Abstract
Introduction
Polybrominated diphenyl ethers (PBDEs) comprise a class of halogenated compounds used extensively as flame retardant chemicals in consumer products resulting in nearly ubiquitous human exposure. Mounting evidence suggests that PBDEs are developmental neurotoxicants; however, associations between early life exposure and child behavior have been largely limited to a single developmental time point.
Methods
The study population consists primarily of white, black and Chinese women who were pregnant on 11 September 2001 and delivered at 1 of 3 downtown New York City hospitals. Maternal–child pairs were followed through age 7 years. Cord blood was collected at delivery and PBDE plasma levels for 210 samples were analyzed by the U.S. Centers for Disease Control and Prevention. The Child Behavior Checklist, a validated maternal-report instrument used for assessing child behavior, was administered annually between the ages of 3 and 7 years. We analyzed the association between natural log-transformed and dichotomized (low vs. high) PBDEs and attention problems using multivariable adjusted negative binomial regression.
Results
We detected 4 PBDE congeners in more than 50% of samples, with concentrations highest for BDE-47 (median ± IQR: 11.2 ± 19.6 ng/g). In adjusted analyses, we detected associations between BDE-47 (1.21, 95% CI: 1.00, 1.47), and BDE-153 (1.18, 95% CI: 1.00, 1.39) in cord plasma and increased attention problems among children at age 4 (n = 109) but not 6 (n = 107) years.
Conclusions
Our findings demonstrate a positive trend between prenatal PBDE exposure and early childhood attention problems, and are consistent with previous research reporting associations between prenatal PBDE exposure and disrupted child behaviors.
Keywords: PBDEs, Flame retardants, Prenatal, Neurodevelopment, Attention
1. Introduction
Attention problems in young children are associated with learning deficits and poor school adjustment. Attention deficit hyperactivity disorder (ADHD), the most frequently diagnosed child neurobehavioral disorder, has a prevalence among United States children aged 5–17 years of approximately 12.3% and 5.5% for boys and girls, respectively (Akinbami et al., 2011; A.P. Association, 2000). Children with a diagnosis of ADHD, including symptoms of inattention, are often at increased risk for poor academic achievement, antisocial behaviors, poor self-esteem and drug abuse later in life (Shaw et al., 2012), precipitating substantial financial costs for affected families and for society (Danckaerts et al., 2010; Doshi et al., 2012). The etiology of attention problems and ADHD is poorly understood. Research supports the role of both genetic (Barkley, 2000) and environmental factors (Banerjee et al., 2007; Polanska et al., 2012), including early life exposure to polybrominated diphenyl ethers (PBDEs), a class of halogenated flame retardants that have been used in large quantities (3–30% by product weight) to reduce the flammability of consumer products (textiles, furniture containing polyurethane foam, plastics, and wire insulation) manufactured and imported into the United States (Abbasi et al., 2015).
PBDEs are not chemically bound to products during manufacture, and thus have a propensity to migrate into environmental media over time, where they can be absorbed by wildlife and humans owing to their lipophilicity (Gill et al., 2004; Watanabe and Sakai, 2003). Incidental ingestion of house dust (Stapleton et al., 2008) and consumption of food (oily fish, meat and dairy products) comprise the primary pathways of human exposure (Schecter et al., 2006), with analyses of cord blood, fetal blood and placental tissue demonstrating that PBDEs readily cross the placenta and enter fetal circulation (Dassanayake et al., 2009; Herbstman et al., 2008; Mazdai et al., 2003). Biomonitoring studies suggest that body burdens are highest among North Americans, with levels in breastmilk found to be 10–100 times higher than levels in European and Asian women (Fangstrom et al., 2008; Lorber, 2008; Zhang et al., 2011). Results from cross-sectional studies suggest that concentrations are highest during early childhood, with evidence indicating peaks around 2 years (Lunder et al., 2010; Toms et al., 2009) and 4–6 years (Sjodin et al., 2014), likely due to increased hand-to-mouth activity and frequent contact with floors during early childhood.
PBDEs have been classified as developmental neurotoxicants based on the results of experimental (Costa and Giordano, 2007; Dingemans et al., 2011) and observational (Herbstman and Mall, 2014; Roth and Wilks, 2014) research. Several studies suggest that PBDEs are associated with developmental profiles characterized by behaviors consistent with ADHD. For example, findings from rodent models demonstrate that compared to controls, animals exposed to PBDE mixtures early in life have significantly impaired sustained attention (Driscoll et al., 2009) and increased hyperactivity (Eriksson et al., 2001). Likewise, results from 4 longitudinal birth cohort studies have identified relationships between pre- and postnatal exposure to PBDEs and symptoms of inattention (Eskenazi et al., 2013; Gascon et al., 2011; Roze et al., 2009), hyperactivity (Chen et al., 2014), or impulsivity (Eskenazi et al., 2013) in early childhood.
Based on these findings, in addition to mounting evidence documenting risks for ecotoxicity (Montano et al., 2013) and other adverse health endpoints (Linares et al., 2015), the Stockholm Convention listed 2 (PentaBDE and OctaBDE) of the 3 (DecaBDE) major commercial mixtures as persistent organic pollutants (POPs) in 2004 (UNEP, 2012). Subsequently, the European Union banned these 2 formulations (Council decision (EC), 2003), which have also been voluntarily phased out of production in the United States (EPA, 2015; G.L.C. Corporation, 2005). Despite this phase out, environmental contamination and human exposure are expected to persist owing to the stability of PBDEs in environmental media and their continued release from existing consumer products (Abbasi et al., 2015).
This birth cohort study was established to examine the impact of prenatal exposure to chemical toxicants, including PBDEs, released in the dust, smoke and fumes following collapse of the World Trade Center (WTC) buildings in the wake of the 11 September 2001 (9/11) terrorist attack. Previous analyses conducted on this cohort have demonstrated that cord plasma PBDE concentrations are consistent with exposure levels detected in other United States birth cohorts (Herbstman et al., 2008), suggesting that a measureable spike in exposure did not result from the WTC event. As such, these samples provide an opportunity to examine associations with exposure levels generalizable to the United States population. Specifically, we hypothesized that prenatal PBDE exposure would be associated with increased attention problems during early childhood.
2. Methods
2.1. Study population and design
The study population consists of women who delivered singleton babies at 1 of 3 downtown New York City hospitals (Beth Israel, St. Vincent's and its affiliated Elizabeth Seton Childbearing Center, and New York University Downtown Hospital) with close proximity to the WTC site (Lederman et al., 2004) between December 12, 2001 and June 26, 2002. Women were approached when they presented for delivery at the hospital and were eligible for participation if they were between the ages of 18–39 years, reported that they smoked <1 cigarette per day during pregnancy, did not use illegal drugs in the preceding year, did not have a preexisting medical condition (diabetes, hypertension, HIV infection, AIDS) and were pregnant on 11 September 2001, based on their estimated last menstrual period. Maternal–child dyads were followed through age 7 years. Institutional Review Board approval was obtained before enrollment began and all women gave written informed consent before delivery. The Centers for Disease Control and Prevention (CDC) were determined not to be engaged in human subjects research because no personally identifiable information was made available to CDC researchers.
