Abstract
We previously observed marked synergy between daptomycin and both rifampin and ampicillin against vancomycin-resistant enterococci (VRE). Because the synergy between daptomycin and ampicillin was observed for 100% of VRE strains with high-level ampicillin resistance (ampicillin MIC of ≥128 μg/ml), we looked for synergy between daptomycin and other β-lactams against 18 strains of methicillin-resistant Staphylococcus aureus (MRSA) by employing a time-kill method using Mueller-Hinton broth supplemented to 50 mg of Ca2+/liter. All strains were resistant to oxacillin (16 of 18 strains were resistant at drug concentrations of ≥256 μg/ml), and all strains were susceptible to daptomycin (the MIC at which 90% of the tested isolates were inhibited was 1 μg/ml). Daptomycin was tested at concentrations of 2, 1, 0.5, 0.25, 0.125, and 0.0625 μg/ml alone or in combination with oxacillin at a fixed concentration of 32 μg/ml. Synergy was found for all 18 strains with daptomycin at one-half the MIC in combination with 32 μg of oxacillin/ml, and synergy was found for 11 of 18 strains (61%) with daptomycin at one-fourth the MIC or less in combination with oxacillin. At 24 h, the daptomycin-oxacillin combination with daptomycin at one-half the MIC showed bactericidal activity against all 18 strains, and the combination with one-fourth the daptomycin MIC showed bactericidal activity against 9 of 18 strains. We also used a novel screening method to look for synergy between daptomycin and other β-lactams. In this approach, daptomycin was incorporated into Ca2+-supplemented Mueller-Hinton agar at subinhibitory concentrations, and synergy was screened by comparing test antibiotic Kirby-Bauer disks on agar with and without daptomycin. By this method, daptomycin with ampicillin-sulbactam, ticarcillin-clavulanate, or piperacillin-tazobactam showed synergy comparable to or greater than daptomycin with oxacillin. For seven of the eight strains tested, time-kill studies confirmed synergy between daptomycin and ampicillin-sulbactam with ampicillin in the range of 2 to 8 μg/ml. The combination of daptomycin and β-lactams may be useful for the treatment of MRSA infection, but further studies are needed to elucidate the mechanisms and to determine the in vivo efficacy of the combination.
Daptomycin is a novel lipopeptide antibiotic with bactericidal activity against a wide range of clinically important gram-positive bacteria, including vancomycin-resistant enterococci (VRE) and methicillin-resistant Staphylococcus aureus (MRSA). In a recent study, we found synergy between daptomycin and both rifampin and ampicillin against VRE (12a). In that work, a screening technique was used to test daptomycin with 18 other antibiotics, and promising combinations were further tested by time-kill studies. In brief, daptomycin was added to Ca2+-supplemented Mueller-Hinton broth at subinhibitory concentrations, and the Etest-determined MIC of individual antibiotics on agar containing daptomycin was compared with the MIC on agar without daptomycin. When MRSA strains were studied with this method, combinations of daptomycin and β-lactams were found to warrant further study. In this report, we present comparative results for several daptomycin-β-lactam combinations by the screening method and confirmative time-kill studies using combinations of daptomycin and oxacillin or ampicillin-sulbactam for 18 strains of MRSA.
MATERIALS AND METHODS
Eighteen randomly selected strains of MRSA were obtained from the clinical microbiology laboratory at Shands Hospital at the University of Florida, Gainesville, between January and March 2002. All isolates were tested for relatedness by pulsed-field gel electrophoresis at the Mayo Medical Laboratories, Rochester, Minn. Five isolates were different from each other and all other strains. The remaining 13 isolates were divided into six unique groups, five pairs and one group of three identical strains, making a total of 11 unique strains. All were identified by MicroScan as MRSA strains, and oxacillin resistance was confirmed by growth on agar containing 6 μg of oxacillin/ml (Becton Dickinson, Cockeysville, Md.). By Etest, the oxacillin MICs for all strains were ≥96 μg/ml (for 16 of the 18 strains, they were ≥256 μg/ml). The daptomycin MICs for all 18 strains were ≤1 μg/ml by both broth and agar dilution (the MIC at which 50% of the isolates were inhibited was 0.5 μg/ml and the MIC at which 90% of the isolates were inhibited was 1.0 μg/ml in both broth and agar). The ampicillin-sulbactam (2:1) MICs for eight unique strains of MRSA in Mueller-Hinton broth were determined by using macrodilution. The MICs of the ampicillin component of ampicillin-sulbactam ranged from 8 to 32 μg/ml. The minimum bactericidal concentrations were generally equal to the MICs and were never more than twice the MIC. S. aureus ATCC 25923 and S. aureus ATCC 29213 were included for quality control testing of daptomycin, and the MICs for these strains were always within the NCCLS acceptable range of 0.25 to 1.0 μg/ml.
