Abstract
The 69 kb plasmid pVPA3-1 was identified in Vibrio parahaemolyticus strain 13-028/A3 that can cause acute hepatopancreatic necrosis disease (AHPND). This disease is responsible for mass mortalities in farmed penaeid shrimp and is referred to as early mortality syndrome (EMS). The plasmid has a GC content of 45.9% with a copy number of 37 per bacterial cell as determined by comparative quantitative PCR analyses. It consists of 92 open reading frames that encode mobilization proteins, replication enzymes, transposases, virulence-associated proteins, and proteins similar to Photorhabdus insect-related (Pir) toxins. In V. parahaemolyticus, these Pir toxin-like proteins are encoded by 2 genes ( pirA- and pirB-like) located within a 3.5 kb fragment flanked with inverted repeats of a transposase-coding sequence (1 kb). The GC content of these 2 genes is only 38.2%, substantially lower than that of the rest of the plasmid, which suggests that these genes were recently acquired. Based on a proteomic analysis, the pirA-like (336 bp) and pirB-like (1317 bp) genes encode for 13 and 50 kDa proteins, respectively. In laboratory cultures of V. parahaemolyticus 13-028/A3, both proteins were secreted into the culture medium. We developed a duplex PCR diagnostic method, with a detection limit of 105 CFU ml−1 and targeting pirA- and pirB-like genes in this strain of V. parahaemolyticus. This PCR protocol can reliably detect AHPND-causing strains of V. parahaemolyticus and does not cross react with non-pathogenic strains or with other species of Vibrio isolated from shrimp ponds.
Keywords: Penaeidae, Early mortality syndrome, Aquaculture, pirAB genes, PCR, Vibrio infection, Plasmid sequence analyses, Extracellular toxins
INTRODUCTION
Since 2009, acute hepatopancreatic necrosis disease (AHPND, also known as early mortality syndrome, EMS) has caused significant mortality, up to 100%, in populations of both Penaeus vannamei and P. monodon cultured in SE Asia and in Mexico. The disease develops quickly, starting approximately 8 d after the ponds are stocked, and severe mortalities occur during the first 20 to 30 d of culture. Histological examination of infected shrimp shows that the disease affects the hepatopancreas, in which the tubule epithelial cells degenerate, round up, detach from the basement membrane, and then slough into the tubule lumen. At terminal stages, the hepatopancreas shows extensive intertubular, hemocytic aggregations. The affected shrimp die from hepatopancreas dysfunction and from secondary Vibrio infections. The inflammatory response that is usually elicited by a pathogen is not evident at the early stage and strongly suggests a toxin etiology. The causative agent is V. parahaemolyticus; the (presumptive) toxins secreted into culture media and cell-free broth can cause AHPND (Tran et al. 2013).
Comparison of genome sequences among strains of V. parahaemolyticus revealed that a plasmid containing genes homologous with Photorhabdus insect-related (Pir) toxin genes was found in all pathogenic strains but was absent in non-pathogenic strains (Kondo et al. 2014). Pir toxins were first identified in P. luminescens, a bacterium that maintains a symbiotic relationship with entomopathogenic nematodes of the family Heterorhabditidae (ffrench-Constant et al. 2000, Duchaud et al. 2003, Waterfield et al. 2005). The Pir toxins act as binary proteins; they are encoded by the pirA and pirB genes, and both proteins are necessary for oral toxicity in moths and mosquitoes (Blackburn et al. 2006, Ahantarig et al. 2009). The pathology of Pir oral toxicity in larvae of the moth Plutella xylostella is in the midgut epithelium, and its toxicity results in severe swelling and shedding of the apical membranes (Blackburn et al. 2006). As shrimp and insects are both arthropods, AHPND-affected shrimp have pathological responses similar to the Pir midgut toxicity in insects; we therefore suspected the involvement of Pir toxin-like proteins in the etiology of AHPND. Here we characterized the plasmid that encodes PirAB-like proteins and developed a PCR method targeting both pirA- and pirB-like genes for screening pathogenic AHPND-strains of V. parahaemolyticus from hatchery and farm environments.
