Abstract
Cells are able to produce and release different types of vesicles, such as microvesicles and exosomes, in the extracellular microenvironment. According to the scientific community, both microvesicles and exosomes are able to take on and transfer different macromolecules from and to other cells, and in this way, they can influence the recipient cell function. Among the different macromolecule cargos, the most studied are microRNAs. MicroRNAs are a large family of non-coding RNAs involved in the regulation of gene expression. They control every cellular process and their altered regulation is involved in human diseases. Their presence in mammalian follicular fluid has been recently demonstrated, and here, they are enclosed within microvesicles and exosomes or they can also be associated to protein complexes. The presence of microvesicles and exosomes carrying microRNAs in follicular fluid could represent an alternative mechanism of autocrine and paracrine communication inside the ovarian follicle. The outcomes from these studies could be important in basic reproductive research but could also be useful for clinical application. In fact, the characterization of extracellular vesicles in follicular fluid could improve reproductive disease diagnosis and provide biomarkers of oocyte quality in ART (Assisted Reproductive Treatment).
Keywords: Extracellular vesicles, Exosomes, MicroRNAs, Ovarian follicle
Introduction
In multicellular eukaryotes, the cells develop different strategies to communicate with each other and to allow the correct hierarchical ordering and organization of all organisms. The established mechanisms of cell communication include direct interaction, such as gap junctions or, alternatively, secretion of extracellular signals. This latter mechanism involves different steps: synthesis of hormones, growth factors, and cytokines released into the extracellular microenvironment, transport to target cells, detection of specific receptors, and finally, change in cellular metabolism, function, proliferation, differentiation, or death. Extracellular signals can act by autocrine, paracrine, or endocrine signaling.
In the last few years, an alternative mechanism by which the cells are able to communicate with each other has come to light. There is growing evidence that cells may produce and release vesicles able to transfer information to other cells and to influence the recipient cell’s function. The extracellular vesicles (EVs) are membrane-contained vesicles released in an evolutionally conserved manner by cells ranging from organisms, such as prokaryotes to higher eukaryotes [1]. Cells are able to produce and release different types of EVs, such as microvesicles, exosomes, apoptotic bodies, and ectosomes that differ in their size, biogenesis, and surface markers [1].
According to the scientific community, both microvesicles and exosomes are able to take on and transfer different macromolecules from and to recipient cells [2, 3]. They can transfer proteins, lipids, and nucleic acids and can influence different physiologic and pathologic processes by modifying the phenotype of the secreting and recipient cells [1, 4–6]. It has been shown in vitro that most cells, such as B and T cells, dendritic cells, mast cells, mesenchymal stem cells, and cancer cells, are able to produce and secrete microvesicles and exosomes and in vivo microvesicles and exosomes have been isolated and characterized from several biological fluids [1, 4–6].
While intensive investigation has targeted their role in different pathologic processes, such as cancer, autoimmune, and degenerative diseases, their physiological functions have been less explored [5]. Despite the great interest for both basic and applied research and the abundance of papers published on this subject, the classification of extracellular vesicles is, to date, unclear. However, we know that exosomes correspond to a homogenous population with a size of 30–100 nm in diameter. They originate from multivesicular bodies (MVBs) containing endosomes that fuse with the plasma membrane releasing the vesicles into the extracellular space [1, 4, 5, 7]. Microvesicles represent a more heterogeneous population with a size of 100–1000 nm in diameter and they bud directly from the plasma membrane [1, 4, 5, 7]. Specific markers absolutely discriminating the two types of vesicles have not yet been identified. To date, we know that exosomes are enriched in tetraspanins, such as CD9, CD63, and CD81, but, on the contrary, microvesicles present no definite unique markers [2]. For this reason, the characterization methods (immunoblotting, immuno-gold labeling combined with electron microscopy and antibody-coupled bead flow cytometry analysis) are not always highly specific.
Moreover, the current techniques to separate exosomes and microvesicles from biological fluids are less than ideal. The current techniques primarily include isolation by differential ultracentrifugation or size exclusion chromatography, but small variations in the purification procedure could result in contaminated samples [7]. In the literature, these issues have sometimes produced conflicting results in attributing a specific molecule cargo to microvesicles or to exosomes [8]. In spite of this shortcoming, it will be necessary to standardize purification and characterization protocols and this field has produced interesting results in basic and applied medical research [7].
Exosomes will be concentrated on in this review because most papers have focused their attention to them. In fact, they probably represent a more homogenous category than microvesicles and most data about molecular cargo identification were shown on exosomes. Among the different biomolecule cargos, microRNAs (miRNAs) are the best characterized, probably because of their high stability and relatively easy extraction as compared to proteins and, above all, because of their basic role in the regulation of gene expression [3]. Regulation of gene expression represents the mechanism able to create cellular complexity in multicellular organisms ensuring correct tissue differentiation. In addition, alteration of the complex processes that govern gene expression are responsible for the onset of pathological phenotypes. Gene expression is the result of coordinated cellular activities and requires transcription factors, chromatin remodeling complexes, and non-coding RNAs (ncRNAs) [9].
