Abstract
Patients affected with severe endometriosis are at significant risk for ovarian tissue damage, which may lead to infertility, reduced response to ovarian stimulation, and occasionally, premature ovarian failure. The risk for a compromised ovarian reserve in young patients is especially high following repeated surgical intervention and in the presence of bilateral endometriomas. In many cases, enhanced loss of ovarian reserve may also result from the damaging effect of the pathologic process on follicle reservoir even without surgical interventions. Women diagnosed with severe endometriosis and those designated for extensive ovarian surgical intervention are frequently not planning to conceive. In light of recent advances in fertility preservation techniques (FPT), such as oocytes and ovarian tissue freezing, as well as their increasing success rates, we critically evaluate the options for FPT in patients suffering from endometriosis. Personalized counseling should be offered to all patients with endometriosis taking into account age, extent of ovarian involvement, current ovarian reserve, previous and impending surgeries for endometriosis, along with current success rates and possible risks associated with FPT.
Keywords: Fertility preservation, Endometriosis, Ovarian reserve, Ovarian follicles, Oocyte-embryo freezing, Ovarian tissue cryopreservation
Introduction
The use of fertility preservation technologies (FPT) has become widely accepted as a standard of care for women facing a high risk of acute premature ovarian failure (POF) due to cancer treatments. This growing experience has greatly increased our knowledge and FPT are currently more versatile and effective. Today, FPT include well-established techniques such as oocytes [1] and embryos freezing [2], as well as ovarian tissue cryopreservation (OTCP). Although substantial evidence to support OTCP effectiveness has accumulated in recent years [3–5], the technique is still considered experimental [6]. The increased awareness and experience utilizing these technologies has led to the acceptance of new indications for FPT, such as genetically related risk for POF or other medical conditions, which considerably limit time available for conception [7, 8].
As such, endometriosis should also be recognized as an indication for FPT: patients with endometriosis-related symptoms, like pelvic pain or the presence of ovarian cysts, frequently undergo extensive pelvic surgery involving the ovaries [9]. The need for surgery and histological evaluation is also justified by recent studies indicating an increased risk for malignancy in patients suffering from endometriosis when large or rapidly growing endometriomas are detected or when suspicious changes in sonographic appearance occur [10]. In such cases, repeated ovarian surgeries may result in irreversible damage to the ovary [11, 12].
Endometriosis is frequently associated with infertility and several biological mechanisms have been proposed. However, the progressive loss of follicle reservoir, which seems related to the pathology per se [13, 14] has a major impact on future fertility and provides a clear motive to consider FPT in patients who are not seeking a pregnancy at present. Together with the worldwide trend of delaying childbearing that by itself is a common indication for assisted reproductive technology (ART) [15] the motivation in favor of FPT is growing. Therefore, in women of young reproductive age with endometriosis who have not yet completed family planning, the potential risk for low ovarian reserve, POF, and future infertility must be raised and carefully assessed, in order to provide an adequate consultation regarding the need for FPT.
This opinion manuscript focuses on the association between endometriosis, ovarian follicle loss, and the risk of impaired ovarian function, as well as the reproductive risk associated with surgery. The advantages and disadvantages of FPT for patients with severe endometriosis involving the ovaries are critically evaluated.
Reduced ovarian reserve in patients affected by endometriosis
The association between endometriosis and infertility is well recognized, and several biological mechanisms have been implicated. These include the following: mechanical obstruction caused by pelvic adhesions, impaired embryo receptivity and implantation, local and systemic inflammatory state with increased concentration of cytokines in peritoneal fluid, and low ovarian reserve [16]. Progressive loss of follicle reservoir is frequently observed and is often responsible for impaired fecundity, poor results achieved by assisted reproduction [17, 18] and increased risk of POF [19]. Accelerated depletion of ovarian reserve is most threatening for patients who are not seeking a pregnancy at present, but are very much interested in preserving chances for conceiving a biological offspring in the future.
Several etiologies are responsible for low ovarian reserve in patients suffering from endometriosis:
Surgery
Severe ovarian damage and extensive follicle loss have been reported after cystectomy for endometriomas [11] resulting in a shorter reproductive life span [20, 21], or even immediate ovarian failure as reported in few cases [19]. Table 1 shows the effect of surgery on ovarian reserve markers.
Table 1.
