Abstract
The common marmoset has been increasingly used for research in the biomedical field; however, there is little information available regarding effective methods of anesthesia in this species. This study retrospectively analyzed 2 regimens of anesthesia induction: intramuscular injection of ketamine followed by inhalation of 5% sevoflurane, and intramuscular injection of midazolam, butorphanol and ketamine followed by inhalation of 5% sevoflurane. Anesthetic depth did not reach the surgical anesthesia stage in 7 out of 99 animals receiving the former regimen, whereas there were only 2 such animals out of 273 receiving the latter regimen. The latter regimen, when followed by maintenance anesthesia with 3% sevoflurane inhalation, was successfully used in various nociceptive procedures. These results indicate that the injection of a combination of midazolam, butorphanol and ketamine followed by inhalation of a high concentration of sevoflurane is effective for anesthesia induction in marmosets.
Keywords: anesthesia induction, balanced anesthesia, multi-modal analgesia, non-human primate, pulse rate
For successful general anesthesia of an animal, the smooth induction of anesthesia is pivotal. One acceptable method to induce anesthesia in cooperative or high-risk animals is to progressively increase the concentration of volatile anesthetics inhaled through a face mask [7, 14, 16]. Using this method, the concentration of the anesthetic is gradually increased starting from 0% of the agent, i.e., 100% carrier gas. If the animal can accept the physical restraint and face mask, this method offers the advantage of being able to accurately control the anesthetic depth during the induction, as well as offers the safety of being able to immediately discontinue the administration of the anesthetics if problems arise. Another acceptable method in cooperative animals is total intravenous anesthesia [19]. Both of these methods require the cooperation of animals and thus cannot be applied to healthy non-cooperative animals.
The common marmoset (Callithrix jacchus) is a non-human primate species with distinctive features, such as small body size (weighing 300 to 450 g) and high fecundity. Marmosets have recently been increasingly used in biomedical research [2, 12, 20]. However, marmosets are extremely sensitive to physical restraint; it has been reported that their resting heart rate measured in their home cage was approximately 230 beats min−1, but increased to approximately 348 (arterial catheter method) or 312 (tail-cuff method) beats min−1 when they were kept in a restraint tube, even when they were accustomed to the apparatus and the procedure [18]. Thus, in common marmosets, minimizing their anxiety and stress is essential for the smooth induction of anesthesia. A practical solution is the combined administration of injectable agents followed by inhalational agents; the former should shorten the time to reach and pass Stage 2 of anesthesia, which is the stage of excitement or involuntary movement [9], and the latter should enable the control of anesthetic depth during the induction and maintenance of anesthesia.
As an injectable anesthetic agent, ketamine has some advantages, such as rapid action, a wide safety margin, and little cardiovascular and respiratory depression [8], and thus is by far the most widely used anesthetic in non-human primate surgical procedures [5]. However, ketamine has limited effects of skeletal muscle relaxation, and its effects differ greatly among individuals [6]. Furthermore, ketamine is more suitable for chemical immobilization than for anesthesia, as increasing its dose only extends the duration of action and does not lead to deeper anesthesia [6]. The aim of this study was to test the working hypothesis that the addition of analgesics and sedatives to ketamine and sevoflurane would improve the induction of anesthesia in marmosets.
This study is based on a retrospective analysis of the records of anesthesia induction in common marmosets, and thus, no ad hoc animal experiments were performed. All the original animal experiments were approved by the ethics committee for primate research of the National Center of Neurology and Psychiatry, Japan, and were conducted in accordance with the institutional guidelines and with the Guidelines for Proper Conduct of Animal Experiments by the Science Council of Japan. The marmosets, ranged from 1 to 10 years old, were caged indoors, with the room lights on from 0700 to 1900 hr, the temperature at 26 to 28°C, the humidity at 40 to 60% and a ventilation rate of 15 air changes per hour. The marmosets were fed 50 g a day of monkey chow (CMS-1M, Clea Japan Inc., Tokyo, Japan) with vitamin supplementation and fruit. Water was provided ad libitum. Marmosets were pair-housed for example in an embryo collection study or single-housed when pairing was not possible for example in an embryo transfer study.
