Abstract
This paper describes the design and evaluation of novel azasterols as potential compounds for the treatment of leishmaniasis and other diseases caused by trypanosomatid parasites. Azasterols are a known class of (S)-adenosyl-l-methionine: Δ24-sterol methyltransferase(24-SMT) inhibitors in fungi, plants, and some parasitic protozoa. The compounds prepared showed activity at micromolar and nanomolar concentrations when tested against Leishmania spp. and Trypanosoma spp. The enzymatic and sterol composition studies indicated that the most active compounds acted by inhibiting 24-SMT. The role of the free hydroxyl group at position 3 of the sterol nucleus was also probed. When an acetate was attached to the 3β-OH, the compounds did not inhibit the enzyme but had an effect on parasite growth and the levels of sterols in the parasite, suggesting that the acetate group was removed in the organism. Thus, an acetate group on the 3β-OH may have application as a prodrug. However, there may be an additional mode(s) of action for these acetate derivatives. These compounds were shown to have ultrastructural effects on Leishmania amazonensis promastigote membranes, including the plasma membrane, the mitochondrial membrane, and the endoplasmic reticulum. The compounds were also found to be active against the bloodstream form (trypomastigotes) of Trypanosoma brucei rhodesiense, a causative agent of African trypanosomiasis.
The parasites that cause leishmaniasis and Chagas disease have ergosterol and other 24-alkylated sterols as the major sterols present in their cells, as found in fungi and plants (7, 26, 33). This contrasts to the situation in mammalian cells, where cholesterol is the only sterol present. Ergosterol and cholesterol have differences in their structure and biosynthesis, which provide potential drug targets.
One principal difference in the biosynthesis of ergosterol and cholesterol is the presence of the 24-alkyl chain in ergosterol and the stigmasterols. This is introduced by the enzyme (S)-adenosyl-l-methionine: Δ24-sterol methyltransferase (24-SMT), which adds a methylene residue to the unsaturated sterol side chain by using S-adenosylmethionine (SAM) as a cofactor (16).
This enzyme has been studied as a drug target in fungi (1, 2, 18, 30) and as a potential herbicide target in plants (20). It has been shown that azasterols, which carry a nitrogen in the side chain of sterols at the 23-, 24-, or 25-position, can inhibit 24-SMT of fungi and plants (17). The azasterols are protonated at physiological pH and are thought to act by mimicking high-energy intermediates in the reaction pathway. In addition, other compounds which carry a positive charge in the sterol side chain, such as sulfonium (1), ammonium (1), and arsonium (20) ions, have been shown to be inhibitors of 24-SMT. However, some of these azasterols and related compounds may have limited use as antimicrobials, as they also inhibit the related enzyme in cholesterol biosynthesis, sterol 24-reductase (6). Presumably this enzyme also passes through positively charged high-energy intermediate. Unfortunately, inhibition of sterol 24-reductase leads to raised levels of toxic biosynthetic intermediates, which give rise to toxicity. Indeed, in the 1960s, when sterol 24-reductase was deliberately targeted with the drug triparanol to lower cholesterol levels, toxic side effects were observed and the drug was withdrawn (25). Therefore, inhibitors of 24-SMT have to be designed to be selective for this enzyme and not sterol 24-reductase.
Inhibition of ergosterol biosynthesis has been shown to be a good drug target in Leishmania spp. and Trypanosoma cruzi, the causative organism of Chagas' disease (26). Relatively little has been done to develop novel 24-SMT inhibitors against Leishmania spp. and T. cruzi. Several inhibitors have been prepared and evaluated against the trypanosomatid Crithidia fasciculate (21). However, the majority of work against these organisms has been carried out with 22,26-azasterol (AZA) (Fig. 1), which has been shown to modify the sterol composition of T. cruzi epimastigotes (the parasite form found in the vector), acting either singly or in combination with other sterol biosynthesis inhibitors (32, 37). In addition, the compound also affects amastigotes (13) (the intracellular form of the parasite) and has been shown to have antiproliferative effects in vitro and in vivo (31). Similarly, the compound is active against Leishmania amazonensis promastigotes and amastigotes (24) and Leishmania donovani promastigotes (8).
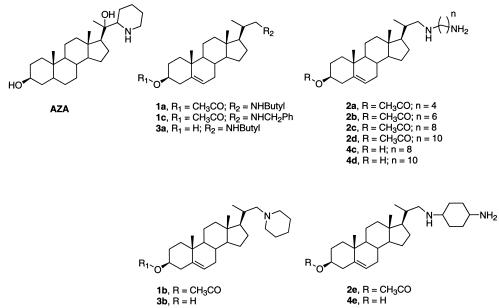
FIG. 1.
Structures of azasterol (AZA) and the prepared molecules.
We wanted to investigate simple azasterols as inhibitors of Leishmania spp. and T. cruzi 24-SMT. In addition, 24-SMT has a binding site for SAM immediately adjacent to the sterol binding site. The SAM binding site can accommodate a positively charged ammonium functionality, a negatively charged carboxylate functionality, and an adenosine functionality. In this paper, we discuss the addition of positively charged ammonium groups to the azasterol to see if additional interactions with the enzyme active site could be discovered. This could give rise to selectivity for 24-SMT compared to the sterol 24-reductase. Earlier enzyme kinetic studies had shown that an unhindered C-3 hydroxyl group was one of the essential factors for substrate-enzyme interactions. It seems that the hydroxyl group of the sterol could be interacting with a tyrosine residue in the sterol binding site (36). Therefore, analogues with and without protection at C-3 of the sterol nucleus were prepared in order to evaluate the role of the substituent at that position. The general structures investigated are shown in Fig. 1.
MATERIALS AND METHODS
Materials.
Chemicals, reagents, and solvents were purchased from Aldrich, Sigma, Lancaster, Merck, or Fisher unless otherwise stated. Leishmania major strain Friedlin was used for cloning. Fetal calf serum was obtained from Gibco. The following species were used for growth assays: L. donovani L82 amastigotes, Trypanosoma cruzi Tulahuan LAC-Z, and Trypanosoma brucei rhodesiense STIB900 (all maintained at the London School of Hygiene and Tropical Medicine) and the MHOM/BR/75/Josefa strain of L. amazonensis (maintained at the Instituto de Biofisica Carlos Chagas Filho).
Chemistry.
The compounds shown in Fig. 1 and Tables 1 and 2 were prepared at the Welsh School of Pharmacy; their preparation will be reported in detail elsewhere.
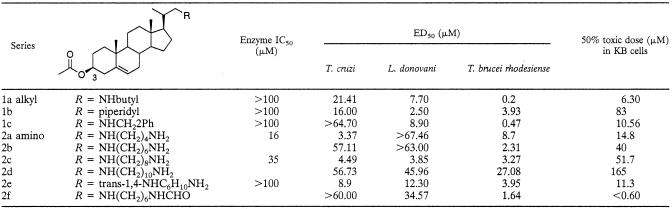
TABLE 1.
Activity of 3β-OH-protected compounds against recombinant L. major 24-SMT and against T. cruzi and L. donovani intracellular amastigotes and T. brucei rhodesiense trypomastigotes
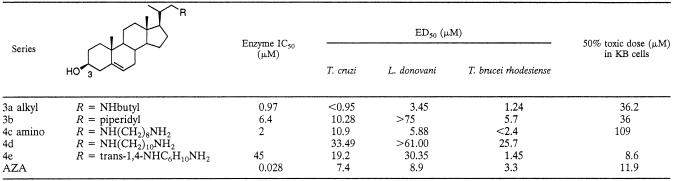
TABLE 2.
