Abstract
Telavancin (TD-6424) is a novel lipoglycopeptide that produces rapid and concentration-dependent killing of clinically relevant gram-positive organisms in vitro. The present studies evaluated the in vivo pharmacodynamics of telavancin in the mouse neutropenic thigh (MNT) and mouse subcutaneous infection (MSI) animal models. Pharmacokinetic-pharmacodynamic studies in the MNT model demonstrated that the 24-h area under the concentration-time curve (AUC)/MIC ratio was the best predictor of efficacy. Telavancin produced dose-dependent reduction of thigh titers of several organisms, including methicillin-susceptible Staphylococcus aureus (MSSA) and methicillin-resistant Staphylococcus aureus (MRSA), penicillin-susceptible and -resistant strains of Streptococcus pneumoniae, and vancomycin-resistant Enterococcus faecalis. The 50% effective dose (ED50) estimates for telavancin ranged from 0.5 to 6.6 mg/kg of body weight (administered intravenously), and titers were reduced by up to 3 log10 CFU/g from pretreatment values. Against MRSA ATCC 33591, telavancin was 4- and 30-fold more potent (on an ED50 basis) than vancomycin and linezolid, respectively. Against MSSA ATCC 13709, telavancin was 16- and 40-fold more potent than vancomycin and nafcillin, respectively. Telavancin, vancomycin, and linezolid were all efficacious and more potent against MRSA ATCC 33591 in the MSI model compared to the MNT model. This deviation in potency was, however, disproportionately greater for vancomycin and linezolid than for telavancin, suggesting that activity of telavancin is less affected by the immune status. The findings of these studies collectively suggest that once-daily dosing of telavancin may provide an effective approach for the treatment of clinically relevant infections with gram-positive organisms.
The emergence of multidrug-resistant gram-positive pathogens, as well as their increasing contribution to nosocomial infections, is a growing medical concern. According to a recent report, methicillin-resistant Staphylococcus aureus, which is now endemic in many U.S. hospitals, accounted for 51% of Staphylococcus aureus isolates from patients with nosocomial infections in intensive care units (2). Resistance to penicillin among strains of Streptococcus pneumoniae is also increasing, with a recent report stating that 20% of Streptococcus pneumoniae isolates were resistant to penicillin (20). Until recently, vancomycin and linezolid, drugs which are either bacteriostatic or slowly bactericidal (9, 15), were the only two approved therapies in the United States which retained activity against resistant gram-positive strains. Daptomycin, a novel bactericidal drug with activity against resistant gram-positive strains (19), has recently received approval. The ongoing threat of vancomycin-resistant enterococci (13) and vancomycin-intermediate susceptible Staphylococcus aureus (18) and recent reports of vancomycin-resistant Staphylococcus aureus (3) further underscore the need for novel, bactericidal therapies active against resistant pathogens.
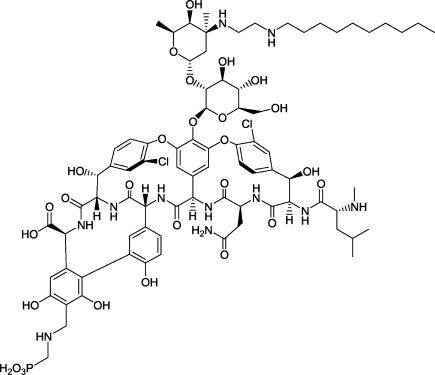
Telavancin (TD-6424) (Fig. 1) is a novel lipoglycopeptide that operates through at least two distinct mechanisms to produce potent and rapid bactericidal activity in vitro against clinically relevant gram-positive pathogens, including Staphylococcus aureus (16; D. V. Debabov, J. Pace, M. Nodwell, S. Trapp, R. Campbell, D. Karr, T. Wu, K. Krause, D. Johnston, C. Lane, D. Schmidt, D. Higgins, B. Christensen, K. Judice, and K. Kaniga, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. C1, p. 1809, 2003). The present studies were conducted to evaluate the in vivo pharmacodynamics of telavancin, in comparison to vancomycin, linezolid, and nafcillin, in the mouse neutropenic thigh (6) and subcutaneous infection (17) models.
FIG. 1.
Chemical structure of telavancin.
MATERIALS AND METHODS
Antimicrobial agents.
Telavancin was synthesized at Theravance, Inc. Linezolid, vancomycin, and nafcillin were either synthesized at Theravance, Inc., or obtained from commercial sources. The purity of all drugs was >90%. Telavancin and linezolid were dissolved in 5% cyclodextrin, whereas vancomycin was dissolved in 5% dextrose in water. All drugs were administered intravenously (i.v.) to mimic the route of administration in a hospital setting.
Bacterial strains.
Four strains of methicillin-resistant Staphylococcus aureus (MRSA), four strains of methicillin-susceptible Staphylococcus aureus (MSSA), one strain of methicillin-resistant Staphylococcus epidermidis (MRSE), one strain of methicillin-susceptible Staphylococcus epidermidis (MSSE), two strains of penicillin-resistant Streptococcus pneumoniae (PRSP), two strains of penicillin-susceptible Streptococcus pneumoniae (PSSP), and one strain of vancomycin-resistant Enterococcus faecalis (VREF) were used in the present studies. MRSA ATCC 33591 (MRSA 33591) and MSSA ATCC 13709 (MSSA 13709) were obtained from the American Type Culture Collection (Manassas, Va.). VREF A256 was a laboratory-engineered strain obtained from the University of California, San Francisco. All other strains (MRSA MCJ25, MRSA SFVA06, MRSA MGH10, MSSA KPB01, MSSA KPB04, MSSA MED415, MRSE SFVA01, MSSE SU03, PRSP SU2, PRSP CHM11, PSSP SU10, and PSSP SU07) were obtained from U.S. hospitals or academic institutions. Staphylococcal and enterococcal overnight cultures were grown in brain heart infusion broth aerobically at 35°C and subcultured in fresh medium to the desired inoculum density. In the case of PSSP and PRSP strains, colonies were swabbed off an initial plate and grown overnight in brain heart infusion broth at 35°C in a 5% CO2 incubator.