Markers of ovarian reserve prior and post surgery
| Authors | No. Pt | AMH (ng/ml) | AFC* | FSH (mIU/ml) | Ovarian volume* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prior | Post | P | Prior | Post | P | Prior | Post | P | Prior | Post | P | ||
| Biacchiardi [22] | 43 | 3.0 ± 0.4 | 1.3 ± 0.3 (9 months) | <0.0001 | 3.3 ± 3.2 | 5.1 ± 3.6 | NS | 6.6 ± 2.0 | 8.0 ± 3.7 | NS | 10.5 ± 0.8 | 8.6 ± 0.9 | <0.0001 |
| Tsolakidis [23] | 10a | 3.9 ± 0.4 | 2.9 ± 0.2 (6 months) | 0.026 | 2 ± 1 | 2.4 ± 08 | NS | 7.2 ± 0.8 | 16.6 ± 3.8 | NS | 89.7 ± 29.6 | 11.5 ± 4.8 | NS |
| Tsolakidis [23] | 10b | 4.5 ± 0.4 | 3.99 ± 0.6 (6 months) | NS | 1.3 ± 0.5 | 4.36 ± 0.8 | 0.02 | 7.7 ± 0.8 | 11.0 ± 2.9 | NS | 7.7 ± 23.6 | 11.0 ± 2.9 | NS |
| Celik [24] | 65 | 1.78 ± 1.71 | 0.72 ± 0.79 (6 months) | <0.001 | 4.9 ± 2.2 | 6.4 ± 2.2 | <0.008 | 6.37 ± 3.04 | 6.67 ± 4.53 | NS | – | – | |
| Hirokawa [25] | 38 | 3.9 ± 2.5 | 2.1 ± 1.6 (1 month) | 0.001 | – | – | – | – | – | – | |||
| Var [26] | 48a | – | – | 5.58 ± 1.13 | 3.67 ± 1.26 | 0.001 | – | – | 13.03 ± 1.13 | 6.27 ± 1.95 | 0.01 | ||
| Var [26] | 48c | – | – | 5.42 ± 0.77 | 4.75 ± 0.6 | 0.02 | 13.56 ± 1.5 | 9.87 ± 2.01 | 0.01 | ||||
*Affected ovary
aLaparoscopic cystectomy
bThree-step procedure
cCoagulation
The amount of healthy tissue inadvertently lost during surgery is larger with excision of bilateral endometriomas and is also related to electrosurgical coagulation and the local inflammation associated with the procedure [27].
The endometrioma cyst wall contains different amounts of follicles, which are lost during surgery. The number of follicles in histological sections obtained from the cyst wall is related to patient’s age: the younger the patient, the larger the number of follicles present in the endometrioma wall. This seems to be correlated not only to the higher density of pre-existing follicles in the ovary, but also to a more “inflammatory typology” of endometriosis at a younger age [28]. Young women also have a higher recurrence rate of their endometriomas (30–50 %) that often leads to repeated ovarian surgery which further compromises ovarian reserve [29].
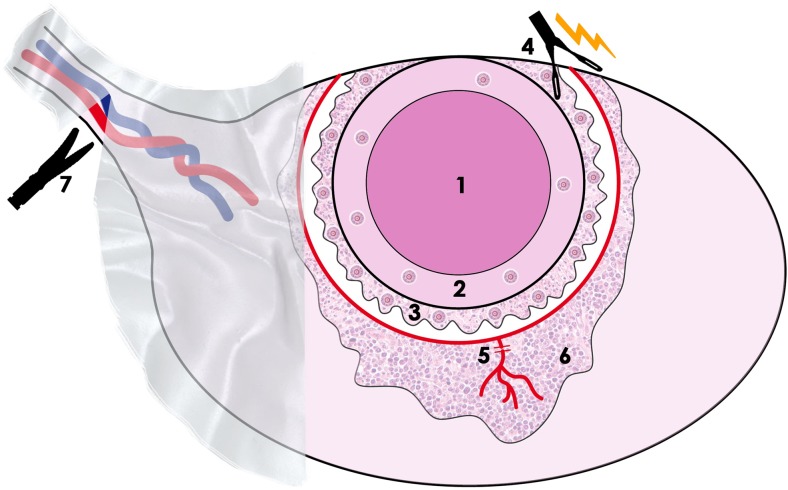
Lastly, extensive adhesiolysis even without direct surgery to the ovaries has been associated with a significant decline in ovarian reserve. This may derive from injury to ovarian vascular bed resulting in reduced ovarian blood supply post-surgery [25]. Table 2 summarizes parameters influencing the effects of surgical intervention on ovarian reserve, these should be taken into account during consultation for FPT. The mechanisms associated with follicle loss during surgery are presented in Fig. 1.
Table 2.
Parameters influencing reduced ovarian reserve by surgery
| I | Mono/bilateral endometriomas Removal of bilateral endometriomas more harmful |
| II | Pre-surgical ovarian reserve Low ovarian reserve and/ or advanced age results in more detrimental impact of surgery on ovarian reserve |
| III | Characteristic and follicle density of the endometrioma’s wall Fibroblastic type: younger patients, high number of follicles Fibrocystic type: older patients have small number of follicles |
| IV | Capability of the surgeon Ability of the surgeon to minimize removal of healthy tissue Identifying endometriotic infiltration extent and lesion borders Minimizing coagulation during the surgery |
| V | Disease recurrence ► Need for repeated surgical interventions |
Fig. 1.