MATERIALS AND METHODS
Bacterial strains and culture
Vibrio parahaemolyticus 13-028/A3 and 13-028/A2 were isolated from the stomachs of AHPND-affected shrimp cultured in Vietnam in 2013. Strain 13-028/A3 was determined to cause this disease through laboratory bioassays (Tran et al. 2013). Strain 13-028/A2 is not AHPND-pathogenic. Tryptic soy broth with 2% NaCl (TSB+) was used for culturing V. parahaemolyticus at 28°C.
For VpPirAB PCR detection, 77 bacterial isolates including pathogenic and non-pathogenic V. parahaemolyticus, V. communis, V. harveyi, V. owensii, and Photobacterium spp. were used for testing specificity. The AHPND-pathogenic strains were isolated from affected shrimp cultured in Mexico and Vietnam. Non-pathogenic strains were isolated from shrimp ponds in Ecuador, Vietnam, Mexico, Peru, India, and USA, where shrimp cultured in these farms did not show clinical signs of AHPND. The pathogenicity of AHPND strains was determined by laboratory infections through immersion or oral feeding, followed by histological examinations described by Tran et al. (2013).
Whole genome sequencing of V. parahaemolyticus
Both V. parahaemolyticus 13-028/A3 and 13-028/A2 were grown in TSB+ at 28°C overnight. Bacterial DNA was extracted and sequenced on an Ion Torrent PGM system (Life Technologies) as described by Tang & Lightner (2014). Draft genome sequences were deposited into GenBank (no. JOKE-00000000.1 for strain 13-028/A3; JOKT00000000.1 for strain 13-028/A2).
Plasmid sequence analyses
By Blast analysis in V. parahaemolyticus 13-028/A3, we found contigs with partial plasmid sequences. To extend and join these contigs, PCRs with primers selected from 5′ and/or 3′ ends of each contig were performed with PuReTaq Ready-To-Go PCR beads (GE Healthcare). Each PCR reaction contained 0.2 μM of each primer, 10 mM Tris-HCl (pH 9.0), 50 mM KCl, 1.5 mM MgCl2, 2.5 U of Taq DNA polymerase, and 1 μl of extracted DNA. The amplicons were purified and sequenced to fill the gaps or to confirm the joining between contigs. The complete nucleotide sequence of pVPA3-1 was deposited in GenBank (accession no. KM067908).
Vector NTI program (Life Technologies) was used to create a circular plasmid map. Translated open reading frames (ORFs) were then compared with known protein sequences using BlastP, and sequences with > 25% homology were considered as matches. The Artemis Comparison Tool was used as a pairwise DNA sequence comparison viewer (Carver et al. 2008).
Quantification of pVPA3-1 by comparative qPCR analyses
All qPCR was carried on the StepOnePlus thermal cycler (Life Technologies) with a PerfeCta SYBR Green FastMix kit (Quanta). Each reaction (total volume: 20 μl) comprised 1-fold SYBR Green qPCR mix, 300 nM both forward and reverse primer targeting toxR ( pirB-like, or ORF14-coding gene), and 1 μl of DNA template. The primer sequences are listed in Table 1. The following cycling conditions were employed: initial denaturation at 95°C for 3 min, followed by 40 cycles of 95°C for 5 s and 60°C for 30 s. For each gene, a standard curve of 6 serial dilutions of extracted DNA (from V. parahaemolyticus 13-028/A3) was run to determine the amplification efficiency for each gene. For each run, a non-template control was included, and each sample was run in duplicate. After quantitative PCR (qPCR) runs, melting curve analyses were performed to verify the presence of the amplicons. The Δ cycle threshold (Ct) values between the pirB-like (or ORF14-coding gene) and toxR gene (single-copy reference standard) were calculated by StepOne vs.2.2.2 software using a comparative Ct method.
Table 1.
