Abstract
Purpose
The aim of this study was to establish a simple tool to predict good-quality embryos in in vitro fertilization (IVF) by using cumulus cells (CCs) or peripheral blood cells (PBCs).
Methods
Mitochondrial DNA was extracted from CCs and PBCs in patients undergoing IVF. Using real-time polymerase chain reaction, mtDNA copy number in a single cell was calculated. Embryo quality was assessed when it was transferred or frozen.
Results
CCs were obtained from 60 oocyte cumulus-cell complexes (OCCCs) in 30 women, and PBCs were collected from 18 women. For the 30 women in the study, the median age was 37 years old (range, 24–43), and the mean body mass index was 21.4 (standard error, 2.0). mtDNA content of CCs and PBCs was highly correlated (Pearson’s r = 0.900, p < 0.0001). The median mtDNA content of CCs for good- and poor-quality embryos was 140 and 57, respectively (p < 0.0001). The median mtDNA content of PBCs for good- and poor-quality embryos was 36 and 13, respectively (p = 0.604). The logistic regression model indicated that mtDNA content in CCs was the only parameter that predicted good-quality embryos (p = 0.020). The receiver operating characteristic curve for obtaining good-quality embryos by mtDNA copy number in CCs had an area under the curve of 0.823, and using a threshold of 86, positive and negative predictive values were 84.4 and 82.1 %, respectively.
Conclusions
The determination of mtDNA content in CCs can be used to predict good-quality embryos.
Keywords: Mitochondrial DNA, Cumulus cells, Blood, In vitro fertilization
Introduction
The average maternal age has increased in developed countries during the last 30 years, and infertility rates have increased [1]. Despite advances in assisted reproductive technology, unresolved issues remain. The most crucial limiting factor determining the outcome of assisted reproduction is poor-quality oocytes due to ovarian aging in patients seeking infertility treatment. Previous studies have demonstrated that oocyte age is associated with mitochondrial dysfunction [2–4]. Mitochondria are maternally inherited organelles that serve as the cell’s powerhouses, using oxidative phosphorylation to supply ATP. Reproductive age is associated with a decrease in the amount of mitochondrial DNA (mtDNA) [5, 6], and decreased mtDNA content leads to poor-quality oocytes and negative fertilization outcomes [7]. However, oocytes themselves cannot be used to predict fertilization outcome owing to ethical concerns.
Cumulus cells (CCs) play a role in oocyte growth and maturation by transmitting signals to the ovary [8, 9], but little is known about the relationship between their mitochondrial status and fertilization outcome. Bonomi et al. reported a relationship between mtDNA copy number of CCs and peripheral blood cells (PBCs) in patients with primary ovarian insufficiency (POI) [10]. Therefore, we conducted a prospective study to identify the relationship between mtDNA content of CCs and PBCs in patients undergoing in vitro fertilization (IVF) or intra-cytoplasmic sperm injection (ICSI) and to develop a non-invasive embryo assessment tool using CCs and PBCs. The outcome of this study was assessed by gross embryo morphology [11, 12].
Materials and methods
This prospective study was reviewed and approved by the institutional review board of Hyogo College of Medicine and the Advanced Fertility Center of Fuchu Nozomi (No. 210, approved August 2013). All subjects gave their written informed consent for CCs and/or blood sampling and genetic analysis.
Embryo assessment
Conventional IVF procedures were performed using previously described routine methods [13]. Embryo assessment was performed on days 3 and 5 for all study groups according to previously published criteria, including cell number, fragmentation, and morphological aspects. Briefly, embryos with 6–8 cells with <20 % fragmentation on day 3 or blastocysts ≧3BB were regarded as good-quality embryos [14] and were transferred or frozen.
Biological materials
All women experiencing unexplained, endometriosis-associated, or tubal factor-related infertility and undergoing IVF at Hyogo College of Medicine or at the Advanced Fertility Center of Fuchu Nozomi between June 2013 and September 2014 were recruited. In subjects undergoing IVF, CCs were removed from oocyte cumulus-cell complexes (OCCCs) using fine needles. In subjects undergoing ICSI, CCs were stripped with hyaluronidase. Collected CCs were frozen at −80 °C for subsequent DNA analysis. Whole peripheral blood cells (PBCs) were collected in EDTA-containing tubes within a day before oocyte retrieval for the determination of mtDNA content. The serum level of estradiol was measured within a day of human chorionic gonadotropin injection for determining the luteinizing hormone (LH) surge. The serum levels of follicle-stimulating hormone (FSH), LH, and anti-Mullerian hormone (AMH) were measured within 7 days after initiation of menstruation.