2.2. Interview
Medical history and delivery information were abstracted from medical records and collected by maternal self-report. Structured interviews were conducted following delivery and prior to hospital discharge by project staff in the mother's preferred language (English, Spanish, or Chinese) to collect demographic information, reproductive history, information on background environmental exposures, occupational history and the location of the residences and workplaces of the mother during the weeks following 11 September 2001.
2.3. Maternal cognitive & psychological tests
Maternal intelligence was assessed during the first study visit, which took place at approximately 12 months of age, using the Test of Non-Verbal Intelligence, Second Edition (TONI-2), a validated instrument for measuring general intelligence independent of language and cultural biases (Brown et al., 1990). Maternal demoralization was measured at delivery and repeatedly at each follow-up period using the Psychiatric Epidemiology Research Instrument Demoralization scale (PERI-D), which provides an indicator of maternal psychological state and is characterized by features of depression, mistrust, helplessness, hopelessness, and poor self-esteem (Dohrenwend et al., 1981).
2.4. Cord plasma PBDE measurement
2.4.1. Cord blood collection
At the time of delivery blood samples were collected from the umbilical cord, transported to our laboratory facilities in Northern Manhattan and processed within hours of collection. The buffy coat, packed red blood cells and plasma were separated and stored at −70 °C. Frozen plasma from 210 samples was shipped to the CDC on dry ice for laboratory analyses. The remaining 119 women that initially enrolled either did not consent to cord blood collection or the sample was inadequate for PBDE analysis.
2.4.2. Laboratory methods
Plasma samples were analyzed for PBDEs at the CDC's Persistent Organic Pollutants Biomonitoring Laboratory at the National Center for Environmental Health. Target analytes included the following PBDE congeners: BDEs 47, 85, 99, 100, 153, 154, and 183. Detailed analytic methods are provided elsewhere (Herbstman et al., 2010; Hovander et al., 2000; Sjodin et al., 2004). Briefly, samples were pretreated and extracted using a solid-phase extraction system and final determination of the target analytes was performed by gas chromatography/isotope dilution high-resolution mass spectrometry. Blank samples were included in each analytical run (3 blanks per 24 samples). The median level detected in blank samples analyzed in parallel to the study samples was subtracted from all sample results. The limits of detection (LODs) were defined as the highest of (i) 3 times the standard deviation of the blank samples and (ii) the instrument detection limit. Individual sample specific LODs were calculated by dividing the detection limit in picograms (pg) with the available sample size in grams (g). Plasma concentrations of co-extracted lipids (total triglycerides and total cholesterol) were determined at CDC using commercially available test kits from Roche Diagnostics Corp (Indianapolis, IN).
2.4.3. PBDE data
Previously published analyses of these cold blood PBDE data have demonstrated that no detectable contamination occurred during sample collection (Herbstman et al., 2010). We lipid adjusted each congener and focused our statistical analyses on the 4 that we detected in more than 50% of our samples (BDEs 47, 99, 100, and 153). We imputed values for undetected concentrations by dividing the LOD by the square root of 2. We examined PBDE data as both natural log-transformed continuous and dichotomized measures, with the latter parametrized into high (highest 20%) and low (lowest 80%) exposure groups in order to examine the effect of exposure at the high end of the distribution relative to the remainder of the study population.
2.5. Child attention assessment
Beginning at child age 3 years, we administered the Child Behavior Checklist (CBCL) annually through age 7 years. The CBCL is a psychometric instrument applied widely in clinical and research settings to assess child behavior and is validated for use among preschool (1.5–5 years) and school-aged (6–18 years) children (Achenback and Rescorla, 2000, 2001). Using a series of questions, the instrument instructs the mother to rate her child's behavior over the prior 6 months using a 3-category scale ranging from not true to often true. The CBCL consists of 10 empirically based syndrome scales composed of sets of co-occurring problems identified by factor analysis. Total scores are calculated by summing the responses for each question within the scale to create a raw score. The instrument also provides T scores, which are standardized to a normative population, however, because T scores are truncated these scores do not allow researchers to differentiate among children with low scores, which are indicative of fewer problems. In this study, we examined the raw scores from the attention problems syndrome subscale, which is derived as the sum of 5 questions or 10 questions, for the preschool and school-aged versions, respectively. The CBCL was administered in English, Spanish or Chinese, depending on the mother's language preference and completion was overseen by research workers trained in neurodevelopmental testing. The majority of follow-up assessments were conducted at the Columbia Center for Children's Environmental Health, however a proportion of the assessments were conducted in the child's home if the parents were unable or unwilling to travel to the center. At ages 5 and 7, the CBCL was administered over the phone, as no in-person visits were conducted.
2.6. Statistical methods
2.6.1. Descriptive statistics
We constructed boxplots and histograms to visually examine variables included in our models, and used analysis of variance (ANOVA) and chi-square tests to descriptively examine stratum-specific continuous and categorical variables, respectively.
2.6.2. Model specification
We conducted multivariable regression analysis to evaluate the relationship between cord plasma PBDE concentrations and attention problems at ages 4 and 6 years, which reflect scores on the preschool and school-aged CBCL, respectively. Although we have longitudinal data, we do not expect the effect of PBDEs on child attention to vary over time, therefore, we did not examine our data following a repeated measures approach. We selected these 2 periods as the focus of our analyses as they reflect the oldest age at which the preschool and school-aged CBCLs were administered in person, rather than over the phone. In our dataset, the CBCL attention problem scores consist of integer values ranging from 0 to 18 with a strong right-skew, which was not adequately corrected for by a natural log-transformation. Based on these characteristics, attention problem scores are best represented as Poisson-distributed count data. However, the variance of these scores exceeded the mean at ages 4 years (mean ± variance: 1.82 ± 3.14) and 6 years (mean ± variance 3.78 ± 11.99), and maximum likelihood tests demonstrated dispersion was significantly greater than 0 (4 year dispersion: 0.27, 95% CI: 0.12, 0.62; 6 year dispersion: 0.49, 95% CI: 0.29, 0.81), indicating our data were significantly over-dispersed. Based on these findings, we fit negative binomial regression models with robust standard errors. Because each mother rated her child's behavior over a 6 month period as instructed by the CBCL, we did not adjust for variation in observation time. We additionally examined our final models stratified by child sex as many childhood behavioral disorders, including ADHD, present with a strong sex-bias.
2.6.3. Covariate selection
We selected covariates for inclusion in multivariable models based on their a priori association with neurodevelopment, including age at testing, sex of the child, ethnicity (Asian, white, black/other), prenatal environmental tobacco smoke (ETS) exposure in the home, and intelligence of the mother (TONI-2). We included maternal demoralization at each follow-up period to control for the potential impact of the mother's psychological state on how she rated her child's behavior (Maoz et al., 2014). If the demoralization measurement was missing at any of the follow-up periods (age 3, n = 2; age 4, n = 3) we imputed a score based on the average of the mother's scores from the two adjacent periods; if two scores were not available, we replaced the missing value with the single score closest in time. We replaced missing maternal intelligence scores (4 year = 17%, 6 year = 21%) with the mean value for all mothers on the TONI-2 and included a missing indicator variable in our final models. We considered inclusion of additional covariates if they changed the regression coefficient for PBDEs by more than 10% when added one at a time to the model including a priori variables. Based on this approach, we additionally included maternal age and marital status in our final models. We assessed statistical significance at a level of 0.05 and conducted all analyses using SAS v9.4 or STATA v13.