Susceptibility testing.
Daptomycin (lot 710403A) was obtained from Cubist Pharmaceuticals, Lexington, Mass. Oxacillin was obtained from Sigma Scientific, St. Louis, Mo. Etest strips were obtained from AB Biodisk, Solna, Sweden. Ampicillin lot 44H0176 was obtained from Sigma Scientific, and sulbactam lot 052301-008-06 was generously provided by Pfizer, Inc., Groton, Conn. All testing was carried out with Mueller-Hinton agar or Mueller-Hinton broth (Becton Dickinson), both supplemented to 50 mg of Ca2+/liter as previously suggested (8).
Antimicrobial agents.
For synergy screening, the MICs of the following antibiotics were measured by Etest with and without daptomycin in the agar: oxacillin, piperacillin, ceftriaxone, cefepime, imipenem, gentamicin, amikacin, azithromycin, tetracycline, chloramphenicol, clindamycin, linezolid, quinupristin-dalfopristin, rifampin, trimethoprim-sulfamethoxazole, vancomycin, amoxicillin-clavulanate, and levofloxacin (AB Biodisk). Cloxacillin, ampicillin-sulbactam, piperacillin-tazobactam, and ticarcillin-clavulanate were tested with Kirby-Bauer disks (Becton Dickinson). Synergy screening was performed as described below.
Synergy screening.
Daptomycin was incorporated into Mueller-Hinton agar supplemented to 50 mg of Ca2+/liter at one-eighth the MIC, one-fourth the MIC, one-half the MIC, the MIC, and two times the MIC in agar for each strain to be tested. Etest strips (or Kirby-Bauer disks) were placed on agar containing no daptomycin or containing daptomycin at one-eighth or one-fourth the MIC. For the plates with either no daptomycin or daptomycin at one-eighth or one-fourth the MIC, six different Etest strips were placed on each 150-mm-diameter plate after inoculation with a suspension equivalent to a McFarland standard of 0.5 prepared by the direct colony suspension method (12). The plates containing one-half, one times, and two times the daptomycin MIC did not have Etest strips placed on them and were included so that an experimentally accurate daptomycin MIC would be obtained within each test run. Etest-determined MICs were read after 20 to 24 h of incubation at 36°C in air. The Etest-determined MIC results for the plates with one-eighth and one-fourth the daptomycin MIC were compared with those for the plates without daptomycin. The decrease in the MIC determined by the Etest at one-fourth or one-eighth the daptomycin MIC in agar was used to calculate the fractional inhibitory concentration (FIC) for the combinations. Synergy was defined as an FIC index of ≤0.5 as conventionally used in checkerboard synergy studies (5). Kirby-Bauer disks were used to test some antibiotics: cloxacillin, ampicillin-sulbactam, piperacillin-tazobactam, and ticarcillin-clavulanate. Here the data were analyzed to compare the relative increases in zone diameter between the different β-lactams on agar with and without daptomycin. A 30-μg daptomycin disk was included in these experiments to represent additivity, since daptomycin in the agar could not be synergistic with the daptomycin in a disk. The paired-differences t test was used to determine the statistical significance of the increase in zone size around Kirby-Bauer disks as the daptomycin concentration was increased (between no daptomycin and one-fourth the MIC or one-fourth and one-half the MIC).
Time-kill study.