Over the last few years, the role of ncRNAs has been investigated more frequently. ncRNAs represent most of the cellular transcriptome (95–98 %) and include long ncRNAs (>200 bp) and small ncRNAs (<200 bp), such as miRNAs, small interfering RNAs (siRNA), and PIWI element-interacting RNAs (piRNA) [9]. miRNAs represent the most studied class of ncRNAs, and they regulate the activity of most protein-coding genes, primarily repressing gene expression at the post transcriptional level. In this way, they are able to control every cellular process and their altered regulation is involved in human diseases [10, 11]. In biological fluid, miRNAs display remarkable stability and resistance to degradation possibly because of their association with protein complexes or because they are enclosed within microvesicles or exosomes. As with cellular miRNAs, circulating miRNAs also show altered expression in different human pathologies [12, 13]. It has been postulated that miRNAs could represent the oldest hormones [13]. As hormones, they should be released by a donor cell secreted by active mechanisms and then they should spread signals that affect cells located in other parts of the organism [13]. Target cells, uptaking the miRNAs, are able to change their genetic program [13, 14]. These observations resulted in a great scientific interest because of their implication in clinical research in that miRNAs could represent noninvasive biomarkers, prognostic factors and also therapeutic targets in cancer or in other complex diseases. Even if most studies concern cancer, miRNAs are becoming a topic of considerable interest also in reproductive biology [15, 16].
Ovarian follicle
Oocyte developmental competence, which is the ability to produce a healthy embryo, develops through protracted and complex processes beginning during embryonic life and ends at the moment that the MII oocyte is ovulated. Successful oocyte activation, after the entrance of the sperm, and then with the fusion of male and female pronuclei, is strongly influenced by several molecules (maternal factors) that the oocyte accumulates during its maturation [17].
During embryonic life, primordial germ cells (PGCs) migrate to the genital ridge where they proliferate by mitosis expanding the size of the germ line and transform from oogonia into primary oocytes. Primary oocytes enter meiosis and become arrested in prophase I at the diplotene stage (dictyate). At this time, the oocyte is enclosed by a specialized lineage of ovarian somatic cells, pre-granulosa cells, to form a primordial follicle [18]. The primordial follicle pool represents the woman’s ovarian reserve.
After puberty, some primary follicles are cyclically recruited and develop through primary, secondary, and antral stages. Most of the antral follicles undergo apoptosis, whereas only few of them continue to grow and, after reaching the LH (luteinizing hormone) surge, are stimulated to resume meiosis. Among them, only the dominant Graffian follicle ovulates to release the mature egg, which is ready for fertilization [18, 19].
Follicle development and oocyte maturation are strictly associated. In fact, the proliferation and the differentiation of somatic follicular cells occur in synchrony with the maturing oocyte and are mediated by a constant exchange of signals between somatic cells and germ cells [18]. The structural and functional modifications of pre-granulosa cells that differentiate into mural granulosa cells (GCs) and cumulus cells (CCs) are controlled by hormonal and oocyte signaling. Pre-granulosa cells are able to maintain the quiescence of primordial follicles, while GCs, after the LH surge, control oocyte maturation. On the other hand, the oocyte, through the synthesis and secretion of GDF9 and BMP15, regulates GC proliferation and differentiation [17, 20]. It has been shown that, in the primordial follicle, FOXL2 (forkhead box L2) regulates the transcription of inhibitor factors that target the oocyte and suppresses its growth [20]. Following the LH surge, GCs synthesize epidermal growth factors, such as amphiregulin (AREG), epiregulin (EREG), and betacellulin (BTC), which are involved in oocyte maturation [21, 22]. Moreover, CCs supply the oocyte with pyruvate, amino acids, and nucleotides that are essential to the oocyte for macromolecule synthesis [23].
The crosstalk between the oocyte and somatic follicular cells occurs mainly by gap junctions established between the follicular somatic cells and between the oocyte and CCs [12]. Proteins forming the gap junction are connexins (Cx). Cx43 and Cx45 are involved in GC connections, while Cx37 and Cx43 are involved in oocyte and CC communication. It has been shown that the different types of gap junctions in crosstalk between the oocyte and CCs have different permeability properties and are thus able to transfer specific signals and molecules [18]. Follicular fluid (FF), accumulated inside the antral follicle, represents an additional means of communication between oocyte and somatic cells.
FF consists of a complex mixture of nucleic acids, proteins, metabolites, and ions, which are secreted by the oocyte, granulose, and theca cells, combined with plasma components that cross the blood–follicular barrier via thecal capillaries [24, 25]. It represents a very important microenvironment for the development of the oocyte and its biochemical composition reflects the physiological status of the follicle. It is common opinion that the analysis of FF components may provide useful information on oocyte quality [24]. In fact, hormones, growth factors, cytokines, and chemokines secreted by follicular cells in FF are able to promote oocyte maturation and alteration of the biochemical composition of FF is related to low quality oocytes. Evaluation of the specific components within FF will help us better understand intra-follicular signaling, as well as, reveal potential biomarkers of oocyte health for women undergoing assisted reproductive treatment (ART).