3. Results
3.1. Study population
The study population consists primarily of white, black and Chinese women and their children. At enrollment mothers had a mean age of 30.2 years and approximately 19% had less than a high school education. On 11 September 2001, the majority of pregnancies were in the first trimester (64%). Table 1 presents characteristics for 1) all participants enrolled in the cohort, 2) the subset of 210 participants with cord plasma PBDE measurements, and 3) the subsets of participants with PBDE measurements and CBCL attention problem scores at ages 4 (n = 109) and 6 years (n = 107). We did not detect significant differences between participants in the full cohort or these subsets for any of the characteristics examined in Table 1, with two exceptions. Mothers included in the 4 year follow-up were significantly older (mean of 31.7 years) compared to mothers initially enrolled (mean 30.2 years, t436 = −2.56, p = 0.01) and mothers with cord PBDE measurements (mean 30.4 years, t317 = −2.18, p = 0.03). Second, TONI-2 intelligence test scores were significantly less likely to be missing for mothers included in the 4 (17%) and 6 (21%) year follow-ups compared to mothers initially enrolled (36%) and those with cord PBDE measurements (39%). Among the 201 cord plasma samples analyzed for PBDEs, at least 50% had detectable levels of BDE-47 (81.4%), BDE-99 (59.5%), BDE-100 (63.6%) and BDE-153 (49.8%). These 4 congeners were moderately to highly correlated (Pearson R2 = 0.45–0.88, p < 0.001), and median concentrations were highest for BDE-47 (11.2 ng/g lipid), followed by BDE-99 (3.2 ng/g lipid), BDE-100 (1.4 ng/g lipid) and BDE-153 (0.7 ng/g lipid). Median attention scores at ages 4 years and 6 years were 1.5 points (range: 0–9) and 3 points (range: 0–18), respectively.
Table 1.
Characteristics of all cohort members, those with cord plasma PBDEs and those with CBCL scores at child ages 4 and 6 years.
| All (n = 329b) |
Cord plasma (n = 210c) |
Age 4 (n = 109) |
Age 6 (n = 107) |
|
|---|---|---|---|---|
| Maternal characteristics at deliverya | ||||
| Age (years) | 30.2 ± 5.2 | 30.4 ± 5.1 | 31.7 ± 4.8 | 31.1 ± 4.8 |
| Education at baseline | ||||
| <High school | 61 (19) | 45 (21) | 14 (13) | 17 (16) |
| High school or GED | 100 (30) | 64 (30) | 29 (27) | 30 (28) |
| College degree | 168 (51) | 101 (48) | 66 (61) | 60 (56) |
| Race/ethnicity | ||||
| Asian | 113 (34) | 85 (41) | 34 (31) | 38 (36) |
| Black | 50 (15) | 27 (13) | 16 (15) | 15 (16) |
| White | 133 (40) | 77 (37) | 50 (46) | 43 (40) |
| Other | 33 (10) | 21 (10) | 9 (8) | 9 (8) |
| Married/living with partner | 265 (81) | 172 (82) | 91 (83) | 89 (83) |
| ETS in the home (yes) | 59 (18) | 36 (17) | 17 (16) | 19 (18) |
| Intelligence (TONI-2) | 95.8 ± 11.4 | 95.9 ± 11.3 | 97.3 ± 13.5 | 97.1 ± 12.9 |
| Missing TONI-2 | 118 (36) | 82 (39) | 19 (17) | 22 (21) |
| Demoralization (PERI-D) | ||||
| Delivery | 0.88 ± 0.45 | 0.86 ± 0.45 | 0.92 ± 0.46 | 0.86 ± 0.40 |
| 4 years | 0.85 ± 0.46 | 0.82 ± 0.42 | 0.84 ± 0.46 | |
| 6 years | 0.73 ± 0.49 | 0.80 ± 0.52 | 0.83 ± 0.53 | |
| Child characteristics | ||||
| Birth weight (g) | 3420 ± 469 | 3399 ± 473 | 3430 ± 477 | 3453 ± 443 |
| Gestational age (days) | 277 ± 9.9 | 276 ± 10.4 | 277 ± 8.7 | 278 ± 8.3 |
| Trimester at WTC disaster | ||||
| Pre-conception | 21 (6) | 18 (9) | 8 (7) | 6 (6) |
| First | 210 (64) | 148 (71) | 73 (68) | 73 (69) |
| Second | 92 (28) | 40 (19) | 25 (23) | 25 (24) |
| Third | 3 (<1) | 3 (1) | 2 (2) | 3 (2) |
| Male | 161 (49) | 105 (50) | 57 (52) | 57 (53) |
| Breastfedd | 24.2 (27.8) | 22.1 (27.2) | 26.3 (28.7) | 24.5 (27.4) |
| Age at 4 year follow-up (days) | 1488 ± 40 | 2261 ± 49 | 1489 ± 36 | |
| Age at 6 year follow-up (days) | 2261 ± 49 | 2259 ± 50 | 2260 ± 50 | |
| Cord PBDE (ng/g lipid) [median, IQR] | ||||
| BDE 47 | 11.2 (19.6) | 11.2 (19.6) | 12.0 (17.5) | 11.4 (15.5) |
| BDE 99 | 3.2 (5.6) | 3.2 (5.6) | 3.5 (5.4) | 3.3 (4.9) |
| BDE 100 | 1.4 (2.1) | 1.4 (2.1) | 1.5 (2.0) | 1.4 (2.1) |
| BDE 153 | 0.7 (2.5) | 0.7 (2.5) | 1.1 (1.0)e | 0.7 (0.9)e |
| Attention problemsf [median, IQR] | ||||
| 4 years | 2 (3) | 1 (3) | 1 (3) | |
| 6 years | 2 (4) | 3 (4) | 3 (4) | |
Abbreviations: BDE, brominated diphenyl ether; CBCL, Child Behavior Checklist; ETC, environmental tobacco smoke; GED, General Education Development test; PBDE, polybrominated diphenyl ether; PERI-D, Psychiatric Epidemiology Research Instrument Demoralization scale; TONI-2, Test of Nonverbal Intelligence, 2nd edition.
Values represent mean ± SD or n (%) unless otherwise noted.
Age 4 years (n = 185); age 6 years (n = 169); demoralization 4 yrs. (n = 196); demoralization 6 yrs (n = 183); BDEs 47, 99 100 (n = 209); BDE 153 (n = 201); attention 4 yrs.: (n = 175); attention 6 yrs. (n = 166).
Demoralization 4 yrs. (n = 123); demoralization 6 yrs. (n = 118); BDEs 47, 99 100 (n = 209); BDE 153 (n = 201); attention 4 yrs.: (n = 109); attention 6 yrs. (n = 107).
Percent of the first year of life that the child was breastfed.
BDE 153 4 yrs. (n = 104); 6 yrs. (n= 103).
Attention problem raw scores derived from the CBCL Attention Problem Syndrome subscale. Higher scores are indicative of worse child behavior.
The lower scores at the earlier period likely reflect lower possible maximum score for the preschool CBCL attention problem scale (maximum = 10) compared to the school-aged attention problem scale (maximum = 20). Mean attention problem scores were not significantly different between boys and girls at ages 4 years (boys, 1.81 vs girls, 1.82) or 6 years (boys, 3.84 vs. girls, 3.71). Median cord plasma PBDE concentrations did not significantly differ between the initial 210 samples analyzed and samples included in the 4 year and 6 year analyses for any of the 4 congeners examined.