Daptomycin MIC measurements and time-kill studies were carried out with Mueller-Hinton broth supplemented to 50 mg of Ca2+/liter by using an inoculum of 9 × 105 to 2.7 × 106 cells/ml prepared by the direct colony suspension method (12). For time-kill synergy studies, the following antibiotic concentrations were prepared: daptomycin at 8, 4, 2, 1, 0.5, 0.25, 0.125, and 0.0625 μg/ml with or without oxacillin at 32 μg/ml. A no-antibiotic growth control was also included. Since the oxacillin MICs for the strains used were so high, the choice of 32 μg/ml was somewhat arbitrary (1). Time-kill studies were carried out with 2-ml volumes of Ca2+-supplemented Mueller-Hinton broth, and 0.1-ml volumes of 1:100, 1:1,000, and 1:10,000 dilutions were subcultured at 0, 4, and 24 h. Synergy was defined as ≥100-fold-greater killing at 24 h by the combination than by the more active of the individual antibiotics alone at the appropriate concentration (5) and also required ≥100-fold killing of the input bacterial CFU. Bactericidal activity (killing) was defined as a ≥103-fold decrease in the number of CFU from input CFU at 4 or 24 h.
RESULTS
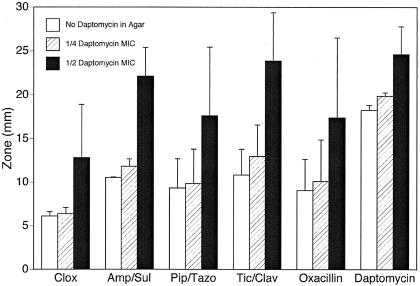
At subinhibitory concentrations of daptomycin, Etest screening results suggested that further studies of daptomycin combined with β-lactams should be pursued. By this method, the FIC index of the combination of oxacillin and daptomycin for 6 of the 18 strains of MRSA (5 of which were clonally unique) was ≤0.5. No other antibiotic combinations had FIC indices of ≤0.5 for more than two strains. Increases in zone size for oxacillin, cloxacillin, ampicillin-sulbactam, piperacillin-tazobactam, and ticarcillin-clavulanate were compared by using Kirby-Bauer disks. A daptomycin disk was included as a control for additivity. Figure 1 shows that when the daptomycin concentration in the agar is raised from one-fourth to one-half the MIC, there are increases in average zone sizes of 7.3 mm for oxacillin, 10.3 mm for ampicillin-sulbactam, 7.8 mm for piperacillin-tazobactam, and 10.9 mm for ticarcillin-clavulanate. In contrast, a daptomycin disk showed only a 4.8-mm increase in zone size after the daptomycin concentration in the agar was increased from one-fourth to one-half the MIC. Since the increased size of the zone around the daptomycin disk on daptomycin-containing agar can only be additive, the greater increases in zone size around the β-lactam disks suggested possible synergy. These differences were highly statistically significant for ampicillin-sulbactam (P < 0.0001, paired-differences t test) and ticarcillin-clavulanate (P < 0.0001), significant for piperacillin-tazobactam (P = 0.05), and not significant for oxacillin (P = 0.15) and cloxacillin (P = 0.21). While analyzing the results of disk synergy testing, we noted that “clonally identical” strains did not always behave identically, particularly for oxacillin, cloxacillin, and piperacillin-tazobactam. We typically observed discrepancies in one to three antibiotic combinations between pairs of “identical” strains. In essence, the clonally related strains showed as much variability in the synergy screening tests as did unrelated strains. However, isolates from all 11 unique clones displayed highly significant increases in zone size with daptomycin in the agar for all β-lactams tested.
FIG. 1.
