Abstract
The effect of the two antibiotics ceftazidime and meropenem on a collection of 46 Burkholderia pseudomallei isolates representing clinical and environmental sources across northern Australia was investigated by using a series of in vitro test methods. The susceptibility testing methods used included Kirby-Bauer disk diffusion, Etest MIC, broth microdilution MIC, and a modification of the microdilution method in which Acanthamoeba cells were added to simulate the effect of a professional phagocytic cell on test outcome. In a semiquantitative validation coculture series, the majority of bacteria were intracellular up to a multiplicity of infection of 10 bacteria to one ameba. The optical density and bacterial count (log10 CFU/ml) correlated across the range tested (r2 = 0.77; P < 0.0001). Susceptibility test results were compared against clinical outcomes. The MICs of ceftazidime were consistently higher than those of meropenem by all three methods. The MICs of both agents were significantly higher when Acanthamoeba trophozoites were added to the broth microdilution method. Conventional and intracellular MIC results were consistent for clinical isolates from the Western Australian outbreak cluster despite the wide variety of clinical outcomes. Further development of the intracellular MIC method is expected to help assess the efficacy of antimicrobial agents on this bacterial species in an intracellular setting.
Burkholderia pseudomallei, the bacterial species responsible for the potentially fatal infection known as melioidosis, is a facultative intracellular pathogen. The ability of B. pseudomallei to survive in macrophages has been known for some time (10) and is used to explain unusual aspects of melioidosis including late onset and recurrent disease. Treatment of the acute, septicemic form of melioidosis often relies on ceftazidime. In a study with a septicemic relapse rate of around 23%, relapse was more common in the patient group not treated with ceftazidime (1). The combined mortality of septicemic and nonsepticemic melioidosis in Australia has been put at 21% (2). There has been growing interest in the use of carbapenems as an alternative antibiotic option for acute B. pseudomallei infection (12, 13). At present, clinical trial data do not conclusively demonstrate an improved outcome when carbapenems are compared with ceftazidime (12), despite the theoretical benefit expected from improved intracellular penetration. In a recent case study, a previously published amebic cellular model of B. pseudomallei infection was used to demonstrate the superior intracellular activity of meropenem over ceftazidime and sulfamethoxazole (7). Although it was found that improved in vitro activity corresponded to the clinical improvement in a single persistent septicemic case, this outcome had alternative explanations based on other aspects of patient care. In the present study we used an amebic intracellular susceptibility method to compare the activity of a carbapenem and ceftazidime on a range of B. pseudomallei isolates from our culture collection.
MATERIALS AND METHODS
Bacterial strains.
Forty-six isolates of B. pseudomallei were used in this study, comprising the three reference strains NCTC 10276, DM 98 (persistently mucoid colony phenotype), and NCTC 13177 (Western Australian outbreak strain [5]); environmental isolates (13); and a geographically representative collection of Australian clinical isolates (36). These strains had already been genotyped by automated EcoRI ribotyping and DNA macrorestriction, as previously described (8). Apart from three groups of genotypically clustered isolates (Table 1), including the Western Australian outbreak group, all isolates belonged to distinct strains. B. pseudomallei isolates were stored in replicate 1-ml containers of 20% glycerol-brain heart infusion broth at −70°C and recovered by subculture onto 5% horse blood agar (Excel Laboratory Products, Bentley, Western Australia, Australia) and incubation in air for 18 h at 37°C. Three non-B. pseudomallei reference strains were used for quality control purposes in the disk diffusion and broth microdilution tests. These were Staphylococcus aureus ATCC 25923, Pseudomonas aeruginosa ATCC 27853, and Escherichia coli ATCC 25922.
TABLE 1.
