Abstract
Purpose
The purpose of this study was to examine the biological variability of follicular fluid (FF) high density lipoprotein (HDL) particle components measured in ipsilateral ovarian follicles.
Methods
We collected FF from two ipsilateral follicles among six women undergoing in vitro fertilization (IVF). We measured concentrations of 19 FF HDL particle components, including HDL cholesterol, free cholesterol, four cholesteryl esters, phospholipids, triglycerides, paraoxonase and arylesterase activities, apolipoproteins A-1 and A-2 (ApoA-1 and ApoA-2), and seven lipophilic micronutrients, by automated analysis and with high-performance liquid chromatography. We assessed biological variability using two-stage nested analysis of variance and compared values with those previously published for contralateral follicles.
Results
For most FF HDL analytes, there was little variability between follicles relative to the variability between women (i.e., %σ2F:%σ2B <0.5). Intraclass correlation coefficients were >0.80 for HDL cholesterol (0.82), phospholipids (0.89), paraoxonase (0.96), and arylesterase (0.91) activities, ApoA-1 (0.89), and ApoA-2 (0.90), and single specimen collections were required to estimate the subject-specific mean, demonstrating sufficient reliability for use as biomarkers of the follicular microenvironment in epidemiologic and clinical studies.
Conclusions
These preliminary results raise the possibility for tighter regulation of HDL in follicles within the same ovary vs. between ovaries. Thus, collection of a single FF specimen may be sufficient to estimate HDL particle components concentrations within a single ovary. However, our results should be interpreted with caution as the analysis was based on a small sample.
Keywords: Biological variability, Follicular fluid (FF), High density lipoprotein (HDL), In vitro fertilization (IVF), Human ovarian follicles
Introduction
It is critical to identify and characterize sources of biological variability in studies of human reproduction, to foster the use of designs and biomarkers that maximize validity and precision using available resources. In terms of female reproduction, substantial variability has been reported between and within women with respect to the laterality of ovulation [1, 2], menstrual cycle length [2], ovarian follicle growth rates, and maximum achieved follicle diameter [3]. More recently, investigators have also begun to characterize the biological variability of proteins, lipids, hormones, and metabolites in human follicular fluid (FF) [4–7]. Given its proximity to a developing oocyte [8], FF is increasingly viewed as a potential source of biomarker information for studying reproductive endpoints [9, 10].
In FF, high density lipoprotein (HDL) is found as the major class of lipoprotein; larger lipoprotein particles are excluded by the follicular basal lamina [11]. Given its potent anti-oxidant activity [12–14], and critical role in delivering cholesterol for steroid hormone synthesis [15, 16], HDL has strong potential as a biomarker of the follicular microenvironment. Studies also identified HDL particle components measured in FF, including cholesterol, ApoA-1 [17], γ-tocopherol, and β-cryptoxanthin [18], as important predictors of embryo quality during in vitro fertilization (IVF). Higher FF apolipoprotein A-1 (ApoA-1) levels were recently associated with poorer embryo quality per oocyte, in women undergoing IVF [19]. These data point to the importance of FF HDL biomarkers in human reproduction and suggest an important role in epidemiologic and clinical investigations of female infertility. Yet, perhaps due to the complexities and invasive nature of FF retrieval, characterization of the biological variability of FF HDL using human data is very limited at present.
To help to address the abovementioned data gap, Bloom et al. recently characterized the biological variability of FF HDL particle components collected from contralateral ovaries [20]. Substantial variability for 19 FF HDL particle components was reported between follicles from contralateral ovaries, as well as between women according to demographic and clinical factors. These results underscored the importance of a “one follicle-one oocyte” design in studies of follicular mechanisms and IVF outcomes. To augment those data, we here made a preliminary characterization of biological variabilities in HDL particle components, using FF collected from ipsilateral follicles, and compared these to our previously reported results for contralateral follicles [20].
Methods
Sample selection
Between April 10, 2010 and June 28, 2011, we recruited a convenience sample of 180 women undergoing IVF treatment with fresh, non-donor oocytes, among women referred to the University of California at San Francisco (UCSF) Center for Reproductive Health for infertility treatment. Overall, 97.8 % of women agreed to participate. The participants provided comprehensive infertility data and health-related behaviors, including current and past cigarette smoking status, through completion of a standard infertility intake form and a study questionnaire. Height and weight were measured to calculate body mass index (BMI; kg/m2). Informed consent was obtained prior to the inclusion to the study. We performed all procedures in accordance with the ethical standards of the UCSF Committee on Human Research and with the 1964 Helsinki declaration and its later amendments. The study protocol was approved by the UCSF Committee on Human Research.