Increase in zone sizes surrounding disks with indicated β-lactams as daptomycin concentration in agar is increased from zero to one-fourth the MIC and one-half the MIC. Mean zone sizes + standard deviations are given in millimeters. Seventeen strains were used for the β-lactam zone assays, and eight strains were used for daptomycin zone assays. The increases in zone sizes for all antibiotics are significantly greater with daptomycin in the agar at one-fourth the MIC than with no daptomycin (all P values were ≤0.006 by the paired-differences t test) and with daptomycin in the agar at one-half the MIC than with no daptomycin (all P values were ≤0.001 by the paired-differences t test). Based on the paired-differences t test, the increases in zone sizes for ampicillin-sulbactam (P < 0.0001) and ticarcillin-clavulanate (P < 0.0001) are significantly greater than the increases in zone sizes around the daptomycin disks when the daptomycin in the agar is increased to one-half from one-fourth the MIC; these results are followed in significance by those for piperacillin-tazobactam (P = 0.05), while the increases are not significant for oxacillin (P = 0.15) and cloxacillin (P = 0.21) (see Materials and Methods for details). Clox, cloxacillin; Amp/Sul, ampicillin-sulbactam; Pip/Tazo, piperacillin-tazobactam; Tic/Clav, ticarcillin-clavulanate.
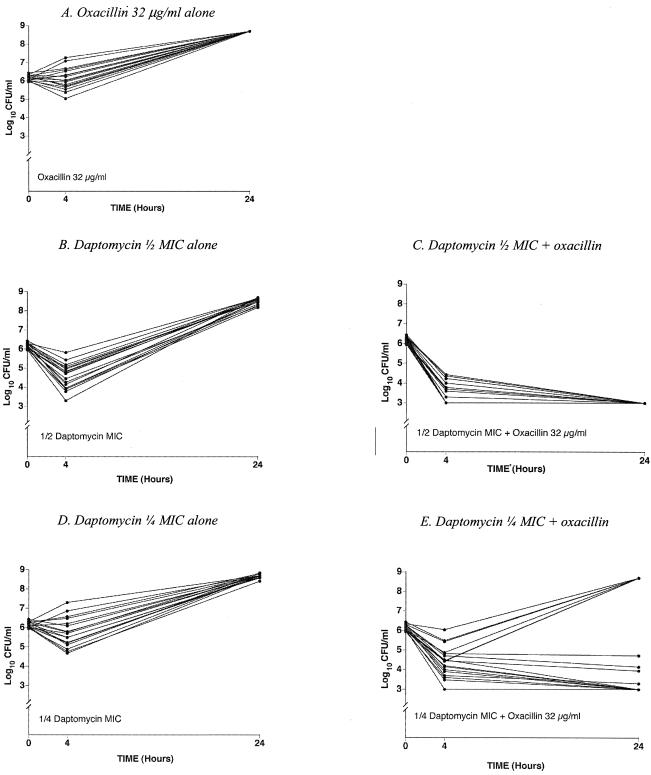
Results of the time-kill studies for daptomycin in combination with oxacillin are shown for 18 strains of MRSA in Fig. 2. As described in Materials and Methods, the combination of daptomycin at one-half the MIC and oxacillin at 32 μg/ml was found to be synergistic for 18 of 18 strains at 24 h. The combination of daptomycin at one-fourth the MIC and oxacillin at 32 μg/ml was found to be synergistic for 11 of 18 (61%) strains at 24 h. Even for the combination with one-eighth the daptomycin MIC, synergy was found for 6 of 18 (33%) strains in comparison to the results with daptomycin or oxacillin alone (data not shown). Killing (i.e., a ≥103-fold decrease from the input CFU at 24 h) was observed for 18 of 18 strains for the daptomycin-oxacillin combination with one-half the daptomycin MIC and for 9 of 18 strains with one-fourth the daptomycin MIC. These data are best appreciated by comparing the results shown in Fig. 2B and C for one-half the daptomycin MIC and those shown in Fig. 2D and E for one-fourth the daptomycin MIC. In the time-kill studies, the behaviors of related clones were identical. The six strains that exhibited regrowth at 24 h after inhibition of growth at 4 h (Fig. 2E) came from four unique clones.
FIG. 2.
Time-kill study results for 18 strains of MRSA (see Materials and Methods for details). (A) Oxacillin at 32 μg/ml alone. At 24 h, all tubes were grossly turbid, a condition represented by a titer of 108.7 CFU/ml. (B) Daptomycin alone at one-half its MIC. (C) Daptomycin at one-half its MIC plus oxacillin at 32 μg/ml. At 24 h, there was no growth on subculture plates, a condition represented by the lowest level of detection, ≤103 CFU/ml. (D) Daptomycin alone at one-fourth its MIC. (E) Daptomycin at one-fourth its MIC plus oxacillin at 32 μg/ml. The growth control for these experiments is not shown but was grossly turbid at 24 h for all strains.