Disk diffusion and Etest results
| Isolate | Inhibition zone diam (mm)
|
Etest MIC (μg/ml)
|
Related isolatesa | ||
|---|---|---|---|---|---|
| Ceftazidime | Meropenem | Ceftazidime | Meropenem | ||
| Reference S. aureus ATCC 25923 | 20 (≥16) | 35 (≥16) | 0.032 | ||
| Reference E. coli ATCC 25922 | 27 (≥16) | 33 (≥16) | 0.008 | ||
| Reference P. aeruginosa ATCC 27853 | 27 (≥16) | 33 (≥16) | 0.19 | ||
| Reference B. pseudomallei strains | |||||
| NCTC 10276 | 25 | 27 | 2 | 1 | |
| DM 98 (mucoid) | 27 | 16 | 4 | 4 | |
| NCTC 13177 | 29 | 27 | 2 | 1 | A |
| Western Australia clinical B. pseudomallei isolates | |||||
| BCC 1 | 28 | 25 | 2 | 1.5 | B |
| BCC 2 | 27 | 25 | 2 | 1.5 | A |
| BCC 3 | 28 | 25 | 2 | 1 | A |
| BCC 4 | 28 | 25 | 2 | 1 | A |
| BCC 8 | 29 | 27 | 3 | 0.75 | A |
| BCC 16 | 27 | 26 | 2 | 1 | B |
| BCC 33 | 28 | 26 | 2 | 1 | A |
| BCC 36 | 29 | 25 | 2 | 1 | A |
| BCC 71 | 27 | 24 | 2 | 1.5 | |
| BCC 74 | 24 | 25 | 2 | 0.75 | |
| BCC 105 | 25 | 22 | 3 | 1.5 | C |
| BCC 107 | 27 | 25 | 3 | 1.5 | |
| BCC 109 | 27 | 26 | 3 | 1.5 | C |
| BCC 110 | 25 | 23 | 3 | 1.5 | C |
| BCC 122 | 25 | 25 | 4 | 1 | |
| Queensland clinical B. pseudomallei isolates | |||||
| BCC 75 | 29 | 25 | 3 | 1 | |
| BCC 76 | 28 | 27 | 3 | 1.5 | |
| BCC 77 | 25 | 26 | 3 | 1 | |
| BCC 78 | 29 | 27 | 3 | 1.5 | |
| BCC 79 | 28 | 25 | 2 | 1 | |
| BCC 80 | 28 | 27 | 3 | 1.5 | |
| BCC 81 | 27 | 26 | 3 | 1 | |
| BCC 83 | 27 | 26 | 3 | 1.5 | |
| BCC 85 | 28 | 27 | 3 | 1.5 | |
| BCC 86 | 29 | 26 | 3 | 1.5 | |
| BCC 87 | 25 | 26 | 4 | 1 | |
| Northern Territory clinical B. pseudomallei isolates | |||||
| BCC 18 | 27 | 27 | 3 | 1.5 | |
| BCC 49 | 26 | 27 | 3 | 1 | |
| BCC 50 | 28 | 26 | 2 | 1.5 | |
| BCC 51 | 27 | 24 | 3 | 1.5 | |
| BCC 52 | 26 | 24 | 3 | 1.5 | |
| BCC 53 | 26 | 26 | 3 | 1 | |
| BCC 69 | 30 | 31 | 2 | 0.5 | |
| BCC 72 | 25 | 24 | 3 | 1.5 | |
| Australian environmental B. pseudomallei isolates | |||||
| BCC 21 | 28 | 23 | 2 | 1.5 | A |
| BCC 22 | 0 | 24 | >256 | 0.38 | |
| BCC 23 | 29 | 26 | 2 | 0.75 | |
| BCC 24 | 30 | 28 | 0.75 | 0.5 | |
| BCC 26 | 26 | 25 | 3 | 1.5 | |
| BCC 27 | 30 | 25 | 1.5 | 1 | |
| BCC 28 | 25 | 25 | 3 | 1.5 | |
| BCC 29 | 26 | 25 | 3 | 0.75 | |
| BCC 30 | 25 | 25 | 2 | 1 | |
| BCC 31 | 28 | 25 | 2 | 1 | |
| BCC 44 | 28 | 26 | 2 | 1.5 | A |
| BCC 45 | 29 | 25 | 2 | 1.5 | A |
| BCC 46 | 29 | 25 | 2 | 1 | A |
Related isolates are groups of epidemiologically related bacteria that cluster by genetic typing.