Clinical protocol and specimen collection
All study participants underwent controlled gonadotropin-induced ovarian stimulation (COS) per standard clinical protocols. Endometrial development and follicle maturation were monitored by regular transvaginal ultrasounds and serum estradiol (E2) measures. Human chorionic gonadotropin (hCG) was administered subcutaneously when a sufficient number of follicles had developed beyond 17 mm diameter. Oocytes were aspirated under conscious sedation using a transvaginal needle (18 gauge) with ultrasound probe approximately 36 h later. We collected ipsilateral follicles from six women, and contralateral follicles were collected from the remaining participants. For each woman, the first follicle was aspirated using a clean, never used needle, and the second follicle was aspirated with a fully washed needle without flushing the follicle. Following collection, each follicle was individually transferred into an empty 10-mL tube and processed. After removal of the oocyte for clinical purposes, each 3.5–5.0-mL FF aspirate was centrifuged to pellet residual granulosa cells. The aspirated FF supernatant was aliquoted (0.6 mL) and frozen at −80 °C. No specimens showed visual evidence of blood contamination before or after centrifugation [21]. We shipped two aliquots from each follicle to the University of Buffalo, State University of New York on dry ice via overnight service for biochemical analyses.
Analytic methods
We used whole FF to determine concentrations of ApoA-1, apolipoprotein A-2 (ApoA-2), and paraoxonase (PON1) activities. ApoA-1 and ApoA-2 levels were analyzed by immunoturbidometric methods using diagnostic kits from Kamiya Biomedical (Seattle, WA) on the Cobas Fara II automated chemistry analyzer (Hoffmann-La Roche, Basel, Switzerland). Arylesterase and paraoxonase activities were also analyzed using the Cobas Fara II as described elsewhere [22]. FF HDL fractions were prepared by selective precipitation to remove trace amounts of apolipoprotein B containing low density lipoproteins [17].
FF HDL particle lipids, including cholesterol, phospholipids, and triglycerides, were measured using diagnostic reagent kits from Sekisui Diagnostics (Lexington, MA) adapted to the Cobas Fara II (Hoffmann-La Roche). Free (unesterified) cholesterol and cholesteryl esters, including cholesteryls palmitate, oleate, linoleate, and arachidonate, were measured by high-performance liquid chromatography (HPLC). Fat-soluble micronutrients, including vitamin A (retinol), vitamin E (α and γ tocopherols), and carotenoids (β-carotene, β-cryptoxanthin, lutein/zeaxanthin, and lycopene) were simultaneously measured by HPLC. The interassay coefficients of variation (CVs) of each analyte ranged from 0.6 % for arylesterase to 7.2 % for β-cryptoxanthin.
Statistical methods
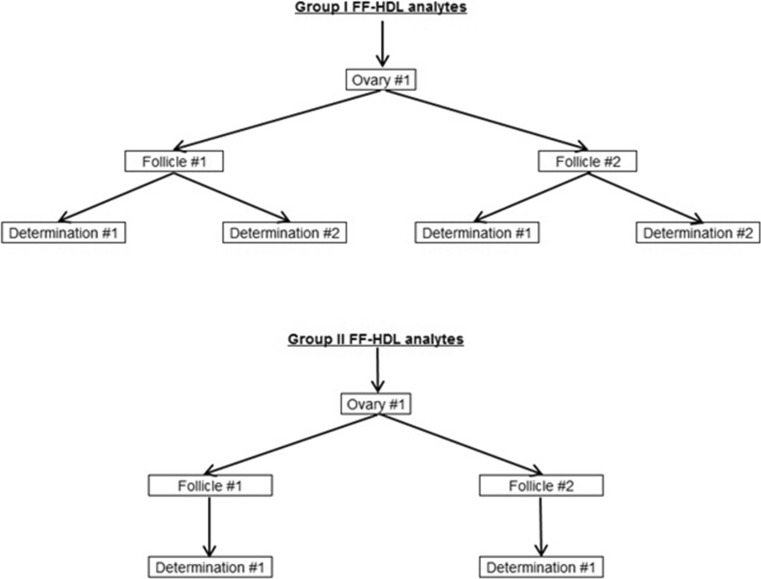
We had sufficient FF volume available to determine HDL cholesterol, phospholipids, triglycerides, paraoxonase and arylesterase activities, ApoA-1, and ApoA-2 in duplicate; we defined these as “group I” analytes (Fig. 1). However, enough FF was available for only a single HPLC determination, including analysis of free cholesterol, cholesteryls palmitate, oleate, linoleate and arachidonate, retinol, β-carotene, β-cryptoxanthin, α-tocopherol, γ-tocopherol, lutein/zeaxanthin, and lycopene, which we designated as “group II” analytes. Prior to statistical analysis, we excluded one free cholesterol observation that was 6.6 interquartile ranges above the 75th percentile of the sample distribution [23], and we applied a natural log transformation to normalize distributions and stabilize variances.
Fig. 1.
