Abstract
Somatic cell nuclear transfer is a useful tool to maintain genetic information of animals. The Gyeongju Donggyeong dog is a breed registered as natural monument in Korea. The unique feature of the Donggyeong dog is its tail, as the Donggyeong dog can be classified as either short tailed or tailless. The aim of this study was to preserve the Donggyeong dog’s unique feature by cloning. Fibroblasts were obtained from a short-tailed Donggyeong dog. In vivo matured oocytes were enucleated, microinjected with a donor cell and fused electrically. Reconstructed embryos were transferred to six recipient dogs. One surrogate became pregnant, and one short-tailed Donggyeong dog was delivered. This study demonstrated that the phenotype of the Donggyeong dog could be conserved by somatic cell nuclear transfer.
Keywords: Gyeongju Donggyeong dog, phenotype, somatic cell nuclear transfer
Since the production of the first cloned dog, Snuppy (Afghan hound) [8], several species, such as the beagle [3], toy poodle [4], retriever [9], border collie [7] and Pekingese [12], have been cloned by somatic cell nuclear transfer (SCNT). Among the many breeds that need to be saved from extinction, the Sapsaree, one of the Korean natural monument dogs, has been produced by SCNT [5]. The Gyeongju Donggyeong dog has been considered a natural monument since 2012 (Cultural Heritage Administration of Korea, number: 540). The name Gyeongju Donggyeong dog originated from the capital of the ancient Silla kingdom in Korea. The Donggyeong dog has the oldest history among the Korean natural monument breeds, and it is referred to in many historic documents, such as Dongkyung jabki (published in AD 1669) and Sungho sasul (published in AD 1740). Despite the Donggyeong dog’s high historical value, only about two hundred individuals remain in Gyeongju, and it is classified as endangered [1, 2, 10]. It is essential to save such a valuable breed from extinction and maintain a pure descent. Accordingly, the aim of this study was to clone the Donggyeong dog by SCNT and observe the similarity of phenotypes between the cloned and cell donor dogs.
In this study, mixed-breed dogs between one to five years were used as oocyte donors and embryo recipients. The study was conducted in accordance with recommendations described in “The Guide for the Care and Use of Laboratory Animals” published by Seoul National University (SNU-141201-4).
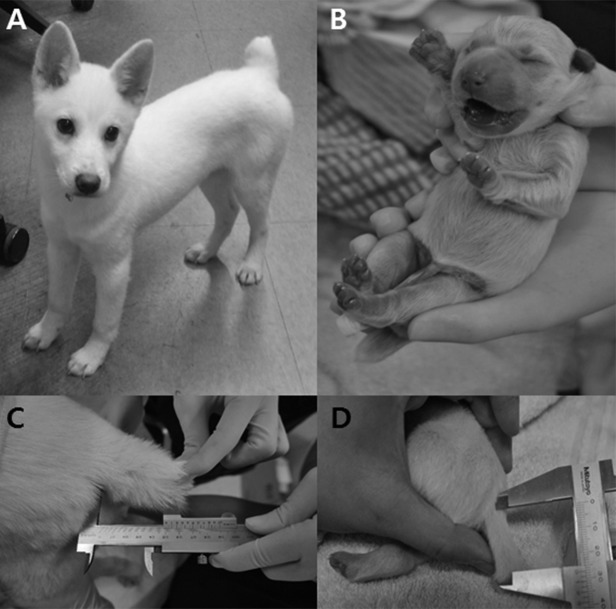
For preparation of donor cells, skin tissue was isolated by an aseptic surgical method from a three-month-old female Donggyeong dog (Fig. 1A). Recovery of in vivo matured oocytes was performed from oviducts approximately 72 hr after ovulation. Prediction of ovulation, preparation of matured oocytes, the process of SCNT and the transfer method for cloned embryos were described previously [6, 8]. Reconstructed embryos (n=98) were transferred into oviducts of six recipient dogs that were naturally synchronized. One recipient dog was confirmed to be pregnant by ultrasonography 26 days after embryo transfer (pregnancy rate: 16.6%) (Table 1). Pregnancy was maintained to term, and one healthy female Donggyeong dog weighing 320 g was delivered by cesarean section 59 days after embryo transfer (Fig. 1B).
Fig. 1.
Pictures of the cell donor and cloned Donggyeong dogs. A) cell donor dog at three months old. B) cloned dog at 1 day after birth. C) tail length of cell donor dog. D) tail length of cloned dog.