Amebas.
The strain used in this study was Acanthamoeba astronyxis CCC 111, which was maintained in peptone glucose yeast extract broth at 20°C. Cells were harvested from tissue culture flasks, centrifuged, and resuspended in sterile 0.89% NaCl solution (Excel).
Antibiotic reagents.
Fresh antibiotic-containing disks and Etest strips were used for susceptibility testing. In order to replicate in-use conditions in the intracellular susceptibility test, commercially available, in-date meropenem and ceftazidime intravenous formulations were used to make up fresh solutions immediately prior to each experiment.
Microtiter coculture of bacteria and amebas.
Bacteria-ameba coculture was conducted under semiquantitative conditions in order to determine the optimal bacterial inoculum to ensure the internalization of bacteria by amebas. A 96-well flat-bottomed microtiter plate was used (Nunc Nalge), with rows A to D for the NCTC 10276 series and rows E to H for the NCTC 13177 series. Each set of four rows was divided into four blocks of wells, each in a pattern of three by four wells. The first block was reserved for saline and tryptic soy broth (TSB; Excel) sterility controls. The second block was loaded with 50 μl of B. pseudomallei cell suspension in TSB, going from undiluted mid-lag phase culture in row A, stepwise to 10−3 mid-lag phase culture in row D. There were two replicates in columns 5 and 6, making a total of three wells at each dilution. A 50-μl aliquot of sterile 0.85% NaCl solution was added to each of these wells to make a total of 100 μl per well. In the third block, the wells were filled with a 50-μl aliquot of Acanthamoeba cell suspension and made up to 100 μl by adding 50 μl of sterile TSB. All wells in the third block contained the same concentration of Acanthamoeba cells. The fourth and final block of wells contained 50 μl of Acanthamoeba cell suspension at constant concentration and 50 μl of B. pseudomallei cell suspension in TSB, following the same dispensing pattern as block B, from undiluted lag phase in row A to 10−3 mid-lag phase culture in row D. The optical density of each well was measured by an automated plate reader at 450-nm absorbance immediately after completion of the well inoculation phase and again after a 24-h incubation at 20°C in air. The change in optical density for each well was determined by subtracting the baseline reading (at time zero) from the 24-h reading. A 10-μl aliquot was taken from below the surface of each well by using a 10-μl displacement pipette and a sterile, aerosol-free tip. This aliquot was dispensed into 0.85% NaCl solution to create a series of dilutions according to the measured optical density of each well. A 50-μl aliquot of the final dilution chosen for each well was spread onto plate count agar (Excel) by using a spiral plater and incubated for 24 h in air at 37°C. Plate counts were then determined and converted to log10 CFU per ml. A 10-μl aliquot from the remaining contents of each well from the first row of each preparation was dispensed onto a fresh glass microscope slide, covered with a glass coverslip, and sealed with clear nail polish. The sealed coverslip preparation was examined on a Bio-Rad MRC 1000/1024 UV laser scanning confocal microscope equipped with a Nikon 60× NA 1.2 water immersion objective. Images were captured at the same magnification by using the monochrome transmission detector on the microscope and focused in a plane between the coverslip and the slide surfaces. Repeat experiments were conducted over a longer (48-h) incubation period and with incubation at 37°C in air.
Conventional susceptibility tests.
Disk diffusion determinations were performed according to a previously described Kirby-Bauer method (11), though disk diffusion has not been formally validated for susceptibility testing of B. pseudomallei by the NCCLS, and an interpretive standard has not been established. Meropenem and ceftazidime were tested. Zones were measured at three evenly spaced diameters with calipers, and the mean diameter was calculated. The interpretation of results was based on the NCCLS zone diameters used for non-Enterobacteriaceae. The MICs of meropenem and ceftazidime were determined by Etest as previously described (4).
Microdilution MIC.