Activity of 3β-OH compounds against recombinant L. major 24-SMT and against T. cruzi and L. donovani amastigotes cultured intracellularly and T. brucei rhodesiense trypomastigotes
Enzyme assays.
In assays of inhibition of 24-SMT, protein extracts from Escherichia coli BL21 (DE3) pLysS/pET28a-HisLmSMT cells were used. Plasmid pET28a-HisLmSMT was obtained by cloning the entire coding sequence of the L. major SMT gene in the pET28a vector (Novagen). L. major recombinant SMT is produced as a His-tagged fusion protein and is overexpressed when induced with isopropylthiogalactopyranoside (IPTG) at 1 mM for 4 h. Cells were disrupted by sonication in a buffer containing 50 mM pH 7.4 Tris-HCl, 2 mM MgCl2, 4 mM CHAPS, 0.5% (vol/vol) Tween 80, and protease inhibitors (three times, 30 s, 50% duty cycle). The sonicate was centrifuged at 12,000 rpm for 30 min at 4°C to obtain the soluble fraction, which contained the active form of the enzyme.
A standard SMT activity assay contained 1 mg of protein in the previously mentioned buffer, 50 mM pH 7.4 Tris-HCl, 2 mM MgCl2, 4 mM CHAPS, 0.5% (vol/vol) Tween 80, 100 μM desmosterol, and 200 μM 14C-labeled SAM, 600,000 dpm per reaction. The inhibitor was resuspended first in a minimal volume of its corresponding solvent and later added to the reaction mixture as an aqueous solution. The reaction was started with the enzyme. Incubations were performed at 30°C for 45 min and terminated with 0.5 ml of 10% KOH dissolved in 80% (vol/vol) methanol. To quantify the efficiency of the extraction, 3H-labeled cholesterol (3 mg, 30,000 dpm per reaction) was added as an internal standard. The methylated sterol product was extracted three times with 1 ml of hexane, and the resulting organic layer was washed once with Tris-HCl buffer to remove the 14C-labeled SAM that was not incorporated; 1 ml of the organic layer was added to 10 ml of Hydrofluor, and the radioactivity was measured in a scintillation counter.
The 50% inhibitory concentration (IC50) values were obtained from plots of percent inhibition versus concentration of inhibitor.
Studies of lipid composition.
L. amazonensis promastigotes were cultivated in LIT medium supplemented with lactalbumin and 10% fetal calf serum (Gibco) (3) at 26°C without agitation. The cultures were initiated with a cell density of 2 × 106 cells per ml, and the drug was added at a cell density of 0.5 × 107 to 1 × 107 cells per ml. Cell densities were measured with an electronic particle counter (model ZBI; Coulter Electronics Inc., Hialeah, Fla.) and by direct counting with a hemacytometer. Cell viability was monitored by trypan blue exclusion with light microscopy.
For the analysis of the effects of drugs on the lipid composition of promastigotes, total lipids from control and drug-treated cells were extracted and fractionated into neutral and polar lipid fractions by silicic acid column chromatography and gas-liquid chromatography (12, 13, 27, 28). The neutral lipid fractions were first analyzed by thin-layer chromatography (on Merck 5721 silica gel plates with heptane-isopropyl ether-glacial acetic acid [60:40:4] as the developing solvent) and conventional gas-liquid chromatography (isothermic separation in a 4-m glass column packed with 3% OV-1 on Chromosorb 100/200 mesh with nitrogen as the carrier gas at 24 ml/min and flame ionization detection in a Varian 3700 gas chromatograph). For quantitative analysis and structural assignments, the neutral lipids were separated in a capillary high-resolution column (Ultra-2 column,25 m by 0.20 mm inner diameter, 5% phenyl-methyl-siloxane, 0.33-μm film thickness) in a Hewlett-Packard 6890 Plus gas chromatograph equipped with an HP5973A mass sensitive detector. The lipids were injected in chloroform, and the column was kept at 50°C for 1 min, and then the temperature was increased to 270°C at a rate of 25°C min−1 and finally to 300°C at a rate of 1°C min−1. The carrier gas (He) flow was kept constant at 0.5 ml min−1. The injector temperature was 250°C, and the detector was kept at 280°C.
Growth inhibition of L. amazonensis promastigotes.
The MHOM/BR/75/Josefa strain of L. amazonensis isolated from a patient with diffuse cutaneous leishmaniasis was used in the present study. It was maintained by passage in BALB/c mice. Promastigotes were cultured in Warren's medium (brain heart infusion plus hemin and folic acid) supplemented with 10% fetal bovine serum at 25°C. The growth curves of promastigotes were initiated with 2 × 106 cell/ml, and the drugs, at different concentrations, were added after 24 h of culture. The number of parasites was determined by daily counting in a Newbauer hemacytometer for 7 days. The 50% effective dose (ED50) was calculated after 96 h of treatment with the function (14) I = ImaxC/(ED50 + C), where I is percent inhibition, Imax is 100% inhibition, C is the concentration of the inhibitor, and ED50 is the concentration required for 50% growth inhibition.
Growth inhibition of L. donovani.
Peritoneal exudate macrophages were harvested from CD1 mice 24 h after starch induction. After washing, the macrophages were dispensed into Lab-tek16-well tissue culture slides and maintained in RPMI 1640 plus 10% heat-inactivated fetal calf serum at 37°C in a 5% CO2-air mixture for 24 h. L. donovani (MHOM/ET/67/L82) amastigotes were harvested from an infected Golden hamster spleen and used to infect the macrophages at a ratio of 7.5 parasites to 1 macrophage. Infected cells were left for a further 24 h and then exposed to drug for a total of 5 days, with the overlay being replaced on day 3 (15). Stock solutions of the test compounds, plus control drugs, were prepared at a concentration of 20 mg/ml in dimethyl sulfoxide. Compounds were tested in a threefold dilution series in quadruplicate at each concentration. The top concentration for each compound was 30 μg/ml, and all concentrations were tested in quadruplicate. On day 5, the overlay was removed, and the slides were fixed (100% methanol) and stained (10% Giemsa, 10 min) before being evaluated microscopically. ED50 and ED90 values were calculated with Msxlfit. The ED50 value for the positive control drug, Pentostam, is usually 3 to 8 μg of antimony(V)/ml.
Growth inhibition of T. cruzi.
Murine (CD1) peritoneal macrophages were harvested 24 h after starch induction and dispensed into 96-well plates at a concentration of 4 x 105/ml. After 24 h, the cells were infected with T. cruzi Tulahuan LAC-Z trypomastigotes, harvested from L6 feeder layer cultures; 24 h later the infected cells were exposed to drug for 3 days, and 50 μl of 500 μM CPRG-1% Nonidet P-40 was added to each well. The plates were read after 2 to 5 h at λ570 (3). ED50 and ED90 values were calculated with Msxlfit.
Growth inhibition of T. brucei rhodesiense.