3.2. Regression models
In adjusted multivariable negative binomial regression models, we detected marginally statistically significant positive associations between natural-log transformed cord plasma BDEs 47, 100 and 153 with attention problem scores among children at age 4 years, but not 6 years (Table 2). At age 4 years, for a one ln-unit increase in PBDE concentration, we found attention problem scores increase by a factor of 1.21 for BDE-47 and BDE-100, 1.08 for BDE-99, and 1.18 for BDE-153, holding all other variables in the model constant. However, the 95% confidence intervals for these models include 1.00 (for BDE-99 and 100) or have a lower-bound estimate at 1.00 (for BDE-47 and BDE-153).
Table 2.
Bivariate and adjusted associations between continuous measurements of cord plasma PBDEs (ng/g lipid) and attention problems on the CBCL at ages 4 and 6 years, as reported by the mother.
| Age 4 CBCL (n = 109) | Age 6 CBCL (n = 107) | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariablea | Univariate | Multivariablea | |||||
| PBDEb | IRR ± SE | 95% CI | IRR ± SE | 95% CI | IRR ± SE | 95% CI | IRR ± SE | 95% CI |
| BDE-47 | 1.20 ± 0.09 | 1.03, 1.40 | 1.21 ± 0.12 | 1.00, 1.47 | 0.86 ± 0.08 | 0.72, 1.03 | 0.91 ± 0.09 | 0.75, 1.10 |
| BDE-99 | 1.07 ± 1.02 | 0.88, 1.29 | 1.08 ± 0.10 | 0.90, 1.28 | 0.92 ± 0.08 | 0.78, 1.08 | 0.95 ± 0.07 | 0.83, 1.10 |
| BDE-100 | 1.15 ± 0.10 | 0.97, 1.36 | 1.21 ± 0.13 | 0.97, 1.50 | 0.86 ± 0.10 | 0.69, 1.07 | 0.90 ± 0.10 | 0.72, 1.13 |
| BDE-153c | 1.09 ± 0.10 | 0.92, 1.30 | 1.18 ± 0.10 | 1.00, 1.39 | 0.95 ± 0.09 | 0.80, 1.14 | 1.03 ± 1.11 | 0.84, 1.26 |
Abbreviations: BDE, brominated diphenyl ether; CBCL, Child Behavior Checklist; IRR, Incidence rate ratio; PBDEs, polybrominated diphenyl ethers; TONI-2; Test of Nonverbal Intelligence, 2nd edition.
Adjusted for: age at exam, sex, ethnicity, environmental tobacco smoke, maternal intelligence (TONI-2), TONI-2 missing indicator, maternal age, marital status and maternal demoralization at exam.
IRR ± SE represent the estimated rate change in attention problem score per ln-unit increase in lipid-adjusted PBDE concentration (ng/g lipid).
Sample size = 104 at age 4 years and 103 at age 6 years due to 4 subjects missing BDE 153 only.
In sex-stratified analyses, we detected positive associations between each of the PBDE congeners examined and attention problem scores among boys (n = 57; IRR range: 1.00–1.28) and girls (n = 52; IRR range: 0.99–1.37) at age 4 years (see Supplemental Fig. 1), however, effect sizes were attenuated and did not reach statistical significance. Results were largely similar to un-stratified models at age 6 years (boys n = 57, girls n = 48), with IRRs ranging from 0.68 to 1.39 (see Supplemental Fig. 2).
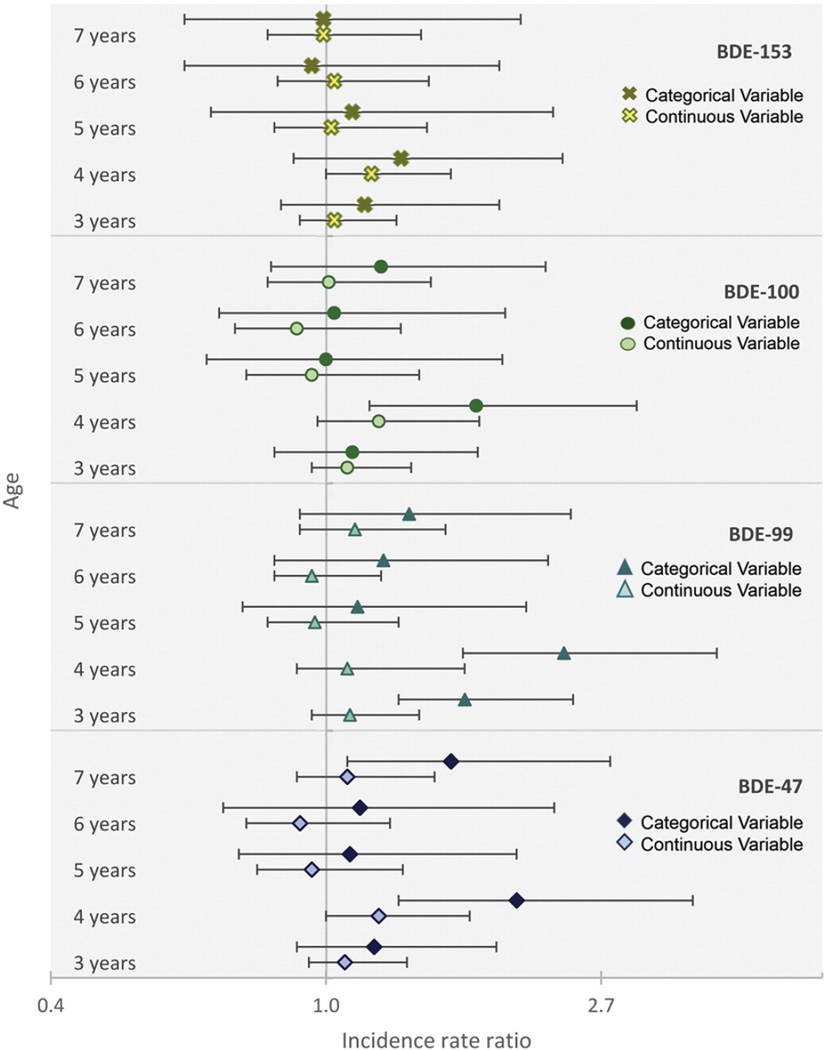
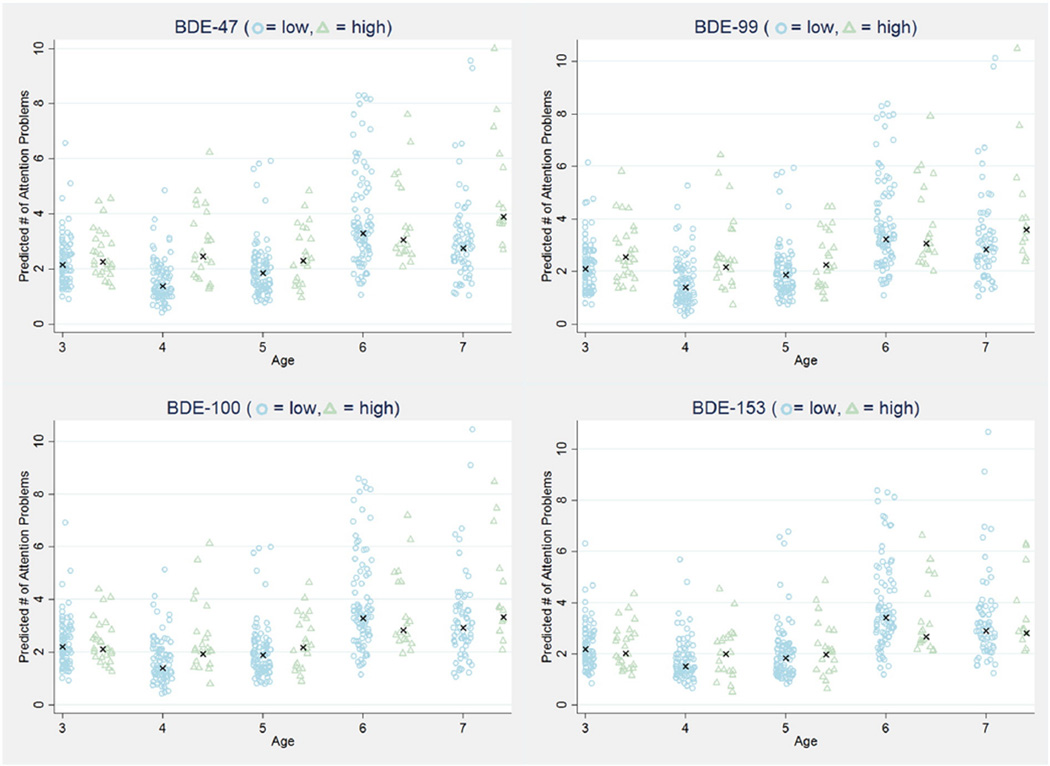
We additionally examined attention problems among children with low (lowest 80%) compared to high (highest 20%) exposure. Fig. 1 presents the IRRs from adjusted models examining cord plasma PBDE exposure parametrized as both a continuous and a categorical variable at each age from 3 years through 7 years. Consistent with our results from models examining continuous PBDE exposure, we found at age 4 years, children with high exposure to BDEs 47, 99 and 100 had 1.7 to 2.4 times the number of attention problems as children with low exposure. Statistically significant associations were additionally detected for BDE-47 at age 7 years (IRR = 1.57, 95% CI = 1.08, 2.30) and BDE-99 at age 3 years (IRR = 1.65, 95% CI = 1.36, 2.09). IRRs and sample sizes for children included at each follow-up period are presented in Supplemental Table 1. As demonstrated by Fig. 2, in models examining the expected number of attention problems, we predict that children with high (highest 20%) PBDE exposure will have more attention problems compared to children with low (lowest 80%) PBDE exposure for each of the 4 congeners examined at each age, except for age 6.
Fig. 1.
Associations (IRRs) between prenatal PBDE exposure and number of attention problems at ages 3–7 years. PBDE concentrations (ng/g lipid) are examined as ln-transformed continuous measures (light markers) and as dichotomous (dark markers) measures comparing the highest 20% of the exposure distribution to the lowest 80%. Error bars represent the 95% confidence limits. Models are adjusted for age at assessment, sex, ethnicity, prenatal environmental tobacco smoke, maternal intelligence (TONI-2), maternal age, marital status and maternal demoralization at the time of the behavioral assessment.
Fig. 2.