PCR primers and their sequences used in this study
| Primer | Sequence (5′ to 3′) | Amplicon size (bp) |
|---|---|---|
| toxR-F | AATCCATGGATTCCACGCGTTATTT | 103 |
| toxR-R | CACCAATCTGACGGAACTGAGATTC | |
| ORF14-F | GGCTCTTTCATAGGTGGTGTCATTC | 103 |
| ORF14-R | CGACTACTATGCCGTTGAGTTGAAG | |
| VpPirB-F | ACTAGGCAAGGCTCATAAATATGACG | 102 |
| VpPirB-R | ATTGCTTCAGGTCCATTGGCAATAA | |
| VpPirA-284F | TGACTATTCTCACGATTGGACTG | 284 |
| VpPirA-284R | CACGACTAGCGCCATTGTTA | |
| VpPirB-392F | TGATGAAGTGATGGGTGCTC | 392 |
| VpPirB-392R | TGTAAGCGCCGTTTAACTCA |
Proteomic analysis
To identify the presumptive toxin(s) causing AH-PND, V. parahaemolyticus 13-028/A3 was grown in the TSB+ media for 24 to 48 h and centrifuged at 6000 × g (15 min) to remove the bacteria, followed by filtering with a 0.2 μm membrane. These cell-free media were precipitated with trichloroacetic acid, resuspended in a sample buffer (Laemmli, 2-fold concentrate), and boiled for 5 min. Samples were spun down and loaded into the 30 μl wells in a 12% precast SDS-PAGE gel (BioRad). A protein gel was run by using a running buffer (25 mM Tris, pH 8.3, 192 mM glycine, 0.1% SDS) at a voltage of 150 V for 1 h. The gel was then stained with Coomassie blue. A single lane of stained gel was excised and tryptically digested at The University of Arizona Proteomic Core facility. The digested peptides were extracted and concentrated; the concentrated peptide solution containing 0.5 μg of proteins was loaded into an HPLC autosampler vial and analyzed by liquid chromatography-mass spectrometry (LC-MS)/MS. The SEQUEST database search algorithm (Thermo Fisher) was used to interpret the MS/MS spectra. All spectra were matched to a protein database created using amino acid sequences from V. parahaemolyticus 13-028/A3 and available V. parahaemolyticus protein databases obtained from NCBI and common contaminants such as trypsin and human keratin peptides. The proteins with 2 or more peptide matches obtained from the SEQUEST database search algorithm are reported.
Duplex PCR for the detection of pirA- and pirB-like genes
For duplex PCR assays detecting pirA- and pirB-like genes, PuReTaq ready-to-go PCR beads were used. The primers used were VpPirA-284F/R and VpPirB-392F/R (see Table 1). Amplifications were performed with the following parameters: initiation denaturation at 94°C for 3 min, followed by 35 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 30 s; and a final extension at 72°C for 7 min. Following PCR, an aliquot of PCR products was analyzed in a 1.5% gel containing ethidium bromide.
To determine the detection limit of this duplex PCR, a V. parahaemolyticus 13-028/A3 culture containing 8.9 × 108 CFU ml−1 was pelleted, resuspended in 1 ml of water, boiled for 10 min, and pelleted. The bacterial DNA in the supernatant was serially diluted and used as templates.
RESULTS
69 kb pVPA3-1 plasmid
A large plasmid (69 168 bp, named pVPA3-1) was identified in the Vibrio parahaemolyticus strain 13-028/A3 that causes AHPND. BlastN analyses also revealed pVPA3-1 in 4 other pathogenic strains from Thailand and Mexico (GenBank nos. JALL00000000.1, BAVF00000000.1, BAVG00000000.1, BAVH00000000.1; Gomez-Gil et al. 2014, Kondo et al. 2014). This plasmid was not found in 4 non-pathogenic strains of V. parahaemolyticus collected in Vietnam (GenBank no. JOKT00000000.1) and Thailand (GenBank nos. BAVI00000000.1, BAVJ00000000.1, BAVK00000000.1). The GC content of pVPA3-1 is 45.9%, similar to the 45.3% found in the V. parahaemolyticus genome.