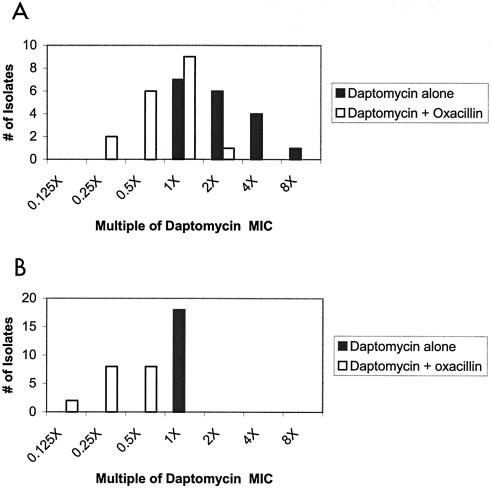
Figure 3 shows the distribution of bactericidal concentrations (expressed as a multiple of the daptomycin MIC) with and without the addition of oxacillin, measured at 4 and 24 h. In essence, the median concentration showing ≥103-fold killing of the input CFU at 4 h was two times the daptomycin MIC when daptomycin was used alone but one times the daptomycin MIC when daptomycin was combined with oxacillin. At 24 h, the median concentration to achieve ≥103-fold killing for daptomycin alone was one times the MIC but was one-fourth the MIC for daptomycin in the presence of oxacillin.
FIG. 3.
Distribution of bactericidal concentrations (expressed as a multiple of the daptomycin MIC) at 4 (A) and 24 (B) h with (□) and without (▪) the addition of oxacillin (see Materials and Methods for details). The distributions of bactericidal activity with and without daptomycin are statistically significantly different at 4 h (P = 0.005) and at 24 h (P < 0.0001) by χ2 analysis.
For the combination of ampicillin-sulbactam and daptomycin, synergy was observed by time-kill studies at one-half the daptomycin MIC for seven of eight clonally unique strains of MRSA and at one-fourth the daptomycin MIC for six of eight strains. Synergy was observed for ampicillin concentrations of 2 to 8 μg/ml for the seven strains for which synergy was observed at one-half the daptomycin MIC and in the same concentration range for the six strains for which synergy was found at one-fourth the daptomycin MIC.
DISCUSSION
In view of the very high level of resistance of these strains of MRSA to oxacillin (MICs are ≥256 μg/ml for 16 of 18 strains), the synergy between daptomycin at one-half the MIC and oxacillin at 32 μg/ml for 18 of 18 strains is surprising. Even at one-fourth and one-eighth the daptomycin MIC, synergy with oxacillin at 32 μg/ml was observed for 61 and 33% of MRSA strains, respectively. In addition, ≥103-fold killing at 24 h of the input CFU was also observed for all strains with the combination of daptomycin at one-half its MIC and oxacillin and for 50% of strains with the combination of daptomycin at one-fourth its MIC and oxacillin. Based on the results (Fig. 1) with subinhibitory concentrations of daptomycin in agar and either disks or Etests to screen for antibiotic synergy, all β-lactams appear to show a marked increase in activity when combined with daptomycin. In a previous study of the activity of daptomycin and ampicillin against VRE, synergy was found against 19 of 19 strains in time-kill studies (12a). While ≥103-fold killing was observed for only 2 of 19 strains of VRE, 10 of 19 strains did show 100-fold killing of the input CFU. Daptomycin-β-lactam synergy appears to be consistent across the gram-positive species tested.
In contrast to the results for VRE, no synergy between daptomycin and rifampin against MRSA strains was observed in the screening study, although all MRSA strains were highly susceptible to rifampin. No synergy between daptomycin and rifampin was found against three laboratory-derived rifampin-resistant MRSA strains (data not shown).