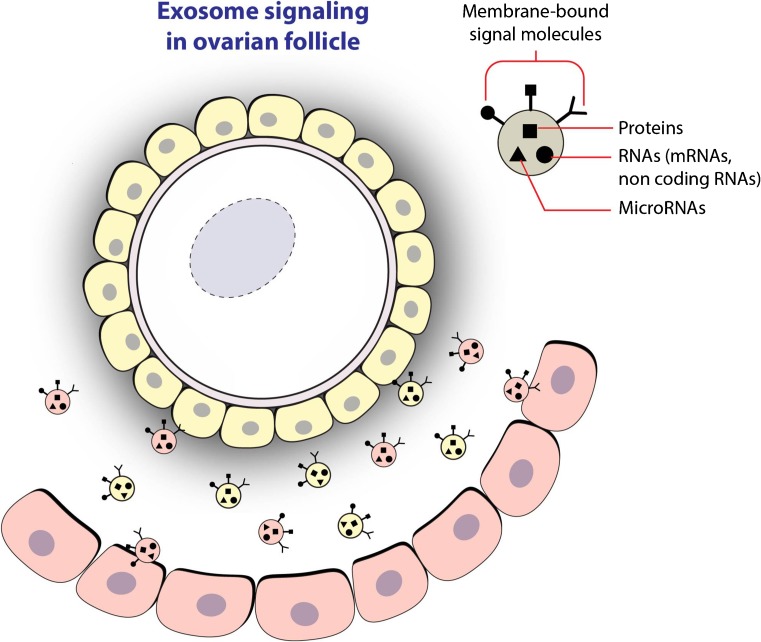
Among the different mechanisms of autocrine and paracrine communication inside the ovarian follicle, an alternative mechanism has recently come to light. By this mechanism, the oocyte and somatic follicular cells could interchange signals, as well as, nutrients. In fact, it has been demonstrated and reported in various papers that microvesicles and exosome carrying miRNAs are in human FF and are also in animal models [26–33] (Fig. 1, Table 1).
Fig. 1.
Exosomes in ovarian follicle. shows the possible exchange of microRNAs, long non-coding RNAs, and proteins among different follicular cells by follicular fluid exosomes
Table 1.
Timetable on identification of microRNAs free and extracellular vesicle cargo in Follicular Fluid
| Publication date | Model | Methods | References | ||
|---|---|---|---|---|---|
| Exosomes | MicroRNA analysis | ||||
| Purification | Characterization | ||||
| March 2012 | Equine | ExoQuick | Electron microscopy–flow cytometry | MicroRNA profiling by real time RT-PCR | [26] |
| July 2013 | Human | None | Deep sequencing and TaqMan miRNA arrays | [42] | |
| July 2013 | Equine | None | Single assays by real time RT-PCR | [43] | |
| October 2013 | Equine | None | Single assays by real time RT-PCR | [44] | |
| November 2013 | Bovine | Exoquick | Electron microscopy–Western blot | MicroRNA profiling by real time RT-PCR | [27] |
| March 2014 | Human | None | Microarray profiling and single assays by real time RT-PCR | [45] | |
| May 2014 | Equine | ExoQuick | None | Single assays by real time RT-PCR | [28] |
| June 2014 | Human | Differential ultracentrifugation | Electron microscopy | microRNA profiling by real time RT-PCR | [29] |
| June 2014 | Human | None | Single assays by real time RT-PCR | [46] | |
| November 2014 | Human | Differential ultracentrifugation | Nanosight– flow cytometry |
MicroRNA profiling by real time RT-PCR | [30] |
| March 2015 | Human and mouse | None | Single assays by real time RT-PCR | [47] | |
| March 2015 | Equine | Exoquick | None | Single assays by real time RT-PCR | [31] |
| May 2015 | Equine | Exoquick | None | Single assays by real time RT-PCR | [32] |
| October 2015 | Human | None | Microarray single assays by real time RT-PCR |
[48] | |
The most significant results of cited papers have been reported in the text
Exosomes: biogenesis, secretion, and targeting
Exosomes originate from MVBs, late endosome-derived cell compartments, which bud off parts of their limiting membrane into their lumen forming intraluminal vesicles. MVBs can either fuse with the lysosome for degradation or with the plasma membrane to release exosomes into the extracellular space [34]. The first evidence of exosome secretion was demonstrated in reticulocytes undergoing maturation into red blood cells and subsequently was described in immune cells where it was shown that exosomes are able to induce antigen presentation [35, 36]. In 2007, Valadi et al. demonstrated that exosomes contain both mRNAs and miRNAs, which can be delivered to another cell and can be functional in this new location. They proposed to call this RNA “exosomal shuttle RNA” [3].
Exosome biogenesis and secretion are multifaceted mechanisms and are different depending on the cell type and cargo sequestered. Exosomes can be formed via at least two distinct pathways. The first pathway utilizes the endosomal sorting complex required for transport (ESCRT) machinery, and the second pathway, independent from ESCRT complexes, involves lipids or tetraspanins [1, 37, 38].
As already mentioned, exosomes are able to transfer macromolecules to target cells, but the mechanisms that select the correct cargo have not yet been characterized. However, it has been demonstrated that the cells are able to modify the number and the cargo of exosomes depending on specific conditions [4]. Moreover, it has been shown that there is a specific repertoire of miRNAs selectively exported to exosomes, whereas others are usually excluded, which indicates that an active sorting mechanism occurs at the RNA level [38]. In a recent review, the authors described some potential modes by which miRNAs can be selected. For example, they suggested that specific nucleotide sequences may be recognized by enzymes or proteins and may guide miRNA incorporation into exosomes and that, alternately, protein complexes may control sorting in a miRNA sequence-independent fashion [39]. For example, the sumoylated heterogeneous nuclear ribonucleoprotein A2B1 recognizes the GGAG motif in the 3′ portion of the miRNA sequence and guides specific miRNAs to be packed into exosomes. Argonaute 2 (AGO 2), one of the components of the miRNA-induced silencing complex (miRISC), could be related to exosomal miRNA sorting. In fact, AGO2 has been identified in exosomes, and the knockout of it could decrease the types or abundance of the preferentially exported miRNAs in human embryonic kidney 293 cell-derived exosomes [39]. A comprehensive list of miRNAs, proteins, and lipids, found incorporated into exosomes and also in other EVs is available in the miRandola (www.atlas.dmi.unict.it/mirandola/index.html) and other databases, such as, ExoCarta (http://www.exocarta.org), EVpedia (http://evpedia.info) or Vesiclepedia (http://www.microvesicles.org/).