To determine the copy number of the pVPA3-1 plasmid, we applied a comparative qPCR to determine the relative quantity of the pirB-like gene (which encodes a PirB toxin-like protein harbored by this plasmid, see ‘Insecticidal toxin genes within the plasmid’) versus the toxR gene. The toxR gene is used as an endogenous control; ToxR protein is a transcriptional regulator for outermembrane proteins and has been used as a species-specific marker (Kim et al. 1999). Only 1 copy of the toxR gene is found in chromosome I in the V. parahaemolyticus strains RIMD 2210633 and BB22OP. The results of a comparative SYBR Green qPCR showed 37 copies of pVPA3-1 plasmid per cell in strain 13-028/A3 (Table 2). There were 7 to 121 copies per cell among various pathogenic strains isolated from Mexico and Vietnam. We also applied another pair of qPCR primers targeting the ORF14-coding gene of the plasmid and found similar results; there were 50 copies of plasmid per cell in strain 13-028/A3 (Table 1). The amplification efficiencies of these 3 qPCRs (targeting toxR, pirB-like, and ORF14-coding genes) were equivalent, 96 to 124%, in 3 runs.
Table 2.
Acute hepatopancreatic necrosis disease (AHPND) pathogenicity (as determined by laboratory infections and/or histological examination), origin, and year of collection of Vibrio parahaemolyticus strains, showing the results of the duplex VpPirAB PCR and plasmid copy numbers. ORF: open reading frame; nd: not determined; –: not detected; Pos: positive reaction by VpPirAB PCR
| Strain | Plasmid copies/bacterium | VpPirAB PCR | Origin (year) | AHPND pathogenicity | |
|---|---|---|---|---|---|
| pirB-like | ORF14 | ||||
| 12-194G | 36 | 33 | Pos | Vietnam (2012) | Pathogenic |
| 13-028/A3 | 37 | 50 | Pos | Vietnam (2013) | Pathogenic |
| 14-188/1 | 32 | 39 | Pos | Vietnam (2014) | Pathogenic |
| 14-188/2 | 13 | 11 | Pos | Vietnam (2014) | Pathogenic |
| 14-188/3 | 121 | 95 | Pos | Vietnam (2014) | Pathogenic |
| 14-188/4 | 9 | 14 | Pos | Vietnam (2014) | Pathogenic |
| 14-188/5 | nd | nd | Pos | Vietnam (2014) | Pathogenic |
| 13-511/A1 | 18 | 25 | Pos | Mexico (2013) | Pathogenic |
| 13-306/D4 | 56 | 54 | Pos | Mexico (2013) | Pathogenic |
| 14-231/B23 | 7 | 11 | Pos | Mexico (2014) | nd |
| 14-231/B25 | nd | nd | Pos | Mexico (2014) | nd |
| 14-231/D74 | 8 | 12 | Pos | Mexico (2014) | nd |
| 13-028/A2 | 0 | 0 | – | Vietnam (2013) | Non-pathogenic |
| 13-488L | nd | nd | – | India (2013) | Non-pathogenic |
| 13-431 | nd | nd | – | USA (2013) | Non-pathogenic |
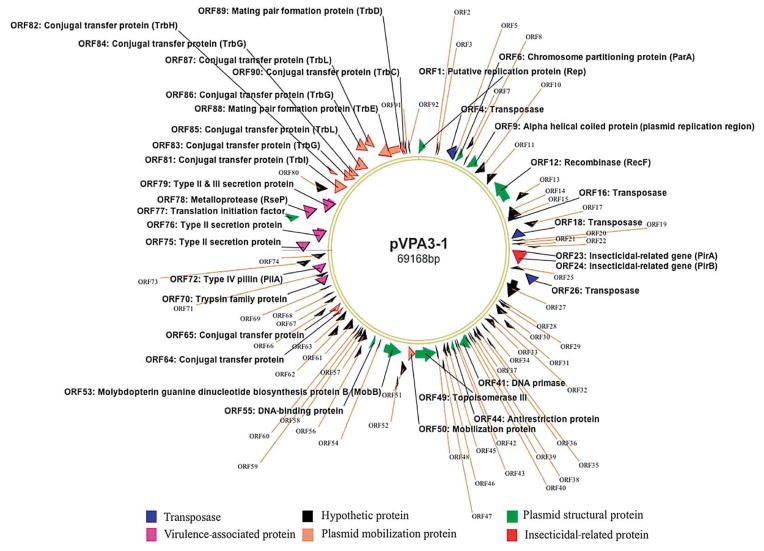
In a BlastN search, only a 1 kb transposase-coding gene showed significant matches to those found in the genome of Vibrio spp. and Shewanella spp. Using the RAST program (Overbeek et al. 2014), 92 ORFs (> 30 amino acids [aa]) were identified within the plasmid, and they can be categorized into 6 groups of genes: transposases, mobilization proteins, plasmid structural proteins, virulence-associated proteins, insecticidal toxin proteins, and hypothetical proteins, which are the largest group consisting of 45 ORFs (Fig. 1). The remaining 8 ORFs had lower or no significant similarities to those in the database, and their functions could not be assigned.