T. brucei rhodesiense STIB900 bloodstream form trypomastigotes were maintained in HMI-18 medium (9) with 15% heat-inactivated fetal calf serum at 37°C in a 5% CO2-air mixture. Trypomastigotes were washed and resuspended in fresh medium at a concentration of 2 × 105/ml. The top concentration for the test compounds was 30 μg/ml. The ED50 for pentamidine is usually between 1.0 and 0.1 ng/ml. Plates were incubated for 72 h at 37°C in a 5% CO2-95% air mixture. At 72 h, the plates were assessed microscopically before Alamar Blue was added (22). Plates were read after 5 to 6 h on a Gemini fluorescent plate reader (Softmax Pro. 3.1.1; Molecular Devices) at EX/EM 530/585 nm with a filter cutoff at 550 nm. ED50 values were calculated with Msxlfit (IDBS).
Cytotoxicity against mammalian cells.
Plates were seeded with 100 μl of human epidermal nasopharyngeal carcinoma KB cells at 4 × 104/ml in RPMI 1640 plus 10% heat-inactivated fetal calf serum and incubated at 37°C in 5% CO2 for 24 h. The overlay was removed and replaced by the drugs to be tested in fresh medium at 300, 30, 3, and 0.3 μg/ml in triplicate at each concentration. The positive control drug was podophyllotoxin (Sigma). Plates were incubated for a further 72 h at 37°C in 5% CO2. The wells were assessed microscopically for cell growth. The overlay was removed and the wells were washed with phosphate-buffered saline (pH 7.0) three times. Then 100 μl of phosphate-buffered saline and 10 μl of Alamar Blue were added per well, and the plates were incubated for 2 to 4 h (37°C, 5% CO2) before reading at EX/EM 530/585 nm (cutoff, 550 nm) in a Gemini plate reader. ED50 and ED90 values were calculated compared to blanks and untreated controls.
Electron microscopy.
Control and treated parasites were fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.2. After fixation, the cells were postfixed in a solution containing 1% OsO4 and 0.8% potassium ferrocyanide in 0.1 M cacodylate buffer, washed in the same buffer, dehydrated in acetone, and embedded in Epon. Ultrathin sections were stained with uranyl acetate and lead citrate and observed in a Zeiss 900 transmission electron microscope.
RESULTS
Enzyme assays.
Compounds were evaluated with cell extracts of the recombinant L. major 24-SMT, which was over expressed in E. coli. The acetoxy derivatives (compounds 1 and 2) showed poor inhibition of the enzyme (Table 1). Only compounds 2a and 2c proved to show inhibition of the enzyme. A remarkable improvement in the inhibition of the enzyme was observed when the unprotected compounds were tested (Table 2). Compound 3a proved to be the best inhibitor among the tested compounds, showing the lowest value for inhibition, with an IC50 of 0.97 μM, close to that obtained by the reference compound AZA. This suggests that the 3β-OH is normally required for inhibition of the enzyme, although in the case of 2a and 2c, where the 3β-OH is protected, this could be compensated for to some extent by interactions of a free amino group in the side chain with the enzyme.
In vitro growth assays.
Compounds were evaluated for activity against the clinically relevant stage of the parasite (Tables 1 and 2). In the case of T. cruzi and L. donovani, the clinically relevant form is the amastigote stage, which is an intracellular form of the parasite. In addition, some of the compounds were tested against the promastigotes (vector form of the parasite) of L. amazonensis. The compounds were also assayed against T. brucei rhodesiense bloodstream form trypomastigotes.
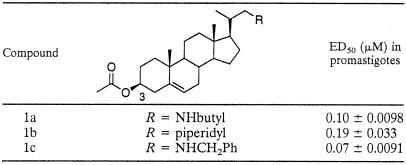
The simple alkyl substituted compounds (1a to c) with the 3β-OH protected showed little activity against T. cruzi, and some of the activity may be attributable to general toxicity (Table 1). However, these compounds were more active against L. donovani and T. brucei rhodesiense, with activity against T. brucei rhodesiense being of particular note for all of the compounds. This inhibition of growth is somewhat surprising given their lack of activity in the enzyme assays (above). This may be explained by the compounds' acting as prodrugs or through some alternative mechanism of action (discussed below). These compounds were also investigated against promastigotes (vector stage) of L. amazonensis, as shown in Table 3, and were found to show potent inhibition of this stage of the life cycle (ED50 between 70 and 190 nM).
TABLE 3.
Activity of 3β-OH-protected compounds against L. amazonensis promastigotes
In the case of the amino derivatives (series 2, Table 1), there is no clear trend for T. cruzi. However, the butyl (2a), octyl (2c), and trans-cyclohexylamino (2e) compounds gave moderate inhibition (ED50 = 3.37, 4.49, and 8.9 μM, respectively). For L. donovani, the butyl and hexyl derivatives were inactive, while the octyl derivative (2c) was again quite active (ED50 = 3.85 μM). Increasing the chain length to the decyl led to loss of activity. The trans-cyclohexylamino (2e) proved to be active once more. All of the compounds with the exception of the butyl and decyl showed good activity against T. brucei rhodesiense, in the region of ED50 = 2 to 4 μM.
Removal of the acetate protecting group (series 3 and 4, Table 2) led to no significant variation of the results obtained previously, with the exception of compound 3a, which showed the highest antiproliferative activity among all the compounds tested against T. cruzi. No modification of the inhibition pattern followed by the 3β-OH protected compounds was observed when the unprotected analogues were tested against T. brucei rhodesiense.
Sterol composition.