Sampling strategy for the measurement of follicular fluid (FF) high density lipoprotein (HDL) analytes measured in ipsilateral follicles, collected from in vitro fertilization patients. Group I analytes included HDL cholesterol, phospholipids, triglycerides, arylesterase, and paraoxonase activities, and apolipoproteins (ApoA-1 and ApoA-2), for which two determinations were made per sampled follicle. Group II analytes included free cholesterol, cholesteryl palmitate, cholesteryl oleate, cholesteryl linoleate, cholesteryl arachidonate, retinol, β-carotene, β-cryptoxanthin, α-tocopherol, γ-tocopherol, lutein/zeaxanthin, and lycopene, for which one determination was made per sampled follicle
We characterized sources of variability due to factors between women (σ2B) and between ipsilateral follicles (σ2F), and due to analytic factors (σ2A), using two-stage nested ANOVA. We specified the models as Yijk = μ + subi + folliclej(i) + ek(ji); where Yijk describes an analyte value for the kth determination (k = 1, 2 for group I analytes; k = 1 for group II analytes) collected from the jth ipsilateral follicle (j = 1, 2) in the ith woman (i = 1, …, 6), μ describes the grand mean for an analyte, subi describes the random effect of the ith woman on the grand mean, folliclej(i) describes the random effect of the jth ipsilateral follicle nested in the ith woman, and ek(ji) describes the random effect of the kth determination nested within the jth ipsilateral follicle sampled from the ith woman.
We defined total variance as σ2T = σ2B + σ2F + σ2A for group I analytes and σ2T = σ2B + σ2F for group II analytes. We were unable to isolate variability due to σ2A from σ2F for group II analytes because of the aforementioned single determination. The relative contributions of each source to the total variance were calculated as proportions multiplied by 100 % (%σ2B, %σ2F, and %σ2A, respectively). For each HDL analyte, we also calculated ratios of variability (RV) between follicles to variability between women (%σ2F:%σ2B) as an indicator of in situ follicle HDL regulation; higher ratio values suggest greater localized control (i.e., within follicle), whereas low values are more indicative of global ovarian regulation (i.e., ovarian circulation). CVs for HDL analytes (l) were calculated as CVl = √σ2l/ , where √σ2l represented the total variance and represented the mean value for an analyte. Intraclass correlation coefficients (ICCs) were estimated as σ2B /σ2T, with 95 % confidence intervals (CIs) estimated using the inverse tan transformation of Smith’s variance [24]. We also assessed the minimum number of specimens (m) necessary to estimate the within-ovary mean for an HDL analyte (l) within 10 % (i.e., ± 10 %) of the “true” value, calculated as m10% = (1.96 × ((CVl)/10))2 [25]. SAS version 9.3 (SAS Institute, Inc. Cary, NC) was used for the analyses.
Results
Mean (standard deviation) age and BMI for six women who provided ipsilateral follicles was 37.3 (4.8) years and 20.6 (1.2) kg/m2, respectively. Except for one study participant, all were non-Asian and had never-smoked. Primary infertility diagnoses were distributed as male factor (n = 1), diminished ovarian reserve (DOR) female factor (n = 3), and non-DOR female factor (n = 2). Half of the participants received a Lupron downregulated COS protocol whereas the remaining half received an antagonist protocol.
Biological variability characteristics for FF group I HDL particle components are described in Table 1. CVs were generally low (ranging from 1.12 % for arylesterase activity to 4.56 % for ApoA-2), although for triglycerides, the CV was high (30.35 %). The relative contribution of %σ2B to RV was higher than %σ2F for all analytes, with a low of RV = 0.03 for paraoxonase activity to a high of 0.33 for triglycerides. The ICC, which describes the proportion of observed variability associated with “true” differences between women exceeded 0.80, and a single specimen was required to estimate the ipsilateral ovary mean within ±10 % of the true value for all group I analytes, except for triglycerides (ICC = 0.74; m10% = 13).
Table 1.
Biological variability characteristics for group I high density lipoprotein (HDL) particle associated follicular fluid (FF) analytes, sampled from ipsilateral follicles in six in vitro fertilization patients
| FF analytes | Mean | SD | CV | σ2 T | %σ2 B | %σ2 F | %σ2 A | RV | m10% | ICC | 95 % CI Low |
95 % CI High |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HDL-cholesterol (mg/dL) | 31.25 | 1.23 | 3.94 | 0.092 | 82.60 | 15.42 | 1.98 | 0.19 | 1 | 0.82 | 0.64 | 1.00 |
| Phospholipids (mg/dL) | 75.46 | 1.16 | 1.54 | 0.063 | 89.12 | 8.02 | 2.86 | 0.09 | 1 | 0.89 | 0.77 | 1.00 |
| Triglycerides (mg/dL) | 5.29 | 1.61 | 30.35 | 0.349 | 73.82 | 24.01 | 2.17 | 0.33 | 13 | 0.74 | 0.47 | 1.00 |
| Arylesterase (kIU/L) | 106.97 | 1.19 | 1.12 | 0.114 | 90.99 | 7.36 | 1.65 | 0.08 | 1 | 0.91 | 0.81 | 1.00 |
| Paraoxonase (IU/L) | 81.53 | 1.73 | 2.12 | 0.247 | 96.20 | 3.20 | 0.60 | 0.03 | 1 | 0.96 | 0.92 | 1.00 |
| ApoA-1 (mg/dL) | 102.22 | 1.23 | 1.20 | 0.074 | 88.89 | 9.27 | 1.84 | 0.10 | 1 | 0.89 | 0.77 | 1.00 |
| ApoA-2 (mg/dL) | 27.25 | 1.24 | 4.56 | 0.074 | 89.68 | 8.81 | 1.51 | 0.10 | 1 | 0.90 | 0.78 | 1.00 |
NOTE: Geometric means and standard deviations (SDs) presented. All values were log-transformed for the analyses
ApoA-1 apolipoprotein A1, ApoA-2 apolipoprotein A2, CI confidence interval, CV coefficient of variation, ICC intraclass correlation coefficient, m 10% minimum number of specimen collections required to estimate the mean within ±10 % of the “true” value, RV % σ2 F: % σ2 B, σ 2 A variability attributed to analytic factors, σ 2 B variability between women, σ 2 F variability between follicles, σ 2 T total variability
In contrast to the group I HDL particle components, CVs for group II HDL analytes were considerably high and ranged from 8.47 % for cholesteryl linoleate to 98.06 % for β-cryptoxanthin (Table 2). Similar to the group I analytes, RV was <0.5 for most group II analytes. Except for free cholesterol (ICC = 0.76) and cholesteryl palmitate (ICC = 0.54), ICCs exceeded 0.80 for all group II analytes. However, the minimum number of specimen collections to estimate the ipsilateral ovary mean was greater than one for all group II analytes, except for cholesteryl linoleate (m10% = 1).
