Abstract
Although DNA encoding antibiotic resistance has been discovered in antibiotic preparations, its significance for the development of antibiotic resistance in bacteria is unknown. No phylogenetic evidence was obtained for recent horizontal transfer of antibiotic resistance genes from antibiotic-producing organisms to bacteria from human or animal sources.
Although the use of antibiotics has been the major weapon in combating infectious diseases, the rapid development of antibiotic resistance has resulted in treatment failures and outbreaks of infections caused by antibiotic-resistant bacteria (2, 5, 10, 11, 15). Numerous studies have proven that the use of an antibiotic is closely related to the rate of resistance to that antibiotic (3, 4). Traditionally, the role of antibiotics in antibiotic-induced antimicrobial resistance is to provide selective pressures to resistant clones. In 1990, Chakrabarty et al. reported the detection of nucleic acids in various antibiotics and the capacity of such nucleic acids to transform bacteria to drug resistance (1). Subsequently, Webb and Davies demonstrated that DNA encoding antibiotic resistance genes that are present in bacteria used in the production of antibiotics can be recovered in antibiotic preparations (9). It was proposed that antimicrobial resistance genes may be coadministered with antibiotics to humans or animals and taken up by bacteria in the hosts, contributing to the rapid development of antibiotic resistance. However, the importance of this second mechanism in the present widespread antibiotic resistance was unknown. In this study, we examined by a comprehensive phylogenetic analysis whether recent horizontal transfer of antibiotic resistance genes from antibiotic-producing organisms to human or animal bacteria has occurred. The phylogenetic relationships of the genes among different bacteria were also studied.
All antibiotic resistance genes that were present in the corresponding antibiotic-producing and non-antibiotic-producing organisms were included in this study (Medline search from 1966 to June 2003 and GenBank search from 1988 to June 2003) (Table 1). Amino acid sequences were used for phylogenetic analysis to minimize the effect of codon usage bias among different bacteria after gene acquisition. All were published sequences downloaded from GenBank (http://www.ncbi.nlm.nih.gov) and were aligned by multiple-sequence alignment with the CLUSTAL W program (8). For phylogenetic tree construction, all sequences found in the corresponding antibiotic-producing organisms and at least 10 sequences from human or animal bacterial isolates closest to those from antibiotic-producing organisms by BLAST search were included. Phylogenetic tree construction was performed with ClustalX version 1.81 (6) and the neighbor-joining method with GrowTree (Genetics Computer Group, Inc., San Diego, Calif.).
TABLE 1.
Antibiotic resistance genes present in both antibiotic-producing organisms and human or animal bacteria
| Antibiotic resistance gene | Antibiotic-producing organism with corresponding gene | Antibiotic(s) produced | Gene(s) in antibiotic-producing organism (accession no.) | Resistance mechanism | Maximum % amino acid identity between antibiotic-producing and other bacteria | Maximum % amino acid identity between different species of non-antibiotic-producing bacteria | Outgroup used in phylogenetic tree construction |
|---|---|---|---|---|---|---|---|
| Erythromycin-resistant methylase (erm)a | Aeromicrobium erythreum | Erythromycin | erm(R) (M11276) | Target modification | <40 | 100 | ksgA (AE000115), kasugamycin methyltransferase |
| Micromonospora griseorubida | Mycinamycin | myr(B) (D14532) | |||||
| Saccharopolyspora erythraea | Erythromycin | erm(E) (M11200, X51891) | |||||
| Streptomyces ambofaciens | Spiramycin | srm(A) (AJ223970) | |||||
| Streptomyces fradiae | Tylosin | tlr(A) (M19269), tlr(D) (X97721) | |||||
| Streptomyces lincolnensis | Lincomycin | lmr(B) (X79146, X62867) | |||||
| Streptomyces mycarofaciens | Midecamycin | mdm(A) (A60725) | |||||
| Streptomyces thermotolerans | Carbomycin | car(B) (M16503) | |||||
