus sp.) | LC055734 | 11,507 | 3,274 | 5,601 | 4,669 | 0 | 0 | 1 | 2 | 0 |
| ptarmigan-OTU4 | Bacteroides sp. | LC055735 | 11,479 | 1,937 | 4,569 | 3,058 | 1 | 0 | 0 | 2 | 0 |
| ptarmigan-OTU5 | Megasphaera sp.b) | LC055736 | 9,501 | 1,382 | 2,420 | 1,272 | 0 | 0 | 0 | 0 | 0 |
| ptarmigan-OTU6 | Bifidobacterium sp. | LC055737 | 6 | 1 | 0 | 0 | 373 | 1,090 | 9,049 | 2,537 | 231 |
| ptarmigan-OTU7 | Bifidobacterium sp. | LC055738 | 8,934 | 963 | 2,736 | 1,421 | 0 | 0 | 0 | 1 | 0 |
| ptarmigan-OTU8 | Slackia sp. (S. equolifaciens) | LC055739 | 5,410 | 623 | 1,169 | 929 | 1 | 0 | 0 | 1 | 0 |
| ptarmigan-OTU9 | Faecalibacterim sp. | LC055740 | 5 | 2 | 0 | 1 | 1,386 | 3,254 | 7,905 | 5,426 | 1,103 |
| ptarmigan-OTU10 | Unidentified species in Ruminococcaceae (Sporobacter termitidis) | LC055741 | 4,127 | 618 | 826 | 664 | 1 | 0 | 71 | 2 | 5 |
| ptarmigan-OTU11 | Asteroleplasma sp. | LC055742 | 2,437 | 824 | 1,193 | 2,533 | 0 | 0 | 0 | 1 | 0 |
| ptarmigan-OTU12 | Unidenfieid species in Clostridiales b) (Roseburia sp.) | LC055743 | 4,885 | 591 | 1,161 | 573 | 7 | 30 | 176 | 54 | 25 |
| ptarmigan-OTU13 | Unidentified species in Ruminococcaceae | LC055744 | 27 | 7 | 11 | 7 | 1,484 | 2,611 | 5,266 | 2,630 | 3,010 |
| ptarmigan-OTU14 | Dialister sp. | LC055745 | 890 | 1,046 | 2,454 | 1,550 | 0 | 0 | 0 | 0 | 0 |
a) Values are numbers of sequence read identified. Total reads for each bird are shown in Supplementary Table 1. b) Similar sequence was detected from Capercaillie [25]. WL4-–WL8, Wild Japanese rock ptarmigans living in Tateyama Mountains. YL1–YL5, Captive bred Svalbard rock ptarmigans at the Preservation and Research Center, The City of Yokohama. Details, see text.
Table 4. OTUs relatively evenly shared by wild and captive bred rock ptarmigans a).
| Seq Code | Suggested species | DDBJ Accession | WL4 | WL5 | WL7 | WL8 | YL1 | YL2 | YL3 | YL4 | YL5 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ptarmigan-OTU31 | Unidentified species in Clostridiales | LC055750 | 2,336 | 185 | 210 | 133 | 45 | 113 | 226 | 121 | 647 |
| ptarmigan-OTU46 | Unidentified species in Clostridiales | LC055752 | 1,003 | 277 | 701 | 546 | 230 | 59 | 0 | 1 | 0 |
| ptarmigan-OTU37 | Unidentified species in Clostridiales | LC055751 | 1,391 | 157 | 474 | 214 | 7 | 0 | 667 | 157 | 465 |
| ptarmigan-OTU76 | Unidentified species in Ruminococcaceae | LC055756 | 1,350 | 102 | 106 | 58 | 3 | 2 | 83 | 11 | 160 |
| ptarmigan-OTU20 | Unidentified species in Enterobacteriaceae | LC055749 | 777 | 423 | 102 | 63 | 34 | 371 | 93 | 189 | 1,907 |
| ptarmigan-OTU88 | Pseudoflavonifractor sp. | LC055757 | 635 | 48 | 126 | 78 | 5 | 20 | 117 | 41 | 36 |
| ptarmigan-OTU56 | Unidentified species in Clostridiales | LC055753 | 41 | 59 | 232 | 243 | 1 | 1,500 | 225 | 65 | 49 |
| ptarmigan-OTU58 | Unidentified species in Lachnospiraceae | LC055754 | 228 | 23 | 61 | 27 | 140 | 307 | 1,473 | 537 | 680 |
| ptarmigan-OTU69 | Unidentified species in Lachnospiraceae | LC055755 | 206 | 12 | 35 | 14 | 79 | 381 | 275 | 376 | 247 |
| ptarmigan-OTU153 | Unidentified species in Coriobacteriaceae | LC055747 | 104 | 14 | 12 | 8 | 12 | 42 | 71 | 18 | 96 |
| ptarmigan-OTU194 | Unidentified species in Clostridiales | LC055748 | 107 | 13 | 7 | 5 | 14 | 178 | 91 | 172 | 179 |
a) Values are numbers of identified sequence reads. Total reads for each bird are appeared in Supplementary Table 1. WL4-WL8, wild Japanese rock ptarmigans living in Tateyama Mountains. YL1-YL5, captive bred Svalbard rock ptarmigans at the Preservation and Research Center, The City of Yokohama. Details, see text.