Table 1. In vivo development of cloned embryos by SCNT using somatic cells derived from donor dog.
| Recipient | No. oocytes donors | Oocyte status a) | No. reconstructed couplets | Pregnancy | No. cloned dogs |
|---|---|---|---|---|---|
| A | 2 | Mature | 15 | – | – |
| B | 1 | Mature | 6 | – | – |
| C | 3 | Mature and early aged | 21 | + | 1 |
| D | 1 | Mature | 9 | – | – |
| E | 3 | Early mature, mature, immature | 29 | – | – |
| F | 2 | Early mature | 18 | – | – |
| Total (n=6) | 12 | 98 | 1 (16.6%) b) | 1 (0.01%) c) | |
a) Status of in vivo oocytes flushed from oviducts approximately 72 hr after ovulation. b) The percentage is based on the total number of recipient dogs. c) The percentage is based on the total number of transferred embryos.
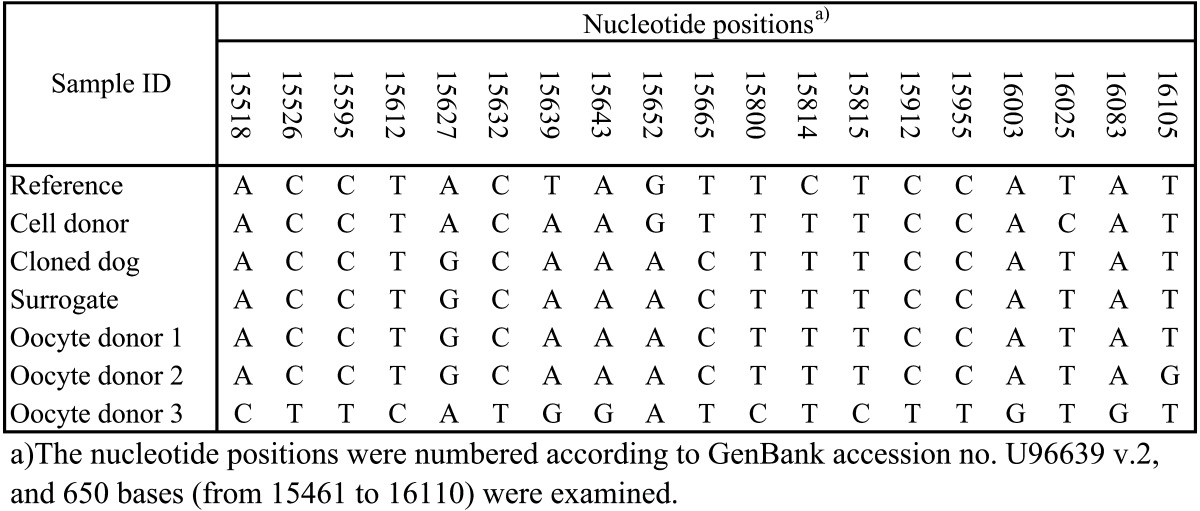
In order to identify the origin of the mitochondrial DNA (mtDNA) in the cloned dog, the genomic DNA was used for canine mtDNA (GenBank accession no U96639 v.2 and 650 bases) analysis. Based on the results, we identified that the cloned dog had identical mtDNA sequences to those of the domestic oocyte donor and surrogate dog (Table 2). To clone the Donggyeong dog, mixed-breed dogs were used as oocyte donors and recipients. Since the oocyte donor dog and recipient dog showed the same information for mtDNA in the mtDNA analysis, it is difficult to distinguish which dog’s mtDNA was transferred to the cloned dog (Table 2). Many studies about dog cloning proved that the cloned dog’s mtDNA was transferred solely from the oocyte donor [4, 5, 7]. Similarly, in the present study, the mtDNA of the cloned dog might have been transferred from the oocyte donor dog. In addition, to determine parentage, DNA extraction and microsatellite analysis with canine-specific markers were performed following the protocol of our previous study [6]. The results of the parentage analysis indicated that the cloned Donggyeong dog was genetically identical to the cell donor Donggyeong dog (Table 3).
Table 2. Sequence alignments within 628 bases of the hypervariable region of mitochondrial DNA.