| Streptomyces venezuelae | Methymycin, neomythymycin, narbomycin, pikromycin | pikR1, pikR2 (AF079138) | |||||
| Aminoglycoside 3′-phosphotransferase [aph(3)] | Bacillus circulans | Butirosin | aph (X033640) | Enzymatic inactivation | <60 | 100 | aphD (X05647), aminoglycoside 6′-phosphotransferase |
| Micromonospora chalcea | Neomycin | aphA-5c (S81599) | |||||
| Streptomyces fradiae | Neomycin | aph (K00432) | |||||
| Streptomyces griseus | Streptomycin | aphE (M37378, X53527) | |||||
| Streptomyces ribosidificus | Ribostamycin | rph (M22126) | |||||
| Aminoglycoside 6′-phosphotransferase [aph(6)] | Streptomyces glaucescens | 5′-Hydroxystreptomycin | strA (X05648) | Enzymatic inactivation | <40 | 100 | aphA7 (M29953), aminoglycoside 3′-phosphotransferase |
| Streptomyces griseus | Streptomycin | aphD (Y00459, X05045) | |||||
| Streptomyces netropsis | Spectinomycin | aph (U70376) | |||||
| Aminoglycoside acetyltransferase (aac) | Micromonosopra chalcea | Neomycin | aacC9 (M55427) | Enzymatic inactivation | <50 | 100 | aphA7 (M29953), aminoglycoside phosphotransferase |
| Saccharothrix mutabilis subsp. capreolus | Capreomycin | cac (U13077) | |||||
| Streptomyces fradiae | Neomycin | aacC8 (M55426) | |||||
| Streptomyces rimosus subsp. paramomycinus | Paromomycin | aacC7 (M22999) | |||||
| Class A beta-lactamase | Amycolatopsis lactamdurans | Cephamycin C | bla (Z13971) | Enzymatic inactivation | <60 | ≥99 | blaOXA-18 (U85514), class D beta-lactamase |
| Streptomyces clavuligerus | Cephamycin C | bla (Z54190) | |||||
| Tetracycline resistance efflux protein | Streptomyces aureofaciens | Chlortetracycline | tcr3 (D38215) | Drug efflux | <40 | 100 | fusA (AE000669), elongation factor EF-G |
| Streptomyces rimosus | Oxytetracycline | otrB (AF079900) | |||||
| Tetracycline resistance ribosomal protection proteinb | Streptomyces rimosus | Oxytetracycline | otrA (X53401) | Target modification | <50 | 100 | cmlA (M64556), chloramphenicol resistance protein |
| Vancomycin resistance protein (vanA) | Amycolatopsis orientalis | Vancomycin | ddlN (AF060799) | Target modification | <63 | ≥99 | carB (U43091), carbomoylphosphate synthetase heavy subunit |
| Vancomycin resistance protein (vanH) | Amycolatopsis orientalis | Vancomycin | vanHaov (AF060799) | Target modification | <64 | ≥98 | hprA (AJ421476), glycerate dehydrogenase |
| Vancomycin resistance protein (vanX) | Amycolatopsis orientalis | Vancomycin | vanXaov (AF060799) | Target modification | <64 | ≥99 | frnI (AF058302), four-carbon starter unit synthase |
| Bacitracin transport protein (bcrA) | Bacillus licheniformis | Bacitracin | bcrA (AF007865) | Drug efflux | <77 | ≥98 | NAc |
| Bacitracin transport protein (bcrB) | Bacillus licheniformis | Bacitracin | bcrB (AF007865) | Drug efflux | <53 | No significant similarity | NA |
| Bacitracin transport protein (bcrC) | Bacillus licheniformis | Bacitracin | bcrC (AF007865) | Drug efflux | <28 | ≥34 | NA |
A total of 13 antibiotic resistance genes that were present in both the corresponding antibiotic-producing and non-antibiotic-producing organisms were found (Table 1). Phylogenetic tree construction was performed on the first 10 genes. For the last three genes, fewer than 10 sequences were found in non-antibiotic-producing organisms and they were analyzed by multiple-sequence alignment alone.
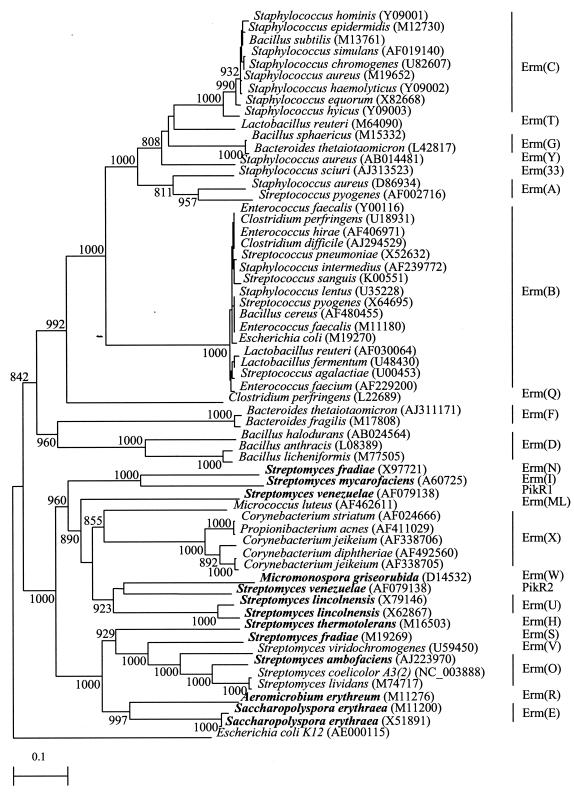
The erm sequences of the macrolide and lincosamide producers were distant from those of human or clinical bacterial isolates (amino acid identities of less than 40%). Recent horizontal transfer of erm genes from the antibiotic producers to human or animal bacteria was not observed. In contrast, the sequences within the human or animal bacteria were more closely related among themselves and showed evidence of frequent horizontal transfer, even between gram-positive and gram-negative bacteria. In particular, transfer among human gut bacteria was very common. There also appeared to be two clusters of genes that evolved independently, one generally from human or animal bacteria and the other generally from environmental bacteria, including the antibiotic producers (Fig. 1).