Predicted number of attention problems for children with high (highest 20%—triangles) and low (lowest 80%—circles) prenatal PBDE exposure at ages 3–7 years. Differences are statistically significant for BDE-47 at ages 4 and 7, BDE-99 at ages 3 and 4, and BDE-100 at age 4 (see Supplemental Table 1). One predicted data point was excluded from these figures because it was over 3× the standard deviation; however, it was considered in statistical testing and in the calculation of the median for each group (indicated by “x”).
4. Discussion
In this study, we observed a general positive trend between cord plasma concentrations of BDEs 47, 99, 100 and 153 with childhood attention problems, except at ages 5 and 6 years. In relation to the CBCL, these two ages are unique as the test transitions from a preschool version (1.5–5 years) to a school age version (6–18 years) at age 6 years. As such, our inconsistent findings may reflect the instruments' reduced capacity to effectively measure behavior patterns during this transition period.
Our results are consistent with findings from 3 prospective birth cohort studies that have previously demonstrated a relationship between PBDE exposure and increased child attention problems. Of these studies, only Eskenazi et al. (2013) assessed attention using maternally reported scores on the CBCL. In this cohort of Mexican-American children from California's Salinas Valley, maternal serum concentrations of Σ 4PBDEs (47, 99, 100 and 153; median: 24.9 ng/g lipid) measured during the 2nd trimester of pregnancy or at delivery was significantly associated with child attention problem scores above the 93rd percentile, a cut-off that captures scores considered to be borderline or clinically significant (OR per 10-fold increase in Σ 4PBDEs 4.6, 95% CI: 0.9–24.5; n = 249), however, no association was detected in models examining scores assessed continuously (β per 10-fold increase in Σ 4PBDEs: 0.1, 95% CI: −0.4, 0.6; n = 249). While mean attention problem scores among the California children were similar (age 5: 2.4 ± 1.6) to those from our NYC-based cohort (age 4: 1.8 ± 1.7), the distribution of scores is not available for comparison. Notably, models examining continuous scores were fit assuming a linear relationship, which may imply that the distribution of their scores were different than in our population.
Eskenazi et al. (2013) also examined a number of other maternally reported behavioral scales, including the Conners' ADHD/DSM-IV Scales (CADS) and Behavioral Assessment System for Children, 2nd edition (BASC-2) and detected statistically significant relationships between maternal Σ 4PBDEs concentrations with symptoms of ADHD measured by the CADS, but not the BASC-2, at age 7 years. Conversely, in a repeated measures analysis maternal serum BDE-47 concentrations (per 10-fold increase; median: 18.9 ng/g lipid) measured during pregnancy (week 16) in a Cincinnati, Ohio-based cohort were positively and significantly associated with child scores on the hyperactivity subscale, but not the attention problem subscale, of the BASC-2 (Chen et al., 2014). Additionally, the California-based cohort and a small European cohort (BDE-47 median: 0.9 ng/g lipid) have reported associations between maternal serum PBDE levels during pregnancy and reduced performance on neuropsychological tests of attention function, including impaired vigilance measured by the Kiddie Continuous Performance Test (Eskenazi et al., 2013) and reduced sustained attention measured by the Test of Everyday Attention for Children (Roze et al., 2009). These findings demonstrate reasonable concordance in associations detected with interviewer-administered instruments and maternal-report instruments.
In addition to these epidemiological studies demonstrating a positive association between PBDE exposure and child inattention, hyperactivity and impulsivity, previous analyses conducted on participants enrolled in this cohort have demonstrated that children with higher cord PBDE concentrations scored lower on tests of mental development (BSID-II) and intelligence (WPSSI-R) (Herbstman et al., 2010). These findings are consistent with a basic tenant of neurobiology, which maintains that attention and cognitive capacity are highly interrelated processes that operate together to retain and store new information. For example, while attention plays a critical role facilitating the judicious selection of salient material for cognitive processing, it is simultaneously constrained by the high demands of memory, such as neural processing speed and storage capacity (Cohen, 2014). Our findings suggest that these circuitous neural pathways may be particularly sensitive to the effects of PBDE exposure.