Fig. 1.
Plasmid pVPA3-1. All predicted open reading frames (ORFs) are shown as arrows in different colors. Direction of arrowhead represents the transcriptional orientation
Transposase genes within the plasmid
There are 4 transposase-coding ORFs: ORF4, −16, −18, and −26. These genes are highly conserved; ORF4 and −16 were 85 to 88% identical to those found in Vibrio spp. and Photobacterium spp. ORF18 and −26 are inverted repeats of the same transposase-coding gene, which is 100% identical to that of Vibrio sp; these 2 ORFs are located up- and downstream of the 3.5 kb fragment containing pirA- and pirB-like genes.
Plasmid mobilization proteins
There are 13 ORFs involved in plasmid conjugation and mobilization: ORF50, −64, −65, and −81 to −90; these ORFs are clustered in a 9 kb region of the plasmid. Four conjugation proteins encoded by ORF81, −82, −85, and −87 showed 28 to 40% identity to those found in plasmids (IncP type) of Photobacterium spp. and Pseudomonas spp. The other 9 proteins were 100% identical to those of Vibrio spp.
Plasmid structural proteins
Eight structural proteins are involved in DNA replication, stability, and maintenance of the plasmid: putative replication protein (ORF1), alpha helical coiled coil protein (ORF9), chromosomal-partitioning protein (ORF6), recombinase (ORF-12), antirestriction (ORF44), topoisomerase III (ORF49), DNA-binding protein (ORF55), and translation initiation factor (ORF77). Five of these had a 100% identity to those of Vibrio spp.
Virulence-associated proteins within the plasmid
The plasmid also contains 6 virulence-associated genes that may be related to the bacterial pathogenicity, including (1) a trypsin (ORF70), (2) 4 type II/III secretion proteins (ORF72, −75, −76, and −79), and (3) a metalloprotease (ORF78). The type II/III proteins are 100% identical to those found in various Vibrio spp. The trypsin is an extracellular protein determined by a proteomic analysis. The metalloprotease has only a 26% identity to that found in Singulisphaera acidiphila.
Insecticidal toxin genes within the plasmid
The pirA- and pirB-like genes were found in a 3.5 kb fragment. This region is flanked with inverted repeats of a transposase-coding gene. The pirA- and pirB-like genes are only 12 bp apart, transcribed in the same orientation, and have a GC content of 38.2%. The PirA-like protein has 111 aa (molecular weight of 13 kDa) and has 28 to 35% identity (E value: 10−4) to the PirA proteins found in Photorhabdus luminescens, P. asymbiotica, Xenorhabdus doucetiae, and Yersinia intermedia.
The PirB-like protein has 438 aa (molecular weight of 50 kDa) and has an Endotoxin_N domain (115 aa) at the N-ter half (aa no. 27–229), followed by a Jacalin_like lectin domain at the C-ter half (aa no. 311–411). BlastP results showed it has 28 to 31% identity (E = 10−45) to the insecticidal toxin PirB, found in a wide range of bacteria, including Alcaligenes faecalis, Y. intermedia, Xenorhadus spp., Photorhabdus spp., and Serratis spp., in which pirAB genes are located within bacterial chromosomes.