FIG. 1.
Phylogenetic tree showing the relationships of erm genes found in the macrolide- and lincosamide-producing organisms (in bold) and those from other bacterial isolates. The tree was inferred from 290 amino acids. Bootstrap values were calculated from 1,000 iterations. The scale bars indicate the estimated number of substitutions per 10 amino acids using the Jukes-Cantor correction. Different classes of erm genes are denoted by vertical lines, and names are on the right. Names and accession numbers are given as cited in the GenBank database.
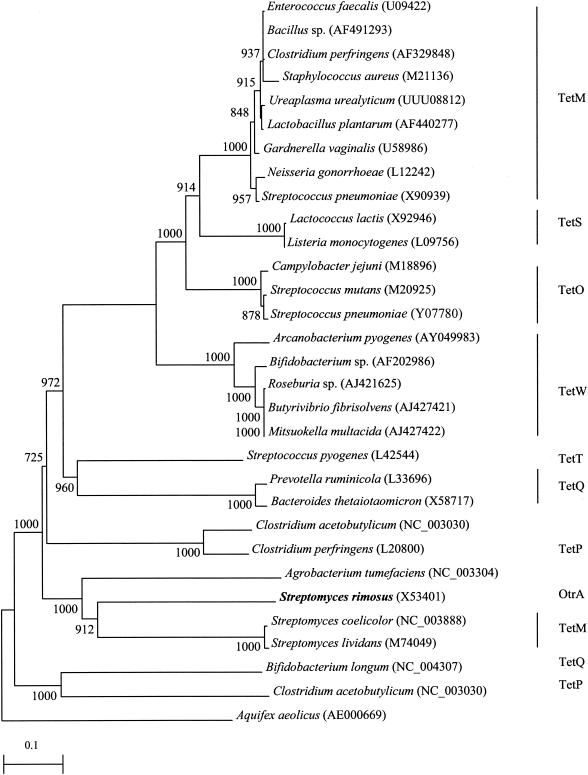
The tetracycline resistance ribosomal protection protein of the tetracycline producer Streptomyces rimosus was also distantly related to those of human or clinical bacterial isolates (amino acid identities of less than 50%), indicating no evidence of recent horizontal gene transfer from the antibiotic producer to human or clinical bacteria. In contrast, closely related genes among human or clinical bacteria were observed. Horizontal gene transfer appeared to have occurred among distantly related bacteria and the female genital tract flora and pathogens (Fig. 2).
FIG. 2.
Phylogenetic tree showing the relationships of tetracycline resistance ribosomal protection proteins of the tetracycline producer S. rimosus (in bold) and those from other bacterial isolates. The tree was inferred from 880 amino acids. Bootstrap values were calculated from 1,000 iterations. The scale bars indicate the estimated number of substitutions per 10 amino acids using the Jukes-Cantor correction. Different classes of tetracycline resistance ribosomal protection proteins are denoted by vertical lines, and names are on the right. Names and accession numbers are given as cited in the GenBank database.
Phylogenetic studies of the other 11 genes showed patterns similar to those reported in detail for erm and tetracycline resistance ribosomal protection protein. The results of their phylogenetic analysis are summarized in Table 1. The maximum amino acid identities of genes among different non-antibiotic-producing bacterial isolates were close to 100% for most genes, but those between antibiotic-producing and human or animal bacteria ranged from <28 to <77%.
From the present study, recent horizontal transfer of antibiotic resistance genes from bacteria that are used for antibiotic production to human or animal bacteria was not observed. Although our results suggest that DNA in antibiotic preparations has not been a very active and widespread mechanism in the dissemination of antibiotic resistance genes, the possibility of its being a minor player cannot be excluded. Nevertheless, our results suggest that DNA decontamination of antibiotic preparations is probably unnecessary. On the other hand, the fact that horizontal transfer was observed among human gastrointestinal tract bacteria, which is in line with our previous findings (7, 12), suggests that this is one of the other possible mechanisms of antibiotic-induced antibiotic resistance (13, 14).