A broth microdilution method was used for meropenem and ceftazidime MIC determinations. An inoculum of 106 CFU of washed B. pseudomallei per ml was used, and the test was conducted in Mueller-Hinton broth (MHB; Excel). The interpretation of results was based on the NCCLS MIC breakpoints for non-Enterobacteriaceae. The Etest-determined MIC was used to establish the midpoint of the twofold antibiotic dilution series for the MIC obtained by the broth microdilution method. A parallel series of antibiotic dilutions was conducted in MHB containing A. astronyxis at a multiplicity of infection (MOI) of 10 bacteria to 1 ameba. Plates were covered and incubated at 37°C in air for 18 h. The MIC was then read visually. Wells containing amebas but no bacteria were included as a negative growth control. Aliquots (10-μl) were examined at a magnification of ×400 under phase contrast to confirm the phagocytosis of bacilli by amebas.
Statistics and data interpretation.
The MIC endpoints were determined by a standard method. Results were analyzed and compared by using statistical software (Prism version 2.01; GraphPad, San Diego, Calif.). Where the endpoints for MICs obtained by microdilution were unclear, the lowest dilution at which any inhibition occurred was taken to indicate the MIC.
RESULTS
Validation of microtiter coculture method.
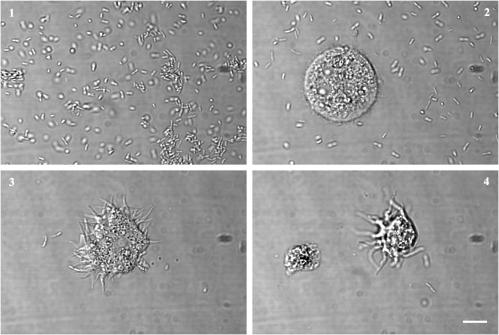
Confocal microscope views of coculture preparations confirmed that the principal contributor to the increased optical density in microtiter coculture wells was bacterial growth (Fig. 1). A comparison of cocultures at four different MOIs demonstrated that the majority of bacteria were intracellular until the MOI reached 10:1. The series of cocultures with B. pseudomallei NCTC 10276 amebas demonstrated a progressive increase in optical density with increasing bacterial inoculum in the bacteria-only and coculture wells (Fig. 2). The ameba-only control wells showed no such change. There was a decrease in optical density in the coculture wells compared with the corresponding bacteria-only wells, consistent with the phagocytosis of bacteria. The optical density increase over 24 h correlated with the final bacterial count attained in each microtiter well (log10 CFU/ml) (r2 = 0.77, P < 0.0001). Longer incubation (48 h) and higher incubation temperature both led to early pellicle growth, which interfered with accurate optical density measurement.
FIG. 1.
Contents of microtiter coculture wells. Differential interference microscope images of microtiter wells containing A. astronyxis and B. pseudomallei NCTC 10276 in coculture after 24 h of incubation at 20°C. The most visibly opaque wells contained bacteria and no amebas, corresponding to the highest MOI (100) (frame 1). At progressively lower MOIs (frames 2 to 4), there were fewer bacteria and more surviving amebas, and intracellular bacteria were evident. Scale bar, 10 μm.
FIG. 2.
Comparison of optical density change over 24 h and final bacterial colony count in microtiter coculture of B. pseudomallei NCTC 10276 with A. astronyxis. x axis, optical density at 450 nm at 24 h of incubation; y axis, log10 CFU of B. pseudomallei per ml at 24 h. Linear regression and 95% confidence limits are indicated (r2 = 0.77, P < 0.0001). OD, optical density.
Disk diffusion.
According to disk diffusion testing, only one isolate was resistant to meropenem, and another one was resistant to ceftazidime. Both of these isolates were sensitive to the other antimicrobial agent. These isolates had susceptibility results that were distinct from those of the other isolates tested (Table 1). The meropenem-resistant isolate had a smaller inhibition zone (15.7 mm) than sensitive isolates (20 to 25 mm) and was a persistently mucoid isolate whose zone diameter was difficult to read. The ceftazidime-resistant isolate had no ceftazidime inhibition zone at all, compared to 24 to 29 mm for ceftazidime-sensitive isolates.