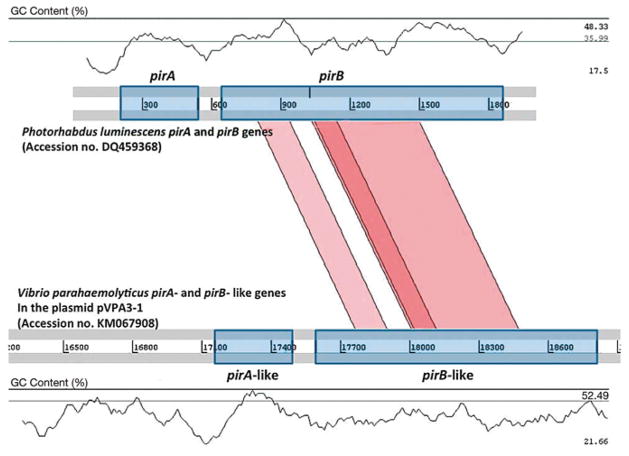
A schematic representation of the comparative analysis between the pirA- and pirB-like genes of V. parahaemolyticus 113-028/A3 (ORF23 and 24 in GenBank no. KM-067908) using Artemis Comparison Tool (ACT) with those of P. luminescens (GenBank no. DQ459368) is shown in Fig. 2. The pirB-like gene of V. parahaemolyticus has a GC content of 37%, similar to 38% in pirB of P. luminescens, but the pirA-like gene of V. parahaemolyticus has a GC content of 43% and is significantly different from the pirA of P. luminescens (35% GC content). The middle region of the PirB-like protein has a higher homology to the PirB protein of P. luminescens.
Fig. 2.
Schematic representation of the comparative analysis of the pirA- and pirB-like genes in Vibrio parahaemolyticus with the pirA and pirB genes of Photorhabdus luminescens. Regions for the pirA and pirB genes are shown with a symmetrical gene order. Translated BLAST (tblastx, score cut-off: 30) was used to align translated genome sequences. The color intensity is proportional to the sequence homology. Nucleotide base pairs are indicated between grey lines and GC contents (%) are shown
Proteomic analysis
By LC-MS/MS, a total of 400 proteins were identified with a 99% protein identification probability from an extracellular medium of V. parahaemolyticus 13-028/A3 grown in TSB+. Among these, 3 proteins were encoded by the pVPA3-1 plasmid; their molecular weights were 13, 38, and 50 kD. The 13 and 50 kDa proteins are PirA- and PirB-like proteins, respectively. The 38 kDa protein is a trypsin (345 aa) encoded by ORF 70, and consists of a signal peptide at aa no. 1–20, a trypsin-like cysteine/serine peptidase domain at aa no. 19–224, and a glygly-CTERM domain at C-ter.
PCR detection of pirA- and pirB-like genes
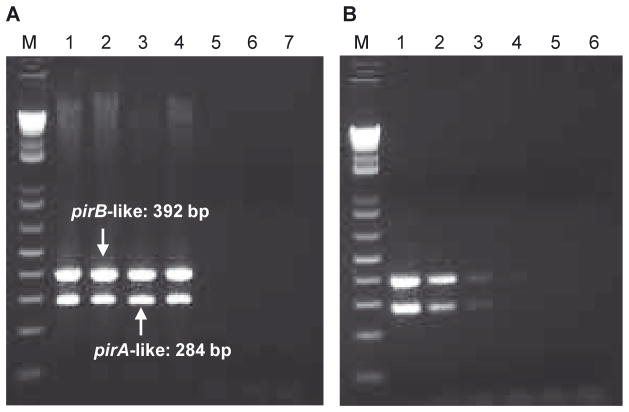
We selected primers from the pirA-and pirB-like genes to screen pathogenic AHPND-strains of V. parahaemolyticus collected from Vietnam and Mexico during 2012 to 2014 (Table 2). The results showed that both PCR amplicons were detected in the pathogenic strains and not found in the non-pathogenic strains (representative strains are shown in Fig. 3A). These 2 PCRs can be performed in the same tube as a duplex reaction and generate 284 bp ( pirA-like) and 392 bp ( pirB-like) amplicons, respectively. When higher concentrations (> 0.5 μg μl−1) of DNA template are used, a 1.4 kb band appears, which is formed from forward primer of pirA-like and reverse primer of pirB-like genes. The detection limit was 105 CFU ml−1 of V. parahaemolyticus 13-028/A3 (Fig. 3B).