In vitro susceptibility testing.
MICs were determined as described by the NCCLS utilizing cation-supplemented Mueller-Hinton broth (14). The MIC was read visually as the lowest drug concentration well with no visible bacterial growth. In a separate study, the influence of protein binding was studied by determining the effect of 95% mouse serum or mouse albumin (40 mg/ml) on the MIC of telavancin. These experiments were conducted using arithmetic dilutions of the drug to improve accuracy (10).
Protein binding determination.
Plasma protein binding was determined by equilibrium dialysis with a Spectrum Multi-Equilibrium Dialyzer. Teflon semimicrocells with approximately 1-ml capacity were used for dialysis using regenerated cellulose membrane disks (Spectrum Laboratories, Rancho Dominguez, Calif.) with a molecular weight cutoff of 12,000 to 14,000. 3H-telavancin and appropriate stock solutions were added to mouse plasma to achieve the final concentrations of 0.1-, 1-, 10-, and 100-μg equivalents of telavancin/ml.
Mouse neutropenic thigh model.
All studies were approved by the Institutional Animal Care and Use Committee (IACUC) at Theravance, Inc.
Animals (female NSA mice; 20 to 25 g) were acquired from Charles River Laboratories (Gilroy, Calif.) and allowed access to food and water ad libitum. Neutropenia was induced with cyclophosphamide (200 mg/kg of body weight, intraperitoneally) administered 4 and 2 days prior to inoculation of bacteria. This treatment regimen induced severe leukopenia and decreased the neutrophil count to approximately 100 cells/mm3. Neutropenia was sustained for >48 h after bacterial inoculation (data not shown).
The bacterial inoculum concentration was ∼106 CFU/ml. In one study, a higher inoculum concentration (∼108 CFU/ml) was used to determine the impact of a higher pretreatment titer on the efficacy of telavancin. Animals were lightly anesthetized with isoflurane (2.5% for induction followed by 1% for maintenance), and 50 μl of the bacterial inoculum was injected into the thigh. One hour after inoculation, animals were treated i.v. with vehicle or various regimens of the test drug. At 0 and 24 h posttreatment, cohorts of animals were euthanized (CO2 asphyxiation) and the thighs were collected aseptically. The thigh was weighed (0.7 to 0.9 g range) and placed into 10 ml of sterile saline and homogenized. For the majority of strains, dilutions of the homogenate were plated onto tryptic soy agar plates, which were incubated overnight. In the case of PSSP and PRSP strains, dilutions of the homogenate were plated onto tryptic soy blood agar plates, which were incubated overnight in a CO2 incubator. In all cases, the titer was expressed as log10 CFU/gram of thigh weight. At the start of treatment (1 h after inoculation) the thigh titer increased by approximately 0.3 log10 CFU/g.
A separate single-dose pharmacokinetic study of telavancin was performed in neutropenic mice infected in the thigh with MRSA 33591. Infected animals were treated, at 1 h postinoculation, with 1, 2.5, 5, and 10 mg/kg, i.v. At various time points postdose, animals were euthanized with CO2 and blood samples were collected by cardiac puncture. Mouse plasma samples were analyzed for drug concentrations by a reversed-phase high-performance liquid chromatography with spectrometry-mass spectroscopy detection. Plasma samples were mixed with internal standard and loaded directly onto a 96-well extraction plate (Oasis HLB). The extracted samples were injected onto an analytical column for chromatographic separation (Merck Chromalith column, 4.6 by 50 mm, at 40°C), and the eluent flowed directly to the mass spectrometer-mass spectroscope for analysis. Mobile phases used were mobile phase A, 0.25% formic acid in water, and mobile phase B, 0.25% formic acid in acetonitrile. The pump program was 25% mobile phase B for 0.5 min, followed by a linear gradient to 50% mobile phase B over 2.0 min, and the flow rate was 0.5 ml/min. The mass spectrometer was operated in positive ion multiple reaction-monitoring mode. The method was linear over the range of 0.25 to 100 μg/ml, and the limit of quantitation was 0.25 μg/ml. Quality control samples were used as a measure of assay performance. The measured concentrations for two-thirds of the quality control samples were within 20% of theoretical concentrations, and the coefficient of variance for replicate samples was <20%.
Mouse subcutaneous infection model.
Animals (female SKH-1 mice, 15 to 25 g) were acquired from Charles River Laboratories (Wilmington, Mass.). Animals were anesthetized with isoflurane (2.5% for induction followed by 1% for maintenance). A small (∼1-cm) dorsal paramedian incision was made, an inoculum-soaked cotton umbilical tape (Ethicon, Sommerville, N.J.) was implanted subcutaneously, and the wound was closed with tissue glue (Veterinary Products Laboratories, Phoenix, Ariz.). Two hours after tape implantation, animals were treated i.v. with vehicle or the appropriate test drug. At 0 and 24 h posttreatment, cohorts of animals were euthanized (CO2 asphyxiation) and the cotton tape and associated tissue were collected aseptically, weighed (range, 80 to 120 mg), and homogenized in 900 μl of sterile saline. Dilutions of the homogenate were plated onto tryptic soy agar plates, which were incubated overnight. The bacterial titers were expressed as log10 CFU/gram of tape weight. At the start of treatment (2 h after inoculation) the tape titer increased by approximately 0.4 log10 CFU/g.