Etest.
Etest results gave meropenem MICs of 0.38 to 4.0 μg per ml, the highest reading being the most resistant isolate by disk diffusion (Table 1). The most meropenem-sensitive isolate was the ceftazidime-resistant isolate, which was also fully ceftazidime resistant by the Etest. The other ceftazidime Etest results were 0.75 to 4.0 μg/ml. The isolate with the highest measurable ceftazidime Etest result also produced the highest Etest meropenem MIC.
Broth microdilution.
MIC results are summarized in Table 2. Ceftazidime Etest MIC results correlated with conventional broth microdilution MIC results but not with the intracellular ceftazidime MICs (Table 3). There was no significant correlation between Etest meropenem MICs and MICs determined by the other two methods. Meropenem Etest results were tightly grouped, reducing the prospects of validating a wide test range. For three isolates, the meropenem microdilution MICs were >16 μg/ml with no clear endpoint but with Etest results of 1.0 to 1.5 μg/ml.
TABLE 2.
Summary of MIC test results by Etest, conventional broth microdilution, and intracellular microdilution for B. pseudomallei susceptibility to ceftazidime and meropenema
| Antibiotic | Method | MIC (μg/ml)
|
|||
|---|---|---|---|---|---|
| MIC50 | MIC90 | Range | Median | ||
| Meropenem | Etest | 1.0 | 1.5 | 1.0-4.0 | 3.0 |
| Meropenem | Conventional | 3.0 | 4.0 | 2.0-6.0 | 3.0 |
| Meropenem | Intracellular | 8.0 | 16.0 | 1.5->32 | 6.0 |
| Ceftazidime | Etest | 3.0 | 4.0 | 1.5->256 | 4.0 |
| Ceftazidime | Conventional | 4.0 | 32.0 | 1.5->32 | 4.0 |
| Ceftazidime | Intracellular | 16.0 | 32.0 | 2.0->32 | 12.0 |
Broth microdilution MICs for control strains were as follows: S. aureus ATCC 25923, 0.25 μg/ml; E. coli ATCC 25922, <0.031 μg/ml; and P. aeruginosa ATCC 27853, 1.0 μg/ml.
MIC50, the MIC at which 50% of the strains are inhibited; MIC90, the MIC at which 90% of the strains are inhibited.
TABLE 3.
Correlation between Etest, conventional broth microdilution, and intracellular microdilution MIC determinations of B. pseudomallei susceptibility to ceftazidime and meropenema
| Antibiotic | Method 1 | Method 2 | Correlation | P | r |
|---|---|---|---|---|---|
| Ceftazidime | Etest | Conventional | Yes | 0.0042 | 0.42 |
| Ceftazidime | Etest | Intracellular | No | ||
| Ceftazidime | Conventional | Intracellular | Yes | 0.0015 | 0.46 |
| Meropenem | Etest | Conventional | No | ||
| Meropenem | Etest | Intracellular | No | ||
| Meropenem | Conventional | Intracellular | Yes | 0.0028 | 0.43 |
Spearman coefficient for non-Gaussian data.
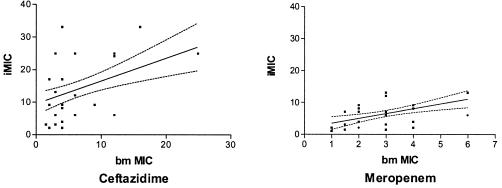
The addition of amebas to the test wells in the broth microdilution series led to significant increases in the MICs of both antibiotics. Microdilution MICs of ceftazidime were significantly higher than those of meropenem with and without amebas in the test wells (Fig. 3). These results represented the results of total bacterial growth following intracellular bacterial survival, which at the starting MOI was largely intracellular (Fig. 1). The conventional broth microdilution MICs of both antibiotics correlated with the intracellular MICs (Fig. 4 and Table 3). Etest MIC results correlated only with conventional ceftazidime microdilution MICs and did not correlate with either the meropenem microdilution MICs or the meropenem and ceftazidime intracellular MICs.