Table 3. Microsatellite genotyping of cell donor, cloned, surrogate and oocytes donor dogs using specific canine DNA markers.
| NAME | Cell donor | Cloned | Surrogate | Oocyte donor 1 |
|---|---|---|---|---|
| PEZ2 | 130 / 130 | 130 / 130 | 126 / 122 | 126 / 126 |
| PEZ10 | 298 / 282 | 298 / 282 | 282 / 282 | 282 / 262 |
| PEZ16 | 298 / 290 | 298 / 290 | 302 / 286 | 302 / 282 |
| CPH4 | 149 / 137 | 149 / 137 | 141 / 141 | 141 / 141 |
| PEZ17 | 222 / 214 | 222 / 214 | 218 / 202 | 210 / 210 |
| CPH12 | 207 / 207 | 207 / 207 | 203 / 193 | 193 / 193 |
It has been reported that a cloned toy poodle had the same coat color as the somatic cell donor dog [4] and that beagles cloned from fetal fibroblasts had similar coat spotting [3]. In the present study, the cloned Donggyeong dog also had a phenotype similar to that of the cell donor dog. The unique feature of the Donggyeong dog is that it can be classified as natural short tailed or tailless. According to information from the Korean Gyeongju Donggyeong Dog Association, the short-tailed Donggyeong dog over twelve months old has a tail length of around 11.38 ± 2.44 cm and five to nine of coccygeal vertebrae in radiographic observations [2, 10]. The tailless Donggyeong dog has one to four of coccygeal vertebrae, and adult dogs (over twelve months old) have a tail length of around 6.3 ± 2.81 cm. In order to identify the number of caudal vertebral bodies, the dorsal radiographic views of the caudal vertebral column in the cloned dog and donor dog were compared. The number of coccygeal vertebral bodies was counted from the sacrum of the dorsal surface of the vertebral body. Based on the dorsal radiographic view, the cell donor dog had six coccygeal vertebral bodies, while the cloned dog had seven coccygeal vertebral bodies (Fig. 2A and 2B). Even though they had different numbers of coccygeal vertebral bodies, they could both be categorized as short-tailed Donggyeong dogs. It has been reported that despite cloned dogs having the same genetic information, they can have different dental development; one cloned dog had normal dental formulas, another cloned dog was missing one permanent molar tooth on the left side, and another cloned dog was missing one permanent molar tooth on the right side [11]. We cannot explain the reason for these differences between a donor dog and a cloned dog, but they might be associated with epigenetic modification during the cloning procedure. Therefore, further studies should be encouraged to analyze the epigenetic effects on the caudal vertebra features of cloned dogs.
Fig. 2.
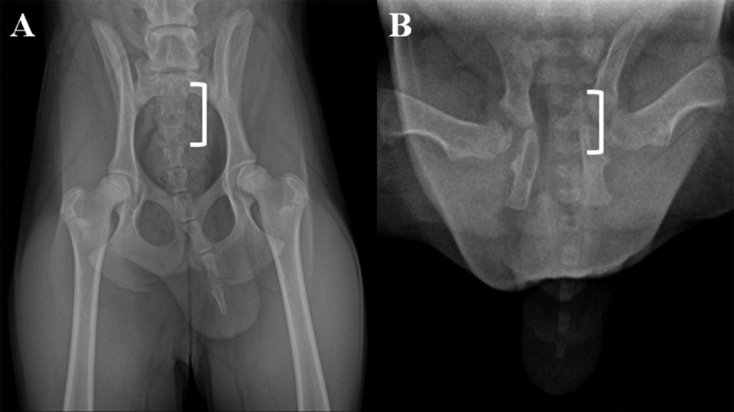
Comparison of the number of coccygeal vertebral bodies of the cloned Donggyeong dog (20 days after birth) and a donor Donggyeong dog (six months old) using digital radiographic views. A) a dorsal radiographic view of a portion of the caudal vertebral column of a cell donor dog is shown to illustrate measurements obtained for the sacrum (white bracket) through to the last coccygeal vertebra. B) dorsal radiographic view of the cloned dog. The coccygeal vertebral number was measured as the number from the dorsal surface of the sacrum. The cell donor dog had six coccygeal vertebral bodies, whereas the cloned dog had seven coccygeal vertebral bodies.
A Donggyeong dog, which is considered an endangered breed and needs to be saved from extinction, was clones using SCNT for the first time. Furthermore, the cloned Donggyeong dog could be classified as short tailed, which is the same as the cell donor Donggyeong dog. The current study demonstrated that SCNT could not only be used for conserving a specific breed of dog but also that it could ensure inheritance of a unique phenotypic feature of a dog breed. To determine the relationship between the coccygeal vertebrae and epigenetic modification, further studies need to analyze epigenetic mechanisms, which can influence coccygeal vertebra development.
