Abstract
Medicinal plants are effective in controlling plasma glucose level with minimal side effects and are commonly used in developing countries as an alternative therapy for the treatment of type 1 diabetes mellitus. The aim of this study is to evaluate the potential antidiabetic and antioxidant impacts of Balanites aegyptiaca and Petroselinum sativum extracts on streptozotocin-induced diabetic and normal rats. The influences of these extracts on body weight, plasma glucose, insulin, total antioxidant capacity (TAC), malondialdehyde (MDA) levels, and liver-pyruvate kinase (L-PK) levels were assessed. Furthermore, the weight and histomorphological changes of the pancreas were studied in the different experimental groups. The herbal preparations significantly reduced the mean plasma glucose and MDA levels and significantly increased the mean plasma insulin, L-PK, and TAC levels in the treated diabetic groups compared to the diabetic control group. An obvious increase in the weight of the pancreas and the size of the islets of Langerhans and improvement in the histoarchitecture were evident in the treated groups compared to untreated ones. In conclusion, the present study provides a scientific evidence for the traditional use of these extracts as antidiabetic and antioxidant agents in type 1 diabetes mellitus.
1. Introduction
Type 1 diabetes mellitus (T1DM) occurs in childhood and is characterized by T-cell autoimmune disease mediated destruction of insulin secreting β cells in the pancreatic islets. This destructive process results in severe insulin deficiency that in turn leads to hyperglycemia [1–3].
T1DM remains a major global health problem, especially in developing countries, in spite of the availability of many antidiabetic drugs because they have limited efficacy and certain adverse effects. This leads to increased demand of research on antidiabetic natural products such as medicinal plants that produce minimal or no side effects [4, 5]. Studies that reveal the mode of action of potential antidiabetic plants will definitely provide a scientific and systematic approach to the use of these plants as hypoglycemic agents [6].
Streptozotocin (STZ) is frequently used to induce experimental T1DM and its diabetic action results from its highly specific cytotoxic action on β cells by increasing production of oxygen free radicals which results in oxidative stress [7, 8].
Balanites aegyptiaca is a widely distributed African plant of medicinal interest. In Egyptian folk medicine, its fruit mesocarp is commonly used as an oral antidiabetic drug [9, 10]. Petroselinum sativum is a cultivated variety of parsley and is traditionally used as carminative, abortifacient, and antihypertensive agent [11]. To the researchers' knowledge, there are no available studies that reveal the antidiabetic and antioxidant influences of Balanites aegyptiaca and Petroselinum sativum extracts on diabetes-induced experimental models or their possible hypoglycemic effects on normal animals. For these reasons, the current study has been undertaken to examine the possible antidiabetic and antioxidant effects of Balanites aegyptiaca fruits and Petroselinum sativum leaf aqueous extracts on STZ-induced diabetic (hyperglycemic) and normal (normoglycemic) rats with a view to transmitting the results for the human use.
2. Methods
2.1. Preparation of Balanites aegyptiaca Fruits and Petroselinum sativum Leaf Aqueous Extracts
Balanites aegyptiaca fruits and Petroselinum sativum leaves were purchased from local markets in Assiut Governorate. The pericarp of Balanites aegyptiaca fruits was cleaned and then prepared in the form of coarse powder. Petroselinum sativum leaves were washed with tap water, dried in the shade for one week, and stored in well-sealed cellophane bags. The dried leaves were powdered to be used for extract preparation. According to Gad and coworkers [6, 12], one kilogram of the dried powdered plant materials was extracted with four liters of boiling distilled water using percolation for 48 hours. The extracts were filtrated. Then, the filtrate was concentrated under reduced pressure by rotatory evaporation using a rotatory evaporator (Heidolph, Germany) at 40°C until the therapeutic residues were obtained. The therapeutic liquids were subjected to lyophilization using the freeze dryer (VirTis, USA) until fine powder materials were obtained and weighed to give 200 grams of dried powdered extract in the case of Balanites aegyptiaca and 150 grams in the case of Petroselinum sativum. Thus, the yielded extract was about 20% w/w and 15% w/w for Balanites aegyptiaca and Petroselinum sativum, respectively. The extracts were dissolved in distilled water before administration to diabetic and normal rats. The extracts were administered orally using an orogastric tube.
2.2. Experimental Animals
This study was conducted on adult male Wistar albino rats. Animals were obtained from the Animal House of the Faculty of Medicine, Assiut University. Their weight ranged between 150 and 200 grams at the beginning of the experiment. Rats were housed in groups in clean cages (five per cage) under standard laboratory conditions, including good aerated room with suitable temperature. Food and water were available ad libitum.