FIG. 3.
Microdilution MICs of ceftazidime and meropenem without amebas (broth microdilution MIC [bm MIC]) and with amebas (intracellular MIC [iMIC]) in the test wells. Results for both drugs were significantly different by a paired t test (for ceftazidime: P < 0.0001, pairing P = 0.0002; for meropenem: P < 0.0001, pairing P = 0.0004).
FIG. 4.
Correlation between conventional (broth microdilution MIC [bm MIC]) and intracellular (iMIC) microdilution MICs of ceftazidime and meropenem. Linear regression lines and 95% confidence intervals are shown.
Clinical outcomes.
The patients from whom B. pseudomallei was isolated represented three jurisdictions in northern Australia. Six patients died, all in the early stages of acute disease. The duration of the hospital stays of the survivors was between 7 days and 8 weeks. The conventional meropenem MIC was consistently within two dilutions of the intracellular MIC for all the isolates representing the Western Australian melioidosis cluster, whether patients died (three patients), recovered (two patients), or had delayed onset infection (two patients). There was no significant positive correlation between the duration of hospital stay and MIC. Only two surviving patients stayed in hospital for more than 4 weeks, and in the case of one of these patients, high-level cotrimoxazole resistance was thought to have been a contributory factor. The meropenem-resistant strain came from a septicemic patient treated successfully with ceftazidime. The ceftazidime-resistant strain was a soil isolate that has not yet been detected in clinical specimens.
DISCUSSION
When ceftazidime was shown to halve the mortality from severe melioidosis in a randomized clinical trial, it became the treatment of choice for the intensive ´ form of the infection (15). Shortly after these trial results were published, carbapenems were shown to have good in vitro activity against B. pseudomallei in a study of acquired antibiotic resistance (3), but it took longer to establish the efficacy of the carbapenem group as a whole against ceftazidime-resistant strains and even longer to test the carbapenems in a clinical trial setting (12, 13). Acquired antibiotic resistance to ceftazidime can be detected by conventional in vitro susceptibility test methods in only a small proportion of strains (3, 9). Of the various susceptibility test methods used to explore the relationship between antibacterial activity and clinical outcome, none has proved ideal, particularly in resolving the issue of how best to prevent late relapse. A promising start has been made on this problem by using a mouse melioidosis model, in which a combination of ceftazidime and cotrimoxazole was found to be more effective than ceftazidime monotherapy (14).
A previous report of an intracellular susceptibility test method led us to seek a method to screen larger numbers of B. pseudomallei strains for intracellular antibacterial susceptibility (7). Results from initial in vitro coculture studies indicated that the opacification of the growth media was due to a combination of intracellular and extracellular growth resulting from bacterium-ameba interactions (6). The validation steps we completed in the present study indicate that while the method aims to assess intracellular antimicrobial efficacy, bacterial growth detected by direct visual inspection is extracellular. The microtiter method must, therefore, be regarded as indirect and semiquantitative. The tendency of B. pseudomallei to form a pellicle when grown for longer periods or at higher incubation temperatures prevented the use of MHB at 37°C for the semiquantitative validation experiment, though this combination was necessary to allow some degree of extrapolation to conventional broth microdilution MIC methods. Although pellicle formation might have interfered with light scattering during optical density measurement, this effect can only occur when an excess of extracellular bacilli is present. In our experience this was an easily visible indication of sub-MIC antibiotic concentrations. While possibly a better indicator of intracellular antimicrobial effect, this method may not be suited to incubation periods longer than 24 h or higher MOIs or incubation temperatures.
Our results confirm that the majority of Australian B. pseudomallei isolates tested are sensitive to both meropenem and ceftazidime in vitro but with generally reduced susceptibility in a cellular environment. Interestingly, the single-agent-resistant strains detected by disk diffusion testing were mucoid variants with a colony phenotype similar in appearance to mucoid P. aeruginosa. It is possible that this phenotype may also cause difficulties for the determination of suitable interpretive standards. The Etest results corroborate the disk diffusion test results and also provide an estimate of the MIC.