Table 2.
Biological variability characteristics for group II high density lipoprotein (HDL) particle associated follicular fluid (FF) analytes sampled from ipsilateral follicles in six in vitro fertilization patients
| FF analytes | Mean | SD | CV | σ2 T | %σ2 B | %σ2 F | RV | m10% | ICC | 95 % CI Low | 95 % CI High |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Free cholesterol (mg/dL)a | 2.10 | 1.08 | 51.49 | 0.045 | 76.42 | 23.58 | 0.31 | 8 | 0.76 | 0.50 | 1.00 |
| Cholesteryl palmitate (mg/dL) | 3.87 | 1.25 | 32.15 | 0.093 | 54.44 | 45.56 | 0.84 | 9 | 0.54 | 0.14 | 0.95 |
| Cholesteryl oleate (mg/dL) | 8.99 | 1.24 | 13.74 | 0.074 | 79.56 | 20.44 | 0.26 | 2 | 0.80 | 0.58 | 1.00 |
| Cholesteryl linoleate (mg/dL) | 14.47 | 1.22 | 8.47 | 0.075 | 79.84 | 20.16 | 0.25 | 1 | 0.80 | 0.59 | 1.00 |
| Cholesteryl arachidonate (mg/dL) | 7.44 | 1.22 | 16.38 | 0.086 | 86.41 | 13.59 | 0.16 | 2 | 0.86 | 0.72 | 1.00 |
| Retinol (μg/mL) | 1.36 | 1.05 | 76.79 | 0.004 | 81.09 | 18.91 | 0.23 | 3 | 0.81 | 0.61 | 1.00 |
| β-carotene (μg/mL) | 1.08 | 1.06 | 98.04 | 0.002 | 96.28 | 3.72 | 0.04 | 5 | 0.96 | 0.92 | 1.00 |
| β-cryptoxanthin (μg/mL) | 1.04 | 1.02 | 98.06 | 0.001 | 95.26 | 4.74 | 0.05 | 13 | 0.95 | 0.90 | 1.00 |
| α-tocopherol (μg/mL) | 4.02 | 1.21 | 30.09 | 0.053 | 86.27 | 13.73 | 0.16 | 2 | 0.86 | 0.71 | 1.00 |
| γ –tocopherol (μg/mL) | 1.20 | 1.08 | 89.90 | 0.011 | 91.32 | 8.68 | 0.10 | 11 | 0.91 | 0.82 | 1.00 |
| Lutein/zeaxanthin (μg/mL) | 1.09 | 1.04 | 95.74 | 0.001 | 90.04 | 9.96 | 0.11 | 5 | 0.90 | 0.79 | 1.00 |
| Lycopene (μg/mL) | 1.06 | 1.01 | 95.97 | 0.001 | 96.79 | 3.21 | 0.03 | 3 | 0.97 | 0.93 | 1.00 |
NOTE: Geometric means and standard deviations (SDs) presented. All values were log-transformed for the analyses. Variability due to analytic factors was captured in conjunction with variability between follicles
a n = 5 due to exclusion of one outlying observation
CI confidence interval, CV coefficient of variation, ICC intraclass correlation coefficient, m 10% minimum number of specimen collections required to estimate the mean within ±10 % of the “true” value, RV % σ2 F: % σ2 B, σ 2 B variability between women, σ 2 F variability between follicles, σ 2 T total variability
Discussion
We determined concentrations of 19 FF HDL particle components in ipsilateral follicles collected from women undergoing IVF and characterized the sources of measurement variability. Variability between follicles (i.e., within ovary) was substantially smaller than variability between women for most HDL particle components. Furthermore, our results indicate that the reliability of group I HDL particle components was suitable for use as biomarkers in epidemiologic and clinical studies, having had ICC ≥0.80 and requiring collection of single specimens to estimate subject-specific means within ovary. However, group II HDL particle components appeared to be less well-suited as biomarkers for epidemiologic or clinical investigations, as most analytes did not meet these requirements.