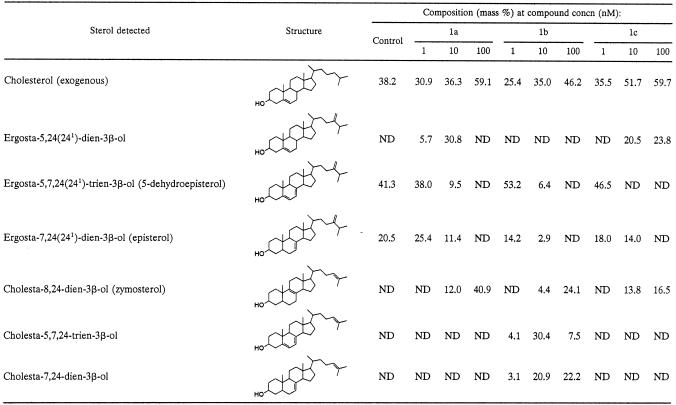
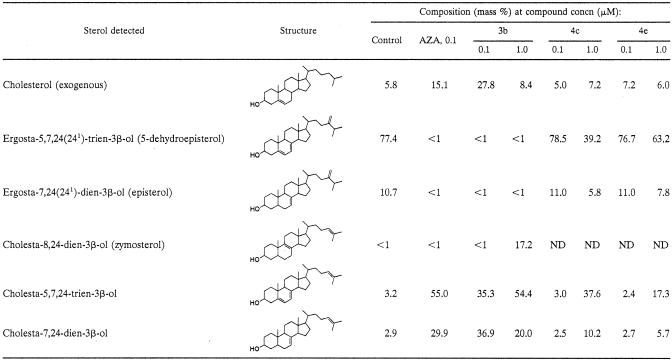
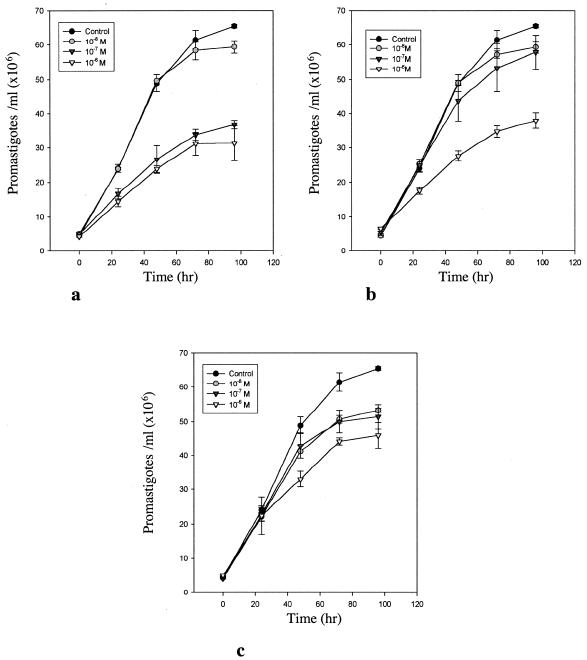
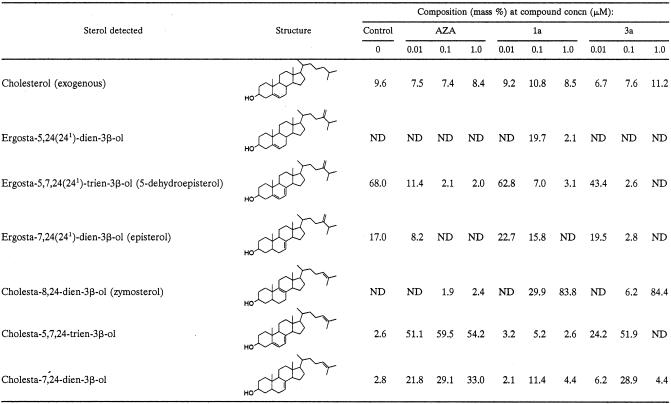
Compounds were also investigated for their effect on the sterol composition of L. amazonensis promastigotes (Tables 4 and 5). The cells were exposed to the indicated drug concentration for 96 h, after which the sterols were extracted and analyzed quantitatively. Compounds 1a to c gave a large reduction in the level of endogenous 24-alkylated sterols (5-dehydroepisterol and episterol) and a concomitant rise in the level of cholesta-type sterols at 10 nM. This shows that 24-SMT is being inhibited by the compounds. When the dose was increased to 100 nM, the endogenous 24-alkylated sterols were not detected (Table 4). The piperidine analogue 3b, in which the 3β-OH was unprotected, still showed a potent inhibition at 0.1 μM, comparable to that achieved by azasterol (Table 5). The octyl (4c) and trans-cyclohexylamino (4e) derivatives, in contrast, gave a much smaller reduction in the levels of 24-alkylated sterols, even at 1.0 μM. To investigate further the relationship between growth inhibition and altered sterol content, the effects of compounds 1a and 3a on both growth and sterol composition of L. amazonensis promastigotes were investigated (Fig. 2 and Table 6). Compound 1a produced growth arrest at 100 nM, while 3a was a slightly weaker inhibitor, giving growth arrest at about 1 μM (Fig. 2). However, when comparing their effect on sterol composition, 3a had a more potent effect on sterol composition at 10 and 100 nM compared to 1a. Thus, 1a is more potent at inhibiting cellular growth than 3a but has a smaller effect on sterol composition.
TABLE 4.
Effects of compounds 1a to 1c on the free sterol composition of L. amazonensis promastigotesa
Sterols were extracted from cells exposed to the indicated drug concentration for 96 h; they were separated from polar lipids by silicic acid column chromatography and analyzed by quantitative capillary gas-liquid chromatography and mass spectrometry. Composition is expressed as mass percentages. ND, not detected.
TABLE 5.
Effects of compounds 3b, 4c, and 4e on the free sterol composition of L. amazonensis promastigotesa
Sterols were extracted from cells exposed to the indicated drug concentration for 96 h; they were separated from polar lipids by silicic acid column chromatography and analyzed by quantitative capillary gas-liquid chromatography and mass spectrometry. Composition is expressed as mass percentages. ND, not detected.
FIG. 2.
Growth inhibition curves of L. amazonensis, showing promastigotes versus time with compounds 1a (a), 3a (b), and azasterol (c).
TABLE 6.
Effects of compounds AZA, 1a, and 3a on the free sterol composition of L. amazonensis promastigotesa
Sterols were extracted from cells exposed to the indicated drug concentration for 96 h; they were separated from polar lipids by silicic acid column chromatography and analyzed by quantitative capillary gas-liquid chromatography and mass spectrometry. Composition is expressed as mass percentages. ND, not detected.
Ultrastructural effects.
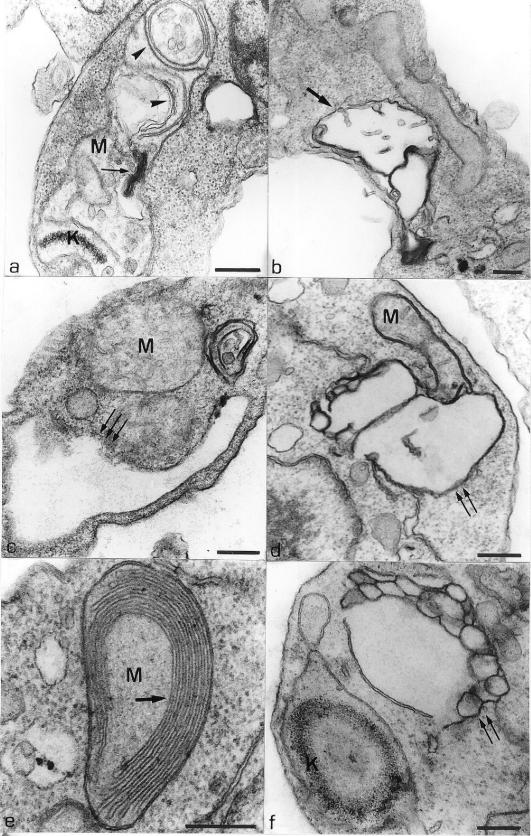
Several alterations were observed in the parasites treated with the compounds 1a, 1b, and 1c. Figure 3 shows a normal promastigote displaying the nucleus and some profiles of the mitochondrion. This image also shows the presence of a glycosome (arrow), acidocalcisome (big arrowhead), and some profiles of the endoplasmic reticulum (small arrowheads). The first alteration was observed in the mitochondrion, which presented an intense swelling, where the matrix became less electron dense (Fig. 4a and b), with the presence of concentric membranes (arrowheads) and myelin-like figures (arrows) (Fig. 4b). Figure 4c shows the presence of a swollen mitochondrial profile. Rupture of the mitochondrion membrane (Fig. 4d, arrows) and an increase in the number of mitochondrial cristae (Fig. 4e) were also observed. Alteration in the structure of kinetoplast DNA (Fig. 4f) was also observed.
FIG. 3.
Electron micrograph of L. amazonensis promastigote without treatment, showing the normal morphology. The presence of a normal mitochondrial profile (M), nucleus (N), glycosome (arrow), acidocalcisome (big arrowhead), and endoplasmic reticulum profile (small arrowheads) is indicated. Bar, 0.5 μm.
FIG.4.