The causes of follicle loss during surgery for endometriosis involve injury to blood vessels and stroma and removal of healthy cortex. 1 Endometrioma. 2 Pseudocapsule of the endometriomal. 3 Healthy cortex containing significant number of follicles stripped with pseudocapsule. 4 Coagulation of vascular bed. 5 Blood vessels injuries. 6 Oedema/inflammation. 7 Adhesiolysis and subsequent injuries of the bloody vessels
Endometrioma/endometriosis
Ovarian endometriosis adversely affects ovarian reserve, as demonstrated by diminished serum anti-Müllerian hormone (AMH), lower antral follicle counts (AFC), lower response to controlled ovarian stimulation (COH), and higher doses of gonadotropins used (Table 3). The tissue surrounding endometriomas often shows morphological alterations that are not present in tissue surrounding benign cysts, such as significant loss of cortical stroma with fibrosis formation and significantly lower follicular density [36–38]. These morphological alterations were never observed in samples from contralateral healthy ovaries. This indicates that the ovarian pathological changes rather than the space-occupying lesion itself are responsible for the decline in ovarian reserve [13] and poor ovarian response in ART cycles [34, 39]. Diminished ovarian reserve has been reported not only in patients suffering from ovarian endometriomas, but also in patients with minimal to mild disease. As such, early menopause has been described in patients with peritoneal endometriosis, with no ovarian involvement [20]. Tissue pathological changes seen in endometriosis, such as focal fibrosis and vascular deficiency have also been observed in the cortex of ovaries exposed to chemotherapy [40] and similarly, no follicles are seen in the fibrotic zone. Thus, this pattern of damage to the entire organ may be common among different harmful processes that, in general, tend to diminish the ovarian reserve [41]. A recent observation explaining reduced ovarian reserve in patients suffering from endometriosis comes from studies on dynamics of follicle populations. The proportion of primordial follicles that are present in ovaries with endometriomas is significantly lower together with a concomitant significant increase in the proportion of non-resting growing follicles [14]. This high proportion of growing follicles observed is in contradiction to the low proportion of growing follicles expected resulting from blood vessels injury. This may represent a persistent process of follicle activation and loss resulting in overall decrease in follicular reserve triggered by the disease. This “burn-out” effect on follicle reservoir was previously shown as a major mechanism leading to chemotherapy-induced ovarian reserve loss and may also be a common pathway of follicle loss post toxic injury [42, 43].
Table 3.
Ovarian reserve in women with and without endometrioma
| AMH | Authors | Endometriosis (No of patients) | Controls (No of patients) | P value |
| Shebl [30] | 2.57 ± 2.0 (153 pt) | 3.46 ± 2.30 (306 pt) | <0.001 | |
| Hwu [11] | 2.34 ± 0.19 (141 pt) | 3.31 ± 0.08 (1.323 pt) | <0.001 | |
| Ercan [31] | 1.62 ± 1.09 (43 pt) | 2.06 ± 0.51 (17 pt) | NS | |
| Uncu [32] | 2.81 mean (30 pt) | 4.20 (30 pt) | 0.02 | |
| AFC | Authors | Affected ovary | Contralateral ovary | P value |
| Almog [33] | 7.5 ± 0.7 (53 pt) | 9.3 ± 0.9 (53 pt) | <0.05 | |
| Biacchiardi [22] | 3.3 ± 3.2 (43 pt) | 8.4 ± 6.0 (43 pt) | <0.0001 | |
| COH results | Authors | Pt with endometriomas (No eggs retrieved) | Control group (No eggs retrieved) | P value |
| Suzuki [34] | 4.40 ± 2.99 (80 cycles) | 5.34 ± 2.99 (283 cycles) | 0.0037 | |
| Kumbak [13] | 13.9 (5–33) (85 pt) | 16.4 (5–37) (83 pt) | 0.03 | |
| Opøien [35] | 8 ± 5.3 (350 pt) | 9.2 ± 5.6 (1.171 pt) | <0.01 |
Age
Oocytes’ decline, both in number and in quality, has been well described and recognized as the main cause of infertility in older patients. The persistent trend towards older age at conception in many countries further deteriorates the chances of women affected by endometriosis, which may present an already diminished ovarian reserve, to conceive and deliver a healthy child.
This point out the urgent need for early counseling regarding fertility preservation: ovarian aging related to advancing age and the acute damage inflicted by surgery are both factors that can be counteracted through FPT.
Counseling patients with endometriosis should highlight the advantages of early conception that will increase success rates and may attenuate disease progression. However, advice on early conception may not be practical for many women. In such cases, women should be presented with the option of ovarian sparing surgery and the most recent techniques of cryopreservation, already widely adopted in oncological patients: freezing oocytes, embryos, or ovarian tissue at a younger age.
Fertility preservation techniques for patients with endometriosis
Ovarian sparing surgery
The presence of large endometrioma frequently necessitates laparoscopic cystectomy. Resection and/or ablation of the cyst are preferable over cyst drainage and coagulation, which are associated with a higher recurrence risk [16]. Accurate evaluation of the possible ovarian damage and appropriate adoption of ovarian sparing surgical techniques are critically required. Thus, skilled surgeons, with expertise in reproductive pathophysiology, should perform operations, in order to prevent unnecessary insult to healthy parenchyma and avoid deep coagulation in the ovaries that might injure blood vessels arising from the hilum [44].