2.3. Induction of Experimental Diabetes
Diabetes was induced in overnight-fasted rats by single intraperitoneal injection of STZ (Sigma-Aldrich Company, USA) freshly dissolved in an ice-cold saline solution (0.9%) in a dose of 80 mg/kg BW [13]. Following STZ injection, rats were fed with glucose solution (5%) for 24 hours to avoid drug-induced hypoglycemia [8]. Blood glucose levels were determined three days after STZ injection using glucometer (Bionime, Taiwan) and the blood samples were taken from the tail vein. Rats with blood glucose range of 150–400 mg/dL were considered diabetic and included in this study [14].
2.4. Experimental Design
The sixty rats were randomly assigned to 6 groups (10 rats each). Three groups were assigned as normoglycemic groups in which the first one is normal control group (NC) and was given nothing except standard rat pellets and water; the second one was a Balanites aegyptiaca extract-treated normal (BAETN) group and was orally given Balanites aegyptiaca fruits aqueous extract (1.5 g/kg BW daily for 45 days) [6]; and the third one is Petroselinum sativum extract-treated normal (PSETN) group and was orally given Petroselinum sativum leaf aqueous extracts (2 g/kg BW daily for 45 days) [15].
The other three groups were assigned as hypoglycemic groups. The first one is diabetic control (DC) group in which rats received a single intraperitoneal injection of STZ. The second one is a Balanites aegyptiaca extract-treated diabetic (BAETD) group in which diabetic rats were treated orally with Balanites aegyptiaca fruits aqueous extract (1.5 g/kg BW daily for 45 days) [6]. The third group is Petroselinum sativum extract-treated diabetic (PSETD) group in which diabetic rats were treated orally with Petroselinum sativum leaf aqueous extracts (2 g/kg BW daily for 45 days) [15].
For all groups, 24 hours after the last dose of treatment, the live body weight (BW) was recorded using an electronic balance (A&D, Japan) and the rats were anesthetized and their blood specimens were individually collected from the retroorbital plexus of veins. The blood was centrifuged for 10 min at 3000 rpm and the sera were stored at −70°C until used. The necessary corrections of plasma parameters with plasma total protein levels (as an index of haemoconcentration) were made to eliminate the haemoconcentration effect [16].
2.5. Tissue Collection and Histomorphometric Study
The rats were anesthetized by ketamine (12 mg/kg BW) and then sacrificed by cervical dislocation. Liver and pancreas tissues were excised and rinsed in ice-cold physiological saline. The liver tissues were homogenized using a homogenizer (Ultra-Turrax T25 Basic, Werke, Germany) in 0.1 M Tris-HCl buffer at pH 7.4. The homogenate was centrifuged at 40000 revolutions/minute for 15 minutes in Heraeus Christ centrifuge to remove the nuclei and the cellular debris. The clear supernatant that contains cytosolic fraction was kept at −70°C to be used for assaying pyruvate kinase later on. The weight of the pancreas was recorded for each animal using a Mettler balance. Pancreatic weight per 100 g BW was calculated in the various experimental groups to make a functional comparison of weight loss and gain. The pancreases of each group were fixed in 10% neutral buffered formalin for 24 hours, and then they were processed for paraffin embedding. 5–7 μm thick paraffin sections were stained with Harris' haematoxylin and eosin [17, 18]. The sections were examined and photographed on microscope with DP72 camera and cell software (Olympus, Japan). Images were labeled using Adobe Photoshop Version 8. Morphometrical studies were performed on the stained histological sections using an image analyzer (Leica Q500 MC, Leica, Germany). The sizes of 100 islets were measured in each group and the mean size was calculated.
2.6. Biochemical Analyses
Plasma glucose, total antioxidant capacity, and malondialdehyde and liver-pyruvate kinase levels were measured colorimetrically using spectrophotometer (Spectronic 21, Milton Roy Company, USA) [19], while the plasma insulin level was measured by ELISA reader (Stat Fax-200, Awareness Technology Company, USA) using specific ELISA kit.
2.7. Statistical Analysis
All data are expressed as mean ± SE deviation. Data were compared among groups using one-way analysis of variance (ANOVA) followed by Tukey posttest using Prism software (v4.00 for Windows, GraphPad Software, San Diego, CA). A P value of less than 0.05 was considered to represent a statistically significant difference.
3. Results and Discussion
3.1. Effects of Balanites aegyptiaca Fruits and Petroselinum sativum Leaf Aqueous Extracts on the Mean BW of the Normal and Diabetic Rats
The mean final BW of the Balanites aegyptiaca extract-treated diabetic (BAETD) and Petroselinum sativum extract-treated diabetic (PSETD) groups were lower than that of the NC group, but the decrease was nonsignificant (Figure 1). Consistent with Jung and his colleagues [20], the diabetic control (DC) group is characterized by a significant reduction in its mean BW as compared with the NC group (Figure 1). Loss of BW could be explained by degradation of structural proteins, increased gluconeogenesis from muscle protein [21], and lipolysis of triglycerides [22] under the effect of experimental diabetes. It is noteworthy that, in the current study, along with reduction of BW in diabetic rats, polyphagia, polyuria, and polydipsia (data not shown) were also observed in parallel with a previous report by [23].