Once produced, exosomes can be secreted constitutively (dendritic cells and epithelial cells) or, alternatively, their secretions could be regulated (mast cells and T cells). For example, in neuronal cells, exosome secretion seems to be induced after stimulation in a Ca2+-dependent manner and secretion can depend on p53 activation in cells undergoing stress [40, 41]. In fact, the p53 response to stress includes not only the production of secreted protein mediators but also the production of exosomes, which can communicate with neighboring cells to influence their behavior [40, 41]. In the extracellular space, exosome interaction with recipient cells can take place by ligand-receptor interactions, by fusion, or by internalization via receptor-mediated endocytosis [34]. Therefore, exosomes can act as paracrine or autocrine mediators and also as endocrine mediators that, through the blood stream, are able to target cells located at distant sites in the body [13].
Exosome signaling in the ovarian follicle
Ovarian follicles represent a very good model to study exosomes and their cargo. First of all, these studies would allow for a better understanding of complex pathways, which regulate follicle maturation and are involved in the production of a fully competent oocyte. With respect to blood, it is extremely difficult to pinpoint the cells producing exosomes and to identify the recipient cells. Ovarian follicles represent a more finite system where it could be easier to understand the source of exosomes, their target cells, and the function. Furthermore, several papers described the role of ovarian miRNAs in biology and in diseases, and a comprehensive review on the subject has recently been published [15].
Nevertheless, today, there are still many points to be explored. In a recent paper, the authors isolated exosomes from human FF by ultracentrifugation and characterized by Nanosight, flow cytometry, and surface markers. They compared upregulated exosomal miRNAs to plasma from the same women [30]. This comparison identified miRNAs specifically synthesized by ovarian follicular cells, excluding those derived from blood. By computational analysis, it was demonstrated that some miRNAs could perform important roles inside the ovarian follicle. Specifically, it was found that miR99a, miR100, miR132, and miR218 could be involved in follicle maturation; miR132, miR212, and miR214 could be able to trigger meiosis resumption by negatively regulating genes encoded for follicle maturation-inhibiting factors and miR29a could be involved in epigenetic modifications [30]. At the same time, other researchers demonstrated the presence of miRNA exosome or microvesicle cargo in human and animal FF (Table 1). In 2012, da Silveira et al., for the first time, described the presence of exosomes and microvesicles containing miRNAs and proteins in equine FF and, more recently, they discussed the role of hormonal-regulated miRNAs within different ovarian follicular cells [26, 31]. In 2013, Sohel et al. separated microvesicles from exosomes in bovine FF by differential ultracentrifugation and ExoQuick precipitation and demonstrated that both are able to transport miRNAs [27]. Moreover, they demonstrated that oocyte growth is affected by specific miRNA expression profiles [27]. Finally, it has recently been demonstrated that microvesicles and exosomes from bovine FF are able to support cumulus expansion [33]. Since 2012, various papers have focused their attention on miRNAs purified from FF in toto (Table 1) [42–48]. They described possible roles of miRNAs in follicle maturation and also explained that an altered expression of miRNAs could be involved in human reproductive diseases. It has been shown that miR132 and miR320 can regulate in vitro steroidogenesis in KGN cell lines and that their altered regulation may be involved in polycystic ovary syndrome (PCOS) [42, 45]. In 2014, a review about the possible role of miRNAs present in ovarian follicle cells in PCOS was published [49]. In addition, it has been demonstrated that different expression levels of miR320 in FF can influence embryo quality and that variation of miRNA profiles in FF and granulose cells are related to aging and oocyte maturation stage [47, 48].
Despite all of this, to date, our knowledge about intercellular communication mediated by exosomes or microvesicles inside the ovarian follicle is limited and many questions remain unanswered. We do not know which macromolecules, in addition to miRNAs, are carried by FF exosomes. In other cellular models, it has been demonstrated that exosomes are able to transfer not only miRNAs but also mRNAs, long ncRNAs, mtDNA, and proteins [1, 4, 34, 50, 51]. It would be interesting to completely characterize the FF exosome cargo because it could provide information on exosome biogenesis, targeting, and cellular effects and may be a source of biomarkers for oocyte quality, reproductive disease diagnosis, or response to hormonal treatment. We know that FF contains different molecules produced by the oocyte and by somatic cells and that there is a close association between FF composition and oocyte quality. The identification and evaluation of FF exosome cargo could allow for the selection of molecules involved in cellular communication and could allow for the possible contaminants derived from lysis or cell death to be avoided. The regulation of biological pathways by ncRNAs represents one of the most important subjects in recent literature [9]. It has been shown that the human oocyte can synthesize three classes of small ncRNAs, which are piRNAs, miRNAs, and endo-siRNAs, but their biological role is controversial and poorly investigated [52]. The isolation and characterization of some of these important regulatory molecules from FF exosomes could allow us to clarify some unexplored aspects of ovarian physiology.