For patients demonstrating preoperative low ovarian reserve indices, those with bilateral ovarian cysts or those suffering from severe endometriosis, no optimal surgical sparing technique has been identified. Due to significant risk of ovarian reserve loss [25], early preoperative referral to a center experienced with FPT is recommended to assess the need for FPT.
Embryo/egg freezing
Controlled ovarian hyperstimulation (COH), oocyte retrieval, IVF, and embryo cryopreservation are the most established FPT. Alternatively, the option to cryopreserve oocytes instead of embryos might be a more realistic option for young women without a partner [45]. It can be considered a good choice in patients affected by endometriosis as it has no effect on future ovarian reserve and is less invasive than other FPT. Nevertheless, several cycles might be required, especially in patients with low ovarian reserve, in order to aspirate an adequate numbers of oocytes (around 12–20) that may provide a realistic chance for future fertility. Number of COH and IVF cycles has not been associated with an increased risk of disease recurrence [46, 47].
Some authors suggest that IVF performance (oocytes quality, fertilization rates, embryo quality, and live birth rates) in the presence of endometriosis is impaired [18, 34] while others show reserved outcomes [35, 48]. Nevertheless, when good quality oocytes are fertilized, or when a large number of embryos is available, implantation rates seem to return to control values [49].
In the presence of ovarian endometrioma, oocyte retrieval is associated with an increased risk of pelvic infection or ovarian abscess formation [50, 51]. These additional risks should be discussed during FP consultation.
The advantage of oocytes collection for FP prior to surgery relates to detrimental effect of surgery on ovarian reserve. In some cases, however, surgical removal of the endometrioma prior to ovarian stimulation should be considered. Studies suggest that surgical therapy may increase fertility in women with advanced endometriosis, proposing early IVF cycles in the immediate post-operative period when the ovaries are disease-free [6, 16]. In addition, oocytes freezing success rate is age related and significantly declines after the age of 36 years [52]; hence, patients with endometriosis are encouraged to freeze oocytes at a younger age if possible and consultation should address to the yield of age-related gamete freezing.
Ovarian tissue cryopreservation
Ovarian tissue cryopreservation (OTCP) is currently used worldwide to preserve fertility in young women facing chemotherapy or radiotherapy who are at high risk of losing ovarian function. OTCP is also practiced in some benign conditions associated with high risk of POF [7].
The technique usually involves one-sided surgical removal of ovarian cortical tissue or complete oophorectomy [53]. The harvested cortical tissue is dissected into thin (1–2 mm) strips measuring 0.5 × 1 cm2, which are frozen for future transplantation. Primordial follicles are located in a poorly vascular environment and are relatively resistant to ischemia. This allows the survival of follicles during the immediate post transplantation ischemic period and may result in successful reestablishment of fertility. In cases of endometriosis, cortical tissue can be harvested and stored, thereby sparing the follicles from a potential future disease progression that could occur in the ovary left in situ. However, this approach is not practical and not recommended. Laparoscopic tissue harvesting is technically more difficult and carries significant additional operative risks in cases of pelvic adhesions. Moreover, removing healthy cortical tissue away from endometriomas can further deteriorate ovarian reserve.
However, during surgical removal of endometrioma, healthy fragments of ovarian cortex can be isolated and cryopreserved. The source of cortical tissue stored might originate from healthy cortical tissue attached to the capsule, which has been removed during the surgery and of cortical fragments that are loosely attached to the ovary at the end of dissection. Ovarian cortical tissue collection can be performed at any center operating for endometriosis and there is no need for patients’ referral since the tissue can be safely transported prior to freezing to fertility preservation center [54].
Data collected from oncological patients who underwent ovarian tissue reimplantation have shown more than 90 % endocrine restoration rate and high pregnancy rate, either spontaneous or following IVF. However, the quantity and quality of follicles as well as reproductive potential of cortical tissue that was attached adjacent to the endometrioma wall should be further studied.
For patients with endometriosis undergoing surgery, consultation on ovarian tissue storing should be individualized according to patients’ age, ovarian reserve status, presence of bilateral ovarian lesions, and repeated surgery. While in some cases healthy cortical tissue is removed unintentionally as a by-product on endometrioma excision, a deliberate removal of normal cortical tissue is far less straightforward and should be viewed as a potential thereat for ovarian reserve. The indication for such intervention is therefore not entirely clear and such option should be critically discussed in light of its potential risks and benefits.
Table 4 summarizes the advantages and disadvantages of the possible method for FP in patients with endometriosis.
Table 4.