Figure 1.
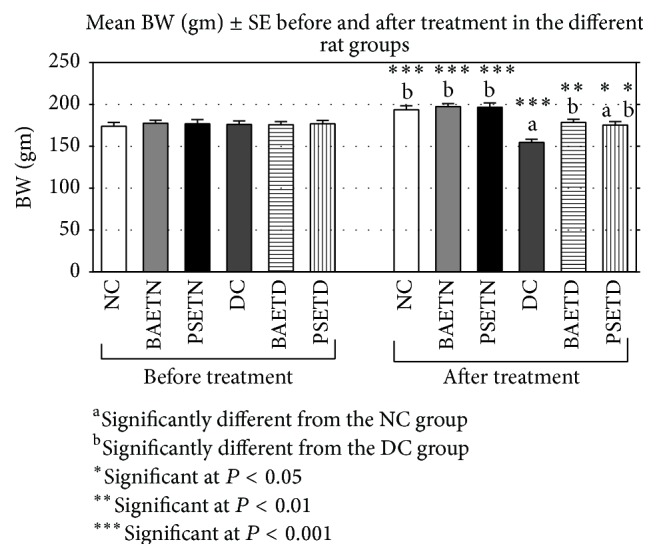
The effect of Balanites aegyptiaca fruits and Petroselinum sativum leaf aqueous extracts on mean BW (gm) in normal and STZ-induced diabetic rats (mean ± SE).
Compared with the DC group, the mean final BW of the BAETD and PSETD groups was significantly improved, but the BAETD group exhibited a better improvement in its mean final BW than that detected in the PSETD group (Figure 1). This effect on BW may be due to the ability of the extracts to reduce hyperglycemia which in turn corrects the aforementioned abnormalities including loss of BW [6]. However, in case of Petroselinum sativum leaf aqueous extracts, the results of the current experiment do not agree with the results from the study conducted by Yanardağ and his colleagues [24]. They showed a significant reduction in BW after treating the diabetic rats with Petroselinum crispum leaf aqueous extracts for 28 days due to the diuretic effect of the extract. This difference between our study and the study of Yanardağ and his colleagues might be due to the differences in the variety of parsley and/or the duration of the experiment.
3.2. Balanites aegyptiaca Fruits and Petroselinum sativum Leaf Aqueous Extracts Significantly (P < 0.001) Reduce the Elevated Plasma Glucose Level in the BAETD Group in Comparison with the DC Group
A nonsignificant difference was found between the mean plasma glucose, insulin, and L-PK levels of the NC group and those of the BAETN and PSETN groups (Figures 2, 3, and 4). These results seem to be consistent with several reports about other plant extracts that failed to produce any changes in their levels in normal treated rats but caused a significant reduction in the blood glucose level and elevation in the blood insulin level in diabetic rats [25–27]. However, the finding of the present study is incompatible with the study of Shaw and his colleagues [5] who demonstrated a fast decline in the blood glucose level of normal rats after starch load with the Balanites extract suggesting that there is enhancement of glucose utilization by a single dose of Balanites. In agreement with Bolkent and colleagues [15, 23], nonsignificant changes were observed in the mean plasma glucose and insulin levels in normal rats that were given Petroselinum sativum leaf aqueous extracts. The absence of significant hypoglycemic activities for Balanites aegyptiaca fruits and Petroselinum sativum leaf aqueous extracts in normal rats in this study is a desirable feature as it has been noted that hypoglycemia causes a cascade of adverse effects [28].
Figure 2.
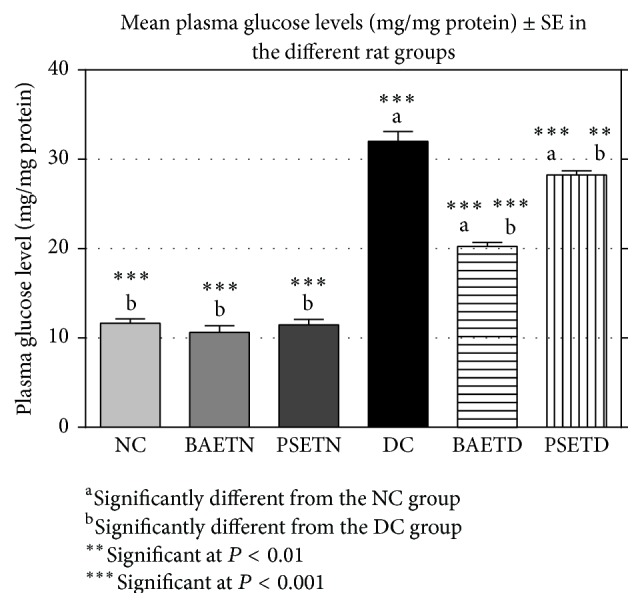
Balanites aegyptiaca fruits and Petroselinum sativum leaf aqueous extracts succeeded significantly (P < 0.001) in reducing the elevated mean plasma glucose level of the treated diabetic group in comparison with the DC group.