(a) Thin section of a promastigote form treated with 0.1 μM compound 1b for 24 h, showing an intense mitochondrial swelling where the matrix became less electron dense. Some concentric membranes (arrowheads) and a myelin-like figure (arrows) in the matrix are observed. (b) Promastigote form treated with 0.1 μM compound 1a for 48 h, showing an intense mitochondrial swelling (arrow). (c) L. amazonensis promastigotes treated with 0.1 μM compound 1a for 48 h, showing an intense mitochondrial swelling and an expansion of the flagellar pocket (arrows). (d) Thin section of a promastigote form treated with 1.0 μM compound 1a for 24 h, showing rupture of the inner mitochondrial membrane (arrows). (e and f) Promastigote forms treated with 1.0 μM compound 1c for 48 h. These images show alterations in the mitochondrion-kinetoplast complex such as an increase in the number of mitochondrial cristae (e, arrows) and a disorganization of the kinetoplast DNA structure (f). Alterations in membranous structures, probably the endoplasmic reticulum, could be observed (f, arrows). K, kinetoplast; M, mitochondrion. Bars, 0.25 μm.
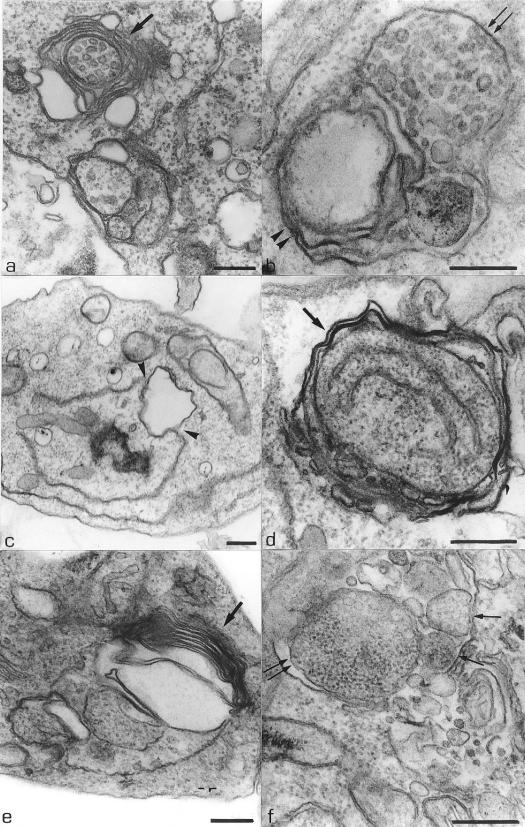
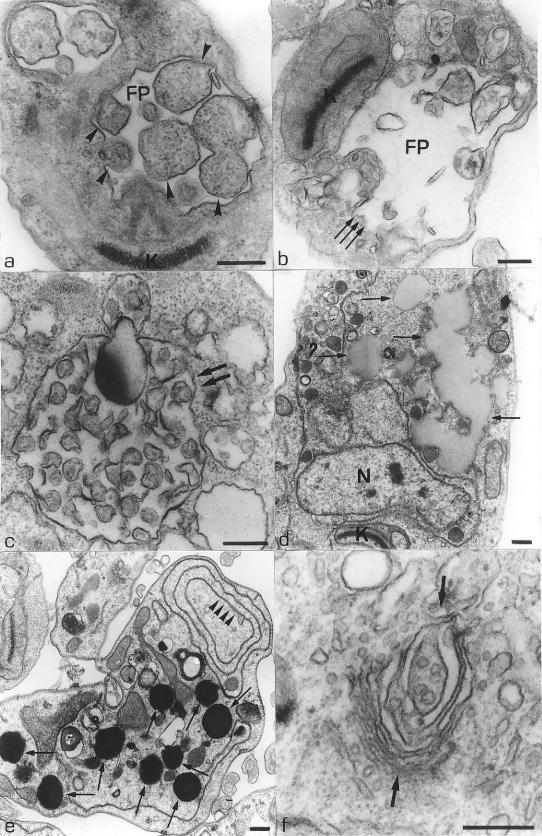
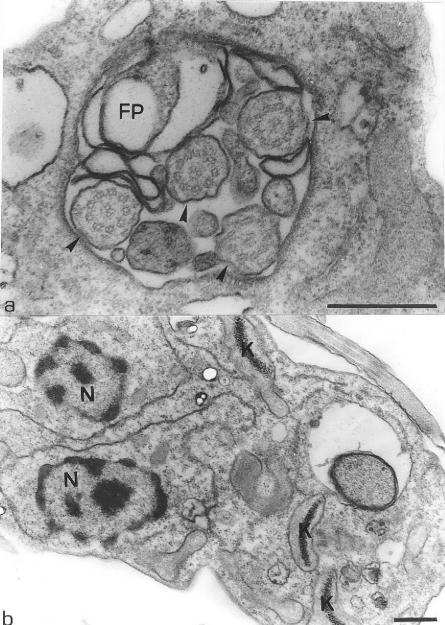
The second alteration observed in the treated parasites was the presence of some multivesicular bodies (Fig. 5a and b, arrows) surrounded by the endoplasmic reticulum (Fig. 5b, arrowheads). Some changes were also observed in the endoplasmic reticulum as the rupture of its membrane (Fig. 4f, arrows; Fig. 5c, arrowheads) and the presence of large myelin-like figures (Fig. 5d and e, arrows), where a part of the cytoplasm was involved by membrane structures forming large vacuoles, which is also observed in Fig. 5f (arrows). Alterations in the flagellar pocket, as the presence of many vesicles with the same appearance of the cytoplasm (Fig. 6a, arrowheads), and an increase in its size (Fig. 4a and 6b, arrows) were also observed. Figure 6c shows a large vacuole containing many membranous structures (arrows). Other modifications observed in the treated parasites included an increase in the number of lipid inclusions (Fig. 6d and e), alterations in the Golgi complex that presented an intense expansion in its cisternae (Fig. 6f, arrows), and the presence of some cells containing four flagella (Fig. 7a, arrowheads), three kinetoplasts, and two nuclei (Fig. 7b).
FIG. 5.
L. amazonensis promastigotes treated with 0.5 μM compound 1b and 1.0 μM compound 1c for 48 h, respectively, showing the presence of multivesicular bodies (arrows) surrounding by the endoplasmic reticulum (b, arrowheads). (c) Thin section of a promastigote form treated with 1.0 μM compound 1a for 24 h, showing rupture of the endoplasmic reticulum membrane (arrowheads). (d and e) Promastigote forms treated with 0.1 μM compound 1a and 1.0 μM compound 1c, respectively, for 48 h. These images show the presence of large myelin-like figures in the cytoplasm surrounded by the endoplasmic reticulum (arrows). (e) Structure that may correspond to the initial phase of the formation of an autophagosome, with profiles of the endoplasmic reticulum involving part of the cytoplasm (arrow). (f) Promastigote form treated with 1.0 μM compound 1c for 48 h, showing some membrane structures surrounded by the endoplasmic reticulum and containing part of the cytoplasm (arrows). Bars, 0.25 μm.
FIG. 6.