Animals were given an intramuscular (IM) injection of either ketamine (30 mg kg−1; Ketalar, Daiichi-Sankyo, Tokyo, Japan) alone or a combination of midazolam (0.30 mg kg−1; Dormicum, Astellas Pharma Inc., Tokyo, Japan), butorphanol (0.015 mg kg−1; Stadol, Bristol-Myers Squibb, Tokyo, Japan) and ketamine (10 mg kg−1). These doses were selected by referring to the literature [15]. A 29-gauge needle attached to a 0.3 ml insulin syringe (Becton, Dickinson and Co., Franklin Lakes, NJ, U.S.A.) was used to deliver the calculated injectable anesthetic agents. As soon as the righting reflex disappeared, 5% sevoflurane (Sevofrane, Maruishi Pharmaceutical Co., Ltd., Osaka, Japan) with carrier gas, i.e., 80–100% oxygen, was administered at 500 ml min−1 through a face mask (Narcobit K-100RG, Natsume Seisakusho Co., Ltd., Tokyo, Japan) [13]. Fore-paw or hind-paw arterial oxygen saturation and pulse rate were monitored using a pulse-oximeter (8600FO, Nonin Medical Inc., Plymouth, MN, U.S.A.) and were recorded on a personal computer. Anesthetic depth was assessed by the pulse rate and the pinch reflex. When the pulse rate dropped below 250 beats min−1, the pinch reflex was examined by manual pinching of the hind limbs. The induction of anesthesia was considered to be successful when the pinch reflex disappeared within 15 min after the IM injection. In all other cases, anesthesia induction was considered to be unsuccessful.
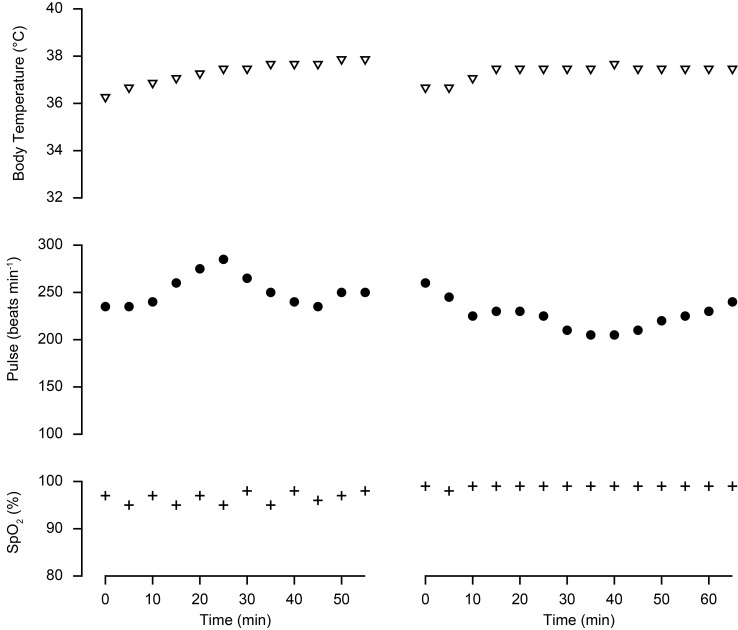
Anesthesia induction was attempted in 372 marmosets with either ketamine-sevoflurane (Ket-Sev) or midazolam-butorphanol-ketamine-sevoflurane (MBK-Sev) regimens. The righting reflex disappeared within a few min in all animals. However, anesthesia induction was not successful in 7 out of 99 animals receiving Ket-Sev and in 2 out of 273 animals receiving MBK-Sev. The frequency of unsuccessful anesthesia induction differed between the 2 regimens (two-tailed Fisher’s exact test, P=0.00176). Exemplified vital signs during the abdominal procedures are shown in Fig. 1. There were no cases of anesthesia-induced accidental death in both groups, indicating that both anesthesia regimens are safe for anesthesia induction in marmosets.
Fig. 1.
Exemplified vital signs during the abdominal procedures. Time 0 indicates the start of monitoring which was about two min after the intramuscular administration of the injectable agents. Left: anesthesia was induced with ketamine and sevoflurane. Right: anesthesia was induced with midazolam, butorphanol, ketamine and sevoflurane.
Ket-Sev has been used for the induction of anesthesia, with the expectation that ketamine would act towards achieving chemical immobilization and sevoflurane would act towards achieving anesthesia. Contrary to this expectation, the Ket-Sev regimen did not induce anesthesia in 7 out of 99 animals. Chemical immobilization using ketamine may be insufficient for the reduction of anxiety in marmosets. If this is the case, it would be better to avoid this regimen, even for non-invasive procedures. On the other hand, the use of midazolam in MBK-Sev regimen is suited to the concept of balanced anesthesia; it allowed reduction of anxiety and stress which then contributed to improve the anesthesia induction.