In the present study, some OTUs were phylogenetically close to Cloacibacillus sp. and Olsenella sp. which, as shown above (Table 3), suggested the existence of wild rock ptarmigan-specific bacteria and thus deserve further elucidation. Indeed, Olsenella sp., which belongs to the order Actinomycetales, could be possibly regarded as health-promoting elements within indigenous GIT bacteriome in ptarmigans, because Olsenella was previously classified as lactobacilli [22], and lactic acid bacteria are usually regarded as health promoting in various animals hosts [21]. Cloacibacillus sp. belongs to the family Synergistaceae, which has been recognized as a toxin-degrading agent in Hawaiian native goats and enables goats to forage alkaloids-rich plants [2]. As mentioned above, food selected by wild Japanese rock ptarmigans contains plant toxins, such as cyclic diterpenes and alkaloids [16]. We foresee that these particular bacteria will be major targets of future isolation work to test their ability to degrade plant toxins. Since the majority of wild Japanese rock ptarmigans are infected with Eimeria protozoa [14], it is very likely that direct inoculation of fecal slurry from wild birds to captive reared nestlings may not be safe. In this context, a sort of bacterial cocktail containing both rock ptarmigan-specific and health-promoting bacteria would be a safer option for the ex situ conservation program.
Supplementary
Acknowledgments
We thank Mr. Tsutomu MATSUDA, President of “Toyama Raicho Kenkyukai” (Toyama Research Association of rock Ptarmigan), for coordinating and supervising of sampling at the Murodo area in Tateyama mountains. We also thank Dr. Emi SUNAGA, Dr. Midori ICHINOSE and Mr Toshiro SHIRAISHI of the Preservation and Research Center, The City of Yokohama, for sampling the cecal feces from captive Svalbard rock ptarmigans. This study was financially supported by Grant-in-Aid for Challenging Exploratory Research (26660219) and Japanese Association of Zoos and Aquariums.
REFERENCES
- 1.Allison M. J., Dawson K. A., Mayberry W. R., Foss J. G.1985. Oxalobacter formigenes gen. nov., sp. nov.: oxalate-degrading anaerobes that inhabit the gastrointestinal tract. Arch. Microbiol. 141: 1–7. doi: 10.1007/BF00446731 [DOI] [PubMed] [Google Scholar]
- 2.Allison M. J., Mayberry W. R., McSweeney C. S., Stahl D. A.1992. Synergistes jonesii, gen. nov., sp.nov.: A Rumen Bacterium That Degrades Toxic Pyridinediols. Syst. Appl. Microbiol. 15: 522–529. doi: 10.1016/S0723-2020(11)80111-6 [DOI] [Google Scholar]
- 3.Altschul S. F., Madden T. L., Schäffer A. A., Zhang J., Zhang Z., Miller W., Lipman D. J.1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25: 3389–3402. doi: 10.1093/nar/25.17.3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett D. C., Tun H. M., Kim J. E., Leung F. C., Cheng K. M.2013. Characterization of cecal microbiota of the emu (Dromaius novaehollandiae). Vet. Microbiol. 166: 304–310. doi: 10.1016/j.vetmic.2013.05.018 [DOI] [PubMed] [Google Scholar]
- 5.Caporaso J. G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F. D., Costello E. K., Fierer N., Peña A. G., Goodrich J. K., Gordon J. I., Huttley G. A., Kelley S. T., Knights D., Koenig J. E., Ley R. E., Lozupone C. A., McDonald D., Muegge B. D., Pirrung M., Reeder J., Sevinsky J. R., Turnbaugh P. J., Walters W. A., Widmann J., Yatsunenko T., Zaneveld J., Knight R.2010. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7: 335–336. doi: 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox N. A., Stern N. J., Hiett K. L., Berrang M. E.2002. Identification of a new source of Campylobacter contamination in poultry: transmission from breeder hens to broiler chickens. Avian Dis. 46: 535–541. doi: 10.1637/0005-2086(2002)046[0535:IOANSO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 7.Dewar M. L., Arnould J. P. Y., Dann P., Trathan P., Groscolas R., Smith S.2013. Interspecific variations in the gastrointestinal microbiota in penguins. Microbiologyopen 2: 195–204. doi: 10.1002/mbo3.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edgar R. C., Haas B. J., Clemente J. C., Quince C., Knight R.2011. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27: 2194–2200. doi: 10.1093/bioinformatics/btr381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fertner M. E., Olsen R. H., Bisgaard M., Christensen H.2011. Transmission and genetic diversity of Enterococcus faecalis among layer chickens during hatch. Acta Vet. Scand. 53: 56. doi: 10.1186/1751-0147-53-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorbach S. L.1996. Chpter 95: Microbiology of the Gastrointestinal Tract. In: Medical Microbiology, 4th ed. (Baron, S. ed.), University of Texas Medical Branch at Galveston, Galveston. Retrieved from http://www.ncbi.nlm.nih.gov/books/NBK7670/#_ncbi_dlg_citbx_NBK7670. [PubMed] [Google Scholar]