Results from experimental studies conducted in murine models indicate that altered maternal thyroid hormone homeostasis may mediate the relationship between prenatal PBDE exposure and disrupted neurodevelopment (Costa et al., 2014). Findings from studies examining these relationships in humans have been inconsistent, although in murine models, PBDEs have been consistently associated with reduced serum thyroxine (T4), unchanged triiodothyronine (T3), and increased thyrotropon (TSH) levels in the exposed dam, fetus and pups (Costa and Giordano, 2007). Multiple biological mechanisms have been proposed to explain these associations, including enhanced metabolism and excretion of T4, interactions with thyroid hormone transport systems, and agonistic or antagonistic behavior at thyroid hormone receptors (Costa and Giordano, 2007). For example, during development, thyroid hormones bind with nuclear thyroid hormone receptors to regulate expression of genes that play critical roles in establishing brain cytoarchitecture, including the progeneration and migration of neurons, myelination of axons, formation of synapses, and pruning of redundant connections (Bernal and Nunez, 1995; Rodier, 1995). The extraordinary precision required by these processes putatively heightens embryonic and fetal brain sensitivity to slight disturbances such as small alterations in thyroid hormone levels (Ahmed et al., 2008). Indeed, findings from a population-based cohort study using Danish nationwide registry data have demonstrated an association between altered thyroid hormone levels and functional changes, including increased risk of ADHD (Anderson et al., 2014, 2015). Our results suggest that future studies designed to examine the mediating role of thyroid hormones are needed to fully understand the pathways underlying our observed associations between prenatal PBDEs and child behavior.
This birth cohort study was established to examine the effects of exposure to dust, smoke and fumes emitted following the collapse of the WTCs on child development. However, in addition to serving as a potential source of chemical toxicants (McGee et al., 2003), the WTC disaster was a traumatic event and acute source of psychosocial stress. Several studies have demonstrated that maternal experiences of stress events characterized by a sudden onset and uncontrollable nature are associated with disrupted neurocognitive and behavioral development (Laplante et al., 2008; McEwen et al., 2015), including increased risk of ADHD (Li et al., 2010). Moreover, research suggests that psychosocial stressors may interact with chemical exposures resulting in enhanced effects on the developing brain (Hougaard and Hansen, 2007; Rauh et al., 2004). Here, we attempted to examine the combined impact of WTC-related stress and prenatal PBDEs on child attention problems but were ultimately unable to disentangle these two exposures. As expected, maternal demoralization measured at delivery was correlated with exposure to the WTC disaster (r spearman = 0.2, p = 0.03), and this correlation decreased with time since the disaster (see Supplemental Fig. 3). Unfortunately, our measure of child behavior is derived from a maternally reported instrument, warranting adjustment for maternal psychological state at the time of testing. As expected, maternal demoralization at delivery was correlated with maternal demoralization at the time of assessment (Pearson r = 0.3–0.5, p < 0.001), precluding our capacity to examine both the direct effect of maternal experiences of WTC-related stress during pregnancy on child behavior and the interaction between stress and PBDE exposure on this outcome. Future studies designed to examine child behavior using neuropsychological tests free from maternal report bias are needed to clarify these relationships.
Additional limitations include our inability to account for other factors that may account for variation in attention problems, such as the child's home environment or the heritability of attention problems. In sex-stratified analyses, we did not detect significant differences of the relationships between PBDEs and attention problems by child sex, however, we may have been unable to detect this interaction owing to sample size limitations.
Strengths of this study include measurement of PBDE levels in cord blood, its prospective design and measurement of the child behavior repeatedly from early to mid-childhood. However, while we observed significant associations between prenatal PBDE concentrations and attention problems measured at 4 years, we did not detect positive associations at age 6 years. The correlation between preschool and school-age attention problems typically ranges from 0.40 to 0.47 (Achenback and Rescorla, 2000) suggesting moderate stability, but additional unmeasured factors may have introduced variation in attention problems at 6 years in the present study, making it more difficult to isolate and detect attention problems associated with prenatal PBDE exposure.
5. Conclusion
This is the first study to examine prenatal exposure to PBDEs with child attention problems throughout early childhood, including both preschool and school age developmental periods. We detected positive associations between cord plasma PBDE levels and attention problems among preschool and school-aged children. These findings support the results of previous epidemiological studies reporting associations between prenatal PBDE exposure and symptoms of inattention, hyperactivity and impulsivity among children. Collectively, these results reinforce the decision to phase-out the use of PBDEs in consumer products and attest to the need to both implement programs for safely disposing of discarded products and for evaluating the safety of replacement flame retardants.
Supplementary Material
Acknowledgments
We thank C. Dodson, W-J Wang, K. Lester, and L. Stricke. This research was supported by the September 11th Fund of the New York Community Trust and 9/11 Neediest Fund; the National Philanthropic Trust; National Institute of Environmental Health Sciences grants ES09089, 5P01 ES09600, and 5R01 ES08977, and U.S. Environmental Protection Agency grant R827027. During preparation of this manuscript W. Cowell was supported by T32ES007322.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ntt.2015.08.009.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Transparency document
The transparency document associated with this article can be found in the online version.
References
- Abbasi G, Buser AM, Soehl A, Murray MW, Diamond ML. Stocks and flows of PBDEs in products from use to waste in the U.S. and Canada from 1970 to 2020. Environ. Sci. Technol. 2015;49:1521–1528. doi: 10.1021/es504007v. [DOI] [PubMed] [Google Scholar]
- Achenback TM, Rescorla LA. Manual for the ASEBA Preschool Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth & Families; 2000. [Google Scholar]
- Achenback TM, Rescorla LA. Manual for the ASEBA School-Age Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth & Families; 2001. [Google Scholar]