When the intended experimental procedure included surgery, e.g., neurosurgery or abdominal surgery, the anesthesia was induced as described above and was followed by additional manipulations, such as endotracheal intubation or intravenous catheterization as required, and the anesthetic status was smoothly shifted to maintenance anesthesia with sevoflurane. The induction of anesthesia, maintenance of anesthesia and recovery from anesthesia were smooth. Thus, MBK-Sev administration appears to achieve a high overall quality of anesthesia.
The safety of sedation and anesthesia varies among small animal species: the anesthetics-related mortality rate is 0.17% (163/98,036) in dogs, 0.24% (189/79,178) in cats, 1.39% (114/8,209) in rabbits and 3.80% (49/1,288) in guinea pigs [3]. This variability is likely to be a result of the number of animals that have been anesthetized, and hence, the amount of data available on that species, and the body size. Although there have apparently been no reports to date on the mortality of marmosets upon anesthesia, considering their risk factors, such as their high stress level and small body size, their mortality rate upon anesthesia is likely to be high among small animal species. Furthermore, a bolus injection of the anesthetic, such as that administered to the animals in this study, is a potential risk factor. Taking these factors into account, the absence of any incidences of accidental death in the 273 marmosets administered with MBK-Sev in this study indicates that this method of anesthesia induction is safe for marmosets.
Induction of anesthesia using MBK-Sev is likely to result in a wider spectrum of analgesic effects than that using Ket-Sev. The visceral analgesic effects of ketamine are controversial [1, 4, 16, 17] and unclear. On the other hand, butorphanol was shown to exert visceral analgesic effects, and thus, MBK-Sev is considered to be better than Ket-Sev for abdominal procedures. Indeed, MBK-Sev was effectively used in non-surgical embryo collection [10] and non-surgical embryo transfer procedures [11] of marmosets; in these studies, the same animals were repeatedly subjected to the manipulations, and yet, they retained good health and successfully achieved pregnancy after embryo transfer. Therefore, MBK-Sev administration enables the well-balanced anesthesia of marmosets.
Multi-modal analgesia, by combining different acting drugs, is expected to augment or complement drug action of each drug and to reduce side effects of each drug, by reduction of each dose and antagonizing actions. Indeed, another advantage of MBK-Sev over Ket-Sev regimen is the reduction of the side effects of ketamine, such as excessive salivation or vomiting.
In conclusion, anesthesia induction in common marmosets using a combination of 0.30 mg kg−1 of midazolam, 0.015 mg kg−1 of butorphanol and 10 mg kg−1 of ketamine, followed by inhalation of 5% sevoflurane should enable us to perform short and non-invasive procedures reliably, and should also enable us to perform long or invasive procedures, such as surgery, in combination with the proper subsequent maintenance anesthesia.
Acknowledgments
This study was supported by a grant from the Takeda Science Foundation.