Figure 3.
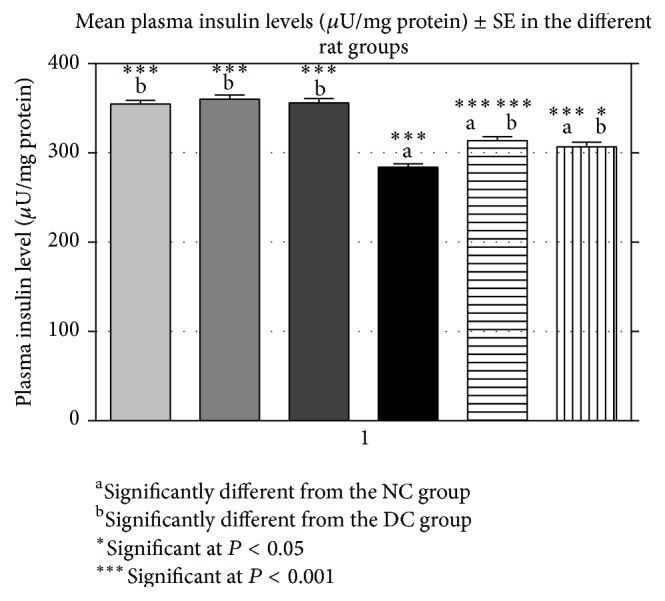
Balanites aegyptiaca fruits and Petroselinum sativum leaf aqueous extracts significantly (P < 0.001) increased the mean plasma insulin of the treated diabetic group in comparison with the DC group.
Figure 4.
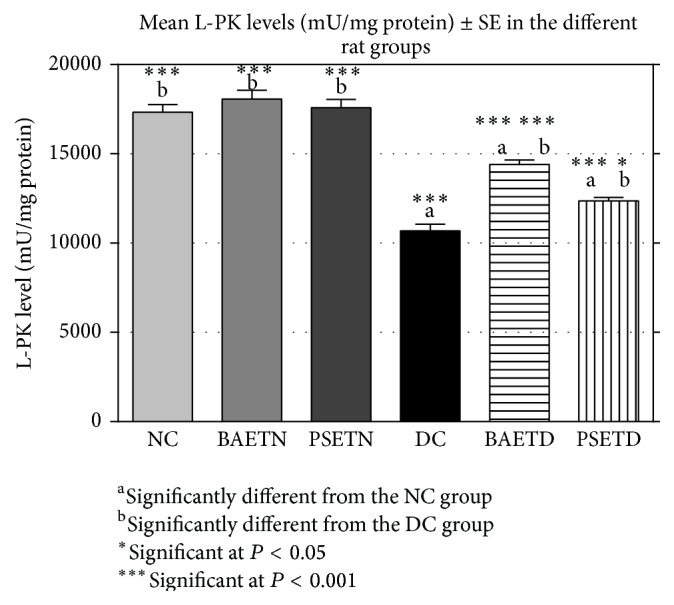
Effect of Balanites aegyptiaca fruits and Petroselinum sativum leaf aqueous extracts on the mean liver PK levels of normal and STZ-induced diabetic rats (mean ± SE).
STZ is the agent of choice for the induction of diabetic metabolic state in experimental animals [8]. In this work, STZ-induced diabetic rats were characterized by a significant increase in their mean plasma glucose level along with a significant decrease in their mean plasma insulin level in comparison with the NC rats as shown in several previous studies [20, 29].
The DC group was characterized by a highly significant (P < 0.001) increase in its mean plasma glucose level and a highly significant decrease in its mean plasma insulin and L-PK levels as compared with the NC group (Figures 2, 3, and 4).
Balanites aegyptiaca fruit aqueous extract succeeded in reducing significantly (P < 0.001) the elevated mean plasma glucose level of the BAETD group in comparison with the DC group. The Balanites aegyptiaca fruit aqueous extract showed more noticeable hypoglycemic effect than that of Petroselinum sativum leaf aqueous extracts (Figure 2). These results are in agreement with several previous studies [6, 10, 30, 31]. It was postulated that the hypoglycemic effect of the Balanites aegyptiaca extract is mediated through insulinomimetic activity [32], stimulation and potentiation of insulin secretion, increased insulin receptors affinity [33], enhancement of hepatic glycogen storage, suppression of hepatic gluconeogenesis, acceleration of glucose metabolism, and inhibition of intestinal glucosidase activity [6]. The hypoglycemic effect of Petroselinum crispum extract is believed to be related to the presence of flavonoids [24]. The antidiabetic potency of flavonoids has been highlighted in many reports and is attributed in part to their antioxidant and hypoglycemic effects.