DNA extraction and measurement of mtDNA copy number
Total DNA from CCs was isolated by using the AllPrep DNA/RNA Micro Kit (Qiagen, US). Total blood DNA was isolated by using the AllPrep DNA/RNA/miRNA Universal Kit (Qiagen, US). CC DNA and blood DNA were prepared at 2 and 10 ng, respectively. mtDNA copy number per single cell was calculated by measuring the total copy number for mtDNA and the control gene RPPH1. Specific probes were used to amplify a fragment of the mitochondrial D-loop region, Hs02596861_s1 MT-7S, and a fragment of RPPH1, Hs03297761_s1 (Applied Biosystems, US).
Real-time PCR
The copy number for mtDNA and RPPH1 in each sample of CCs and PBCs was quantified using real-time polymerase chain reaction according to the standard conditions for Taqman (SDS-7900HT; Applied Biosystems). Standard curves were based on purified plasmid DNA corresponding to Hs02596861_s1 MT-7S and Hs03297761_s1 (Applied Biosystems).
The array is shown below.
Hs02596861_s1 (human mitochondrial MT-7S), NC_012920.1
AAGTGTGTTAATTAATTAATGCTTGTAGGACATAATAATAACAATTGAATGTCTGCACAGCCACTTTCCACACAGACATCATAACAAAAAATTTCCACCAAACCCCCCCTCCCCCGCTTCTGGCCACAGCACTTAAACACATCTCTGCCAAACCCCAAAAACAAAGAACCCTAACACCAGCCTAACCAGATTTCAAATTTTATCTTTTGGCGGTAGCACTTTTAACAGTCACCCCCCAACTAACACATTATTTTCCCCTCCCACTCCCATACTACTAATCTCATCAATACAACCCCCGC
Hs03297761_s1 (human RPPH1), NR_002312.1
ATAGGGCGGAGGGAAGCTCATCAGTGGGGCCACGAGCTGAGTGCGTCCTGTCACTCCACTCCCATGTCCCTTGGGAAGGTCTGAGACTAGGGCCAGAGGCGGCCCTAACAGGGCTCTCCCTGAGCTTCGGGGAGGTGAGTTCCCAGAGAACGGGGCTCCGCGCGAGGTCAGACTGGGCAGGAGATGCCGTGGACCCCGCCCTTCGGGGAGGGGCCCGGCGGATGCCTCCTTTGCCGGAGCTTGGAACAGACTCACGGCCAGCGAAGTGAGTTCAATGGCTGAGGTGAGGTACCCCGCAGGGGACCTCATAACCCAATTCAGACTACTCTCCTCCGCCCATT
Statistical analysis
Correlations were estimated using Pearson’s correlation coefficients. Normal distributions are reported as means and the standard error of the mean. Non-normal distributions are reported as medians and the 25–75 % quartile. A measure of the central tendencies of two groups of non-normal distributions was conducted using the Mann-Whitney test. Multivariate analysis was conducted using a logistic regression model. A p value <0.05 was considered significant. Receiver operating characteristic (ROC) curves were analyzed to predict good-quality embryos. ROC curves were derived by plotting the relationship between the specificity and sensitivity at various cut-off levels. The accuracy of mtDNA copy number of CCs or PBCs as a diagnostic tool to separate good-quality embryos from bad-quality embryos was measured using the area under the ROC curve (AUC). When the AUC was over 0.80, the accuracy was considered to be good. All the analyses were performed using XLSTAT 2014 (Addinsoft, Paris, France).
Results
Cumulus cells were obtained from 60 OCCCs during an IVF procedure in 30 women, and blood was collected from 18 of 30 women. For ovarian stimulation, the long GnRH agonist protocol was generally used among enrolled patients. The short protocol was used for two patients who responded poorly to the long protocol, but their oocytes did not become fertilized. The influence of the protocol type was not evaluated in this study. The median age of the 30 enrolled women was 37 years old. The demographics and clinical measures of the enrolled patients are described in Table 1.
Table 1.