REFERENCES
- 1.Alam S., Saito Y., Kosaka Y.1996. Antinociceptive effects of epidural and intravenous ketamine to somatic and visceral stimuli in rats. Can. J. Anaesth. 43: 408–413. doi: 10.1007/BF03011723 [DOI] [PubMed] [Google Scholar]
- 2.Baker H., Ridley R.1986. Use of the common marmoset (Callithrix jacchus) in psychopharmacological research. pp. 41–73. In: Working Methods in Neuropsychopharmacological Research (Joseph, M. K. and Waddington, L. J. eds.), Manchester University Press, Manchester. [Google Scholar]
- 3.Brodbelt D. C., Blissitt K. J., Hammond R. A., Neath P. J., Young L. E., Pfeiffer D. U., Wood J. L.2008. The risk of death: the confidential enquiry into perioperative small animal fatalities. Vet. Anaesth. Analg. 35: 365–373. doi: 10.1111/j.1467-2995.2008.00397.x [DOI] [PubMed] [Google Scholar]
- 4.Corssen G., Domino E. F.1966. Dissociative anesthesia: further pharmacologic studies and first clinical experience with the phencyclidine derivative CI-581. Anesth. Analg. 45: 29–40. doi: 10.1213/00000539-196601000-00007 [DOI] [PubMed] [Google Scholar]
- 5.Fortman J. D., Hewett T. A., Bennett B. T.2002. The laboratory nonhuman primate, 1st ed., CRC Press, Boca Raton. [Google Scholar]
- 6.Green C. J., Knight J., Precious S., Simpkin S.1981. Ketamine alone and combined with diazepam or xylazine in laboratory animals: a 10 year experience. Lab. Anim. 15: 163–170. doi: 10.1258/002367781780959107 [DOI] [PubMed] [Google Scholar]
- 7.Harvey R. C.1992. Precautions when using mask induction. Vet. Clin. North Am. Small Anim. Pract. 22: 310–311. doi: 10.1016/S0195-5616(92)50616-7 [DOI] [PubMed] [Google Scholar]
- 8.Haskins S. C., Farver T. B., Patz J. D.1985. Ketamine in dogs. Am. J. Vet. Res. 46: 1855–1860. [PubMed] [Google Scholar]
- 9.Hewer C. L.1937. The stages and signs of general anaesthesia. BMJ 2: 274–276. doi: 10.1136/bmj.2.3996.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishibashi H., Motohashi H. H., Kumon M., Yamamoto K., Okada H., Okada T., Seki K.2013. Ultrasound-guided non-surgical embryo collection in the common marmoset. Reprod. Biol. 13: 139–144. doi: 10.1016/j.repbio.2013.02.002 [DOI] [PubMed] [Google Scholar]
- 11.Ishibashi H., Motohashi H. H., Kumon M., Yamamoto K., Okada H., Okada T., Seki K.2013. Efficient embryo transfer in the common marmoset monkey (Callithrix jacchus) with a reduced transfer volume: a non-surgical approach with cryopreserved late-stage embryos. Biol. Reprod. 88: 115. doi: 10.1095/biolreprod.113.109165 [DOI] [PubMed] [Google Scholar]
- 12.Mansfield K.2003. Marmoset models commonly used in biomedical research. Comp. Med. 53: 383–392. [PubMed] [Google Scholar]
- 13.Matsuda Y., Ohsaka K., Yamamoto H., Jiyouraku K., Natsume K., Hirabayashi S., Kounoike M., Inoue M.2007. NARCOBIT: a newly developed inhalational anesthesia system for mice. Exp. Anim. 56: 131–137. doi: 10.1538/expanim.56.131 [DOI] [PubMed] [Google Scholar]
- 14.Paddleford R. R.1992. Advantages and guidelines for mask induction. Vet. Clin. North Am. Small Anim. Pract. 22: 308–309. doi: 10.1016/S0195-5616(92)50615-5 [DOI] [PubMed] [Google Scholar]
- 15.Popilskis S. J., Lee D. R., Elmore D. B.2008. Anesthesia and analgesia in nonhuman primates. pp. 335–363. In: Anesthesia and Analgesia in Laboratory Animals (Fish, R. E., Brown, M. J., Danneman, P. J. and Karas, A. Z. eds.), Academic Press, London. [Google Scholar]
- 16.Sawyer D. C.1982. The induction period. pp. 13–43. In: The Practice of Small Animal Anesthesia (Sawyer, D. C. ed.), W.B. Saunders, Philadelphia. [Google Scholar]
- 17.Sawyer D. C., Rech R. H., Durham R. A.1991. Does ketamine provide adequate visceral analgesia when used alone or in combination with acepromazine, diazepam, or butorphanol in cats? Vet. Anaesth. Analg. 18: 381. doi: 10.1111/j.1467-2995.1991.tb00585.x [DOI] [Google Scholar]
- 18.Schnell C. R., Wood J. M.1993. Measurement of blood pressure and heart rate by telemetry in conscious, unrestrained marmosets. Am. J. Physiol. 264: H1509–H1516. [DOI] [PubMed] [Google Scholar]
- 19.Short C. E., Bufalari A.1999. Propofol anesthesia. Vet. Clin. North Am. Small Anim. Pract. 29: 747–778. doi: 10.1016/S0195-5616(99)50059-4 [DOI] [PubMed] [Google Scholar]
- 20.’t Hart B. A., Abbott D. H., Nakamura K., Fuchs E.2012. The marmoset monkey: a multi-purpose preclinical and translational model of human biology and disease. Drug Discov. Today 17: 1160–1165. doi: 10.1016/j.drudis.2012.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]