Daptomycin is believed to act by Ca2+-dependent insertion of its acyl tail into the gram-positive cell membrane, which is followed by the development of potassium efflux channels, depolarization of the membrane, and cell death (15, 16). A recent study has shown that membrane depolarization and potassium leakage correlate well with decreased viability at concentrations above the daptomycin MIC (14). Sieradzki and Tomasz found that inhibitors of the early stages of peptidoglycan synthesis (e.g., fosfomycin, β-chloro-d-alanine, and d-cycloserine) markedly decreased the MIC of methicillin for the COL strain of MRSA from ≥800 μg/ml to as low as 6 to 50 μg/ml depending on the specific second antibiotic studied (13). They proposed that inhibition of the early stages of peptidoglycan synthesis resulted in the production of altered peptidoglycan precursors, which led to decreased effectiveness of penicillin binding protein 2′ in cross-linking peptidoglycan. At its MIC, daptomycin is known to shut down macromolecular synthesis, so subinhibitory concentrations may have significant effects on peptidoglycan precursor levels. If insertion of daptomycin into the cell membrane at subinhibitory concentrations altered peptidoglycan precursors in a manner analogous to that of fosfomycin, β-chloro-d-alanine, or d-cycloserine, penicillin binding protein 2′ might be unable to perform its cross-linking function in the presence of oxacillin. Another possibility is that subinhibitory concentrations of daptomycin somehow affect the levels or functionality of one or more factors essential for methicillin resistance (fems) or auxillary (aux) factors required for the full expression of mecA.
Alternatively, the activity of daptomycin might involve blocking the induction of mecA by oxacillin itself. It is well known that mecA is normally only expressed in ∼1 in 105 cells, but its expression rapidly increases when β-lactams bind to the surface receptors for the derepression of mecA (10). If daptomycin were to block induction of mecA at subinhibitory concentrations, an apparent increase in killing might be observed. Finally, we cannot exclude the possibility that oxacillin somehow enhances the activity of daptomycin.
The consistency of both bactericidal activity and synergy makes the combination of daptomycin and β-lactams attractive for the treatment of MRSA infection. It is reasonable to ask whether the antibiotic levels tested in this work are clinically achievable. For daptomycin at a dose of 4 mg/kg of body weight, the peak and trough concentrations are approximately 56 and 7.5 μg/ml of plasma, respectively (J. Silverman [Cubist Pharmaceuticals], data on file), and daptomycin is ∼92% protein bound, giving trough-free drug concentrations of ∼0.6 μg/ml. Thus, trough-free levels of daptomycin are in the same range as those studied here. Since oxacillin is >90% protein bound, it is unlikely that trough-free drug levels of 32 μg/ml (as used in this study) could be achieved in a clinical setting. However, depending on renal clearance, levels of ampicillin and piperacillin in blood of 75 to 80 μg/ml (with proportional concentrations of sulbactam and tazobactam) are achievable with high-dose continuous infusions (4, 6, 9), and levels of ampicillin as high as 103 to 130 μg/ml have been reported (11). Protein binding levels are reported to be 18% for both ampicillin and amoxicillin (7), 16% for piperacillin (2), 38% for sulbactam (3), and 20 to 23% for tazobactam (2). In this study, synergy was found against seven of eight MRSA strains by time-kill studies with daptomycin and ampicillin-sulbactam with ampicillin concentrations in the range of 2 to 8 μg/ml.
We conclude that the combination of daptomycin and β-lactams may be useful in the treatment of MRSA infections but that further in vitro and in vivo studies should be carried out before clinical use can be recommended.
Acknowledgments
We gratefully acknowledge the support of the Department of Pathology, Immunology and Laboratory Medicine, University of Florida, and the staff of the Clinical Microbiology Laboratory, Shands Hospital, University of Florida. We thank Jared Silverman, Cubist Pharmaceuticals, for his detailed review of this manuscript and many helpful suggestions. We thank Claire Noegel for preparation of this manuscript.
This study was supported in part by a grant from Cubist Pharmaceuticals.