3.3. Balanites aegyptiaca Fruits and Petroselinum sativum Leaf Aqueous Extracts Significantly (P < 0.001) Increased the Plasma Insulin Levels of the BAETD Group Compared with the DC Group
Balanites aegyptiaca fruits and Petroselinum sativum leaf aqueous extracts significantly (P < 0.001) increased the mean plasma insulin (Figure 3) of the treated diabetic group in comparison with the DC group. In agreement with El-Bayomy and colleagues [34, 35], administration of Balanites aegyptiaca fruit aqueous extracts to diabetic rats significantly increased their mean plasma insulin levels (Figure 3). These in vivo studies ran in parallel with an in vitro study conducted by Abdel-Moneim [33] which indicated that Balanites aegyptiaca stimulated the β cells of pancreatic islets to secrete insulin, potentiated the glucose stimulation of insulin secretion, and increases the number and affinity of insulin receptors and postreceptors in β cells. Yanardağ and his coworkers [24] suggested that Petroselinum crispum leaf extract did not increase insulin release from β cells of the pancreas and that inhibition of gluconeogenesis and direct stimulation of glycolysis may be involved in the mechanism of its hypoglycemic action. The apparent differences in the results of the present study and the previous one could be explained on the basis of the dissimilarity in the examined varieties of parsley that might affect the presence and/or concentrations of the active constituents. In addition, the methods of extraction are also different: evaporation under reduced pressure followed by lyophilization in this study versus evaporation under reduced pressure only in the preceding one.
3.4. Balanites aegyptiaca Fruits and Petroselinum sativum Leaf Aqueous Extracts Significantly (P < 0.001) Increase the Mean L-PK Levels of the BAETD Group in Comparison with the DC Group
L-PK catalyzes the conversion of phosphoenolpyruvate to pyruvate and this represents the last irreversible steps of glycolysis in the liver [36]. It was suggested that the increase in the mean level of the L-PK is directly responsible for increased utilization of glucose in the liver [37] and it occurred most probably as a result of the insulinotropic action of the extract [38] by stimulating the transcription of the gene encoding L-PK. The rise in the insulin level plays an indirect role in L-PK activation by antagonizing the action of glucagon to stimulate protein kinase A-mediated phosphorylation of the enzyme [39].
In the present study, we observed a significant decrease in the mean L-PK level of diabetic rats compared with normal untreated rats (Figure 4). This might be due to the reduction in the transcription of gene encoding L-PK in the insulinopenic diabetic state [39].
BAETD group showed a significant increase in its mean L-PK level in comparison with the DC group (Figure 4). This increase was greater than that detected in the PSETD group. Similar to the present study, other herbal extracts had been shown to improve the diabetic condition by increasing L-PK levels in diabetic rats [30, 38]. In contrast, Kamel [10] found that oral administration of both saponin mixture and polysaccharides (isolated from Balanites aegyptiaca mesocarp aqueous extract) after six hours did not exert any effect on liver hexokinase (another key regulatory enzyme of glycolysis) activity in diabetic mice.
With respect to the influence of Petroselinum sativum leaf aqueous extracts on the mean level of the L-PK, no data are available about their effect on this enzyme or even any one of the glycolytic enzymes. Nevertheless, the finding of this study supports the hypothesis that direct stimulation of glycolysis by Petroselinum crispum extract may be involved in the mechanism of its hypoglycemic action [24].
It was suggested that the increase in the mean level of the L-PK following extracts' dosing occurs most probably as a result of the insulinotropic action of the extract [38] by stimulating the transcription of the gene encoding L-PK. The rise in the insulin level plays an indirect role in L-PK activation by antagonizing the action of glucagon to stimulate protein kinase A-mediated phosphorylation of the enzyme [39].
3.5. Balanites aegyptiaca Fruits and Petroselinum sativum Leaf Aqueous Extracts Significantly (P < 0.001) Increased Plasma TAC Levels and Significantly (P < 0.01) Decreased Plasma MDA Levels of the BAETD Group Compared with the DC Group
In the present study, the mean plasma TAC level in the DC group was significantly decreased in comparison with the NC group (Figure 5). This finding coincides with the other studies which reported that diabetes was associated with reduced levels of antioxidant activity in the blood and tissues of diabetic animals [40, 41]. The reduced mean plasma TAC level in the DC group might indicate that there was an imbalance between free radical formation and antioxidant protection as a consequence of increased utilization of endogenous antioxidants in response to the elevated levels of free radicals [16].
Figure 5.