Fertility preservation techniques for patients with endometriosis—pro and cons
| Embryo/oocyte cryopreservation | |
| Pro | Cons |
| Documented results especially when embryos are frozen | Risk of infections related to oocyte retrieval and abscess formation |
| No risk of procedure-related ovarian reserve depletion | Poor quality oocytes, embryos (controversial data) |
| The pick-up may avoid contact of the oocyte with the detrimental effect of the peritoneal fluid | Need of ovarian stimulation that might cause the progression of the disease (controversial data) |
| Patients suffering from endometriosis are frequent costumers of ART procedures | Need of repeated IVF cycles in order to collect an adequate number of oocytes that can be stored |
| Ovarian tissue cryopreservation | |
| Pro | Cons |
| Highly effective technique for fertility preservation | Laparoscopic procedure in these patients may be more difficult and risky |
| Easily performed during the surgical intervention for the disease | Storing tissue surrounding cyst or pseudocapsule—number and quality of follicles are questionable |
| No need of ovarian stimulation | Storing healthy tissue remote from cyst may result in ovarian damage and reduced ovarian reserve |
| Frozen tissue is spared from potential destruction in cases of disease recurrence | No results, so far, have been shown in this class of patients |
Approach to patients and conclusive remarks
The long-term insult to ovaries resulting in diminished ovarian reserve in patients suffering from severe endometriosis is now widely recognized. Ovarian surgery for endometriosis, especially bilateral ovarian surgery and repeated operations, are associated with a substantial reduction of follicular reserve. Pregnancy at an early age is recommended and the importance of not delaying first pregnancy should be raised during consultation. However, when this is not a realistic option, patients should be counseled regarding present and future ability to conceive. Consultation should be guided by current ovarian reserve, disease extent, progression rate, need for ovarian surgeries, and high recurrence rate. FPT should also be considered in patients suffering from mild endometriosis when reduced ovarian reserve is identified and at older reproductive age. Patients with good ovarian reserve, who are not suffering from bilateral ovarian endometriosis with no impending extensive or repeated ovarian surgery and those affected by minimal/mild endometriosis can be conservatively monitored up to their mid-30s. Thereafter, conservative surveillance option should be reconsidered if patients did not conceive as FPT effectiveness significantly declines. A combined follow-up and treatment by highly trained endoscopy surgeons and teams experienced in FPT, will provide an up-to-date approach for the benefit of the patients as they are most capable of providing balanced information on success rates and possible associated risks.
Freezing embryos or unfertilized oocytes seems to be the most convenient technique of fertility preservation for women suffering from endometriosis. It does not affect ovarian reserve and offers a real chance of future pregnancy when a good amount of oocytes or embryos has been stored.
Even in young patients, several cycles might be needed in order to collect an adequate number of oocytes (12–20). For patients designated for an extensive or bilateral pelvic surgery, several ART cycles before surgery may provide a sufficient number of oocytes/embryos for freezing. This holds truth for patients at an older reproductive age or those already demonstrating low ovarian reserve. In young patients, both surveillance and oocyte freezing are acceptable options. In any case, the risk associated with oocyte retrieval should be considered.
The benefits of storing ovarian tissue harvested during surgery for endometriosis have never been tested. Although the technique was proven to be effective in providing good chances for future fertility in cancer patients, currently, there is insufficient information regarding concentration and quality of oocytes surrounding endometrioma wall and more studies are indicated. At present, only healthy ovarian tissue should be stored for fertility preservation. Thus, the benefits and disadvantages should be critically discussed, taking into consideration the risk for enhanced ovarian reserve lose during surgery. Following surgery for endometriosis, young patients not wishing to conceive immediately may consider several COH cycles in order to freeze oocytes. These recommendations are based on existing literature data and experience. Continuous data collection together with a growing experience with FPT, will enable further clarification of current indications for fertility preservation in the context of endometriosis, taking both economic burden and known success rates into consideration.
Acknowledgment
The authors would like to thank Dr. Moran Shapira for her editing assistance.
Compliance with ethical standards
Source of Funding
None.
Footnotes
Capsule Personalized counseling should be offered to all patients with endometriosis taking into account age, extent of ovarian involvement, current ovarian reserve, previous and impending surgeries for endometriosis, along with current success rates and possible risks associated with FPT.