Fig. 3.
PCR detection of pirA- and pirB-like genes. (A) Amplifications with pathogenic acute hepatopancreatic necrosis disease (AHPND)-Vibrio parahaemolyticus strains and non-pathogenic strains. Pathogenic strains collected from Mexico (Lane M: 1 kb plus DNA ladder; Lane 1: strain 13-511/A1; Lane 3: strain 13-306/D4, see Table 2) and Vietnam (Lane 2: strain 13-028/A3; Lane 4: strain 12-194G); non-pathogenic strains from shrimp farms in Vietnam (Lane 5: strain 13-028/A2), India (Lane 6: strain 13-488L), and USA (Lane 7: strain 13-431). (B) Determination of sensitivity by serially diluting V. parahaemolyticus 13-028/A3 DNA; bacterial concentrations were as follows: 1 × 107 (Lane 1), 1 × 106 (Lane 2), 1 × 105 (Lane 3), 1 × 104 (Lane 4), and 1 × 103 CFU ml−1 (Lane 5), and a non-template control (Lane 6). Lane M: 1 kb plus DNA ladder
This duplex PCR appeared to be specific for pathogenic strains of V. parahaemolyticus (Table 2), and it did not cross react with non-pathogenic strains of V. parahaemolyticus (24 isolates were tested), V. communis (4 isolates), V. harveyi (14 isolates), V. owensii (2 isolates) and Photobacterium spp. (1 isolate) isolated from shrimp farms in Vietnam, Ecuador, Peru, Mexico, India, and the US (Texas).
DISCUSSION
A plasmid that contains 2 toxin genes was found in the pathogenic AHPND-strains of Vibrio parahaemolyticus but was absent in non-pathogenic strains. These 2 genes are similar to the Pir toxins produced by other species of bacteria. These PirAB-like proteins are secreted into the bacterial media, and the cell-free culture media can cause AHPND (Tran et al. 2013), suggesting that these PirAB-like proteins may be the causative agents for AHPND. This is the first report describing the PirAB-like proteins in Vibrio species.
The modes of action for PirAB-like proteins have yet to be determined. However, they apparently differ from PirAB toxins that affect insects. Insecticidal PirAB proteins are known to produce both hemolymph and oral toxicity similar to those reported for the δ-endotoxins of Bacillus thuringiensis (Bt toxin; Waterfield et al. 2005, Blackburn et al. 2006). The insecticidal PirAB toxins primarily affect the midgut, whereas the shrimp AHPND toxins affect the hepatopancreas. Injection of V. parahaemolyticus 13-028/A3 into the shrimp hemolymph did not cause AHPND pathology, and oral (or immersion) exposure appears to be required for this disease to develop. Another difference is that pirA and pirB genes are localized within the bacterial chromosome in other bacteria, whereas the pirA- and pirB- like genes in AHPND-pathogenic strains of V. parahaemolyticus are harbored in a plasmid.
Within the plasmid, these genes are flanked by a transposase-coding sequence which is a mobile genetic element and can induce horizontal gene transfer; this can result in the need for frequent screening of pathogenic strains in shrimp culture systems. This is because these pirA- and pirB-like genes can potentially be spread through transposition, conjugation, and plasmid uptake, allowing non-pathogenic strains to become pathogenic. The potential for such transfers increases when bacteria are densely colonized, either in the shrimp stomach/hepatopancreas or in pond biofilms.
Although the presence of the PirAB toxin-like proteins in the cell-free media suggests that these are putative toxins that cause AHPND in shrimp, the toxicity of these proteins was not tested in this study. Future work is planned to produce AHPND pathology by injecting purified PirAB toxin-like proteins into shrimp.