According to proteomic studies, exosomal proteins include both conserved proteins, which are identified in almost all exosomes no matter what their origin, and cell-type-specific proteins [4]. It has been shown that different cytokines, lacking an N-terminal signal peptide, can be secreted by exosomes [53]. It has long been known that cytokines are involved in ovarian physiology regulating cellular proliferation/differentiation, follicular survival/atresia, and oocyte maturation [54, 55]. Cytokines have been considered as attractive biomarkers of oocyte maturational status and of successful assisted reproductive outcome. However, our understanding of cytokines and their interactions remains incomplete, so it would be interesting to evaluate the presence of cytokines in FF exosomes.
Other proteomic studies demonstrated that exosomes contain proteins involved in cellular signaling pathways, such as the Wnt family. Specifically, it has been shown that there is an evolutionarily conserved functional role (present in Drosophila and in human cells) of extracellular vesicular transport of Wnt proteins [56]. Exosomes carry Wnts on their surface to induce Wnt signaling activity in target cells [56]. It has been amply demonstrated that Wnts and Frizzled (Fz) receptors are expressed at specific stages of follicular growth and luteinization and are important for the growth and development of ovarian follicles [57].
Other important areas that should be explored regarding FF exosomes include FF exosomes produced by somatic follicular cells or by the oocyte or both and what are the target cells. Comparing the miRNome of the oocyte and FF, we found that some miRNAs are shared between the oocyte and FF while others are exclusively present inside the oocyte [unpublished data]. It is possible to speculate that miRNAs, exclusively present in the MII oocyte, could be used during the first phases of embryo development. Intriguingly, we found miR371, a miR involved in pluripotency and in reprogramming are highly expressed in the human MII oocyte [unpublished data, 58]. On the contrary, FF miRNAs, produced by somatic follicular cells or by the oocyte, should explicate their role during follicular maturation.
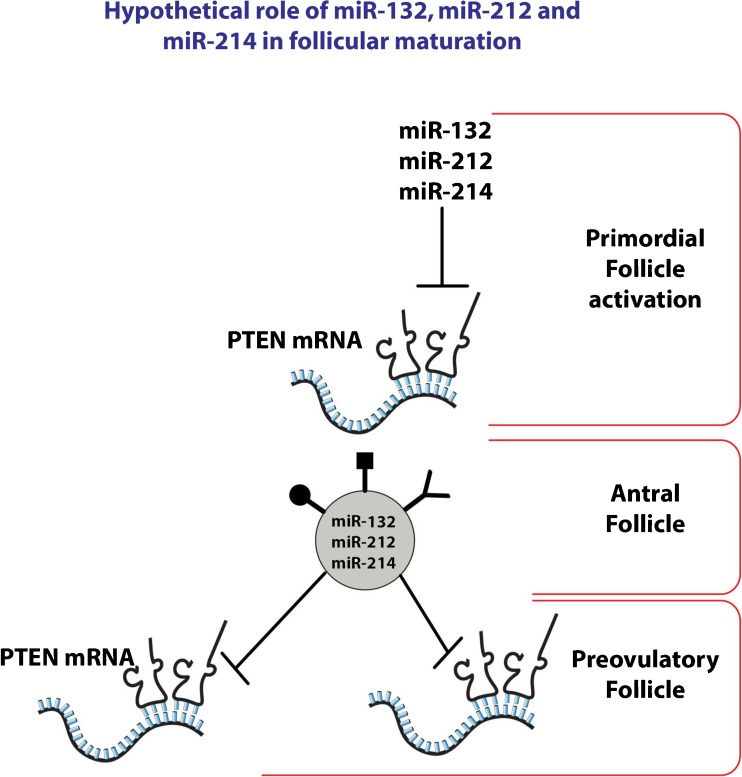
The last point that has long been debated in the literature concerning research on cell–cell communication by EVs is whether the secretory molecules are really physiologically functional or if, at least sometimes, these could represent a suitable mechanism to eliminate unnecessary material [14]. It was recently reported that CD9 and CD82 tetraspanins are able to regulate Wnt signaling, which reduced the intracellular pool of β-catenin. This reduction is due to the increase of exosome-associated transport of β-catenin outside the cell [59]. This finding has suggested that exosomal packaging and release of cytosolic proteins could represent a new way to downregulate the activity of intracellular signaling pathways [59]. Interestingly, miR132, miR212, miR214, highly expressed in FF exosomes, are able to target PTEN [30]. PTEN silencing (possibly mediated by FF miRNAs) and the resulting AKT activation, switch on the phosphatidylinositol 3-kinase (PI3K) signaling pathway. The PI3K pathway is involved in different steps of follicular maturation. In fact, it controls primordial follicle survival and activation, regulates cyclic follicular recruitment, causes ovulation in granulose cells, and stimulates meiosis resumption in the oocyte [60]. It is possible to speculate that miRNAs targeting PTEN could be initially used by the oocyte to activate the primordial follicle, which could subsequently be sequestered by exosomes, sent to FF and, in a time-dependent manner, could be able to induce the PI3K pathway in GCs and in the oocyte (Fig. 2).
Fig. 2.
Hypothetical role of miR132, miR212, and miR214 in follicular maturation. miRNAs targeting PTEN could be initially used by the oocyte to activate the primordial follicle, which could subsequently be sequestered by exosomes and sent to FF and then, in a time-dependent manner, could be able to induce the PI3K pathway in GCs and in the oocyte
Conclusion
The discovery of EVs circulating in biological fluids and the ability to carry specific signals to the other cells raised the increasing attention of the scientific community, especially for the important clinical application of early diagnosis and therapies based on drug delivery. Important results have been obtained by studying different types of cancer and degenerative diseases even though it will be necessary to perform further studies to clarify some points before clinical application. It is vital to have more accurate methods available to discriminate the different subpopulations of EVs and to verify their specific cargo. Moreover, we need greater knowledge about the mechanisms that allow for the correct selection of the cargo and about the recognition signals between vesicles and target cells.