Experimental treatments.
In dose-fractionation studies, the total dose of telavancin was administered either as a single dose or split into two divided doses (administered every 12 h [q 12 h]), three divided doses (q 8 h), or four divided doses (q 6 h). In all other studies, telavancin was administered as a single dose (q 24 h) at doses ranging from 0.1 to 80 mg/kg, i.v. Vancomycin and linezolid were administered i.v. twice in 24 h (q 12 h). Nafcillin was administered i.v. four times in 24 h (q 6 h). Dosing volume was 100 μl/mouse. The dosing frequency of the three comparator drugs (vancomycin, linezolid, and nafcillin) was chosen based on published findings on the pharmacodynamics and optimal pharmacokinetic-pharmacodynamic (PK-PD) targets of glycopeptides (primarily area under the curve [AUC]/MIC ratio), oxazolidinones (AUC/MIC ratio and time spent above the MIC) and beta-lactams (time spent above the MIC) (1, 4, 5).
Data analysis.
Data are expressed as means ± 1 standard deviation (SD).
Dose-response curves are fitted with a four-parameter logistic equation using GraphPad Prism for Microsoft Windows (version 3.00; GraphPad Software, San Diego, Calif.). The equation used is as follows: y = Min + (Max − Min)/(1 + 10[log ED50 − x] × Hillslope), where x is the logarithm of dose and y is the response (in log10 CFU per gram). y starts at a minimum (Min) (fixed to the 24-h vehicle control response) and approaches asymptotically to a maximum (Max) with a sigmoidal shape.
The 50% effective dose (ED50) was defined as the dose required to produce 50% of the maximum response. ED50 estimates are expressed as means with 95% confidence intervals (CI). A two-tailed Students t test was used to compare ED50 estimates between treatments. P < 0.05 was considered to be statistically significant. The log10 stasis dose (EDstasis) was defined as the dose producing no net change in titer as measured in the thigh compared to pretreatment titer. A 1-log10 kill dose (ED1-log kill) was defined as the dose required to produce a decrease in titer of 1 log CFU/g from pretreatment controls. Similarly, ED2-log kill and ED3-log kill were defined as the doses required to produce decreases in titer of 2 and 3 log10 CFU/g.
The pharmacokinetic parameters of telavancin were determined using a two-compartment i.v. bolus model (model 7) using WinNonlin (version 4.1.0; Pharsight, Mountain View, Calif.). The AUC from the time of dosing to the last measurable concentration was calculated by the linear trapezoidal rule. From the single-dose pharmacokinetic data, the three PK-PD parameters (AUC/MIC ratio, maximum concentration of drug in serum [Cmax]/MIC ratio, and time spent above the MIC) were estimated, using total and free-drug concentrations, under different dose-fractionation regimens (q 24 h [one dose], q 12 h [two doses], q 8 h [three doses], or q 6 h [four doses]). These parameters were then correlated with corresponding efficacy data (log10 CFU/gram) using WinNonlin Pharmacodynamic model 108—the inhibitory effect sigmoid Emax model.
RESULTS
In vitro inhibitory activity of telavancin.
Table 1 lists the MICs of telavancin, vancomycin, nafcillin, and linezolid against the various strains. The data show that telavancin is approximately 10- to 30-fold more potent than vancomycin and linezolid against the four strains of Streptococcus pneumoniae. Against the four MRSA strains, the activity of telavancin was modestly better than or comparable to that of vancomycin and linezolid, whereas nafcillin was ineffective as expected. VREF A256 was susceptible to telavancin and linezolid. However, this organism was resistant to vancomycin. Against the remaining strains, telavancin was either as potent as or slightly more potent than the comparator drugs.
TABLE 1.
MICs of various drugs for gram-positive bacterial strains
| Organism | MIC (μg/ml)
|
|||
|---|---|---|---|---|
| Telavancin | Vancomycin | Linezolid | Nafcillin | |
| MRSA 33591 | 1 | 2 | 2 | 64 |
| MRSA MCJ-25 | 0.5 | 1 | 2 | 32 |
| MRSA SFVA06 | 2 | 2 | 2 | 64 |
| MRSA MGH 10 | 0.5 | 1 | 4 | 32 |
| MSSA 13709 | 1 | 1 | 4 | 0.25 |
| MSSA KPB-01 | 1 | 1 | 4 | 0.25 |
| MSSA KPB-04 | 0.5 | 1 | 2 | 0.25 |
| MSSA MED-415 | 0.5 | 1 | 4 | 0.25 |
| MRSE SFVA-01 | 1 | 2 | 2 | 4 |
| MSSE SU-03 | 1 | 2 | 4 | 0.25 |
| PRSP SU-02 | 0.062 | 0.25 | 0.5 | 4 |
| PRSP CHM-11 | 0.062 | 0.25 | 1 | 4 |
| PSSP SU-10 | 0.062 | 0.5 | 1 | ≤0.031 |
| PSSP SU-07 | 0.062 | 0.5 | 2 | ≤0.031 |
| VREF A256 | 4 | >64 | 2 | 4 |
In the presence of 95% mouse serum and mouse albumin (40 mg/ml), the MIC of telavancin was 2 and 4 μg/ml against MRSA 33591, which represents a 5- and 10-fold increase over that observed in broth medium alone (0.4 μg/ml).
Protein binding.
Telavancin exhibited concentration-independent protein binding in mouse plasma that ranged from 94 to 96%.
Single-dose pharmacokinetics of telavancin in infected neutropenic mice.