Active follicular regulation, with constitutive modification of the HDL particle [26, 27], is suggested by previous studies of mammalian ovaries. Tight biochemical controls between follicles were implied by interfollicular communication among cohorts of developing follicles within the ovary [28], which typically is mediated by endocrine and intraovarian factors [29]. Accordingly, variability between ipsilateral follicles was low relative to variability between women for most of the HDL particle components we measured, suggesting coordinated interfollicular HDL metabolism within ovary. In contrast, previous data for contralateral follicles suggested a larger contribution from extra-ovarian sources for most HDL particle components [20], possibly associated with the mesenteric circulation/blood supply. For example, the RV of phospholipids (0.55 vs. 0.09), ApoA-1 (0.51 vs. 0.10), and ApoA-2 (1.21 vs. 0.10) in the contralateral follicles was substantially higher than those measured in ipsilateral follicles, respectively, implying a greater contribution of between contralateral follicle variability than between ipsilateral follicle variability, possibly due to ovary-specific blood supplies. A conclusive explanation for the different contributions made by sources of variability between follicles in HDL particle components from ipsilateral and contralateral follicles is not known. Still, our preliminary data are consistent with a greater degree of regulation within than between ovaries for specific HDL particle components, and thus, fewer FF specimen collections may be sufficient to estimate HDL particle components concentrations within a single ovary.
We also conducted a reliability analysis of ipsilateral FF HDL particle components to evaluate their potential use as biomarkers in epidemiologic and clinical studies [30]. The CV describes the variability around a subject-specific mean; CV <10 % is generally considered as a clinical threshold for biomarker reliability [31]. In our study of HDL particle components measured in ipsilateral follicles, the CVs of most group I HDL analytes were well below 10 %, with the exception of triglycerides (30.35 %). Previously, a similar observation was seen for triglycerides sampled from contralateral follicles (i.e., CV = 22.52 %) [20]. Also similar to the contralateral analyses, CVs exceeded 10 % for all group II HDL analytes, except for cholesteryl linoleate (8.47 %) [20]. The higher CVs for group II HDL analytes compared to group I analytes in our study might be due to their substantially lower mean concentrations compared to group I, yet variance estimates of measured analytes in both groups were similar. Therefore, our results suggest the challenge in providing a reliable estimate for group II HDL particle component means, regardless of the ovary sampled.
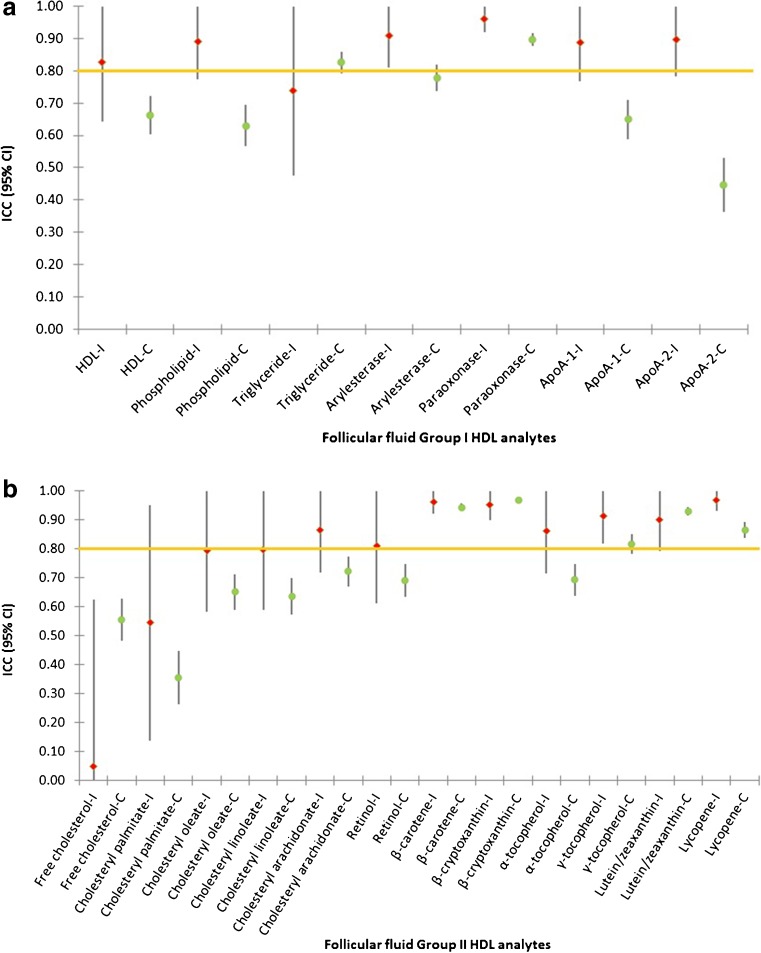
The ICC estimates the correlation between two replicate specimens, with an ICC ≥0.80 generally considered as a minimum reliability for use in epidemiologic studies [24]; an ICC <0.80 will require a sample size increase of more than 20 % for equivalent statistical power. In our study, ICCs were ≥0.80 for most HDL analytes, except triglycerides (0.74), free cholesterol (0.76), and cholesteryl palmitate (0.54). In contralateral follicles, ICCs exceeded 0.80 for only triglycerides, paraoxonase activity, β-carotene, β-cryptoxanthin, γ-tocopherol, lutein/zeaxanthin, and lycopene (Fig. 2) [20]. Thus, FF collection in ipsilateral follicles rather than contralateral follicles appears to be a better choice for epidemiologic investigations, if the study question addresses the impact of a large panel of FF HDL particle components.