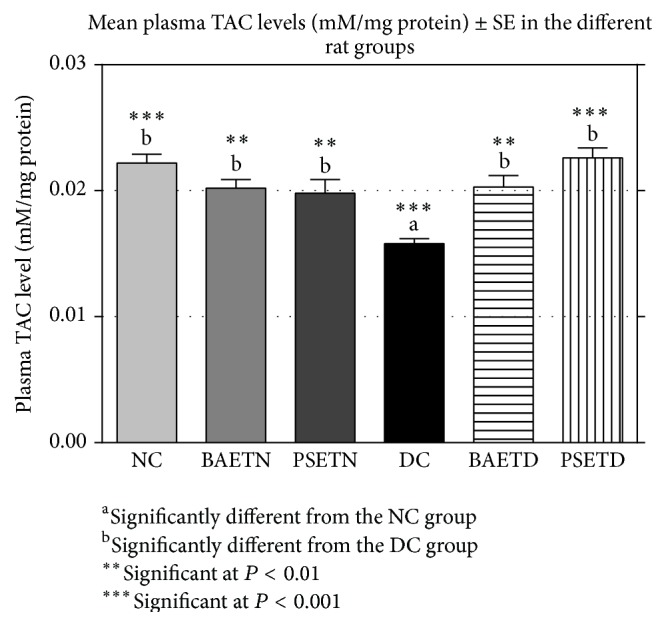
Oral administration of either Balanites aegyptiaca fruit aqueous extract or Petroselinum sativum leaf aqueous extracts to the diabetic rats produced a significant (P < 0.01) increase in their mean plasma TAC levels when compared with the diabetic control rats.
STZ treatment significantly increased lipid peroxides and decreased antioxidant enzyme activities in the plasma of rats, confirming that STZ-induced diabetes is accompanied by increased generation of ROS [42], which in turn induced lipid peroxidation [43]. The results of the present experiment agreed with the previous data as well as with the hypothesis of Meenu and his colleagues [44] who postulated that hyperglycemia resulted in the generation of free radicals, which can exhaust antioxidant defence, thereby leading to the disruption of cellular function and oxidative damage to the membranes, and enhance the susceptibility to lipid peroxidation.
Oral administration of either Balanites aegyptiaca fruit aqueous extract or Petroselinum sativum leaf aqueous extract to the diabetic rats produced a significant (P < 0.01) increase in their mean plasma TAC levels (Figure 5) and a significant (P < 0.01) decrease in their mean plasma MDA levels (Figure 6) when compared with the diabetic control rats. Supplementation of Petroselinum sativum leaves aqueous extract to the diabetic rats resulted in a greater enhancement in their mean plasma TAC and MDA levels than Balanites aegyptiaca fruit aqueous extract.
Figure 6.
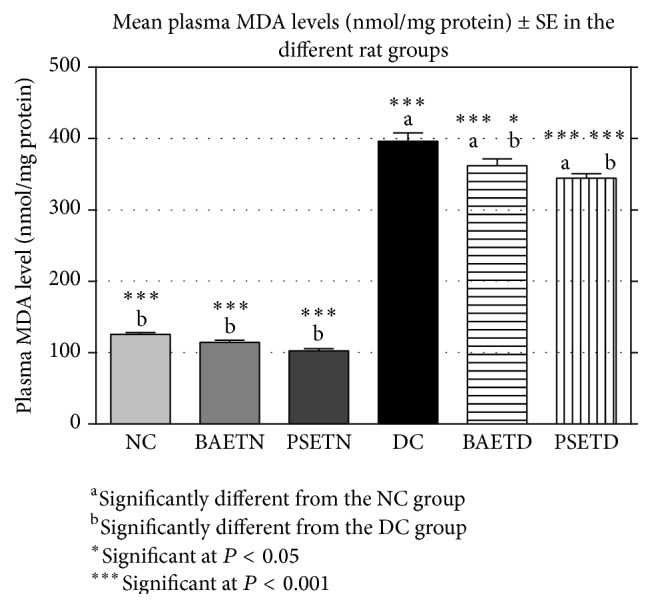
Oral administration of either Balanites aegyptiaca fruit aqueous extract or Petroselinum sativum leaf aqueous extract to the diabetic rats produced a significant (P < 0.01) decrease in their mean plasma MDA levels when compared with the diabetic control rats.
In the present study, the mean plasma MDA level was decreased significantly in the PSETD group compared with the DC group. This is consistent with a significant reduction in the elevated plasma level of MDA in the cisplatin and CCl4 poisoned rats following administration of Petroselinum sativum leaf extracts [12, 45]. The in vivo studies were confirmed by another in vitro study carried out by Al-Mamary [46] who found that Petroselinum sativum leaves juice inhibited liver homogenate oxidation mediated by the ferric sulphate/ascorbate system.
The antioxidant activities of Balanites aegyptiaca fruits and Petroselinum sativum leaf aqueous extracts in diabetic rats that were observed in this study may be secondary to their hypoglycemic effects as well as the presence of some phytochemical antioxidant agents. It was postulated that these activities may be due to enhancement of antioxidant enzyme synthesis by acting on the antioxidant response elements in the enhancer region at the promoter site of the gene that codes for the enzymes [47].
3.6. Histomorphological Study
In the BAETN and PSETN groups, the mean weight of pancreatic tissue and the size of the islets of Langerhans were nonsignificantly different from those of the NC group. As far as the researchers are concerned, there were no published studies in the literature focusing on the influence of any of these extracts on the pancreas weight in normal laboratory animals. Nevertheless, evidence from the histopathological part of this study indicated that both extracts were unable to significantly increase the size of the pancreatic islets in the treated normal rats which indicates that the growth stimulation effect of both herbal extracts can only appear in diabetic rats, not in normal ones.