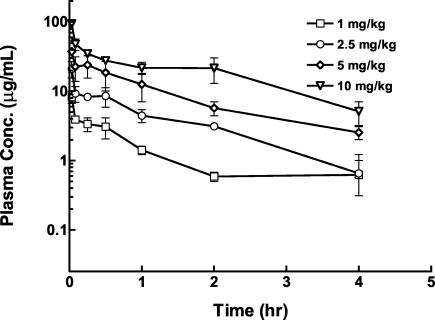
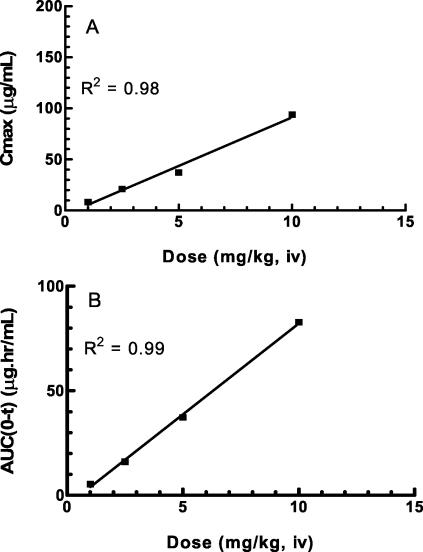
The time-concentration profile for single doses of telavancin is shown in Fig. 2. Both Cmax and AUC increased in proportion to the dose (Fig. 3). A two-compartment model with a terminal half-life of 1.0 to 1.4 h best described the pharmacokinetics of telavancin.
FIG. 2.
Single-dose concentration-versus-time pharmacokinetic profile for various doses of telavancin in neutropenic mice infected in the thigh with MRSA 33591. The abscissa shows the time, and the ordinate shows the plasma drug concentration (Conc.) (n = 3 per group). Data are expressed as means ± 1 SD (error bars).
FIG. 3.
Relationship between telavancin dose and Cmax (A) and dose versus AUC (B) for various doses of telavancin. The values are means (n = 3).
Effects of dose fractionation on the efficacy of telavancin in the mouse neutropenic thigh model.
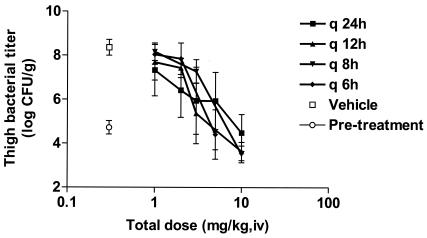
Dose fractionation studies were performed with telavancin in the mouse neutropenic thigh model, using MRSA 33591 as the test organism, to determine the relationship between dosing interval and efficacy. The total 24-h dose of telavancin (1, 2, 3, 5, or 10 mg/kg, i.v.) was administered either as a single dose (q 24 h), split into two divided doses administered q 12 h, split into three divided doses administered q 8 h, or split into four divided doses administered q 6 h.
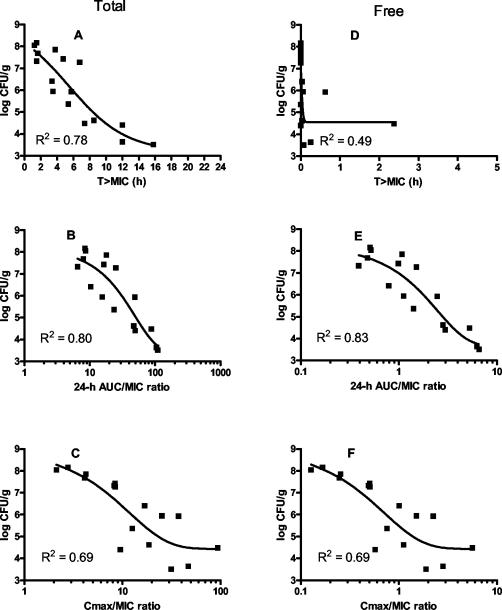
The pretreatment bacterial titer in the thigh was 4.7 ± 0.3 log CFU/g. In vehicle-treated controls, thigh titer increased to 8.4 ± 0.3 log CFU/g in 24 h. Telavancin produced comparable dose-dependent reductions in thigh bacterial titer at each of the four dosing regimens (Fig. 4). As shown in Fig. 5, both AUC/MIC and time spent above the MIC were equally good predictors of efficacy when total drug concentrations were considered, whereas AUC/MIC was the best predictor of efficacy when free drug concentrations were used (assuming protein binding of 94%).
FIG. 4.
Effects of telavancin against MRSA 33591 on titer in thighs from mice treated with various doses of telavancin administered in one, two, three, or four divided doses. The abscissa shows the total 24-h dosage, and the ordinate shows the titer observed in thighs (n = 5 to 6 for vehicle and 5 to 10 for telavancin). Data are expressed as means ± 1 SD (error bars).
FIG. 5.
Relationship between titer observed in thigh samples and the three following pharmacodynamically linked variables: time above the MIC (A and D), 24-h AUC/MIC ratio (B and E), and Cmax/MIC ratio (C and F) using total (A, B, and C) and free-drug (D, E, and F) concentrations. The organism studied was MRSA 33591.
Pharmacodynamic effects of telavancin against multiple gram-positive organisms in the mouse neutropenic thigh model.
Table 2 lists the growth of various strains at 24 h in vehicle-treated animals. The data show that all strains of MRSA, MSSA, and PSSP and one strain of PRSP (PRSP CHM 11) grew very robustly (growth in 24 h, >2.5 log10 CFU/g), whereas growth was modest (growth in 24 h, <2.5 log10 CFU/g) with the remaining organisms (PRSP SU02, MRSE SFVA01, MSSE SU03, and VREFs A256).
TABLE 2.