With regard to the EVs in the ovarian follicle, our knowledge is limited and more effort is needed to shed light on the still numerous open questions. We do not identify the cells of ovarian follicles that are able to produce and release vesicles and the cells are able to uptake them. We do not know which molecules, in addition to miRNAs (proteins, long ncRNAs, and DNA), are present inside FF EVs. With regard to their role in folliculogenesis and their involvement in reproductive pathological conditions, any data will have to be validated by future studies, possibly by using follicular in vitro maturation systems. In fact, the ovarian follicle is the fundamental reproductive unit and studies focused on the different isolated follicular components (CCs, GCs, oocyte, FF) are able to provide only partial information. Nevertheless, in the field of reproductive biology, the potential of these new studies in basic, clinical, and biotechnological research appears to have been accepted [61]. The possibility of clinical applications is extremely interesting. For basic researchers, the final goal is to clarify the unknown points within complex pathways regulating follicular growth that create a healthy oocyte able to generate a new life.
Acknowledgments
The author wishes to thank M Vento and M Purrello with whom she shares her research work. She wishes to thank R Battaglia and the lab staff for their contribution and the Scientific Bureau of the University of Catania for language support.
Compliance with ethical standards
Conflict of interest
The author declares that she has no competing interests.
Footnotes
Capsule This review summarizes the current state of knowledge about extracellular vesicles in follicular fluid and elaborates on their their potential roles in follicular differentiation and oocyte maturation.
References
- 1.Yáñez-Mó M, Siljander PR, Andreu Z, Zavec AB, Borràs FE, Buzas EI, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015 doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee Y, El Andaloussi S, Wood MJ. Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum Mol Genet. 2012;21(R1):R125–34. doi: 10.1093/hmg/dds317. [DOI] [PubMed] [Google Scholar]
- 3.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 4.Lo Cicero A, Stahl PD, Raposo G. Extracellular vesicles shuffling intercellular messages: for good or for bad. Curr Opin Cell Biol. 2015 doi: 10.1016/j.ceb.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Corrado C, Raimondo S, Chiesi A, Ciccia F, De Leo G, Alessandro R. Exosomes as intercellular signaling organelles involved in health and disease: basic science and clinical applications. Int J Mol Sc. 2013 doi: 10.3390/ijms14035338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ragusa M, Barbagallo D, Purrello M. Exosomes: nanoshuttles to the future of BioMedicine. Cell Cycle. 2015 doi: 10.1080/15384101.2015.1006535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antonyak MA, Cerione RA. Emerging picture of the distinct traits and functions of microvesicles and exosomes. Proc Natl Acad Sci U S A. 2015;112(12):3589–90. doi: 10.1073/pnas.1502590112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanada M, Bachmann MH, Hardy JW, Frimannson DO, Bronsart L, Wang A, et al. Contag CH Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proc Natl AcadSci U S A. 2015;112(12):E1433–42. doi: 10.1073/pnas.1418401112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu XD. Non-coding RNA: a new frontier in regulatory biology. Natl Sci Rev. 2014;1:190–204. doi: 10.1093/nsr/nwu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 11.Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10:704–14. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, Lee MJ, et al. The microRNA spectrum in 12 body fluids. Clin Chem. 2010;56:1733–41. doi: 10.1373/clinchem.2010.147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cortez MA, Bueso-Ramos C, Ferdin J. Lopez-Berestein G. CalinGA. MicroRNAs in body fluids--the mix of hormones and biomarkers. Nat Rev ClinOncol: Sood AK; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kosaka N, Yoshioka Y, Hagiwara K, Tominaga N, Katsuda T, Ochiya T. Trash or treasure: extracellular microRNAs and cell-to-cell communication. Front Genet. 2013 doi: 10.3389/fgene.2013.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGinnis LK, Luense LJ, Christenson LK. MicroRNA in ovarian biology and disease. Cold Spring HarbPerspect Med. 2015 doi: 10.1101/cshperspect.a022962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Fang Y, Liu Y, Yang X. MicroRNAs in ovarian function and disorders. J Ovarian Res. 2015;8:51. doi: 10.1186/s13048-015-0162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hutt KJ, Albertini DF. An oocentric view of folliculogenesis and embryogenesis. Reprod Biomed Online. 2007;14:758–64. doi: 10.1016/S1472-6483(10)60679-7. [DOI] [PubMed] [Google Scholar]
- 18.Zuccotti M, Merico V, Cecconi S, Redi CA, Garagna S. What does it take to make a developmentally competent mammalian egg? Hum Reprod Update. 2011 doi: 10.1093/humupd/dmr009. [DOI] [PubMed] [Google Scholar]