(a and b) L. amazonensis promastigotes treated with 1.0 μM and 0.1 μM compound 1a, respectively, for 48 h. These images show the presence of large vesicles in the flagellar pocket (a, arrowheads) and an intense dilatation of the flagellar pocket (b, arrows). (c) Parasite treated with 0.1 μM compound 1c for 48 h, showing the presence of a large vacuole containing some membrane profiles (arrows). (d and e) Thin sections of promastigote forms treated with 1.0 μM compound 1b for 72 h and 96 h, respectively. These images show an intense accumulation of lipid inclusions in the cytoplasm (arrows). The presence of profiles of the endoplasmic reticulum forming true vacuoles (arrowheads) is observed (e). (f) Electron micrograph of a promastigote form treated with 1.0 μM compound 1a for 24 h, showing an intense dilatation of the cisternae of the Golgi complex (arrows). FP, flagellar pocket; K, kinetoplast; N, nucleus. Bars, 0.25 μm.
FIG. 7.
L. amazonensis promastigotes treated with 0.1 μM compound 1c for 48 h. The presence of four flagella (a, arrowheads), three kinetoplasts, and two nuclei (b) in the some section plane suggests that cell division is blocked. FP, flagellar pocket; K, kinetoplast; N, nucleus. Bars, 0.5 μm.
DISCUSSION
Compounds were screened against the L. major enzyme and in whole-cell assays. The compounds showed antiproliferative effects against T. cruzi, L. donovani, L. amazonensis, and T. brucei rhodesiense at micromolar and nanomolar concentrations, similar to the data obtained for other inhibitors of the enzyme 24-SMT isolated from fungi and parasitic protozoa (1, 2, 17, 20, 30). The analysis of the sterol composition of treated Leishmania parasites indicates that the compounds are inhibiting 24-SMT in the parasites.
The effect of adding an amino group to the side chain as a partial mimic of S-adenosylmethionine did not have a major effect on the activity of the compounds. Thus, compounds of series 2 (with amino substituents) had enzyme inhibition and antiparasite activity similar to that of compounds of series 1 (without amino substituents) and similarly for series 4 compared to series 3.
Interesting results were found with the compounds of series 1 in which the 3β-OH was blocked as an acetate. These compounds did not inhibit the L. major enzyme (Table 1), but when cultured in L. amazonensis promastigotes, induced a marked reduction of the levels of 24-alkylated sterols at 10 nM and a much larger effect at 100 nM, with almost complete loss of 24-alkyl sterols; these effects correlated well with their action on the survival and growth of the L. amazonensis promastigotes. The acetate derivative 1 showed antiproliferative effects comparable to those of the nonacetylated derivative 3 against intracellular L. donovani and T. cruzi parasites as well as against the bloodstream form of T. brucei rhodesiense. One explanation is that the acetate groups are being cleaved in the organism, either in the medium or intracellularly, whereas they are stable in the buffer used for the enzyme assay. Once the acetate is cleaved, the azasterol will then inhibit 24-SMT, giving rise to the change in sterol composition.
These findings suggest that the acetate derivatives could be acting as prodrugs. Interestingly in the case of L. donovani, the acetate derivatives 1 and 2 generally show slightly higher antiproliferative activity (i.e., lower ED50) than the corresponding free OH compounds (compare 1b with 3b, 2c with 4c, 2d with 4d, and 2e with 4e). This result may be due to the ester group giving better delivery of compounds to the Leishmania amastigotes, which requires the compounds to traverse three membranes: the macrophage membrane, the parasitophorus vacuole membrane, and the amastigote plasma membrane. Perhaps the higher ED50 values found for the unprotected compounds (series 3 and 4, Table 2) when tested against L. donovani could be explained on the basis of the difficult permeation of these compounds into the cells. It could be that the ester group is required by these molecules to get inside the cells. However, the higher antiproliferative activity (Fig. 2) of compound 1a compared to 3a, despite the fact that the former is less effective in reducing the level of 24-alkyl sterols (Table 6), indicated that the acetate derivative could have an additional mode of antiparasitic activity.
Unfortunately, the presence of the ester group in position 3 of the sterol appears to be responsible for the toxicity of the protected compounds against mammalian KB cells. Presumably the higher bioavailability conferred by the ester group makes compounds interfere with some other vital processes in the cell.
Among the compounds in series 4, compound 4e seems not to be acting by inhibition of the enzyme, again suggesting an additional mechanism of action. This assumption is supported by the enzyme and sterol composition studies (Table 5), which revealed that the exposure of the parasite cells to this compound did not result in any alteration of the normal sterol composition. Although the octyl derivative 4c had shown good inhibition of the enzyme (Table 2), it did not reduce the level of endogenous sterols in the exposed cells at 0.1 μM, and only 50% reduction was reached when the dose was increased to 1.0 μM (Table 5). Thus, it is not possible to conclude that the mode of action of this compound is by inhibition of the enzyme 24-SMT.
It is very interesting that the compounds showed activity against the bloodstream form of T. brucei rhodesiense. In the procyclic (insect) form of T. brucei rhodesiense, the parasite has ergosterol as the main sterol present and contains all the requisite biosynthetic machinery, such as 24-SMT, to make these compounds. However, in the trypomastigote (mammalian) stage, the parasite has low-density lipoprotein receptors and is assumed to acquire all the sterols (such as cholesterol) from the host (4, 5). However, these compounds were found to be active against T. brucei rhodesiense. There are several possible reasons. (i) T. brucei rhodesiense alkylates sterol precursors taken up from the human host. This is found in Pneumocystis carinii and is an essential part of metabolism (30). (ii) A residual amount of biosynthesis of ergosterol is required in T. brucei rhodesiense. In other organisms, such as pathogenic yeasts, ergosterol (or another 24-alkylated sterol) is essential, even in small amounts, and is proposed to have a “sparking” role (19, 30, 32). This is a requirement for a minute amount of sterol of specific structure for the cell to proliferate. (iii) The compounds are acting on a molecular target other than 24-SMT and could include disruption of the T. brucei rhodesiense plasma membrane, although there is selectivity of these compounds to the parasite. Studies are needed to investigate this further.
L. amazonensis promastigotes treated with different concentrations of compounds 1a, 1b, and 1c presented several alterations in their ultrastructure. The main alteration was observed in the mitochondrion-kinetoplast complex, which showed intense mitochondrial swelling and some alterations in the mitochondrial membrane, such as its rupture at some sites, and an increase in the number of cristae. These alterations in the mitochondrion were observed in a previous study when L. amazonensis (35) and T. cruzi (10, 11) were incubated with other ergosterol biosynthesis inhibitors, such as terbinafine, ketoconazole, and 22,26-azasterol. The presence of these changes in the mitochondrion of trypanosomatids treated with different ergosterol biosynthesis inhibitors suggests that the presence of ergosterol or 5-dehydroepisterol is essential for the preservation of a normal structural organization of the mitochondrial membrane in these parasites. In T. cruzi epimastigotes, a large concentration of endogenous and exogenous sterol in the mitochondrion was reported, (23), suggesting that ergosterol and/or other 24-alkylated sterols are essential and that decreased availability of such sterols will affect mitochondrial functions. This observation contrasts to those showing that there are no sterols in the inner mitochondrial membrane of mammalian cells.