Point dose estimates of telavancin required to attain different pharmacodynamic endpoints against gram-positive bacterial strains in the MNT model
| Organism | 24-h growth (log CFU/g)a | Doses (mg/kg) of telavancin required to attainb:
|
||||
|---|---|---|---|---|---|---|
| ED50 | EDstasis | ED1-log kill | ED2-log kill | ED3-log kill | ||
| MRSA 33591 | 3.7 | 2.5 | 6.3 | 27.5 | — | — |
| MRSA MCJ25 | 3.3 | 3.1 | 4.4 | 8.9 | 37.1 | — |
| MRSA SFVA06 | 2.8 | 3.7 | 3.3 | 6.1 | 14.4 | — |
| MRSA MGH 10 | 4.0 | 4.4 | 8.5 | 29.5 | — | — |
| MSSA 13709 | 3.5 | 1.7 | 2.5 | 5.5 | 58.9 | — |
| MSSA KPB01 | 3.7 | 2.2 | 6.3 | 47.8 | — | — |
| MSSA KPB04 | 4.1 | 1.7 | 6.3 | 32.3 | — | — |
| MSSA MED 415 | 4.2 | 2.0 | 2.8 | 4.8 | 10.7 | 50 |
| MRSE SFVA01 | 1.0 | 1.0 | 0.7 | 1.4 | 6.7 | — |
| MSSE SU03 | 2.0 | 1.2 | 0.9 | 2.9 | 23.9 | — |
| PRSP SU2 | 2.2 | 0.5 | 0.4 | 0.6 | 1.1 | 3.5 |
| PRSP CHM11 | 3.5 | 2.9 | 3.3 | 5.4 | 10.9 | 50 |
| PSSP SU10 | 3.3 | 1.8 | 1.7 | 2.6 | 4.6 | 12.0 |
| PSSP SU07 | 4.1 | 2.0 | 2.8 | 5.2 | 12.8 | 50 |
| VREFs A256 | 2.0 | 6.6 | 50 | — | — | — |
Calculated as the difference between the 24-h titer in vehicle-treated animals and the pretreatment titer.
Refer to Materials and Methods for definition of pharmacodynamic endpoints. —, endpoint not achieved at the doses tested.
Telavancin, administered as a single dose in 24 h, produced a dose-dependent reduction of titer against all strains. Table 2 summarizes the point-dose estimates required to attain different pharmacodynamic endpoints. The ED50 point estimates ranged from 0.5 to 6.6 mg/kg, i.v. The maximal killing (defined as the reduction in thigh titer from pretreatment values) was ≥3 log10 CFU/g (for PRSP SU2, PRSP CHM11, PSSP SU10, and PSSP SU-7), between 2 and 3 log10 CFU/g (for MRSA MCJ25, MRSA SFVA06, MSSA 13709, MRSE SFVA01, and MSSE SU03), between 1 and 2 log10 CFU/g (for MRSA 33591, MRSA MGH 10, MSSA KPB01, and MSSA KPB04) or less than 1 log10 (for VRE A256).
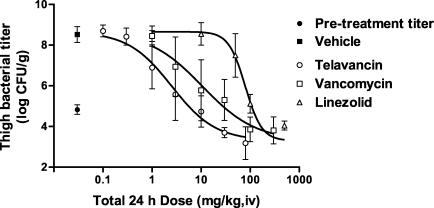
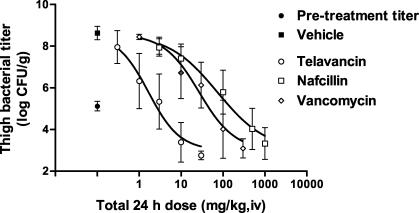
The efficacies of three comparator drugs (vancomycin, linezolid, and nafcillin) against MRSA 33591 and/or MSSA 13709 strains were evaluated and compared to that of telavancin. The dose-response curves of telavancin and the comparators are shown in Fig. 6 and 7 and the point dose estimates for the different endpoints are summarized in Table 3. Against MRSA 33591, telavancin (ED50 = 2.5 [2.1 to 3.1] mg/kg, i.v.) was 4- and 32-fold more potent (P < 0.05) than vancomycin (ED50 = 10.6 [6.4 to 17.6] mg/kg, i.v.) and linezolid (ED50 = 79.4 [68.8 to 91.5] mg/kg, i.v.), respectively. Against MSSA 13709, telavancin (ED50 = 1.7 [1.3 to 2.2] mg/kg, i.v.) was 16- and 43-fold more potent (P < 0.05) than vancomycin (ED50 = 27.0 [18.4 to 39.7] mg/kg, i.v.) and nafcillin (ED50 = 73.6 [51.3 to 105.7] mg/kg, i.v.), respectively. Against VREF A256, the potency of telavancin (ED50 = 6.6 [2.9 to 14.7] mg/kg, i.v.) and linezolid (ED50 = 17.1 [7.0 to 41.6] mg/kg, i.v.) were not significantly different (P > 0.05) from each other, whereas vancomycin was inactive at 100 mg/kg, i.v.
FIG. 6.
Efficacies of telavancin, vancomycin, and linezolid against MRSA 33591 in the mouse neutropenic thigh model. The abscissa shows the total 24-h dosage, and the ordinate shows the titer observed in thighs. Vehicle (n = 16) and telavancin (n = 5 to 16 per dose) were administered q 24 h, whereas vancomycin (n = 5 per dose) and linezolid (n = 6 per dose) were administered q 12 h. n = 16 for the control pretreatment group. Data are expressed as means ± 1 SD (error bars).
FIG. 7.