Acknowledgments
This work was supported by CP Foods, Bangkok, Thailand. Mass spectrometry and proteomics data were acquired by the Arizona Proteomics Consortium supported by NIEHS grant ES06694 to the SWEHSC, NIH/NCI grant CA023074 to the AZCC, and by the BIO5 Institute of the University of Arizona. The Thermo Fisher LTQ Orbitrap Velos mass spectrometer was provided by grant 1S10 RR028868-01 from NIH/NCRR.
LITERATURE CITED
- Ahantarig A, Chantawat N, Waterfield NR, ffrench-Constant R, Kittayapong P. PirAB toxin from Photorhabdus asymbiotica as a larvicide against Dengue vectors. Appl Environ Microbiol. 2009;75:4627–4629. doi: 10.1128/AEM.00221-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn MB, Farrar RR, Novak NG, Lawrence SD. Remarkable susceptibility of the diamondback moth (Plutella xylostella) to ingestion of Pir toxins from Photorhabdus luminescens. Entomol Exp Appl. 2006;121:31–37. [Google Scholar]
- Carver T, Berriman M, Tivey A, Patel C, et al. Artemia and ACT: viewing, annotating and comparing sequences stored in a relational database. Bioinformatics. 2008;24:2672–2676. doi: 10.1093/bioinformatics/btn529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchaud E, Rusniok C, Frangeul L, Buchrieser C, et al. The genome sequence of the entomopathogenic bacterium Photorhabdus luminescens. Nat Biotechnol. 2003;21:1307–1313. doi: 10.1038/nbt886. [DOI] [PubMed] [Google Scholar]
- ffrench-Constant RH, Waterfield N, Burland V, Pema NT, Daborn PJ, Bowen D, Blattner FR. A genomic sam-ple sequence of the entomopathogenic bacterium Photorhabdus luminescens W14: potential implications for virulence. Appl Environ Microbiol. 2000;66:3310–3329. doi: 10.1128/aem.66.8.3310-3329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Gil B, Soto-Rodriguez S, Lozano R, Betancourt-Lozano M. Draft genome sequence of Vibrio parahaemolyticus strain M0605, which causes severe mortalities of shrimps in Mexico. Genome Announc. 2014;2:e00055–14. doi: 10.1128/genomeA.00055-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YB, Okuda J, Matsumoto C, Takahashi N, Hashimoto S, Nishibushi M. Identification of Vibrio parahaemolyticus strains at species level by PCR targeted to the toxR gene. J Clin Microbiol. 1999;37:1173–1177. doi: 10.1128/jcm.37.4.1173-1177.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo H, Tinwongger S, Proespraiwong P, Mavichak R, Unajak S, Nozaki R, Hirono I. Draft genome sequences of six strains of Vibrio parahaemolyticus isolated from early mortality syndrome/acute hepatopancreatic necrosis disease shrimp in Thailand. Genome Announc. 2014;2:e00221–14. doi: 10.1128/genomeA.00221-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeek R, Olsen R, Pusch GD, Olson GJ, et al. The SEED and the Rapid Annotations of microbial genomics using Subsystems Technology (RAST) Nucleic Acids Res. 2014;42:D206–D214. doi: 10.1093/nar/gkt1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang KFJ, Lightner DV. Homologues of insecticidal toxin complex genes within a genomic island in the marine bacterium Vibrio parahaemolyticus. FEMS Microbiol Lett. 2014;361:34–42. doi: 10.1111/1574-6968.12609. [DOI] [PubMed] [Google Scholar]
- Tran L, Nunan L, Redman RM, Mohney LL, Pantoja CR, Fitzsimmons F, Lightner DV. Determination of the infectious nature of the agent of acute hepatopancreatic necrosis syndrome affecting penaeid shrimp. Dis Aquat Org. 2013;105:45–55. doi: 10.3354/dao02621. [DOI] [PubMed] [Google Scholar]
- Waterfield N, Kamita SG, Hammock BD, ffrench-Constant R. The Photorhabdus Pir toxins are similar to a developmentally regulated insect protein but show no juvenile hormone esterase activity. FEMS Microbiol Lett. 2005;245:47–52. doi: 10.1016/j.femsle.2005.02.018. [DOI] [PubMed] [Google Scholar]