Acknowledgments
This study was supported by IPET (#311062-04-3-SB010), the RDA (PJ010928032015), NATURE CELL (#2014-0082), the Research Institute for Veterinary Science of Seoul National University, the BK21 PLUS program, Natural Balance Korea and TS Corporation.
REFERENCES
- 1.Choi S. G.2010. Studies on the origin and breed characteristics of Gyeongju Donggyeong dog. Ph.D. Dissertation. Daegu University, Korea.
- 2.Choi S. G., Sung K. C., Lee E. W.2010. Study on anatomical characteristics by radiographic evaluation of the coccyx and pelvis in the Gyeongju Donggyeong dogs. Korean J. Res. Gyeongju 19: 163–173. [Google Scholar]
- 3.Hong S. G., Jang G., Kim M. K., Oh H. J., Park J. E., Kang J. T., Koo O. J., Kim D. Y., Lee B. C.2009. Dogs cloned from fetal fibroblasts by nuclear transfer. Anim. Reprod. Sci. 115: 334–339. doi: 10.1016/j.anireprosci.2008.12.005 [DOI] [PubMed] [Google Scholar]
- 4.Jang G., Hong S. G., Oh H. J., Kim M. K., Park J. E., Kim H. J., Kim D. Y., Lee B. C.2008. A cloned toy poodle produced from somatic cells derived from an aged female dog. Theriogenology 69: 556–563. doi: 10.1016/j.theriogenology.2007.11.002 [DOI] [PubMed] [Google Scholar]
- 5.Jang G., Hong S., Kang J., Park J., Oh H., Park C., Ha J., Kim D., Kim M., Lee B.2009. Conservation of the Sapsaree (Canis familiaris), a Korean Natural Monument, using somatic cell nuclear transfer. J. Vet. Med. Sci. 71: 1217–1220. doi: 10.1292/jvms.71.1217 [DOI] [PubMed] [Google Scholar]
- 6.Jang G., Kim M. K., Oh H. J., Hossein M. S., Fibrianto Y. H., Hong S. G., Park J. E., Kim J. J., Kim H. J., Kang S. K., Kim D. Y., Lee B. C.2007. Birth of viable female dogs produced by somatic cell nuclear transfer. Theriogenology 67: 941–947. doi: 10.1016/j.theriogenology.2006.11.006 [DOI] [PubMed] [Google Scholar]
- 7.Kim G. A., Oh H. J., Park J. E., Kim M. J., Park E. J., Lim S. H., Kang S. K., Jang G., Lee B. C.2013. Employing mated females as recipients for transfer of cloned dog embryos. Reprod. Fertil. Dev. 25: 700–706. doi: 10.1071/RD11221 [DOI] [PubMed] [Google Scholar]
- 8.Lee B. C., Kim M. K., Jang G., Oh H. J., Yuda F., Kim H. J., Shamim M. H., Kim J. J., Kang S. K., Schatten G., Hwang W. S.2005. Dogs cloned from adult somatic cells. Nature 436: 641. doi: 10.1038/436641a [DOI] [PubMed] [Google Scholar]
- 9.Oh H. J., Hong S. G., Park J. E., Kang J. T., Kim M. J., Kim M. K., Kang S. K., Kim D. Y., Jang G., Lee B. C.2009. Improved efficiency of canine nucleus transfer using roscovitine-treated canine fibroblasts. Theriogenology 72: 461–470. doi: 10.1016/j.theriogenology.2009.03.016 [DOI] [PubMed] [Google Scholar]
- 10.Park C. E., Lee E. W., Sung K. C., Choi S. G.2010. Investigation of candidated genes for molecular characterization of Donggyeong dog populations (Gyeongju). Korean J. Vet. Serv. 33: 361–366. [Google Scholar]
- 11.Park J. E., Kim M. K., Kang J. T., Oh H. J., Hong S. G., Kim D. Y., Jang G., Lee B. C.2010. Growth and hematologic characteristics of cloned dogs derived from adult somatic cell nuclear transfer. Cell Reprogram 12: 141–150. doi: 10.1089/cell.2009.0044 [DOI] [PubMed] [Google Scholar]
- 12.Park J., Oh H., Hong S., Kim M., Kim G., Koo O., Kang S., Jang G., Lee B.2011. Effective donor cell fusion conditions for production of cloned dogs by somatic cell nuclear transfer. Theriogenology 75: 777–782. doi: 10.1016/j.theriogenology.2010.10.016 [DOI] [PubMed] [Google Scholar]