Fig. 2.
Intraclass correlation coefficients (ICCs) with 95 % confidence intervals for follicular fluid high density lipoprotein (HDL) particle components measured in specimens collected from ipsilateral (−I) and contralateral ovaries (−C). a Group I HDL analytes include HDL cholesterol, phospholipids, triglycerides, arylesterase, and paraoxonase activities, and apolipoproteins (ApoA-1 and ApoA-2), and b group II HDL analytes include free cholesterol, cholesteryl palmitate, cholesteryl oleate, cholesteryl linoleate, cholesteryl arachidonate, retinol, β-carotene, β-cryptoxanthin, α-tocopherol, γ-tocopherol, lutein/zeaxanthin, and lycopene
For the purposes of epidemiologic and clinical investigations, it is critical to identify the number of specimens required to estimate subject-specific means. For most group I HDL analytes, collection of a single specimen was sufficient to estimate the mean within ±10 % of the true subject-specific mean, except for triglycerides (m10% = 13). On the other hand, Group II analytes required collection of a greater and varying number of specimens, ranging from a single specimen for cholesteryl linoleate to 13 specimens for β-cryptoxanthin. The large m10% for most group II analytes, despite ICCs ≥0.80, indicates a greater degree of variability between follicles for those analytes. A similar pattern was previously reported for group I and group II HDL particle components in contralateral follicles, yet the number of required specimen collections was generally greater than for ipsilateral follicles [32]. Still, a larger number of ipsilateral than contralateral specimen collections is required for triglycerides (13 vs. 6) and β-cryptoxanthin (13 vs. 10). Again, these results suggest that selection of either ipsilateral or contralateral follicles should be determined by specific HDL analytes of interest in studies utilizing FF HDL biomarkers.
Our study has several limitations, and thus, the results should be interpreted with caution. Most importantly, we had a very limited sample size as ipsilateral follicles were collected from only six women. Thus, our estimates of biologic variability were imprecise, and we were unable to characterize FF HDL analytes by relevant demographic and clinical factors, such as infertility diagnosis (e.g., diminished ovarian reserve) and COS protocol relevant to IVF. A larger sample size will be needed to more definitively investigate the clinical relevance of these results for IVF. It is reported that levels of HDL components measured in mammalian FF depend on follicle size [33], yet diameter data were unavailable to us for incorporation into the analysis. However, we collected only follicles greater than 17 mm diameter, which would minimize the impact of follicle size on HDL concentrations. In addition, insufficient remaining sample volumes for our HPLC analysis prevented isolation of group II analyte variability due to analytic factors from between-follicle variability. Still, given reported analytical performance of the HPLC assays elsewhere [20], and previous estimates of analytical variability in serum [34], we believe that the variability due to analytic factors was likely to be small.
Conclusions
In conclusion, our study demonstrated smaller between ipsilateral follicles variability for most FF HDL particle components than our previously reported results for follicles from contralateral ovaries, potentially reflecting a higher degree of follicular control within ovary. These findings illustrate the need to conduct variability and reliability studies prior to selecting FF biomarkers for epidemiologic and clinical investigations. To our knowledge, this is the first study investigating the biological variability of FF HDL particle components within the same ovary. Our study results are restricted by the aforementioned limitations, particularly the small sample size, and so require further confirmation using a larger sample size to provide for a more conclusive interpretation of clinical impacts. However, these preliminary results offer useful insight into the use of FF HDL particle components as biomarkers for epidemiologic and clinical investigations.
Acknowledgments
The authors would like to thank Ying Wang, PhD for providing critical feedback for this manuscript.
Compliance with ethical standards
Funding
This work was funded through grant R21 AG031957-01A2, provided by the National Institute on Aging, National Institutes of Health.
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All procedures were performed in accordance with the ethical standards of the UCSF Committee on Human Research and with the 1964 Helsinki declaration and its later amendments. The study protocol was approved by the UCSF Committee on Human Research.
Informed consent
Informed consent was obtained prior to participation in the study.
Footnotes
Capsule The variability of FF HDL analytes from ipsilateral follicles was smaller than variability that was previously reported in follicles from contralateral ovaries.