Acknowledgments
This work was partly supported by the University Development Fund, University Research Grant Council, and the Committee of Research and Conference Grants, The University of Hong Kong.
REFERENCES
- 1.Chakrabarty, A. N., S. G. Dastidar, M. Ganguli, and D. Chattopadhyay. 1990. ′DNA' as contaminants in antibiotics and its capacity to transform bacteria to drug resistance. Indian J. Exp. Biol. 28:58-62. [PubMed] [Google Scholar]
- 2.Chiu, S. S., P. L. Ho, F. K. Chow, K. Y. Yuen, and Y. L. Lau. 2001. Nasopharyngeal carriage of antimicrobial-resistant Streptococcus pneumoniae among young children attending 79 kindergartens and day care centers in Hong Kong. Antimicrob. Agents Chemother. 45:2765-2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cristino, J. M. 1999. Correlation between consumption of antimicrobials in humans and development of resistance in bacteria. Int. J. Antimicrob. Agents 12:199-202. [DOI] [PubMed] [Google Scholar]
- 4.Granizo, J. J., L. Aguilar, J. Casal, C. Garcia-Rey, R. Dal-Re, and F. Baquero. 2000. Streptococcus pneumoniae resistance to erythromycin and penicillin in relation to macrolide and beta-lactam consumption in Spain (1979-1997). J. Antimicrob. Chemother. 46:767-773. [DOI] [PubMed] [Google Scholar]
- 5.Ho, P. L., K. Y. Yuen, W. C. Yam, S. S. Y. Wong, and W. K. Luk. 1995. Changing patterns of susceptibilities of blood, urinary and respiratory pathogens in Hong Kong. J. Hosp. Infect. 31:305-317. [DOI] [PubMed] [Google Scholar]
- 6.Jeanmougin, F., J. D. Thompson, M. Gouy, D. G. Higgins, and T. J. Gibson. 1998. Multiple sequence alignment with ClustalX. Trends Biochem. Sci. 10:403-405. [DOI] [PubMed] [Google Scholar]
- 7.Lee, R. A., P. C. Y. Woo, A. P. C. To, S. K. P. Lau, S. S. Y. Wong, and K. Y. Yuen. 2003. Geographical difference of disease association in Streptococcus bovis bacteremia. J. Med. Microbiol. 52:903-908. [DOI] [PubMed] [Google Scholar]
- 8.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Webb, V., and J. Davies. 1993. Antibiotic preparations contain DNA: a source of drug resistance genes? Antimicrob. Agents Chemother. 37:2379-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong, S. S. Y., P. L. Ho, P. C. Y. Woo, and K. Y. Yuen. 1999. Bacteremia caused by staphylococci with inducible vancomycin heteroresistance. Clin. Infect. Dis. 29:760-767. [DOI] [PubMed] [Google Scholar]
- 11.Wong, S. S. Y., T. K. Ng, W. C. Yam, D. N. C. Tsang, P. C. Y. Woo, S. K. S. Fung, and K. Y. Yuen. 2000. Bacteremia due to Staphylococcus aureus with reduced susceptibility to vancomycin. Diagn. Microbiol. Infect. Dis. 36:261-268. [DOI] [PubMed] [Google Scholar]
- 12.Woo, P. C. Y., A. P. C. To, H. Tse, S. K. P. Lau, and K. Y. Yuen. 2003. Clinical and molecular epidemiology of erythromycin resistant beta-hemolytic Lancefield group G streptococci causing bacteremia. J. Clin. Microbiol. 41:5188-5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woo, P. C. Y., A. P. C. To, S. K. P. Lau, and K. Y. Yuen. 2003. Facilitation of horizontal transfer of antimicrobial resistance by transformation of antibiotic-induced cell-wall-deficient bacteria: a hypothesis. Med. Hypotheses 61:503-508. [DOI] [PubMed] [Google Scholar]
- 14.Woo, P. C. Y., S. S. Y. Wong, P. N. L. Lum, W. T. Hui, and K. Y. Yuen. 2001. Cell-wall-deficient bacteria and culture-negative febrile episodes in bone-marrow-transplant recipients. Lancet 357:675-679. [DOI] [PubMed] [Google Scholar]
- 15.Yuen, K. Y., W. H. Seto, W. T. Hui, and P. Y. Chau. 1990. Multi-resistant Streptococcus pneumoniae in Hong Kong. J. Antimicrob. Chemother. 25:721-723. [DOI] [PubMed] [Google Scholar]