References
- 1.Practice Committee of the American Society for Reproductive Medicine Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013;99(1):37–43. doi: 10.1016/j.fertnstert.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 2.Bedoschi G, Oktay K. Current approach to fertility preservation by embryo cryopreservation. Fertil Steril. 2013;99(6):1496–1502. doi: 10.1016/j.fertnstert.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donnez J, Dolmans MM. Transplantation of ovarian tissue. Best Pract Res Clin Obstet Gynaecol. 2014;28(8):1188–1197. doi: 10.1016/j.bpobgyn.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Meirow D, Rahanani H, Biedermann H. Ovarian tissue cryopreservation and transplantation: a realistic effective technology for fertility preservation methods. Mol Biol. 2014;1154:455–473. doi: 10.1007/978-1-4939-0659-8_21. [DOI] [PubMed] [Google Scholar]
- 5.Jensen AK, Kristensen SG, Macklon KT, Jeppesen JV, Fedder J, Ernst E, et al. Outcomes of transplantations of cryopreserved ovarian tissue to 41 women in Denmark. Hum Reprod. 2015;30(12):2838–2845. doi: 10.1093/humrep/dev230. [DOI] [PubMed] [Google Scholar]
- 6.Practice Committee of the American Society for Reproductive Medicine Ovarian tissue cryopreservation: a committee opinion. Fertil Steril. 2014;101(5):1237–1243. doi: 10.1016/j.fertnstert.2014.02.052. [DOI] [PubMed] [Google Scholar]
- 7.Jadoul P, Dolmans MM, Donnez J. Fertility preservation in girls during childhood: is it feasible, efficient and safe and to whom should it be proposed? Hum Reprod Update. 2010;16(6):617–630. doi: 10.1093/humupd/dmq010. [DOI] [PubMed] [Google Scholar]
- 8.Grynberg M, Bidet M, Benard J, Poulain M, Sonigo C, Cédrin-Durnerin I, Polak M. Fertility preservation in Turner syndrome. Fertil Steril. 2015 [DOI] [PubMed]
- 9.Pearce CL, Templeman C, Rossing MA, Lee A, Near AM, Webb PM, et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case–control studies. Lancet Oncol. 2012;13:285–294. doi: 10.1016/S1470-2045(11)70404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nezhat F, Apostol R, Mahmoud M, El Daouk M. Malignant transformation of endometriosis and its clinical significance. Fertil Steril. 2014;102(2):342–344. doi: 10.1016/j.fertnstert.2014.04.050. [DOI] [PubMed] [Google Scholar]
- 11.Hwu YM, Wu FS-Y, Li S-H, Sun F-J, Lin M-H, Lee RK-K. The impact of endometrioma and laparoscopic cystectomy on serum anti-Mullerian hormone levels. Reprod Biol Endocrinol. 2011;9:80. doi: 10.1186/1477-7827-9-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo SW. Recurrence of endometriosis and its control. Hum Reprod Update. 2009;15:441–461. doi: 10.1093/humupd/dmp007. [DOI] [PubMed] [Google Scholar]
- 13.Kumbak B, Kahraman S, Karlikaya G, Lacin S, Guney A. In vitro fertilization in normoresponder patients with endometriomas: comparison with basal simple ovarian cysts. Gynecol Obstet Investig. 2008;65(3):212–216. doi: 10.1159/000112310. [DOI] [PubMed] [Google Scholar]
- 14.Kitajima M, Dolmans MM, Donnez O, Masuzaki H, Soares M, Donnez J. Enhanced follicular recruitment and atresia in cortex derived from ovaries with endometriomas. Fertil Steril. 2014;101(4):1031–1037. doi: 10.1016/j.fertnstert.2013.12.049. [DOI] [PubMed] [Google Scholar]
- 15.Mills M, Rindfuss RR, McDonald P. teVelde E. ESHRE Reproduction and Society Task Force. Why do people postpone parenthood? Reasons and social policy incentives. Hum Reprod Update. 2011;17(6):848–860. doi: 10.1093/humupd/dmr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Practice Committee of the American Society for Reproductive Medicine Endometriosis and infertility: a committee opinion. Fertil Steril. 2012;98:591–598. doi: 10.1016/j.fertnstert.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 17.Lemos NA, Arbo E, Scalco R, Weiler E, Rosa V, Cuhna-Filho JS. Decreased anti-Mullerian hormone and altered ovarian follicular cohort in infertile patients with mild/minimal endometriosis. Fertil Steril. 2008;89:1064–1068. doi: 10.1016/j.fertnstert.2007.04.048. [DOI] [PubMed] [Google Scholar]
- 18.Lin XN, Wei ML, Tong XM, Xu WH, Zhou F, Huang QX, et al. Outcome of in vitro fertilization in endometriosis-associated infertility: a 5-year database cohort study. Chin Med J. 2012;25:2688–2693. [PubMed] [Google Scholar]
- 19.Busacca M, Riparini J, Somigliana E, Oggioni G, Izzo S, Vignali M, et al. Postsurgical ovarian failure after laparoscopic excision of bilateral endometriomas. Am J Obstet Gynecol. 2006;195(2):421–425. doi: 10.1016/j.ajog.2006.03.064. [DOI] [PubMed] [Google Scholar]
- 20.Coccia ME, Rizzello F, Mariani G, Bulletti C, Palagiano A, Scarselli G. Ovarian surgery for bilateral endometriomas influences age at menopause. Hum Reprod. 2011;26(11):3000–3007. doi: 10.1093/humrep/der286. [DOI] [PubMed] [Google Scholar]