MIC testing of agents against B. pseudomallei raises issues of accuracy, significance, and relevance. Broth microdilution, particularly when applied to ceftazidime MIC determination, was prone to difficulty in establishing a clear endpoint. The Etest, on the other hand, gave a clear endpoint except in the case of the ceftazidime-resistant isolate for which the MIC was immeasurably high. In other cases the Etest put the MIC lower. Ceftazidime MICs obtained by the Etest correlated with MICs obtained by the microdilution method. The Etest meropenem MICs were so tightly grouped between 0.5 and 1.5 μg/ml (excepting the resistant isolate) that correlation with microdilution MIC results was not expected.
The addition of a cellular component to the broth microdilution MIC method raised the MIC of both antibiotics for the majority of isolates tested. The differential was greatest for ceftazidime and may have been underestimated by the convention of using the lowest inhibitory value for statistical determinations. Nevertheless, the majority of meropenem MICs obtained by both methods were below 10 μg/ml, while the majority of ceftazidime MIC results were below 10 μg/ml only when amebas were absent. This suggests that the action of the carbapenem antibiotic is affected less than that of the cephalosporin by the addition of amebas. If these results are genuinely representative of bacterium-eukaryotic cell interactions in a clinical setting, they suggest that the majority of isolates we tested are capable of functional resistance to ceftazidime and, to a lesser extent, to meropenem in a cellular environment. As intra-amebic antibiotic levels were not measured and antibiotic-mediated effects on the amebas were not sought, caution should be exercised in attributing the results of this preliminary study solely to differences in intracellular antibiotic action.
The range of clinical outcomes in the patients whose isolates we tested could not have been predicted by the disk diffusion, Etest, or broth microdilution methods of determining MICs. Though both the conventional and intracellular MIC methods suggest that meropenem might be more effective than ceftazidime in vitro, this was not clearly reflected by the clinical outcomes. The one group of seven patients infected by a single strain of B. pseudomallei from a probable point source illustrates the importance of the host response in determining clinical outcome (of five who had early onset infection, two died quickly, and neither of the two who had late onset infection died). The remaining patients represent the two centers with the most melioidosis patients and cover a range of antibiotic regimes. No subgroup was large enough to allow analysis of the possible predictive value of the intracellular MIC. The complexities of intensive (acute) and convalescent or eradication (maintenance) regimens need to be investigated systematically in a series of in vitro and clinical studies. In particular, the intracellular MIC needs investigation as a predictor of clinical outcomes in a much larger patient group, especially in studies of potentially synergistic combinations of meropenem or ceftazidime used with supplementary eradication agents.
In conclusion, this comparative study of the susceptibility of a B. pseudomallei culture collection to meropenem and ceftazidime found a reduced susceptibility to both agents when an intracellular susceptibility test method was used. Comparison of test results with clinical outcomes points to host factors as the predominant determinant of outcome. This study provides preliminary data indicating a degree of internal consistency between the differing methods chosen and some potentially interesting differences. Though it is too early to say how intracellular susceptibility testing of B. pseudomallei might be applied in clinical practice, further refinement of an intracellular method may produce valuable insights into the effect of important therapeutic agents on this species in a cellular milieu.
Acknowledgments
We thank our laboratory colleagues in Western Australia, the Northern Territory, and Queensland for supplying some of the isolates tested, our clinical colleagues for patient outcome data, and Adam Merritt for assistance with reference strains.
We also acknowledge Lotterywest for support of the Lotteries Confocal Microscopy Unit during this study.
F.R. was supported in part by an unrestricted research grant from Zeneca Australia Pty. The company did not exert any control over the design or execution of this project or this report.