References
- 1.Fukuda M, Fukuda K, Andersen CY, Byskov AG. Contralateral selection of dominant follicle favours pre-embryo development. Hum Reprod. 1996;11:1958–62. doi: 10.1093/oxfordjournals.humrep.a019524. [DOI] [PubMed] [Google Scholar]
- 2.Ecochard R, Gougeon A. Side of ovulation and cycle characteristics in normally fertile women. Hum Reprod. 2000;15:752–5. doi: 10.1093/humrep/15.4.752. [DOI] [PubMed] [Google Scholar]
- 3.Mikolajczyk RT, Stanford JB, Ecochard R. Multilevel model to assess sources of variation in follicular growth close to the time of ovulation in women with normal fertility: a multicenter observational study. Reprod Biol Endocrinol. 2008;6. [DOI] [PMC free article] [PubMed]
- 4.Singh AK, Chattopadhyay R, Chakravarty B, Chaudhury K. Markers of oxidative stress in follicular fluid of women with endometriosis and tubal infertility undergoing IVF. Reprod Toxicol. 2013;42:116–24. doi: 10.1016/j.reprotox.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Revelli A, Piane LD, Casano S, Molinari E, Massobrio M, Rinaudo P. Follicular fluid content and oocyte quality: from single biochemical markers to metabolomics. Reprod Biol Endocrinol. 2009;7:40. doi: 10.1186/1477-7827-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benedict MD, Missmer SA, Vitonis AF, Cramer DW, Meeker JD. Cotinine concentrations in follicular fluid as a measure of secondhand tobacco smoke exposure in women undergoing in vitro fertilization: inter-matrix comparisons with urine and temporal variability. Chemosphere. 2011;84:110–6. doi: 10.1016/j.chemosphere.2011.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zamah AM, Hassis ME, Albertolle ME, Williams KE. Proteomic analysis of human follicular fluid from fertile women. Clin Proteomics. 2015;12:5. doi: 10.1186/s12014-015-9077-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oktem O, Urman B. Understanding follicle growth in vivo. Hum Reprod. 2010;25:2944–54. doi: 10.1093/humrep/deq275. [DOI] [PubMed] [Google Scholar]
- 9.Pasqualotto EB, Agarwal A, Sharma RK, Izzo VM, Pinotti JA, Joshi NJ, et al. Effect of oxidative stress in follicular fluid on the outcome of assisted reproductive procedures. Fertil Steril. 2004;81:973–6. doi: 10.1016/j.fertnstert.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 10.Mehdizadeh A, Rahimipour A, Farzadi L, Darabi M, Shahnazi V, Shaaker M, et al. Correlation between the level of cholesteryl ester transfer protein in follicular fluid with fertilization rates in IVF/ICSI cycles. Iran J Reprod Med. 2011;9:193–8. [PMC free article] [PubMed] [Google Scholar]
- 11.Le Goff D. Follicular fluid lipoproteins in the mare: evaluation of HDL transfer from plasma to follicular fluid. Biochim Biophys Acta. 1994;1210:226–32. doi: 10.1016/0005-2760(94)90125-2. [DOI] [PubMed] [Google Scholar]
- 12.Ansell BJ, Navab M, Hama S, Kamranpour N, Fonarow G, Hough G, et al. Inflammatory/antiinflammatory properties of high-density lipoprotein distinguish patients from control subjects better than high-density lipoprotein cholesterol levels and are favorably affected by simvastatin treatment. Circulation. 2003;108:2751–6. doi: 10.1161/01.CIR.0000103624.14436.4B. [DOI] [PubMed] [Google Scholar]
- 13.Kontush A, Chantepie S, Chapman MJ. Small, dense HDL particles exert potent protection of atherogenic LDL against oxidative stress. Atertio Thromb Vasc Biol. 2003;23:1881–8. doi: 10.1161/01.ATV.0000091338.93223.E8. [DOI] [PubMed] [Google Scholar]
- 14.Negre-Salvayre A, Dousset N, Ferretti G, Bacchetti T, Curatola G, Salvayre R. Antioxidant and cytoprotective properties of high-density lipoproteins in vascular cells. Free Radic Biol Med. 2006;41:1031–40. doi: 10.1016/j.freeradbiomed.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Azhar S, Tsai L, Medicherla S, Chandrasekher Y, Giudice L, Reaven E. Human granulosa cells use high density lipoprotein cholesterol for steroidogenesis. J Clin Endocrinol Metab. 1998;83:983–91. doi: 10.1210/jcem.83.3.4662. [DOI] [PubMed] [Google Scholar]
- 16.Li XL, Peegel H, Menon KMJ. Regulation of high density lipoprotein receptor messenger ribonucleic acid expression and cholesterol transport in theca-interstitial cells by insulin and human chorionic gonadotropin. Endocrinology. 2001;142:174–81. doi: 10.1210/endo.142.1.7865. [DOI] [PubMed] [Google Scholar]