- 21.Roman H, Tarta O, Pura I, Opris I, Bourdel N, Marpeau L, et al. Direct proportional relationship between endometrioma size and ovarian parenchyma inadvertently removed during cystectomy, and its implication on the management of enlarged endometriomas. Hum Reprod. 2010;25:1428–1432. doi: 10.1093/humrep/deq069. [DOI] [PubMed] [Google Scholar]
- 22.Biacchiardi CP, Piane LD, Camanni M, Deltetto F, Delpiano EM, Marchino GL, et al. Laparoscopic stripping of endometriomas negatively affects ovarian follicular reserve even if performed by experienced surgeons. Reprod BioMed Online. 2011;23:740–746. doi: 10.1016/j.rbmo.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 23.Tsolakidis D, Pados G, Vavilis D, Athanatos D, Tsalikis T, Giannakou A, et al. The impact on ovarian reserve after laparoscopic ovarian cystectomy versus three-stage management in patients with endometriomas: a prospective randomized study. Fertil Steril. 2010;94:71–77. doi: 10.1016/j.fertnstert.2009.01.138. [DOI] [PubMed] [Google Scholar]
- 24.Celik HG, Dogan E, Okyay E, Ulukus C, Saatli B, Uysal S, et al. Effect of laparoscopic excision of endometriomas on ovarian reserve: serial changes in the serum antimullerian hormone levels. Fertil Steril. 2012;97:1472–1478. doi: 10.1016/j.fertnstert.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 25.Hirokawa W, Iwase A, Goto M, Takikawa S, Nagatomo Y, Nakahara T, et al. The post-operative decline in serum anti-Mullerian hormone correlates with the bilaterality and severity of endometriosis. Hum Reprod. 2011;26(4):904–910. doi: 10.1093/humrep/der006. [DOI] [PubMed] [Google Scholar]
- 26.Var T, Batioglu S, Tonguc E, Kahyaoglu I. The effect of laparoscopic ovarian cystectomy versus coagulation in bilateral endometriomas on ovarian reserve as determined by antral follicle count and ovarian volume: a prospective randomized study. Fertil Steril. 2011;95:2247–2250. doi: 10.1016/j.fertnstert.2011.03.078. [DOI] [PubMed] [Google Scholar]
- 27.Li CZ, Liu B, Wen ZQ, Sun Q. The impact of electrocoagulation on ovarian reserve after laparoscopic excision of ovarian cyst: a prospective clinical study of 191 patients. Fertil Steril. 2009;92:1428–1435. doi: 10.1016/j.fertnstert.2008.08.071. [DOI] [PubMed] [Google Scholar]
- 28.Romualdi D, Franco Zannoni G, Lanzone A, Selvaggi L, Tagliaferri V, Gaetano Vellone V, et al. Follicular loss in endoscopic surgery for ovarian endometriosis: quantitative and qualitative observations. Fertil Steril. 2011;96(2):374–378. doi: 10.1016/j.fertnstert.2011.05.078. [DOI] [PubMed] [Google Scholar]
- 29.Tandoi I, Somigliana E, Riparini J, Ronzoni S, Vigano P, Candiani M. High rate of endometriosis recurrence in young women. J Pediatr Adolesc Gynecol. 2011;24(6):376–379. doi: 10.1016/j.jpag.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 30.Shebl O, Ebner T, Sommergruber M, Sir A, Tews G. Anti Muellerian hormone serum levels in women with endometriosis: a case–control study. Gynecol Endocrinol. 2009;25(11):713–716. doi: 10.3109/09513590903159615. [DOI] [PubMed] [Google Scholar]
- 31.Ercan CM, Sakinci M, Duru NK, Alanbay I, Karasahin KE, Baser I. Antimullerian hormone levels after laparoscopic endometrioma stripping surgery. Gynecol Endocrinol. 2010;26:468–472. doi: 10.3109/09513591003632134. [DOI] [PubMed] [Google Scholar]
- 32.Uncu G, Kasapoglu I, Ozerkan K, Seyhan A, Oral Yilmaztepe A, Ata B. Prospective assessment of the impact of endometriomas and their removal on ovarian reserve and determinants of the rate of decline in ovarian reserve. Hum Reprod. 2013;28(8):2140–2145. doi: 10.1093/humrep/det123. [DOI] [PubMed] [Google Scholar]
- 33.Almog B, Shehata F, Sheizaf B, Tulandi T. Effect of different types of ovarian cyst on antral follicle count. Fertil Steril. 2010;94:2338–2339. doi: 10.1016/j.fertnstert.2010.01.074. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki T, Izumi S, Matsubayashi H, Awaji H, Yoshikata K, Makino T. Impact of ovarian endometrioma on oocytes and pregnancy outcome in vitro fertilization. Fertil Steril. 2005;83:908–913. doi: 10.1016/j.fertnstert.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 35.Opøien HK, Fedorak P, Omland AK, Abyholm T, Bjerke S, Ertzeid G, et al. In vitro fertilization is a successful treatment in endometriosis-associated infertility. Fertil Steril. 2012;97(4):912–918. doi: 10.1016/j.fertnstert.2012.01.112. [DOI] [PubMed] [Google Scholar]
- 36.Kitajima M, Defrère S, Dolmans MM, Colette S, Squifflet J, Van Langendonckt A, et al. Endometriomas as a possible cause of reduced ovarian reserve in women with endometriosis. Fertil Steril. 2011;96(3):685–691. doi: 10.1016/j.fertnstert.2011.06.064. [DOI] [PubMed] [Google Scholar]