Another important alteration is the presence of membrane changes, including elaborated structures in the cytoplasm, appearing as myelin-like figures, with the involvement of the endoplasmic reticulum. The presence of these structures suggests the occurrence of an autophagic process, with the formation of structures known as autophagosomes. These structures were also observed when promastigote forms of L. amazonensis were treated with 22,26-azasterol (35). In addition, we observed rupture of the endoplasmic reticulum cisternae, which may be related to the absence of sterols essential to the maintenance of the membrane structure in this organelle. The presence of membrane profiles in the flagellar pocket of parasites treated with different drugs is characteristic of an exocytic process, and we cannot exclude the possibility that they result from the secretion into this region of abnormal lipids, which accumulate as a consequence of the drug action, and the assembly of the concentric membranes and myelin-like figures, probably due to their different physical properties (29).
In previous studies with L. amazonensis promastigotes cultured in the presence of 12-O-tetradecanoyl phorbol-13-acetate, an activator of proteinase kinase C, an increase in the exocytic process was observed with the appearance of membranous structures within the flagellar pocket (34), similar to those observed in the parasites treated with different ergosterol biosynthesis inhibitors. In L. amazonensis treated with ketoconazole and terbinafine, two inhibitors of ergosterol biosynthesis, the presence of myelin-like figures, membrane profiles in the flagellar pocket, and multivesicular bodies, presenting intense reaction to acid phosphatase activity were also observed (35). Other changes observed in the treated parasites included an increase in the number of lipid inclusions, which indicates that the treated parasites accumulate abnormal lipids, as confirmed by analysis of the sterol composition, an intense expansion of the flagellar pocket and of the Golgi complex cisternae, and the presence of four flagella, three kinetoplasts, and two nuclei, which is characteristic of cell division-arrested cells.
We conclude that the depletion of essential sterols of parasites of the genus Leishmania is probably the main cause of several alterations in its ultrastructure that lead to growth inhibition and, finally, cell lysis, although we cannot exclude other effects causing ultrastructural alterations.
Overall, it seems that the simple alkyl derivatives 1a-c and their analogues with a free 3-OH, compounds 3a and b, show good antiparasitic activity, with inhibition values of the same order or better than that of the reference compound AZA. From the enzymatic studies, there is enough evidence to conclude that the presence of a free hydroxyl group at position 3 of the sterol nucleus is required for effective binding to the enzyme. However, addition of an ester functionality at the 3β-OH may give rise to a prodrug effect, allowing better delivery of compounds, although additional modes of action of these ester derivatives are possible.
The length and nature of the side chain of the sterol appear to have some influence on the in vitro activity of the compounds with an amino group in the side chain. It has been shown that the butyl and octyl amino derivatives, compounds 2b, 2c, and 4c, have optimum side chains for activity.
In addition, we have demonstrated that the azasterols are active against T. brucei rhodesiense and represent an interesting lead compound for this organism.
Acknowledgments
We acknowledge NIPA laboratories, the European Union (INCO-DC programme, project number ERBIC18CT980371), and the UNDP/World Bank/WHO Programme for Research and Training in Tropical Diseases (T.D.R.) for financial support. The EPSRC National Mass Spectrometry Centre in Swansea is acknowledged for accurate mass spectrometry.
REFERENCES
- 1.Ator, M. A., S. J. Schmidt, J. L. Adams, and R. E. Dolle. 1989. Mechanism and inhibition of Delta-24-sterol methyltransferase from Candida albicans and Candida tropicalis. Biochemistry 28:9633-9640. [DOI] [PubMed] [Google Scholar]
- 2.Ator, M. A., S. J. Schmidt, J. L. Adams, R. E. Dolle, L. I. Kruse, C. L. Frey, and J. M. Barone. 1992. Synthesis, specificity, and antifungal activity of inhibitors of the Candida albicans Δ-24-sterol methyltransferase. J. Med. Chem. 35:100-106. [DOI] [PubMed] [Google Scholar]
- 3.Buckner, F. S., C. L. M. J. Verlinde, A. C. La Flamme, and W. C. Van Voorhis. 1996. Efficient technique for screening drugs for activity against Trypanosoma cruzi parasites expressing beta-galactosidase. Antimicrob. Agents Chemother. 40:2592-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coppens, I., P. Bastin, T. Levade, and P. J. Courtoy. 1995. Activity, pharmacological inhibition and biological regulation of 3-hydroxy-3-methylglutaryl coenzyme-A reductase in Trypanosoma brucei. Mol. Biochem. Parasitol. 69:29-40. [DOI] [PubMed] [Google Scholar]
- 5.Coppens, I., and P. J. Courtoy. 2000. The adaptative mechanisms of Trypanosoma brucei for sterol homeostasis in its different life-cycle environments. Annu. Rev. Microbiol. 54:129-156. [DOI] [PubMed] [Google Scholar]
- 6.Counsell, R. E., P. D. Klimstra, L. N. Nysted, and R. E. Ranney. 1965. Hypocholesterolemic agents. V. Isomeric azacholesterols. J. Am. Chem. Soc. 87:45-48. [DOI] [PubMed] [Google Scholar]
- 7.Goad, L. J., G. G. Holz, and D. H. Beach. 1984. Sterols of Leishmania species. Implications for biosynthesis. Mol. Biochem. Parasitol. 10:161-170. [DOI] [PubMed] [Google Scholar]
- 8.Haughan, P. A., M. L. Chance, and L. J. Goad. 1995. Effects of an azasterol inhibitor of sterol 24-transmethylation on sterol biosynthesis and growth of Leishmania donovani promastigotes. Biochem. J. 308:31-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirumi, H., and K. Hirumi. 1989. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum-protein without feeder cell-layers. J. Parasitol. 75:985-989. [PubMed] [Google Scholar]
- 10.Lazardi, K., J. A. Urbina, and W. Desouza. 1990. Ultrastructural alterations induced by 2 ergosterol biosynthesis inhibitors, ketoconazole and terbinafine, on epimastigotes and amastigotes of Trypanosoma (Schizotrypanum) cruzi. Antimicrob. Agents Chemother. 34:2097-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lazardi, K., J. A. Urbina, and W. Desouza. 1991. Ultrastructural alterations induced by ICI-195,739, a bis-triazole derivative with strong antiproliferative action against Trypanosoma (Schizotrypanum) cruzi. Antimicrob. Agents Chemother. 35:736-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liendo, A., K. Lazardi, and J. A. Urbina. 1998. In-vitro antiproliferative effects and mechanism of action of the bis-triazole D0870 and its S(-) enantiomer against Trypanosoma cruzi. J. Antimicrob. Chemother. 41:197-205. [DOI] [PubMed] [Google Scholar]