Demographics and clinical measures of enrolled patients (n = 30)
| Age (years; median, 25–75 % quartile) | 37 (34–41) |
| Duration of infertility (years; median, 25–75 % quartile) | 2 (1–2) |
| BMI (mean ± SEM) | 21.6 ± 2.4 |
| Estradiol (pg/ml; mean ± SEM) | 2861 ± 3313 |
| FSH (mIU/ml; mean ± SEM) | 6.5 ± 3.7 |
| AMH (ng/ml; mean ± SEM) | 2.8 ± 3.1 |
| Total oocyte retrieved (median, 25–75 % quartile) | 7 (2–12) |
| N of subjected OCCCs per individual in this trial | |
| 1 | 9 |
| 2 | 13 |
| 3 | 7 |
| 4 | 1 |
BMI body mass index, SEM standard error of the mean, N number, OCCC oocyte cumulus-cell complex
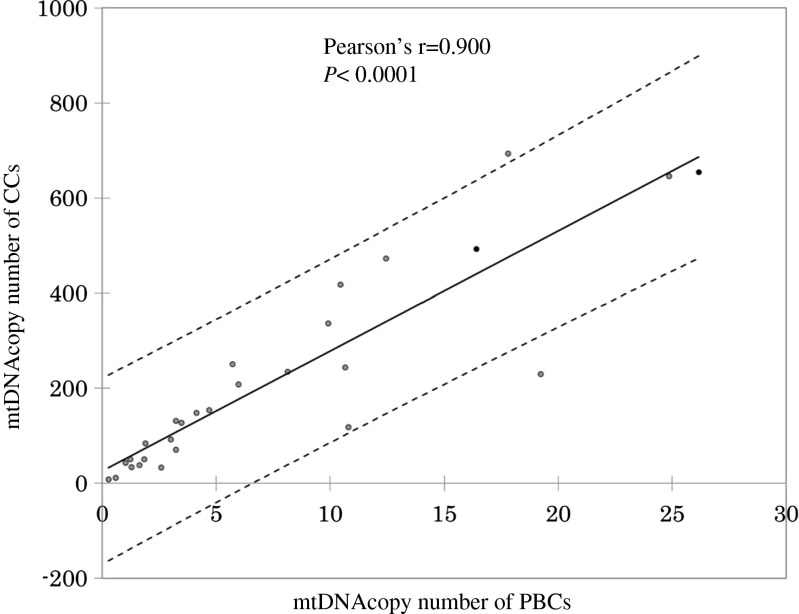
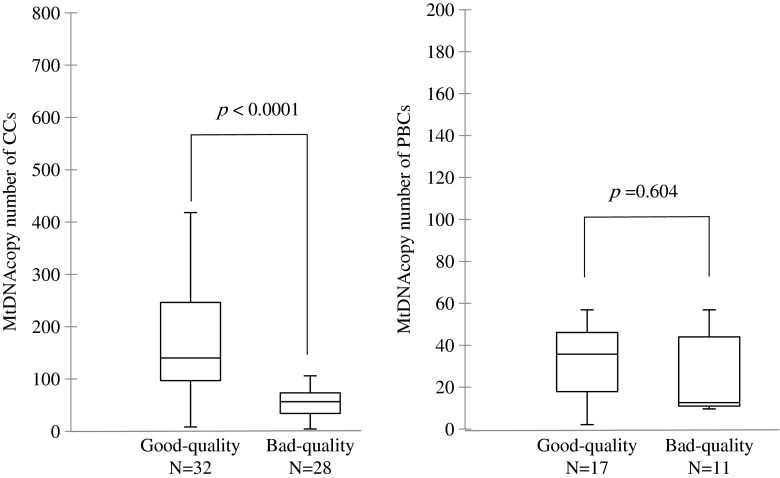
Blood and CC mtDNA content were highly correlated (Pearson’s r = 0.900, p < 0.0001) (Fig. 1). The median mtDNA content of CCs for good- and poor-quality embryos was 140 (25–75 % quartile, 97–264) and 57 (25–75 % quartile, 34–74), respectively (Fig. 2). Increased mtDNA copy number was observed in good-quality embryos (p < 0.0001). Median mtDNA content of PBCs for good- and poor-quality embryos was 36 (25–75 % quartile, 18–46) and 13 (25–75 % quartile, 11–44), respectively (p = 0.604).
Fig. 1.