- 37.Maneschi F, Marasa L, Incandela S, Mazzarese M, Zupi E. Ovarian cortex surrounding benign neoplasms: a histologic study. Am J Obstet Gynecol. 1993;169(2 Pt 1):388–393. doi: 10.1016/0002-9378(93)90093-X. [DOI] [PubMed] [Google Scholar]
- 38.Muzii L, Bianchi A, Crocè C, Manci N, Benedetti Panici P. Laparoscopic excision of ovarian cysts: is the stripping technique a tissue sparing procedure? Fertil Steril. 2002;77:609–614. doi: 10.1016/S0015-0282(01)03203-4. [DOI] [PubMed] [Google Scholar]
- 39.Somigliana E, Infantino M, Benedetti F, Arnoldi M, Calanna G, Ragni G. The presence of ovarian endometriomas is associated with a reduced responsiveness to gonadotropins. Fertil Steril. 2006;86(1):192–196. doi: 10.1016/j.fertnstert.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 40.Meirow D, Dor J, Kaufman B, Shrim A, Rabinovici J, Schiff E, et al. Cortical fibrosis and blood-vessels damage in human ovaries exposed to chemotherapy. Potential mechanisms of ovarian injury. Hum Reprod. 2007;22(6):1626–1633. doi: 10.1093/humrep/dem027. [DOI] [PubMed] [Google Scholar]
- 41.Sanchez AM, Viganò P, Somigliana E, Panina-Bordignon P, Vercellini P, Candiani M. The distinguishing cellular and molecular features of the endometriotic ovarian cyst: from pathophysiology to the potential endometrioma-mediated damage to the ovary. Hum Reprod Update. 2014;20(2):217–230. doi: 10.1093/humupd/dmt053. [DOI] [PubMed] [Google Scholar]
- 42.Kalich-Philosoph L, Roness H, Carmely A, Fishel-Bartal M, Ligumsky H, Paglin S, et al. Cyclophosphamide triggers follicle activation and “burnout”; AS101 prevents follicle loss and preserves fertility. Sci Transl Med. 2013;5(185):185ra62. doi: 10.1126/scitranslmed.3005402. [DOI] [PubMed] [Google Scholar]
- 43.Roness H, Gavish Z, Cohen Y, Meirow D. Ovarian follicle burn-out: a universal phenomenon? Cell Cycle. 2013;12(20):3245–3246. doi: 10.4161/cc.26358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muzii L, Marana R, Angioli R, Bianchi A, Cucinella G, Vignali M, et al. Histologic analysis of specimens from laparoscopic endometrioma excision performed by different surgeons: does the surgeon matter? Fertil Steril. 2011;95:2116–2119. doi: 10.1016/j.fertnstert.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 45.Cobo A, Diaz C. Clinical application of oocyte vitrification: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2011;96(2):277–285. doi: 10.1016/j.fertnstert.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 46.D’Hooghe TM, Denys B, Spiessens C, Meuleman C, Debrock S. Is the endometriosis recurrence rate increased after ovarian hyperstimulation? Fertil Steril. 2006;86(2):283–290. doi: 10.1016/j.fertnstert.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 47.Benaglia L, Somigliana E, Santi G, Scarduelli C, Ragni G, Fedele L. IVF and endometriosis-related symptom progression: insights from a prospective study. Hum Reprod. 2011;26(9):2368–2372. doi: 10.1093/humrep/der208. [DOI] [PubMed] [Google Scholar]
- 48.Reinblatt SL, Ishai L, Shehata F, Son WY, Tulandi T, Almog B. Effects of ovarian endometrioma on embryo quality. Fertil Steril. 2011;95:2700–2702. doi: 10.1016/j.fertnstert.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 49.Garcia-Velasco JA, Arici A. Is the endometrium or oocyte/embryo affected in endometriosis? Hum Reprod. 1999;14(Suppl 2):77–89. doi: 10.1093/humrep/14.suppl_2.77. [DOI] [PubMed] [Google Scholar]
- 50.Yaron Y, Peyser MR, Samuel D, Amit A, Lessing JB. Infected endometriotic cysts secondary to oocyte aspiration for in-vitro fertilization. Hum Reprod. 1994;9:1759–1760. doi: 10.1093/oxfordjournals.humrep.a138789. [DOI] [PubMed] [Google Scholar]
- 51.Elizur SE, Lebovitz O, Weintraub AY, Eisenberg VH, Seidman DS, Goldenberg M, et al. Pelvic inflammatory disease in women with endometriosis is more severe than in those without. Aust N Z J Obstet Gynaecol. 2014;54(2):162–165. doi: 10.1111/ajo.12189. [DOI] [PubMed] [Google Scholar]
- 52.Cil AP, Bang H, Oktay K. Age-specific probability of live birth with oocyte cryopreservation: an individual patient data meta-analysis. Fertil Steril. 2013;100(2):492–499. doi: 10.1016/j.fertnstert.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meirow D. Fertility preservation in cancer patients using stored ovarian tissue: clinical aspects. Curr Opin Endocrinol Diabetes Obes. 2008;15(6):536–547. doi: 10.1097/MED.0b013e32831a44a8. [DOI] [PubMed] [Google Scholar]
- 54.Dittrich R, Lotz L, Keck G, Hoffmann I, Mueller A, Beckmann MW, et al. Live birth after ovarian tissue auto-transplantation following overnight transportation before cryopreservation. Fertil Steril. 2012;97(2):387–390. doi: 10.1016/j.fertnstert.2011.11.047. [DOI] [PubMed] [Google Scholar]