- 13.Liendo, A., G. Visbal, M. M. Piras, R. Piras, and J. A. Urbina. 1999. Sterol composition and biosynthesis in Tryanosoma cruzi amastigotes. Mol. Biochem. Parasitol. 104:81-91. [DOI] [PubMed] [Google Scholar]
- 14.Martin, M. B., J. S. Grimley, J. C. Lewis, H. T. Heath, B. N. Bailey, H. Kendrick, V. Yardley, A. Caldera, R. Lira, J. A. Urbina, S. N. J. Moreno, R. Docampo, S. L. Croft, and E. Oldfield. 2001. Bisphosphonates inhibit the growth of Trypanosoma brucei, Trypanosoma cruzi, Leishmania donovani, Toxoplasma gondii, and Plasmodium falciparum: a potential route to chemotherapy. J. Med. Chem. 44:909-916. [DOI] [PubMed] [Google Scholar]
- 15.Neal, R. A., and S. L. Croft. 1984. An in vitro system for determining the activity of compounds against the intracellular amastigote form of Leishmania donovani. J. Antimicrob. Chemother. 14:463-475. [DOI] [PubMed] [Google Scholar]
- 16.Nes, W. D. 2000. Sterol methyl transferase: enzymology and inhibition. Mol. Cell. Biol. Lipids 1529:63-88. [DOI] [PubMed] [Google Scholar]
- 17.Oehlschlager, A. C., R. H. Angus, A. M. Pierce, H. D. Pierce, and R. Srinivasan. 1984. Azasterol inhibition of delta-24-sterol methyltransferase in Saccharomyces cerevisiae. Biochemistry 23:3582-3589. [DOI] [PubMed] [Google Scholar]
- 18.Oehlschlager, A. C., S. M. Singh, and S. Sharma. 1991. Squalene synthetase inhibitors. Synthesis of sulfonium ion mimics of the carbocationic intermediates. J. Org. Chem. 56:3856-3861. [Google Scholar]
- 19.Parks, L. W., S. J. Smith, and J. H. Crowley. 1995. Biochemical and physiological-effects of sterol alterations in yeast: a review. Lipids 30:227-230. [DOI] [PubMed] [Google Scholar]
- 20.Rahier, A., J. C. Genot, F. Schuber, P. Benveniste, and A. S. Narula. 1984. Inhibition of S-adenosyl-L-methionine sterol-C-24-methyltransferase by analogs of a carbocationic ion high-energy intermediate-Structure activity relationships for C-25 heteroatoms (N,As,S) substituted triterpenoid derivatives. J. Biol. Chem. 259:5215-5223. [PubMed] [Google Scholar]
- 21.Rahman, M. D., and R. A. Pascal. 1990. Inhibitors of ergosterol biosynthesis and growth of the trypanosomatid protozoan Crithidia fasciculata. J. Biol. Chem. 265:4989-4996. [PubMed] [Google Scholar]
- 22.Raz, B., M. Iten, Y. Grether-Buhler, R. Kaminski, and R. Brun. 1997. The Alamar Blue assay to determine drug sensitivity of African trypanosomes (T. b. rhodesiense and T. b. gambiense) in vitro. Acta Trop. 68:139-147. [DOI] [PubMed] [Google Scholar]
- 23.Rodrigues, C. O., R. Catisti, S. A. Uyemura, A. E. Vercesi, R. Lira, C. Rodriguez, J. A. Urbina, and R. Docampo. 2001. The sterol composition of Trypanosoma cruzi changes after growth in different culture media and results in different sensitivity to digitonin-permeabilization. J. Eukaryot. Microbiol. 48:588-594. [DOI] [PubMed] [Google Scholar]
- 24.Rodrigues, J. C. F., M. Attias, C. Rodriguez, J. A. Urbina, and W. de Souza. 2002. Ultrastructural and biochemical alterations induced by 22,26-azasterol, a delta(24[25])-sterol methyltransferase inhibitor, on promastigote and amastigote forms of Leishmania amazonensis. Antimicrob. Agents Chemother. 46:487-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roux, C. 1964. Action tératogène du triparanol chez l'animal. Arch. Fr. Pediatr. 21:451-464. [PubMed] [Google Scholar]
- 26.Urbina, J. A. 1997. Lipid biosynthesis pathways as chemotherapeutic targets in kinetoplastid parasites. Parasitology 114:S91-S99. [PubMed] [Google Scholar]
- 27.Urbina, J. A., G. Payares, L. M. Contreras, A. Liendo, C. Sanoja, J. Molina, M. Piras, R. Piras, N. Perez, P. Wincker, and D. Loebenberg. 1998. Antiproliferative effects and mechanism of action of SCH 56592 against Trypanosoma (Schizotrypanum) cruzi: In vitro and in vivo studies. Antimicrob. Agents Chemother. 42:1771-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urbina, J. A., G. Payares, J. Molina, C. Sanoja, A. Liendo, K. Lazardi, M. M. Piras, R. Piras, N. Perez, P. Wincker, and J. F. Ryley. 1996. Cure of short- and long-term experimental Chagas' disease using D0870. Science 273:969-971. [DOI] [PubMed] [Google Scholar]
- 29.Urbina, J. A., S. Pekerar, H. B. Le, J. Patterson, B. Montez, and E. Oldfield. 1995. Molecular order and dynamics of phosphatidylcholine bilayer-Membranes in the presence of cholesterol, ergosterol and lanosterol-a comparative-study using H-2-NMR, C-13-NMR and P-31-NMR spectroscopy. Biochim. Biophys. Acta Biomembr. 1238:163-176. [DOI] [PubMed] [Google Scholar]
- 30.Urbina, J. A., G. Visbal, L. M. Contreras, G. McLaughlin, and R. Docampo. 1997. Inhibitors of delta(24(25)) sterol methyltransferase block sterol synthesis and cell proliferation in Pneumocystis carinii. Antimicrob. Agents Chemother. 41:1428-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urbina, J. A., J. Vivas, K. Lazardi, J. Molina, G. Payares, M. M. Piras, and R. Piras. 1996. Antiproliferative effects of delta 24(25) sterol methyl transferase inhibitors on Trypanosoma (Schizotrypanum) cruzi: in vitro and in vivo studies. Chemotherapy 42:294-307. [DOI] [PubMed] [Google Scholar]
- 32.Urbina, J. A., J. Vivas, G. Visbal, and L. M. Contreras. 1995. Modification of the sterol composition of Trypanosoma (Schizotrypanum) cruzi epimastigotes by Delta(24(25))-sterol methyl transferase inhibitors and their combinations with ketoconazole. Mol. Biochem. Parasitol. 73:199-210. [DOI] [PubMed] [Google Scholar]
- 33.Vanden Bossche, H. November/December 1993. Antiprotozoal activity of ergosterol biosynthesis inhibitors: focus on Trypanosomatidae and Plasmodiidae. Microbiol. Eur. 1993:20-28. [Google Scholar]
- 34.Vannier-Santos, M. A., A. Martiny, J. R. Meyer-Fernandes, and W. de Souza. 1995. Leishmanial protein kinase C modulates host cell infection via secreted acid phosphatase. Eur. J. Cell Biol. 67:112-119. [PubMed] [Google Scholar]
- 35.Vannier-Santos, M. A., J. A. Urbina, A. Martiny, A. Neves, and W. Desouza. 1995. Alterations induced by the antifungal compounds ketoconazole and terbinafine in Leishmania. J. Eukaryot. Microbiol. 42:337-346. [DOI] [PubMed] [Google Scholar]
- 36.Venkatramesh, M., D. A. Guo, Z. H. Jia, and W. D. Nes. 1996. Mechanism and structural requirements for transformation of substrates by the (S)-adenosyl-L-methionine: Delta(24(25))-sterol methyl transferase from Saccharomyces cerevisiae. Biochim. Biophys. Acta Lipids Lipid Metab. 1299:313-324. [DOI] [PubMed] [Google Scholar]
- 37.Vivas, J., J. A. Urbina, and W. deSouza. 1997. Ultrastructural alterations in Trypanosoma (Schizotrypanum) cruzi induced by Delta(24(25)) sterol methyl transferase inhibitors and their combinations with ketoconazole. Int. J. Antimicrob. Agents 8:1-6. [DOI] [PubMed] [Google Scholar]