REFERENCES
- 1.Chaowagul, W., Y. Suputtamongkol, D. A. Dance, A. Rajchannvong, J. Pattara-arechachai, and N. J. White. 1993. Relapse in melioidosis: incidence and risk factors. J. Infect. Dis. 168:1181-1185. [PubMed] [Google Scholar]
- 2.Currie, B. J., D. A. Fisher, D. M. Howard, J. N. C. Burrow, S. Selvanayagam, P. L. Snelling, N. M. Anstey, and M. Mayo. 2000. The epidemiology of melioidosis in Australia and Papua New Guinea. Acta Trop. 74:121-127. [DOI] [PubMed] [Google Scholar]
- 3.Dance, D. A., V. Wuthiekanun, W. Chaowagul, and N. J. White. 1989. The antimicrobial susceptibility of Pseudomonas pseudomallei. Emergence of resistance in vitro and during treatment. J. Antimicrob. Chemother. 24:295-309. [DOI] [PubMed] [Google Scholar]
- 4.Huang, M., P. N. Baker, S. Bannerjee, and F. C. Tenover. 1992. Accuracy of the E test for determining antimicrobial susceptibilities of staphylococci, enterococci, Campylobacter jejuni, and gram-negative bacteria resistant to antimicrobial agents. J. Clin. Microbiol. 30:3243-3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inglis, T. J. J., S. C. Garrow, C. Adams, M. Henderson, M. Mayo, and B. J. Currie. 1999. Acute melioidosis outbreak in Western Australia. Epidemiol. Infect. 123:437-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inglis, T. J. J., P. Rigby, T. A. Robertson, N. S. Dutton, M. Henderson, and B. J. Chang. 2000. Interaction between Burkholderia pseudomallei and Acanthamoeba species results in coiling phagocytosis, endamebic bacterial survival, and escape. Infect. Immun. 68:1681-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inglis, T. J. J., C. L. Golledge, A. Clair, and J. Harvey. 2001. Case report: recovery from persistent septicemic melioidosis. Am. J. Trop. Med. 65:76-82. [DOI] [PubMed] [Google Scholar]
- 8.Inglis, T. J. J., L. O'Reilly, N. Foster, A. Clair, and J. Sampson. 2002. Comparison of rapid, automated ribotyping and DNA macrorestriction analysis of Burkholderia pseudomallei. J. Clin. Microbiol. 40:3198-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jenney, A. W., G. Lum, D. A. Fisher, and B. J. Currie. 2001. Antibiotic susceptibility of Burkholderia pseudomallei from tropical northern Australia and implications for therapy of melioidosis. Int. J. Antimicrob. Agents. 17:109-113. [DOI] [PubMed] [Google Scholar]
- 10.Jones, A. L., T. J. Beveridge, and D. E. Woods. 1996. Intracellular survival of Burkholderia pseudomallei. Infect. Immun. 64:782-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Committee for Clinical Laboratory Standards. 1993. Performance standards for antimicrobial disk susceptibility tests. Approved standard M2-A5. National Committee for Clinical Laboratory Standards, Villanova, Pa.
- 12.Simpson, A. J., Y. Suputtamongkol, M. D. Smith, B. J. Angus, A. Rajanuwong, V. Wuthiekanun, P. A. Howe, A. L. Walsh, W. Chaowagul, and N. J. White. 1999. Comparison of imipenem and ceftazidime as therapy for severe melioidosis. Clin. Infect. Dis. 29:381-387. [DOI] [PubMed] [Google Scholar]
- 13.Smith, M. D., V. Wuthiekanun, A. L. Walsh, and N. J. White. 1996. In vitro action of carbapenem antibiotics against β-lactamase susceptible and resistant strains of Burkholderia pseudomallei. J. Antimicrob. Chemother. 37:611-615. [DOI] [PubMed] [Google Scholar]
- 14.Ulett, G. C., R. Hirst, B. Bowden, K. Powell, and R. Norton. 2003. A comparison of antibiotic regimens in the treatment of acute melioidosis in a mouse model. J. Antimicrob. Chemother. 51:77-81. [DOI] [PubMed] [Google Scholar]
- 15.White, N. J., D. A. Dance, W. Chaowagul, Y. Wattanagoon, V. Wuthiekanun, and N. Pitakwachara. 1989. Halving of mortality of severe melioidosis by ceftazidime. Lancet ii:697-701. [DOI] [PubMed] [Google Scholar]