The correlation of mtDNA copy number between PBCs and CCs (n = 18). mtDNA mitochondrial DNA, PBCs peripheral blood cells, CCs cumulus cells
Fig. 2.
MtDNA copy number for CCs (n = 60) and PBCs (n = 18) and the association with embryo quality. Median mtDNA contents of CCs of good- and bad-quality embryos were 140 (25–75 % quartile, 97–264) and 57 (25–75 % quartile, 34–74), respectively. The median mtDNA contents of PBCs for good- and bad-quality embryos were 36 (25–75 % quartile, 18–46) and 13 (25–75 % quartile, 11–44), respectively. The p value was calculated using the Mann-Whitney U test. mtDNA mitochondrial DNA, PBCs peripheral blood cells, CCs cumulus cells
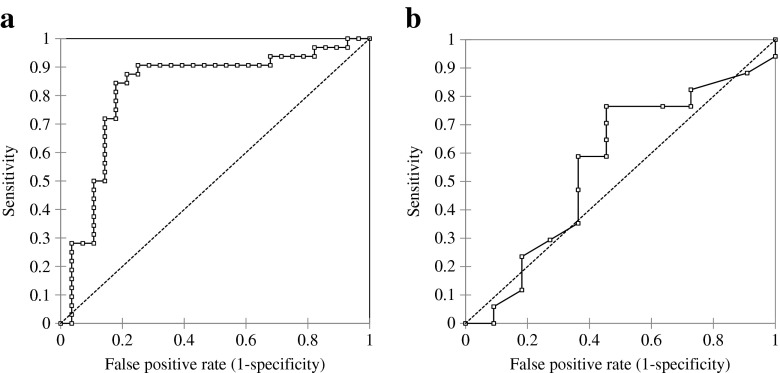
The ROC curve for obtaining good-quality embryos using CC mtDNA copy number had an AUC of 0.823 (95 % confidential interval [95 % CI], 0.710–0.935), and using a threshold of 86, sensitivity, specificity, positive, and negative predictive values were 84.4, 82.1, 84.4, and 82.1 %, respectively (Fig. 3a). The AUC of PBCs was 0.561 (95 % CI, 0.342–0.781). Using a threshold of 12, the sensitivity, specificity, positive, and negative predictive values were 76.5, 54.5, 72.2, and 60.0 %, respectively (Fig. 3b).
Fig. 3.
Receiver operating characteristic (ROC) curves of mtDNA copy number of CCs and PBCs for the prediction of obtaining good-quality embryos. ROC curves were derived by plotting the relationship between the specificity and sensitivity at various cut-off levels. a The area under the curve (AUC) of CCs was 0.823 (95 % confidential interval [95 % CI], 0.710–0.935). The best compromise between true and false positives was achieved using a threshold of 86 according to the ROC curve analysis. Sensitivity, specificity, positive, and negative predictive values were 84.4, 82.1, 84.4, and 82.1 %, respectively. b The AUC of PBCs was 0.561 (95 % CI, 0.342–0.781). Using a threshold of 12, sensitivity, specificity, positive, and negative predictive values were 76.5, 54.5, 72.2, and 60.0 %, respectively
Among variables including mtDNA content of CCs and PBCs, age, estradiol, FSH, and AMH, only the mtDNA content of CCs was significantly associated with good-quality embryos according to the logistic regression model (p = 0.020).
Among 21 patients who provided more than two OCCCs in this study, 14 patients had both good- and poor-quality embryos. Twenty-eight (78 %) of 36 OCCCs were correctly classified into two groups of quality using a threshold of 86 for the mtDNA content of CCs.
Discussion
We performed a search in PubMed and Google using the words “cumulus cells” and “mitochondrial DNA.” Based on an absence of reports, this is the first report in the literature to demonstrate that the mtDNA content of CCs predicts whether good-quality embryos will be obtained for IVF and that the mtDNA content of CCs and PBCs is relevant in patients undergoing IVF. CC mtDNA copy number was a reliable diagnostic tool for predicting embryo quality, with an AUC of 0.823, indicating high predictive power.