REFERENCES
- 1.Adam, D., K. Wilhelm, and V. Chysky. 1981. Antibiotic concentrations in blood and tissue. Intraoperative ischemia as a model for the determination of tissue concentrations using mezlocillin and oxacillin as examples. Arzneim.-Forsch. 31:1972-1976. [PubMed] [Google Scholar]
- 2.Bergan, T. 1981. Overview of acylureidopenicillin pharmacokinetics. Scand. J. Infect. Dis. Suppl. 29:33-48. [PubMed] [Google Scholar]
- 3.de la Pena, A., and H. Derendorf. 1999. Pharmacokinetic properties of beta-lactamase inhibitors. Int. J. Clin. Pharm. Ther. 37:63-75. [PubMed] [Google Scholar]
- 4.Dickinson, G. M., D. G. Droller, R. L. Greenman, and T. A. Hoffman. 1981. Clinical evaluation of piperacillin with observations on penetrability into cerebrospinal fluid. Antimicrob. Agents Chemother. 20:481-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eliopoulos, G. M., and R. C. Moellering, Jr. 1996. Antimicrobial combinations, p. 330-396. In V. Lorian (ed.), Antibiotics in laboratory medicine. Williams & Wilkins, Baltimore, Md.
- 6.Grant, E. M., J. L. Kuti, D. P. Nicolau, C. Nightingale, and R. Quintiliani. 2002. Clinical efficacy and pharmacoeconomics of a continuous-infusion piperacillin-tazobactam program in a large community teaching hospital. Pharmacotherapy 22:471-483. [DOI] [PubMed] [Google Scholar]
- 7.Hall, L. M., L. H. Hall, and L. B. Kier. 2003. QSAR modeling of beta-lactam binding to human serum proteins. J. Comput.-Aided Mol. Des. 17:103-118. [DOI] [PubMed] [Google Scholar]
- 8.Jones, R. N., and A. L. Barry. 1987. Antimicrobial activity and spectrum of LY146032, a lipopeptide antibiotic, including susceptibility testing recommendations. Antimicrob. Agents Chemother. 31:625-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin, C., A. Cotin, A. Giraud, M. Beccani-Argème, P. Alliot, M.-N. Mallet, and M. Argème. 1998. Comparison of concentrations of sulbactam-ampicillin administered by bolus injections or bolus plus continuous infusion in tissues of patients undergoing colorectal surgery. Antimicrob. Agents Chemother. 42:1093-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKinney, T. K., V. K. Sharma, W. A. Craig, and G. L. Archer. 2001. Transcription of the gene mediating methicillin resistance in Staphylococcus aureus (mecA) is corepressed but not coinduced by cognate mecA and β-lactamase regulators. J. Bacteriol. 183:6862-6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mekonen, E. T., G. A. Noskin, D. M. Hacek, and L. R. Peterson. 1995. Successful treatment of persistent bacteremia due to vancomycin-resistant, ampicillin-resistant Enterococcus faecium. Microb. Drug Resist. 1:249-253. [DOI] [PubMed] [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, fifth ed. Approved standard M7-45(20). National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 12a.Rand, K. H., and H. Houck. 2004. Daptomycin synergy with rifampicin and ampicillin against vancomycin-resistant enterococci. J. Antimicrob. Chemother. 53:530-532. [DOI] [PubMed] [Google Scholar]
- 13.Sieradzki, K., and A. Tomasz. 1997. Suppression of beta-lactam antibiotic resistance in a methicillin-resistant Staphylococcus aureus through synergic action of early cell wall inhibitors and some other antibiotics. J. Antimicrob. Chemother. 39(Suppl. A):47-51. [DOI] [PubMed] [Google Scholar]
- 14.Silverman, J. A., N. G. Perlmutter, and H. M. Shapiro. 2003. Correlation of daptomycin bactericidal activity and membrane depolarization in Staphylococcus aureus. Antimicrob. Agents Chemother. 47:2538-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tally, F. P., and M. F. DeBruin. 2000. Development of daptomycin for gram-positive infections. J. Antimicrob. Chemother. 46:523-526. [DOI] [PubMed] [Google Scholar]
- 16.Thorne, G. M., and J. Alder. 2002. Daptomycin: a novel lipopeptide antibiotic. Clin. Microbiol. Newsl. 24:33-40. [Google Scholar]