- 19.Sun QY, Miao YL, Schatten H. Towards a new understanding on the regulation of mammalian oocyte meiosis resumption. Cell Cycle. 2009;8:2741–7. doi: 10.4161/cc.8.17.9471. [DOI] [PubMed] [Google Scholar]
- 20.Reddy P, Zheng W, Liu K. Mechanisms maintaining the dormancy and survival of mammalian primordial follicles. Trends Endocrinol Metab. 2010 doi: 10.1016/j.tem.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Fragouli E, Lalioti MD, Wells D. The transcriptome of follicular cells: biological insights and clinical implications for the treatment of infertility. Hum Reprod Update. 2014 doi: 10.1093/humupd/dmt044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zamah AM, Hsieh M, Chen J, Vigne JL, Rosen MP, Cedars MI, et al. Human oocyte maturation is dependent on LH-stimulated accumulation of the epidermal growth factor-like growth factor, amphiregulin. Hum Reprod. 2010 doi: 10.1093/humrep/deq212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russell DL, Robker RL. Molecular mechanisms of ovulation: co-ordination through the cumulus complex. Hum Reprod Update. 2007;13:289–312. doi: 10.1093/humupd/dml062. [DOI] [PubMed] [Google Scholar]
- 24.Revelli A, Delle Piane L, Casano S, Molinari E, Massobrio M, Rinaudo P. Follicular fluid content and oocyte quality: from single biochemical markers to metabolomics. Reprod Biol Endocrinol. 2009 doi: 10.1186/1477-7827-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodgers RJ, Irving-Rodgers HF. Formation of the ovarian follicular antrum and follicular fluid. Biol Reprod. 2010;82:1021–9. doi: 10.1095/biolreprod.109.082941. [DOI] [PubMed] [Google Scholar]
- 26.da Silveira JC, Veeramachaneni DN, Winger QA, Carnevale EM, Bouma GJ. Cell-secreted vesicles in equine ovarian follicular fluid contain miRNAs and proteins: a possible new form of cell communication within the ovarian follicle. Biol Reprod. 2012 doi: 10.1095/biolreprod.111.093252. [DOI] [PubMed] [Google Scholar]
- 27.Sohel MM, Hoelker M, Noferesti SS, Salilew-Wondim D, Tholen E, Looft C, et al. Exosomal and non-exosomal transport of extra-cellular microRNAs in follicular fluid: implications for bovine oocyte developmental competence. PLoS One. 2013 doi: 10.1371/journal.pone.0078505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.da Silveira JC, Carnevale EM, Winger QA, Bouma GJ. Regulation of ACVR1 and ID2 by cell-secreted exosomes during follicle maturation in the mare. Reprod Biol Endocrinol. 2014 doi: 10.1186/1477-7827-12-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diez-Fraile A, Lammens T, Tilleman K, Witkowski W, Verhasselt B, De Sutter P, et al. Age-associated differential microRNA levels in human follicular fluid reveal pathways potentially determining fertility and success of in vitro fertilization. Hum Fertil (Camb) 2014 doi: 10.3109/14647273.2014.897006. [DOI] [PubMed] [Google Scholar]
- 30.Santonocito M, Vento M, Guglielmino MR, Battaglia R, Wahlgren J, Ragusa M, et al. Molecular characterization of exosomes and their microRNA cargo in human follicular fluid: bioinformatic analysis reveals that exosomal microRNAs control pathways involved in follicular maturation. Fertil Steril. 2014 doi: 10.1016/j.fertnstert.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 31.da Silveira JC, de Andrade GM, Nogueira MF, Meirelles FV, Perecin F. Involvement of miRNAs and cell-secreted vesicles in mammalian ovarian antral follicle development. Reprod Sci. 2015 doi: 10.1177/1933719115574344. [DOI] [PubMed] [Google Scholar]
- 32.da Silveira JC, Winger QA, Bouma GJ, Carnevale EM. Effects of age on follicular fluid exosomal microRNAs and granulosa cell transforming growth factor-? signalling during follicle development in the mare. Reprod Fertil Dev. 2015 doi: 10.1071/RD14452. [DOI] [PubMed] [Google Scholar]
- 33.Hung WT, Hong X, Christenson LK, McGinnis LK. Extracellular vesicles from bovine follicular fluid support cumulus expansion. Biol Reprod. 2015 . pii: biolreprod.115.132977. [DOI] [PMC free article] [PubMed]
- 34.Urbanelli L, Magini A, Buratta S, Brozzi A, Sagini K, Polchi A, et al. Signaling pathways in exosomes biogenesis. Secretion and Fate Genes. 2013 doi: 10.3390/genes4020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J Biol Chem. 1987;262:9412–20. [PubMed] [Google Scholar]
- 36.Raposo G. NijmanHW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, GeuzeHJ.B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–72. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2088;319:1244–7. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 38.Van Niel G, Charrin S, Simoes S, Romao M, Rochin L, Saftig P, et al. The tetraspaninCD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev Cell. 2011;21:708–21. doi: 10.1016/j.devcel.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang J, Li S, Li L, Li M, Guo C, Yao J, et al. Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinforma. 2015;13(1):17–24. doi: 10.1016/j.gpb.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH, et al. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosc. 2010 doi: 10.1523/JNEUROSCI.5699-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu X, Harris SL, Levine AJ. The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res. 2006;66(9):4795–801. doi: 10.1158/0008-5472.CAN-05-4579. [DOI] [PubMed] [Google Scholar]