As shown previously by Bangar and colleagues [48, 49], we found a significant decrease in the mean weight of the pancreas of the diabetic rats compared with the mean weight of the pancreas of untreated normal rats (Figure 7).
Figure 7.
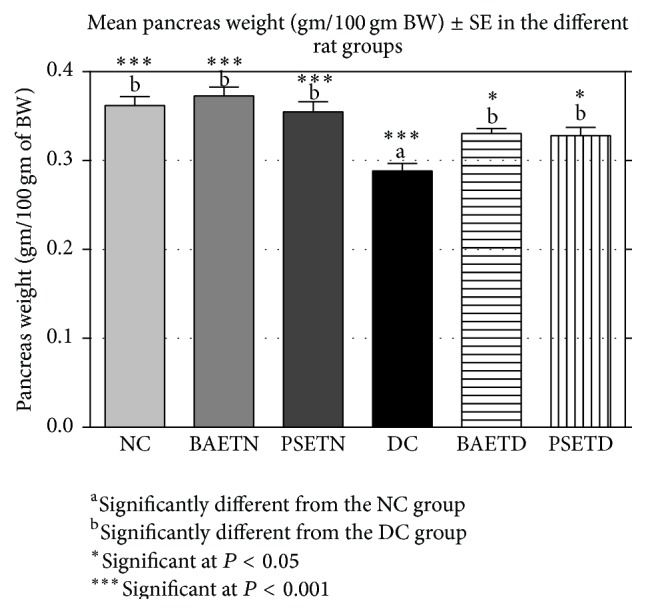
Effects of Balanites aegyptiaca fruits and Petroselinum sativum leaf aqueous extracts on the mean pancreas weight (gm/100 gm of BW) in normal and STZ-induced diabetic rats (mean ± SE).
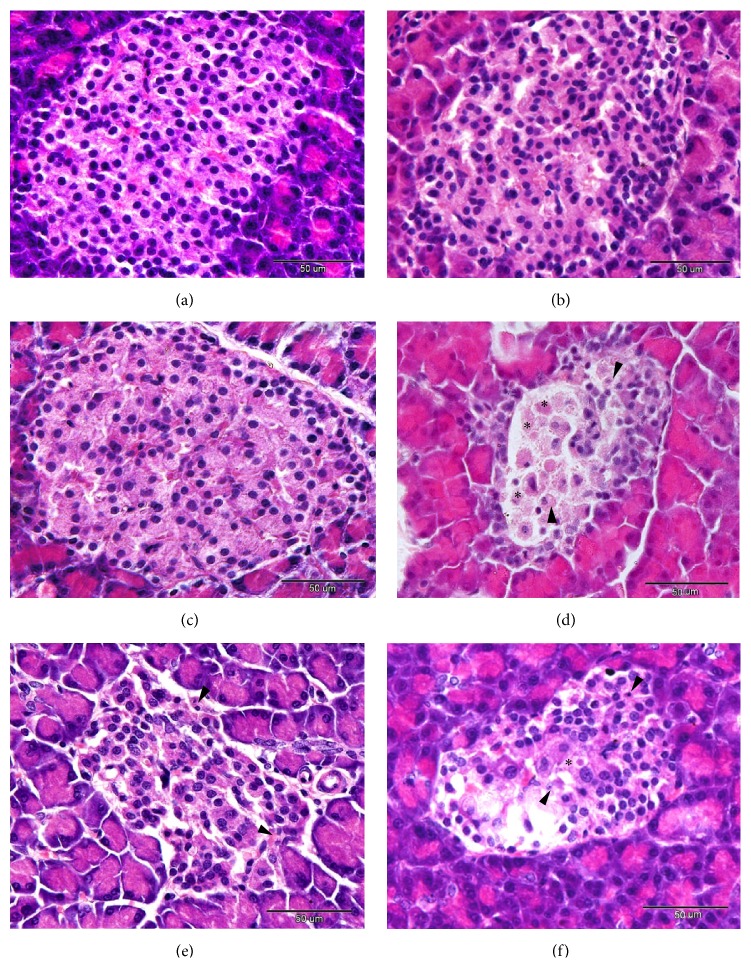
Some researchers used the size of pancreatic islets as an index for the evaluation of the antidiabetic effects of different extracts [48, 50]. In addition, we found that the reduction in the mean weight of the pancreas was accompanied by a significant decline in the size of pancreatic islets in STZ-induced diabetic rats and by an obvious degeneration and necrosis of β cells (Figure 9(d)). A previous study conducted by Heidari and his colleagues [51] attributed the reduction in the weight of the pancreas to the disruption and the disappearance of pancreatic islets and the selective destruction of β cells by the diabetogenic action of STZ.
Figure 9.