- Ahmed OM, El-Gareib AW, El-Bakry AM, Abd El-Tawab SM, Ahmed RG. Thyroid hormones states and brain development interactions. Int. J. Dev. Neurosci. 2008;26:147–209. doi: 10.1016/j.ijdevneu.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Akinbami L, Liu X, Pastor P, Reuben C. Attention deficity hyperactivity disordorder among children aged 5–17 years in the United States, 1998–2009, NCHS Data Brief, no 70. Hyattsville, MD: National Center for Health Statistics; 2011. [PubMed] [Google Scholar]
- Anderson S, Laurberg P, Wu C. Attention deficit hyperactivity disorder and autism spectrum disorder in children born to mothers with thyroid dysfuncion: a Danish nationwide cohort study. BJOG. 2014;121:1365–1374. doi: 10.1111/1471-0528.12681. [DOI] [PubMed] [Google Scholar]
- Anderson S, Olsen J, Laurberg P. Fetal programming by maternal thyroid disease. Clin. Endocrinol. 2015 doi: 10.1111/cen.12744. (Epub Adead of Print). [DOI] [PubMed] [Google Scholar]
- A.P. Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, DC: American Psychological Association Press; 2000. (text rev.). [Google Scholar]
- Barkley RA. Genetics of childhood disorders: XVII. ADHD, part 1: the executive functions and ADHD. J. Am. Acad. Child Adolesc. Psychiatry. 2000;39:1064–1068. doi: 10.1097/00004583-200008000-00025. [DOI] [PubMed] [Google Scholar]
- Banerjee TD, Middleton F, Faraone SV. Environmental risk factors for attention-deficit hyperactivity disorder. Acta Paediatr. 2007;96:1269–1274. doi: 10.1111/j.1651-2227.2007.00430.x. [DOI] [PubMed] [Google Scholar]
- Bernal J, Nunez J. Thyroid hormones and brain development. Eur. J. Endocrinol. 1995;133:390–398. doi: 10.1530/eje.0.1330390. [DOI] [PubMed] [Google Scholar]
- Brown L, Sherbenou R, Johnson S. Test of Non-Verbal Intelligence: A Language-Free Measure of Cognitive Ability. Austen, TX: PRO-ED, Inc.; 1990. [Google Scholar]
- Chen A, Yolton K, Rauch SA, Webster GM, Hornung R, Sjodin A, Dietrich KN, Lanphear BP. Prenatal polybrominated diphenyl ether exposures and neurodevelopment in U.S. children through 5 years of age: the HOME study. Environ. Health Perspect. 2014;122:856–862. doi: 10.1289/ehp.1307562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA. The Neuropsychology of Attention. 2nd. Springer US; 2014. [Google Scholar]
- Costa LG, Giordano G. Developmental neurotoxicity of polybrominated diphenyl ether (PBDE) flame retardants. Neurotoxicology. 2007;28:1047–1067. doi: 10.1016/j.neuro.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, de Laat R, Tagliaferri S, Pellacani C. A mechanistic view of polybrominated diphenyl ether (PBDE) developmental neurotoxicity. Toxicol. Lett. 2014;230:282–294. doi: 10.1016/j.toxlet.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Council Decision (EC) no. 11/2003, OJ L 42 of 6 February 2003, Amending for the 24th Time Council Directive 76/769/EEC Relating to Restrictions on the Marketing and Use of Certain Dangerous Substances and Preparations (Pentabromodiphenyl Ether, Octabromodiphenyl Ether) 2003 [Google Scholar]
- Danckaerts M, Sonuga-Barke EJ, Banaschewski T, Buitelaar J, Dopfner M, Hollis C, Santosh P, Rothenberger A, Sergeant J, Steinhausen HC, et al. The quality of life of children with attention deficit/hyperactivity disorder: a systematic review. Eur. Child Adolesc. Psychiatry. 2010;19:83–105. doi: 10.1007/s00787-009-0046-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassanayake RM, Wei H, Chen RC, Li A. Optimization of the matrix solid phase dispersion extraction procedure for the analysis of polybrominated diphenyl ethers in human placenta. Anal. Chem. 2009;81:9795–9801. doi: 10.1021/ac901805d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemans MM, van den Berg M, Westerink RH. Neurotoxicity of brominated flame retardants: (in)direct effects of parent and hydroxylated polybrominated diphenyl ethers on the (developing) nervous system. Environ. Health Perspect. 2011;119:900–907. doi: 10.1289/ehp.1003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohrenwend BP, Dohrenwend BS, Warheit GJ, Bartlett GS, Goldsteen RL, Goldsteen K, Martin JL. Stress in the community: a report to the President's Commission on the Accident at Three Mile Island. Ann. N. Y. Acad. Sci. 1981;365:159–174. doi: 10.1111/j.1749-6632.1981.tb18129.x. [DOI] [PubMed] [Google Scholar]
- Doshi JA, Hodgkins P, Kahle J, Sikirica V, Cangelosi MJ, Setyawan J, Erder MH, Neumann PJ. Economic impact of childhood and adult attention-deficit/hyperactivity disorder in the United States. J. Am. Acad. Child Adolesc. Psychiatry. 2012;51:990–1002. e2. doi: 10.1016/j.jaac.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Driscoll LL, Gibson AM, Hieb A. Chronic postnatal DE-71 exposure: effects on learning, attention and thyroxine levels. Neurotoxicol. Teratol. 2009;31:76–84. doi: 10.1016/j.ntt.2008.11.003. [DOI] [PubMed] [Google Scholar]
- EPA. DecaBDE Phase-out Initiative. United States Environmental Protection Agency: Chemical Safety and Pollution Prevention. Washington D.C.: United States; 2015. [Google Scholar]
- Eriksson P, Jakobsson E, Fredriksson A. Brominated flame retardants: a novel class of developmental neurotoxicants in our environment? Environ. Health Perspect. 2001;109:903–908. doi: 10.1289/ehp.01109903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Chevrier J, Rauch SA, Kogut K, Harley KG, Johnson C, Trujillo C, Sjodin A, Bradman A. In utero and childhood polybrominated diphenyl ether (PBDE) exposures and neurodevelopment in the CHAMACOS study. Environ. Health Perspect. 2013;121:257–262. doi: 10.1289/ehp.1205597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fangstrom B, Athanassiadis I, Odsjo T, Noren K, Bergman A. Temporal trends of polybrominated diphenyl ethers and hexabromocyclododecane in milk from Stockholm mothers, 1980–2004. Mol. Nutr. Food Res. 2008;52:187–193. doi: 10.1002/mnfr.200700182. [DOI] [PubMed] [Google Scholar]
- Gascon M, Vrijheid M, Martinez D, Forns J, Grimalt JO, Torrent M, Sunyer J. Effects of pre and postnatal exposure to low levels of polybromodiphenyl ethers on neurodevelopment and thyroid hormone levels at 4 years of age. Environ. Int. 2011;37:605–611. doi: 10.1016/j.envint.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Gill U, Chu I, Ryan JJ, Feeley M. Polybrominated diphenyl ethers: human tissue levels and toxicology. Rev. Environ. Contam. Toxicol. 2004;183:55–97. doi: 10.1007/978-1-4419-9100-3_3. [DOI] [PubMed] [Google Scholar]
- G.L.C. Corporation. Great Lakes Chemical Corporation Completes Phase-out of two Flame Retardants. Indianapolis, IN: Great Lakes Chemical Corporation; 2005. [Google Scholar]
- Maoz H, Goldstein T, Goldstein BI, Axelson DA, Fan J, Hickey MB, Monk K, Sakolsky D, Diler RS, Brent D, et al. The effects of parental mood on reports of their children's psychopathology. J. Am. Acad. Child Adolesc. Psychiatry. 2014;53 doi: 10.1016/j.jaac.2014.07.005. (1111-22 e5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbstman JB, Sjodin A, Apelberg BJ, Witter FR, Halden RU, Patterson DG, Panny SR, Needham LL, Goldman LR. Birth delivery mode modifies the associations between prenatal polychlorinated biphenyl (PCB) and polybrominated diphenyl ether (PBDE) and neonatal thyroid hormone levels. Environ. Health Perspect. 2008;116:1376–1382. doi: 10.1289/ehp.11379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbstman JB, Sjodin A, Kurzon M, Lederman SA, Jones RS, Rauh V, Needham LL, Tang D, Niedzwiecki M, Wang RY, et al. Prenatal exposure to PBDEs and neurodevelopment. Environ. Health Perspect. 2010;118:712–719. doi: 10.1289/ehp.0901340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbstman JB, Mall JK. Developmental exposure to polybrominated diphenyl ethers and neurodevelopment. Curr. Environ. Health Rep. 2014;1:101–112. doi: 10.1007/s40572-014-0010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hougaard KS, Hansen AM. Enhancement of developmental toxicity effects of chemicals by gestational stress. A review. Neurotoxicol. Teratol. 2007;29:425–445. doi: 10.1016/j.ntt.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Hovander L, Athanasiadou M, Asplund L, Jensen S, Wehler EK. Extraction and cleanup methods for analysis of phenolic and neutral organohalogens in plasma. J. Anal. Toxicol. 2000;24:696–703. doi: 10.1093/jat/24.8.696. [DOI] [PubMed] [Google Scholar]