- 42.Sang Q, Yao Z, Wang H, Feng R, Wang H, Zhao X, et al. Identification of microRNAs in human follicular fluid: characterization of microRNAs that govern steroidogenesis in vitro and are associated with polycystic ovary syndrome in vivo. J Clin Endocrinol Metab. 2013 doi: 10.1210/jc.2013-1715. [DOI] [PubMed] [Google Scholar]
- 43.Schauer SN, Sontakke SD, Watson ED, Esteves CL, Donadeu FX. Involvement of miRNAs in equine follicle development. Reproduction. 2013 doi: 10.1530/REP-13-0107. [DOI] [PubMed] [Google Scholar]
- 44.Donadeu FX. SchauerSN. Domest Anim Endocrinol: Differential miRNA expression between equine ovulatory and anovulatory follicles; 2013. [DOI] [PubMed] [Google Scholar]
- 45.Roth LW, Mc Callie B, Alvero R, Schoolcraft WB, Minjarez D, Katz-Jaffe MG. Altered microRNA and gene expression in the follicular fluid of women with polycystic ovary syndrome. J Assist Reprod Genet. 2014 doi: 10.1007/s10815-013-0161-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yin M, Wang X, Yao G, Lü M, Liang M, Sun Y, et al. Transactivation of micrornA-320 by microRNA-383 regulates granulosa cell functions by targeting E2F1 and SF-1 proteins. J Biol Chem. 2014 doi: 10.1074/jbc.M113.546044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feng R, Sang Q, Zhu Y, Fu W, Liu M, Xu Y, et al. MiRNA-320 in the human follicular fluid is associated with embryo quality in vivo and affects mouse embryonic development in vitro. Sci Rep. 2015 doi: 10.1038/srep08689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moreno JM, Núñez MJ, Quiñonero A, Martínez S, de la Orden M, Simón C, et al. Follicular fluid and mural granulosa cells microRNA profiles vary in in vitro fertilization patients depending on their age and oocyte maturation stage. Fertil Steril. 2015 doi: 10.1016/j.fertnstert.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 49.Sørensen AE, Wissing ML, Salö S, Englund AL, Dalgaard LT. MicroRNAs related to polycystic ovary syndrome (PCOS) Genes (Basel) 2014 doi: 10.3390/genes5030684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Q, Shao Y, Zhang X, Zheng T, Miao M, Qin L, et al. Plasma long noncoding RNA protected by exosomes as a potential stable biomarker for gastric cancer. Tumour Biol. 2015 doi: 10.1007/s13277-014-2807-y. [DOI] [PubMed] [Google Scholar]
- 51.Falcieri E, Battistin L, Agnati LF, Stocchi V. C2C12 myoblasts release micro-vesicles containing mtDNA and proteins involved in signal transduction. Exp Cell Res. 2010;316:1977–84. doi: 10.1016/j.yexcr.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 52.Watanabe T, Totoki Y, Toyoda A, Kaneda M, Kuramochi-Miyagawa S, Obata Y, et al. Endogenous siRNAs from naturally formed dsRNAs regulate transcripts in mouse oocytes. Nature. 2008 doi: 10.1038/nature06908. [DOI] [PubMed] [Google Scholar]
- 53.Kandere-Grzybowska K, Letourneau R, Kempuraj D, Donelan J, Poplawski S, Boucher W, et al. IL-1 induces vesicular secretion of IL-6 without degranulation from human mast cells. J Immunol. 2003;171:4830–6. doi: 10.4049/jimmunol.171.9.4830. [DOI] [PubMed] [Google Scholar]
- 54.Zolti M, Ben-Rafael Z, Meirom R, Shemesh M, Bider D, Mashiach S, et al. Cytokine involvement in oocytes and early embryos. Fertil Steril. 1991;56:265–72. doi: 10.1016/s0015-0282(16)54483-5. [DOI] [PubMed] [Google Scholar]
- 55.Field SL, Dasgupta T, Cummings M, Orsi NM. Cytokines in ovarian folliculogenesis, oocyte maturation and luteinisation. Mol Reprod Dev. 2014. [DOI] [PubMed]
- 56.Gross JC, Chaudhary V, Bartscherer K, Boutros M. Active Wnt proteins are secreted on exosomes. Nat Cell Biol. 2012;14:1036–45. doi: 10.1038/ncb2574. [DOI] [PubMed] [Google Scholar]
- 57.Richards JS, Russell DL, Ochsner S, Hsieh M, Doyle KH, Falender AE, et al. Novel signaling pathways that control ovarian follicular development, ovulation, and luteinization. Recent Prog Horm Res. 2002;57:195–220. doi: 10.1210/rp.57.1.195. [DOI] [PubMed] [Google Scholar]
- 58.Lichner Z, Páll E, Kerekes A, Pállinger E, Maraghechi P, Bosze Z, et al. The miR-290-295 cluster promotes pluripotency maintenance by regulating cell cycle phase distribution in mouse embryonic stem cells. Differentiation. 2011 doi: 10.1016/j.diff.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 59.Chairoungdua A, Smith DL, Pochard P, Hull M, Caplan MJ. Exosome release of beta-catenin: a novel mechanism that antagonizes Wnt signaling. J. Cell Biol. 2010;190:1079–91. doi: 10.1083/jcb.201002049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zheng W, Nagaraju G, Liu Z, Liu K. Functional roles of the phosphatidylinositol 3-kinases (PI3Ks) signaling in the mammalian ovary. Mol Cell Endocrinol. 2012 doi: 10.1016/j.mce.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 61.Barkalina N, Jones C, Wood MJ, Coward K. Extracellular vesicle-mediated delivery of molecular compounds into gametes and embryos: learning from nature. Hum Reprod Update. 2015 doi: 10.1093/humupd/dmv027. [DOI] [PubMed] [Google Scholar]