Efficacies of telavancin, vancomycin, and nafcillin against MSSA 13709 in the mouse neutropenic thigh model. The abscissa shows the total 24-h dosage, and the ordinate shows the titer observed in thighs. Vehicle (n = 15) and telavancin (n = 9 to 10 per dose) were administered q 24 h, vancomycin (n = 6 per dose) was administered q 12 h, and nafcillin (n = 9 to 10 per dose) was administered q 6 h. n = 15 for the control pretreatment group. Data are expressed as means ± 1 SD (error bar).
TABLE 3.
Point dose estimates needed against infection caused by MRSA 33591 in MNT and MSI modelsa
| Drug | Doseb (mg/kg) needed in model
|
ED50 ratio (MNT/MSI) | ED1-log kill ratio (MNT/MSI) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MNT
|
MSI
|
||||||||
| EDstasis | ED50 | ED1-log kill | EDstasis | ED50 | ED1-log kill | ED2-log kill | |||
| Telavancin | 6.3 | 2.5 | 27.5 | 0.8 | 0.9 | 2.1 | 10 | 2.7 | 13 |
| Vancomycin | 34.6 | 10.6 | 199.5 | 2.8 | 2.8 | 3.9 | 10 | 3.8 | 51 |
| Linezolid | 109.6 | 79.4 | >500 | 2.3 | 2.3 | 2.6 | >500 | 34.5 | >192 |
Refer to Materials and Methods for definition of pharmacodynamic endpoints. MNT, mouse neutropenic thigh; MSI, mouse subcutaneous infection.
Dose administered i.v.
Effect of inoculum size on the efficacy of telavancin against MRSA 33591 in the mouse neutropenic thigh model.
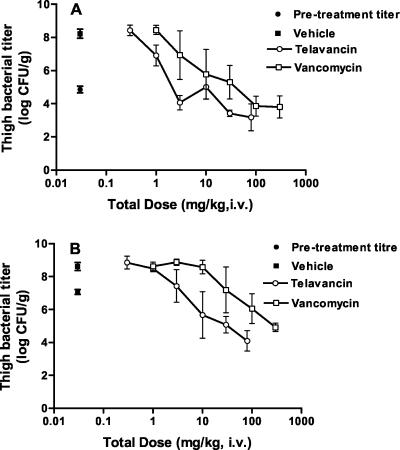
These experiments were performed to determine the impact of pretreatment titer on the potency and efficacy of telavancin. The pretreatment thigh bacterial titers were 4.9 ± 0.2 log10 CFU/g and 7.1 ± 0.15 log10 CFU/g, respectively, in the animals inoculated with low and high inocula. In vehicle-treated controls, the titers after 24 h were 8.2 ± 0.3 log10 CFU/g and 8.6 ± 0.3 log10 CFU/g, respectively, in the animals inoculated with low and high inocula. Treatment with telavancin and vancomycin produced dose-dependent reductions in thigh bacterial titer in both low- and high-inoculum groups (Fig. 8). The estimated ED50s (95% CI) for telavancin and vancomycin were 1.2 (0.9 to 1.7) and 10.1 (3.9 to 25.7) mg/kg, i.v., respectively, in the low-inoculum group and 7.1 (4.0 to 12.4) and 61.2 (27.9 to 134.4) mg/kg, i.v., respectively, in the high-inoculum group. Telavancin was approximately eightfold more potent (P < 0.05) than vancomycin in the low- and high-inoculum groups. Point dose estimates required to achieve the different pharmacodynamic endpoints are shown in Table 4. At the doses tested, telavancin and vancomycin produced a maximal kill of 1.8 and 1.1 log10 CFU, respectively, with the low inoculum and 3.0 and 2.2 log10 CFU, respectively, with the higher inoculum.
FIG. 8.
Efficacies of telavancin and vancomycin against MRSA 33591 in the murine neutropenic thigh model at different starting inocula. (A) Control inoculum; (B) high inoculum. Vehicle (n = 5) and telavancin (n = 5 per dose) were administered q 24 h, whereas vancomycin (n = 5 per dose) was administered q 12 h (n = 5 for control pretreatment group). The abscissa shows the total (24-h) dosage, and the ordinate shows the bacterial titer in the thigh. Values represent means ± 1 SD (error bar).
TABLE 4.
Point dose estimates required to achieve different pharmacodynamic endpoints against MRSA 33591 at different pretreatment titers in the mouse neutropenic thigh model
| Pretreatment titer and drug | Dose (mg/kg) for achieving pharmacodynamic endpointa
|
||||
|---|---|---|---|---|---|
| ED50 | EDstasis | ED1-log kill | ED2-log kill | ED3-log kill | |
| 4.9 log10 CFU/g (control) | |||||
| Telavancin | 1.2 | 1.8 | 4.7 | — | — |
| Vancomycin | 10.1 | 28.8 | 100 | — | — |
| 7.1 log10 CFU/g (high) | |||||
| Telavancin | 7.1 | 3.5 | 9.1 | 22.4 | 80 |
| Vancomycin | 61.2 | 42.6 | 87.1 | 288.4 | — |
Doses administered i.v. —, endpoint not achieved at the doses tested.
Pharmacodynamic effects of telavancin against MRSA in the mouse subcutaneous infection model.
In this model, the pretreatment titer was 7.2 ± 0.4 log10 CFU/g. In vehicle-treated animals, the titer after 24 h was 8.7 ± 0.7 log10 CFU/g. Telavancin, vancomycin, and linezolid produced dose-dependent reductions in titer. The estimated ED50s (95% CI) for telavancin, vancomycin, and linezolid were 0.9 (0.6 to 1.3), 2.8 (2.1 to 3.9), and 2.3 (1.5 to 3.6) mg/kg, i.v., respectively. Telavancin was approximately threefold more potent (P < 0.05) than both vancomycin and linezolid. The point dose estimates required to produce different pharmacodynamic endpoints are shown in Table 3. Also shown in Table 3 is a comparison of the efficacious doses of telavancin, vancomycin and linezolid in the two models, expressed as the ratios of ED50 or ED1-log kill in the two models. The data show that the deviation in potency between the two models is of a smaller magnitude for telavancin compared to vancomycin and linezolid.