- 17.Browne RW, Shelly WB, Bloom MS, Ocque AJ, Sandler JR, Huddleston HG, et al. Distributions of high-density lipoprotein particle components in human follicular fluid and sera and their associations with embryo morphology parameters during IVF. Hum Reprod. 2008;23:1884–94. doi: 10.1093/humrep/den183. [DOI] [PubMed] [Google Scholar]
- 18.Browne RW, Bloom MS, Shelly WB, Ocque AJ, Huddleston HG, Fujimoto VY. Follicular fluid high density lipoprotein-associated micronutrient levels are associated with embryo fragmentation during IVF. J Assist Reprod Genet. 2009;26:557–60. doi: 10.1007/s10815-009-9367-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valckx SDM, De Pauw I, De Neubourg D, Inion I, Berth M, Fransen E, et al. BMI-related metabolic composition of the follicular fluid of women undergoing assisted reproductive treatment and the consequences for oocyte and embryo quality. Hum Reprod. 2012;27:3531–9. doi: 10.1093/humrep/des350. [DOI] [PubMed] [Google Scholar]
- 20.Bloom MS, Kim K, Fujimoto VY, Browne RW. Variability in the components of high-density lipoprotein particles measured in human ovarian follicular fluid: a cross-sectional analysis. Fertil Steril. 2014;101:1431–U59. doi: 10.1016/j.fertnstert.2014.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levay PF, Huyser C, Fourie FLR, Rossouw DJ. The detection of blood contamination in human follicular fluid. J Assist Reprod Genet. 1997;14:212–7. doi: 10.1007/BF02766112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Browne RW, Koury ST, Marion S, Wilding G, Muti P, Trevisan M. Accuracy and biological variation of human serum paraoxonase 1 activity and polymorphism (Q192R) by kinetic enzyme assay. Clin Chem. 2007;53:310–7. doi: 10.1373/clinchem.2006.074559. [DOI] [PubMed] [Google Scholar]
- 23.Kitchens LJ. Exploring statistics: a modern introduction to data analysis and inference. Pacific Grove: Duxbury Press; 1998. p. 940. [Google Scholar]
- 24.Lachin JM. The role of measurement reliability in clinical trials. Clin Trials. 2004;1:553–66. doi: 10.1191/1740774504cn057oa. [DOI] [PubMed] [Google Scholar]
- 25.Fraser CG, Harris EK, Petersen PH. Generation and application of data on biological variation in clinical chemistry. Crit Rev Clin Lab Sci. 1989;27:409–30. doi: 10.3109/10408368909106595. [DOI] [PubMed] [Google Scholar]
- 26.Fujimoto VY, Kane JP, Ishida BY, Bloom MS, Browne RW. High-density lipoprotein metabolism and the human embryo. Hum Reprod Update. 2010;16:20–38. doi: 10.1093/humupd/dmp029. [DOI] [PubMed] [Google Scholar]
- 27.van Montfoort APA, Plosch T, Hoek A, Tietge UJF. Impact of maternal cholesterol metabolism on ovarian follicle development and fertility. J Reprod Immunol. 2014;104:32–6. doi: 10.1016/j.jri.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Ginther OJ, Kastelic JP, Knopf L. Intraovarian relationships among dominant and subordinate follicles and the corpus luteum in heifers. Theriogenology. 1989;32:787–95. doi: 10.1016/0093-691X(89)90467-6. [DOI] [PubMed] [Google Scholar]
- 29.Baker SJ, Srsen V, Lapping R, Spears N. Combined effect of follicle-follicle interactions and declining follicle-stimulating hormone on murine follicle health in vitro. Biol Reprod. 2001;65:1304–10. doi: 10.1095/biolreprod65.4.1304. [DOI] [PubMed] [Google Scholar]
- 30.Taioli E, Kinney P, Zhitkovich A, Fulton H, Voitkun V, Cosma G, et al. Application of reliability models to studies of biomarker validation. Environ Health Perspect. 1994;102:306–9. doi: 10.1289/ehp.94102306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schulte PA, Rothman N, Schottenfeld D. Design considerations in molecular epidemiology. In: Schulte PA, Perera FP, editors. Molecular epidemiology: principles and practices. San Diego: Academic Press; 1993. pp. 159–98. [Google Scholar]
- 32.Kim K, Bloom MS, Fujimoto VY, Browne RW. Number of specimens required to estimate average follicular fluid high-density lipoprotein particle components in women undergoing in vitro fertilization (letter) Fertil Steril. 2014;101 doi: 10.1016/j.fertnstert.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 33.Nandi S, Kumar VG, Manjunatha BM, Gupta PSP. Biochemical composition of ovine follicular fluid in relation to follicle size. Dev Growth Differ. 2007;49:61–6. doi: 10.1111/j.1440-169X.2007.00901.x. [DOI] [PubMed] [Google Scholar]
- 34.Browne RW, Bloom MS, Schisterman EF, Hovey K, Trevisan M, Xu C, et al. Analytical and biological variation of biomarkers of oxidative stress during the menstrual cycle. Biomarkers. 2008;13:160–83. doi: 10.1080/13547500701775563. [DOI] [PMC free article] [PubMed] [Google Scholar]