- 11.Hayaishi S., Kawamoto Y.2006. Low genetic diversity and biased distribution of mitochondrial DNA haplotypes in the Japanese macaque (Macaca fuscata yakui) on Yakushima Island. Primates 47: 158–164. doi: 10.1007/s10329-005-0169-1 [DOI] [PubMed] [Google Scholar]
- 12.Herlemann D. P. R., Labrenz M., Jürgens K., Bertilsson S., Waniek J. J., Andersson A. F.2011. Transitions in bacterial communities along the 2000 km salinity gradient of the Baltic Sea. ISME J. 5: 1571–1579. doi: 10.1038/ismej.2011.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hugenholtz P., Tyson G. W., Webb R. I., Wagner A. M., Blackall L. L.2001. Investigation of candidate division TM7, a recently recognized major lineage of the domain Bacteria with no known pure-culture representatives. Appl. Environ. Microbiol. 67: 411–419. doi: 10.1128/AEM.67.1.411-419.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishihara S., Shiibashi T., Sato Y., Murata K., Nogami S.2006. Two Eimeria species isolated from wild Japanese rock ptarmigans (Lagopus mutus japonicus) in Japan. J. Vet. Med. Sci. 68: 991–993. doi: 10.1292/jvms.68.991 [DOI] [PubMed] [Google Scholar]
- 15.Ito H., Kotake T., Masai M.1996. In vitro degradation of oxalic acid by human feces. Int. J. Urol. 3: 207–211. doi: 10.1111/j.1442-2042.1996.tb00518.x [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi A., Nakamura H.2011. Seasonal change of food items of the Japanese Rock Ptarmigan. Jpn. J. Ornithol. 60: 200–215. doi: 10.3838/jjo.60.200 [DOI] [Google Scholar]
- 17.Lozupone C., Knight R.2005. UniFrac: a new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 71: 8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masella A. P., Bartram A. K., Truszkowski J. M., Brown D. G., Neufeld J. D.2012. PANDAseq: paired-end assembler for illumina sequences. BMC Bioinformatics 13: 31. doi: 10.1186/1471-2105-13-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ministry of the Environment2013. National conservation program for Japanese rock ptarmigans. http://www.env.go.jp/press/files/jp/24426.pdf (In Japanese).
- 20.Murai A., Murakami M., Sakai H., Shimizu H., Murata K., Yanai T.2011. Glomerular lipidosis accompanied by renal tubular oxalosis in wild and laboratory-reared Japanese rock ptarmigans (Lagopus mutus japonicus). Avian Dis. 55: 709–713. doi: 10.1637/9752-040611-Case.1 [DOI] [PubMed] [Google Scholar]
- 21.Ohashi Y., Ushida K.2009. Health-beneficial effects of probiotics: Its mode of action. Anim. Sci. J. 80: 361–371. doi: 10.1111/j.1740-0929.2009.00645.x [DOI] [PubMed] [Google Scholar]
- 22.Olsen I., Johnson J. L., Moore L. V. H., Moore W. E. C.1991. Lactobacillus uli sp. nov. and Lactobacillus rimae sp. nov. from the human gingival crevice and emended descriptions of lactobacillus minutus and Streptococcus parvulus. Int. J. Syst. Bacteriol. 41: 261–266. doi: 10.1099/00207713-41-2-261 [DOI] [PubMed] [Google Scholar]
- 23.Sato Y.1986. Pseudomonas aeruginosa Infection with Granulomas in a Japanese Ptarmigan. J. Jpn. Vet. Med. Assoc. 39: 516–519. doi: 10.12935/jvma1951.39.516 [DOI] [Google Scholar]
- 24.Waite D. W., Taylor M.2015. Exploring the avian gut microbiota: current trends and future directions. Front. Microbiol. 6: 673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagstaff D. J.2008. International Poisounous Plant Checklist. CRC Press, Boca Raton. [Google Scholar]
- 26.Wienemann T., Schmitt-Wagner D., Meuser K., Segelbacher G., Schink B., Brune A., Berthold P.2011. The bacterial microbiota in the ceca of Capercaillie (Tetrao urogallus) differs between wild and captive birds. Syst. Appl. Microbiol. 34: 542–551. doi: 10.1016/j.syapm.2011.06.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.