Oocytes are surrounded by CCs, and their interaction is critical for oocyte maturation, fertilization, and cleavage [15]. CCs deliver metabolic support to oocytes in glycolysis and the tricarboxylic acid cycle [16]. Increased levels of ATP during oocyte maturation were demonstrated in mice when oocytes were surrounded by CCs [17]. At fertilization, mitochondrion-derived ATP is essential for sperm-triggered oscillations [18]. Oocyte aging has been proposed to occur in association with mitochondrial dysfunction resulting from the accumulation of point mutations and decreased mtDNA content [5, 19]. Several studies demonstrated an aberrant gene expression profile in aged CCs [20, 21]. However, little information is available concerning mitochondrial dysfunction in aged CCs. Tsai et al. reported that a mitochondrial DNA 4977-bp deletion in CCs was found in 35 non-pregnant women, but not in 16 pregnant women who had undergone IVF or ICSI. The deletion was more prevalent in women aged 34 years or older [22].
Reproductive age is a well-known parameter influencing embryo quality and fertilization outcome [1]. However, reproductive age was not associated with good-quality embryos in the multivariate analysis conducted in this study, in which 18 women (67 %) were aged 35 years or older. Therefore, in the present population, mtDNA content probably reflects oocyte aging more accurately than the reproductive age.
Among 11 patients with POI reported by Bonomi M et al. [10] and 30 patients with unexplained infertility in the current study, the relationship between mtDNA copy number for CCs and PBCs was significant, with Pearson’s r values of 0.742 (p = 0.008) and 0.900 (p < 0.0001), respectively. However, the mtDNA content of PBCs was not predictive for good-quality embryos in this study. Moreover, the mtDNA content in CCs differed even within the same individual and was associated with the quality of embryo.
Limitations of the study included that the blood sampling for mtDNA of PBCs was conducted only once in each individual. In addition, the sample size of PBCs was probably not large enough to determine whether it could be used to predict embryo quality.
A simple diagnostic tool for ovarian aging helps young women pursuing careers to plan a family, allows women with infertility to have easy access to the best treatment, and provides young women with planned chemotherapy to initiate a fertility preservation program. Measuring blood mtDNA content is simple, rapid, and minimally invasive, and it is worthwhile to study its use in the development of diagnostic tool for ovarian aging.
Conclusion
To develop a rapid and accurate non-invasive embryo assessment tool, we evaluated mtDNA content in CCs and PBCs in association with gross embryo morphology [11, 12]. We demonstrated that mtDNA content in CCs could be a new marker for good-quality embryos. However, the sample size was small. For routine clinical use, a larger study should further investigate an endpoint of a successful pregnancy outcome.
Compliance with ethical standards
This prospective study was reviewed and approved by the institutional review board of Hyogo College of Medicine and the Advanced Fertility Center of Fuchu Nozomi (No. 210, approved August 2013). All subjects gave their written informed consent for CCs and/or blood sampling and genetic analysis.
Footnotes
Capsule The determination of mtDNA content in CCs can be used to predict good-quality embryos.
References
- 1.Balasch J. Ageing and infertility: an overview. Gynecol Endocrinol. 2010;26:855–60. doi: 10.3109/09513590.2010.501889. [DOI] [PubMed] [Google Scholar]
- 2.Keefe DL, Niven-Fairchild T, Powell S, Buradagunta S. Mitochondrial deoxyribonucleic acid deletions in oocytes and reproductive aging in women. Fertil Steril. 1995;64:577–83. [PubMed] [Google Scholar]
- 3.Simsek-Duran F, Li F, Ford W, Swanson RJ, Jones HW, Jr Castora FJ. Age-associated metabolic and morphologic changes in mitochondria of individual mouse and hamster oocytes. PLoS One. 2013;8 doi: 10.1371/journal.pone.0064955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Blerkom J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion. 2011;11:797–813. doi: 10.1016/j.mito.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Chan CC, Liu VW, Lau EY, Yeung WS, Ng EH, Ho PC. Mitochondrial DNA content and 4977 bp deletion in unfertilized oocytes. Mol Hum Reprod. 2005;11:843–6. doi: 10.1093/molehr/gah243. [DOI] [PubMed] [Google Scholar]