DISCUSSION
Telavancin is a novel antibiotic which exerts its antibacterial activity against gram-positive organisms through at least two distinct mechanisms (16; Debabov et al., 43rd ICAAC). In vitro studies have demonstrated that telavancin, unlike linezolid and vancomycin, produces rapid and concentration-dependent killing of susceptible and resistant gram-positive bacteria, including MRSA 33591 and MSSA 13709, the two strains used in the present studies (16).
The studies reported in this communication were conducted to determine the efficacy of telavancin against relevant gram-positive organisms in two distinct animal models, which differ primarily in the status of the immune system. In the mouse neutropenic thigh model, the immune system is suppressed by pretreatment of the animals with cyclophosphamide. Consequently, given the minor contribution of the immune system in this in vivo setting, the antibacterial efficacy and potency of compounds are largely driven by the intrinsic microbiological properties of the molecule. In contrast, the animals in the subcutaneous infection model are immunocompetent. Thus, the antibacterial efficacy and potency of compounds in this model are assisted by host defense systems. The relative potency of a given molecule in the two models could possibly be used as a measure of the intrinsic antibacterial activity of the molecule.
In vitro susceptibility studies demonstrated that telavancin was active and potent against all gram-positive strains that were tested, including those which were highly resistant to nafcillin (all MRSA strains) and vancomycin (VREFs A256). In particular, telavancin, unlike vancomycin and linezolid, was potent against both susceptible and resistant strains of Streptococcus pneumoniae. The increment in the MIC of telavancin in the presence of 95% serum or a 40-mg/ml concentration of albumin against MRSA 33591 was consistent with our finding that the drug is highly protein bound. The observed increase in MIC (5- to 10-fold) was, however, somewhat lower than that predicted for a molecule that is 95% protein bound. A possible explanation for this finding is that telavancin operates, at least in part, through a membrane effect that is less affected by the extent of protein binding.
It is now well recognized that attainment of optimal efficacy and therapeutic index for any given antibacterial agent requires a careful understanding of its PK-PD properties (4, 8, 11, 12). Indeed, a rational PK-PD approach to selection of dosing frequency has led to improved effectiveness and/or safety of beta-lactams and aminoglycosides (7). Dose-fractionation efficacy studies are a useful indirect way to elucidate PK-PD relationships for antibacterials (4). Using this approach, we demonstrated that the efficacies of telavancin in the neutropenic thigh model were comparable at four different dosing intervals. Consistent with this data was the finding that AUC/MIC ratio was the PK-PD variable that best predicted efficacy. It is possible, however, that the PK-PD relationships may be different in alternate infection models and other bacterial strains. Nevertheless, given that telavancin produces concentration-dependent killing in vitro, our hypothesis is consistent with the current dogma that the in vivo efficacy of such agents is driven largely by total drug exposure (4, 8). It also implies that once-daily dosing would be the preferred regimen for attaining optimal efficacy in clinical studies. This contention has been borne out in phase 1 studies that have shown that telavancin, at doses of 7.5 to 15 mg/kg, i.v., produces impressive serum bactericidal titers up to 24 h following a single i.v. dose (S. Barriere, J. P. Shaw, J. Seroogy, K. Kaniga, J. Pace, K. Judice, and T. Mant, Abstr. 13th Eur. Congress Clin. Microbiol. Infect. Dis., abstr. P-1214, 2003).
Telavancin was efficacious and potent against all the strains tested, including those that were highly resistant to nafcillin (MRSA 33591) and vancomycin (VREF A256). Although the absolute potency of telavancin, expressed as ED50, appeared to be similar across strains, the magnitude of kill did differ between strains. The magnitude and potency of kill were significant for the four strains of Streptococcus pneumoniae and majority of staphylococcal strains but moderate for certain staphylococcal strains (MSSA KPB01, and MRSA KPB01) and enterococci (VREFs A256). There was no clear relationship between the killing potency and 24-h growth or MIC for the different strains. It is likely that other variables, such as the presence or absence of key host factors, affect the susceptibility of the organism to the drug in the in vivo setting.
Telavancin was significantly more potent than comparator drugs (vancomycin, nafcillin, and linezolid) on an ED50 and EDstasis basis against MRSA 33591 and/or MSSA 13709 in the neutropenic thigh model. These apparent differences in relative in vivo potencies cannot be explained on the basis of MICs alone. For example, in the case of MRSA 33591, the MICs of telavancin and linezolid vary by a factor of two whereas the in vivo potencies differ by approximately 30-fold. A more plausible explanation for this finding is that the intrinsic bactericidal activity of molecules strongly contributes to their efficacy in this model. It should be noted, however, that since the pharmacokinetics of the comparator drugs was not assessed in the present study, no conclusions can be drawn on the relative potency of the three drugs in terms of drug exposure. However, as shown in Fig. 5E, the 24-h AUC/MIC ratio (free drug) of telavancin required for stasis against MRSA 33591 is 3, which is approximately 10-fold lower than that reported for vancomycin and linezolid (5), implying that telavancin is intrinsically more potent. The present study has also shown that the efficacies and relative potencies of telavancin and vancomycin are independent of the inoculum titer. However, for both vancomycin and telavancin, there is a modest inoculum effect given that the absolute dose required to achieve different pharmacodynamic endpoints varies at different pretreatment titers.