- Laplante DP, Brunet A, Schmitz N, Ciampi A, King S. Project Ice Storm: prenatal maternal stress affects cognitive and linguistic functioning in 5 1/2-year-old children. J. Am. Acad. Child Adolesc. Psychiatry. 2008;47:1063–1072. doi: 10.1097/CHI.0b013e31817eec80. [DOI] [PubMed] [Google Scholar]
- Lederman SA, Rauh V, Weiss L, Stein JL, Hoepner LA, Becker M, Perera FP. The effects of the world trade center event on birth outcomes among term deliveries at three lower Manhattan hospitals. Environ. Health Perspect. 2004;112:1772–1778. doi: 10.1289/ehp.7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Olsen J, Vestergaard M, Obel C. Attention-deficit/hyperactivity disorder in the offspring following prenatal maternal bereavement: a nationwide follow-up study in Denmark. Eur. Child Adolesc. Psychiatry. 2010;19:747–753. doi: 10.1007/s00787-010-0113-9. [DOI] [PubMed] [Google Scholar]
- Linares V, Belles M, Domingo JL. Human exposure to PBDE and critical evaluation of health hazards. Arch. Toxicol. 2015;89:335–356. doi: 10.1007/s00204-015-1457-1. [DOI] [PubMed] [Google Scholar]
- Lorber M. Exposure of Americans to polybrominated diphenyl ethers. J. Expo. Sci. Environ. Epidemiol. 2008;18:2–19. doi: 10.1038/sj.jes.7500572. [DOI] [PubMed] [Google Scholar]
- Lunder S, Hovander L, Athanassiadis I, Bergman A. Significantly higher polybrominated diphenyl ether levels in young U.S. children than in their mothers. Environ. Sci. Technol. 2010;44:5256–5262. doi: 10.1021/es1009357. [DOI] [PubMed] [Google Scholar]
- Mazdai A, Dodder NG, Abernathy MP, Hites RA, Bigsby RM. Polybrominated diphenyl ethers in maternal and fetal blood samples. Environ. Health Perspect. 2003;111:1249–1252. doi: 10.1289/ehp.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee JK, Chen LC, Cohen MD, Chee GR, Prophete CM, Haykal-Coates N, Wasson SJ, Conner TL, Costa DL, Gavett SH. Chemical analysis of World Trade Center fine particulate matter for use in toxicologic assessment. Environ. Health Perspect. 2003;111:972–980. doi: 10.1289/ehp.5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gray J, Nasca C. Recognizing resilience: learning from the effects of stress on the brain. Neurobiol. Stress. 2015;1:1–11. doi: 10.1016/j.ynstr.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montano M, Gutleb AC, Murk AJ. Persistent toxic burdens of halogenated phenolic compounds in humans and wildlife. Environ. Sci. Technol. 2013;47:6071–6081. doi: 10.1021/es400478k. [DOI] [PubMed] [Google Scholar]
- Polanska K, Jurewicz J, Hanke W. Exposure to environmental and lifestyle factors and attention-deficit/hyperactivity disorder in children—a review of epidemiological studies. Int. J. Occup. Med. Environ. Health. 2012;25:330–355. doi: 10.2478/S13382-012-0048-0. [DOI] [PubMed] [Google Scholar]
- Rauh VA, Whyatt RM, Garfinkel R, Andrews H, Hoepner L, Reyes A, Diaz D, Camann D, Perera FP. Developmental effects of exposure to environmental tobacco smoke and material hardship among inner-city children. Neurotoxicol. Teratol. 2004;26:373–385. doi: 10.1016/j.ntt.2004.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier PM. Developing brain as a target of toxicity. Environ. Health Perspect. 1995;103(Suppl. 6):73–76. doi: 10.1289/ehp.95103s673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth N, Wilks MF. Neurodevelopmental and neurobehavioural effects of polybrominated and perfluorinated chemicals: a systematic review of the epidemiological literature using a quality assessment scheme. Toxicol. Lett. 2014;230:271–281. doi: 10.1016/j.toxlet.2014.02.015. [DOI] [PubMed] [Google Scholar]
- Roze E, Meijer L, Bakker A, Van Braeckel KN, Sauer PJ, Bos AF. Prenatal exposure to organohalogens, including brominated flame retardants, influences motor, cognitive, and behavioral performance at school age. Environ. Health Perspect. 2009;117:1953–1958. doi: 10.1289/ehp.0901015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter A, Papke O, Harris TR, Tung KC, Musumba A, Olson J, Birnbaum L. Polybrominated diphenyl ether (PBDE) levels in an expanded market basket survey of U.S. food and estimated PBDE dietary intake by age and sex. Environ. Health Perspect. 2006;114:1515–1520. doi: 10.1289/ehp.9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw M, Hodgkins P, Caci H, Young S, Kahle J, Woods AG, Arnold LE. A systematic review and analysis of long-term outcomes in attention deficit hyperactivity disorder: effects of treatment and non-treatment. BMC Med. 2012;10:99. doi: 10.1186/1741-7015-10-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjodin A, Jones RS, Lapeza CR, Focant JF, McGahee EE, 3rd, Patterson DG., Jr Semiautomated high-throughput extraction and cleanup method for the measurement of polybrominated diphenyl ethers, polybrominated biphenyls, and polychlorinated biphenyls in human serum. Anal. Chem. 2004;76:1921–1927. doi: 10.1021/ac030381+. [DOI] [PubMed] [Google Scholar]
- Sjodin A, Jones RS, Caudill SP, Wong LY, Turner WE, Calafat AM. Polybrominated diphenyl ethers, polychlorinated biphenyls, and persistent pesticides in serum from the national health and nutrition examination survey, 2003–2008. Environ. Sci. Technol. 2014;48:753–760. doi: 10.1021/es4037836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Kelly SM, Allen JG, McClean MD, Webster TF. Measurement of polybrominated diphenyl ethers on hand wipes: estimating exposure from hand-to-mouth contact. Environ. Sci. Technol. 2008;42:3329–3334. doi: 10.1021/es7029625. [DOI] [PubMed] [Google Scholar]
- Toms LM, Sjodin A, Harden F, Hobson P, Jones R, Edenfield E, Mueller JF. Serum polybrominated diphenyl ether (PBDE) levels are higher in children (2–5 years of age) than in infants and adults. Environ. Health Perspect. 2009;117:1461–1465. doi: 10.1289/ehp.0900596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNEP. Listing of POPs Under the Stockholm Convention. Chatelaine, Switzerland: The Stockholm Convention, United Nations Environmental Programme; 2012. [Google Scholar]
- Watanabe I, Sakai S. Environmental release and behavior of brominated flame retardants. Environ. Int. 2003;29:665–682. doi: 10.1016/s0160-4120(03)00123-5. [DOI] [PubMed] [Google Scholar]
- Zhang L, Li J, Zhao Y, Li X, Yang X, Wen S, Cai Z, Wu Y. A national survey of polybrominated diphenyl ethers (PBDEs) and indicator polychlorinated biphenyls (PCBs) in Chinese mothers' milk. Chemosphere. 2011;84:625–633. doi: 10.1016/j.chemosphere.2011.03.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.