- 6.Murakoshi Y, Sueoka K, Takahashi K, Sato S, Sakurai T, Tajima H, et al. Embryo developmental capability and pregnancy outcome are related to the mitochondrial DNA copy number and ooplasmic volume. J Assist Reprod Genet. 2013;30:1367–75. doi: 10.1007/s10815-013-0062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santos TA, El Shourbagy S, St John JC. Mitochondrial content reflects oocyte variability and fertilization outcome. Fertil Steril. 2006;85:584–91. doi: 10.1016/j.fertnstert.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 8.Huang Z, Wells D. The human oocyte and cumulus cells relationship: new insights from the cumulus cell transcriptome. Mol Hum Reprod. 2010;16:715–25. doi: 10.1093/molehr/gaq031. [DOI] [PubMed] [Google Scholar]
- 9.Uyar A, Torrealday S, Seli E. Cumulus and granulosa cell markers of oocyte and embryo quality. Fertil Steril. 2013;99:979–97. doi: 10.1016/j.fertnstert.2013.01.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonomi M, Somigliana E, Cacciatore C, Busnelli M, Rossetti R, Bonetti S, et al. Blood cell mitochondrial DNA content and premature ovarian aging. PLoS One. 2012;7 doi: 10.1371/journal.pone.0042423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hill GA, Freeman M, Bastias MC, Rogers BJ, Herbert CM, 3rd, Osteen KG, et al. The influence of oocyte maturity and embryo quality on pregnancy rate in a program for in vitro fertilization-embryo transfer. Fertil Steril. 1989;52:801–6. doi: 10.1016/s0015-0282(16)61034-8. [DOI] [PubMed] [Google Scholar]
- 12.Shulman A, Ben-Nun I, Ghetler Y, Kaneti H, Shilon M, Beyth Y. Relationship between embryo morphology and implantation rate after in vitro fertilization treatment in conception cycles. Fertil Steril. 1993;60:123–6. doi: 10.1016/s0015-0282(16)56048-8. [DOI] [PubMed] [Google Scholar]
- 13.Teranishi A, Kuwata A, Fumino T, Hamai H, Shigeta M. A theoretical model for single blastocyst transfer. J Assist Reprod Genet. 2009;26:327–34. doi: 10.1007/s10815-009-9321-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gardner DK, Schoolcraft WB. In vitro culture of human blastocysts. In: Jansen R, Mortimer D, editors. Toward reproductive certainty: fertility and genetics beyond. Carnforth, UK.: Parthenon Publishing; 1999. pp. 378–88. [Google Scholar]
- 15.Dumesic DA, Meldrum DR, Katz-Jaffe MG, Krisher RL, Schoolcraft WB. Oocyte environment: follicular fluid and cumulus cells are critical for oocyte health. Fertil Steril. 2015;103:303–16. doi: 10.1016/j.fertnstert.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Sugiura K, Pendola FL, Eppig JJ. Oocyte control of metabolic cooperativity between oocytes and companion granulosa cells: energy metabolism. Dev Biol. 2005;279:20–30. doi: 10.1016/j.ydbio.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 17.Dalton CM, Szabadkai G, Carroll J. Measurement of ATP in single oocytes: impact of maturation and cumulus cells on levels and consumption. J Cell Physiol. 2014;229:353–61. doi: 10.1002/jcp.24457. [DOI] [PubMed] [Google Scholar]
- 18.Dumollard R, Marangos P, Fitzharris G, Swann K, Duchen M, Carroll J. Sperm-triggered [Ca2+] oscillations and Ca2+ homeostasis in the mouse egg have an absolute requirement for mitochondrial ATP production. Development. 2004;131:3057–67. doi: 10.1242/dev.01181. [DOI] [PubMed] [Google Scholar]
- 19.Bentov Y, Casper RF. The aging oocyte—can mitochondrial function be improved? Fertil Steril. 2013;99:18–22. doi: 10.1016/j.fertnstert.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 20.McReynolds S, Dzieciatkowska M, McCallie BR, Mitchell SD, Stevens J, Hansen K, et al. Impact of maternal aging on the molecular signature of human cumulus cells. Fertil Steril. 2012;98:1574–80. doi: 10.1016/j.fertnstert.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Al-Edani T, Assou S, Ferrières A, Bringer Deutsch S, Gala A, Lecellier CH, et al. Female aging alters expression of human cumulus cells genes that are essential for oocyte quality. Biomed Res Int. 2014;2014:964614. doi: 10.1155/2014/964614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsai HD, Hsieh YY, Hsieh JN, Chang CC, Yang CY, Yang JG, et al. Mitochondria DNA deletion and copy numbers of cumulus cells associated with in vitro fertilization outcomes. J Reprod Med. 2010;55:491–7. [PubMed] [Google Scholar]