Telavancin, vancomycin, and linezolid were all efficacious in the subcutaneous infection model, and their antibacterial potencies were greater in this model compared to those observed in the neutropenic thigh model. This deviation in potency between the two models was, however, greatest for linezolid and much smaller for vancomycin and telavancin. These data suggest that the antibacterial activity of telavancin is less affected by the immune status of the animal and may be a reflection of the potent and rapid bactericidal properties of telavancin in vitro. Other explanations such as differential distribution of drug in the thigh and subcutaneous infection sites cannot be excluded at this point. Another caveat is that the PK-PD parameters for telavancin and the comparators in the two models could be dissimilar given that the models use different strains of mice and have different sites of infection.
Overall, the data generated in the present studies demonstrate that telavancin is a potent and efficacious antibacterial molecule in vivo against a range of susceptible and resistant gram-positive organisms. Telavancin, which is currently being evaluated in clinical studies, has the potential to be a valuable addition to the therapeutic armamentarium for the treatment of infections with gram-positive organisms.
REFERENCES
- 1.Andes, D., and W. A. Craig. 2001. Animal model pharmacokinetics and pharmacodynamics: a critical review. Int. J. Antimicrob. Agents 19:261-268. [DOI] [PubMed] [Google Scholar]
- 2.CDC NNIS System. 2001. National Nosocomial Infections Surveillance (NNIS) system report, data summary from January 1992-June 2001, issued August 2001. Am. J. Infect. Control 29:404-421. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2002. Staphylococcus aureus resistant to vancomycin-United States, 2002. Morbid. Mortal. Wkly. Rep. 51:565-567. [PubMed] [Google Scholar]
- 4.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic paramaters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-12. [DOI] [PubMed] [Google Scholar]
- 5.Craig, W. A. 2003. Basic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect. Dis. Clin. N. Am. 17:479-501. [DOI] [PubMed] [Google Scholar]
- 6.Craig, W. A., J. Redington, and S. C. Ebert. 1991. Pharmacodynamics of amikacin in vitro and in mouse thigh and lung infections. J. Antimicrob. Chemother. 27(Suppl. C):29-40. [DOI] [PubMed] [Google Scholar]
- 7.Drusano, G. L. 1990. Human pharmacodynamics of beta-lactams, aminoglycosides and their combination. Scand. J. Infect. Dis. Suppl. 74:235-248. [PubMed] [Google Scholar]
- 8.Drusano, G. L. 1988. Role of pharmacokinetics in the outcome of infections. Antimicrob. Agents Chemother. 32:289-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gradelski, E., L. Valera, L., B. Kolek, D. Bonner, and J. Fung-Tomc. 2001. Comparative killing kinetics of the novel des-fluoro(6) quinolone BMS-284756, fluoroquinolones, vancomycin and beta-lactams. Int. J. Antimicrob. Agents 18:43-48. [DOI] [PubMed] [Google Scholar]
- 10.Leggett, J. E., and W. A. Craig. 1989. Enhancing effect of serum ultrafiltrate on the activity of cephalosporins against gram-negative bacilli. Antimicrob. Agents Chemother. 33:35-40.2496656 [Google Scholar]
- 11.Liu, P., M. Muller, and H. Derendorf. 2002. Rational dosing of antibiotics: the use of plasma concentrations versus tissue concentrations. Int. J. Antimicrob. Agents 19:285-290. [DOI] [PubMed] [Google Scholar]
- 12.Mouton, J. W., M. N. Dudley, O. Cars, H. Derendorf, and G. L Drusano. 2002. Standardization of pharmacokinetic/pharmacodynamic (PK/PD) terminology for anti-infective drugs. Int. J. Antimicrob. Agents 19:355-358. [DOI] [PubMed] [Google Scholar]
- 13.Murray, B. E. 2000. Vancomycin-resistant enterococcal infections. N. Engl. J. Med. 342:710-721. [DOI] [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 5th ed. NCCLS document M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 15.Norrby, R. 2001. Linezolid-a review of the first oxazolidinone. Expert Opin. Pharmacother. 2:293-302. [DOI] [PubMed] [Google Scholar]
- 16.Pace, J. L., K. Krause, D. Johnston, D. Debabov, T. Wu, L. Farrington, C. Lane, D. L. Higgins, B. Christensen, K. J. Judice, and K. Kaniga. 2003. In vitro activity of TD-6424 against Staphylococcus aureus. Antimicrob. Agents Chemother. 47:3602-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Renneberg, J., and M. Walder. 1988. A mouse model for simultaneous pharmacokinetic and efficacy studies of antibiotics at sites of infection. J. Antimicrob. Chemother. 22:51-60. [DOI] [PubMed] [Google Scholar]
- 18.Smith, T. L., M. L. Pearson, K. R. Wilcox, C. Cruz, M. V. Lancaster, B. Robinson-Dunn, F. C. Tenover, M. J. Zervos, J. D. Band, E. White, W. R. Jarvis, et al. 1999. Emergence of vancomycin resistance in Staphylococcus aureus. N. Engl. J. Med. 340:493-501. [DOI] [PubMed] [Google Scholar]
- 19.Tally, F. P, and M. F. DeBruin. 2000. Development of daptomycin for gram-positive infections. J. Antimicrob. Chemother. 46:523-526. [DOI] [PubMed] [Google Scholar]
- 20.Whitney, C. G., M. M. Farley, J. Hadler, L. H. Harrison, C. Lexau, A. Reingold, L. Lefkowitz, P. R. Cieslak, M. Cetron, E. R. Zell, J. H. Jorgensen. and A Schuchat. 2000. Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States. N. Engl. J. Med. 343:1917-1924. [DOI] [PubMed] [Google Scholar]