((a)–(d)) A photomicrograph of the pancreas of NC group (a), BAETN group (b), and the PSETN group (c). The diabetic pancreas (d) showed severe necrotic and degenerative changes (arrowheads). Residues of the cytoplasm and the nuclei were also seen (asterisks). In the BAETD and PSETD groups ((e) and (f), resp.), the degenerative and necrotic changes were markedly lower than that observed in the DC group, H&E staining. Scale bar = 50 μm.
Administration of Balanites aegyptiaca fruits and Petroselinum sativum leaf aqueous extracts significantly increased the mean weight of the pancreas in STZ-induced diabetic rats (Figure 7) and the mean size of the islets of Langerhans (Figure 8) as compared with the DC group. This has been associated with improvement in the histopathological pictures of their pancreas. To our knowledge, all the previous studies have not focused on the changes in the pancreas weight following administration of either Balanites aegyptiaca extracts or Petroselinum sativum extracts on the diabetic animal models. However, the data of the current work are in agreement with many researchers who reported that several other plant extracts succeeded in restoring the pancreas weight of diabetic rats by repairing or regenerating pancreatic β cells [48, 52].
Figure 8.
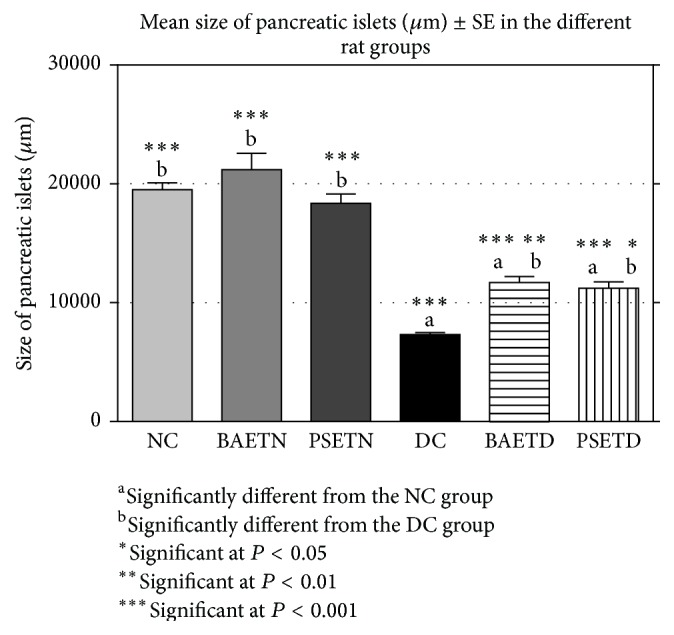
Administration of Balanites aegyptiaca fruits and Petroselinum sativum leaf aqueous extracts to the diabetic rats exhibited a significant increase in the mean size of the islets of Langerhans as compared with the DC group.
The histopathological pictures of the pancreas in the BAETN and PSETN groups were similar to that of the NC group. Severe degenerative and necrotic changes were observed in the islets cells of the DC group when compared with the control group (Figures 9(a) and 9(d)). In addition, the nuclei of the pancreatic cells showed pyknosis and karyolysis. Disappearance of some cells nuclei and residues of the nuclei and destroyed cells was also seen. Following the administration of Balanites aegyptiaca fruits and Petroselinum sativum leaf aqueous extract to the diabetic rats, most of the cells appeared normal (Figures 9(b) and 9(e)), while in the PSETD group, few degenerative and necrotic changes were still found in the pancreatic islets cells (Figures 9(c) and 9(f)). The pancreatic tissue in the BAETD group showed a better improvement than that observed with the PSETD group.
The exact mechanism by which the extracts regenerated islet β cells and increased the size of pancreatic islets at the cellular level has not been examined in this study, but some hypothesis can be put forward. It is well known that β cell apoptosis is a common feature of T1DM [53]. In fact, both Balanites aegyptiaca [54] and Petroselinum sativum [11], like many herbal extracts, are rich in flavonoids. The flavonoid genistein reduced β cell apoptosis in pancreatic islets, increased the number of insulin-positive β cells in the islets, promoted islet β cell survival, and preserved islet mass [55]. It is clear by now that alternative approaches for treatment of T1DM include the stimulation of regeneration of endogenous pancreatic β cells. Three different challenges to meet these alternative approaches include protection of the new β cells from autoimmunity and allorejection [56], the endogenous replenishment of pancreatic β cells [57], and the transdifferentiation from pancreatic acinar cells [58]. It was revealed that flavonoid rich fractions of Oreocnide integrifolia leaves have an enhancing influence on islet neogenesis and greater β cell regeneration in pancreatectomized BALB/c mice [59].
4. Conclusion
This study showed that Balanites aegyptiaca and Petroselinum sativum aqueous extracts have antidiabetic and antioxidant effects on the diabetic rats. These extracts can be potentially used with insulin therapy to minimize its side effects and to improve the treatment of T1DM and probably other oxidative stress-associated diseases.
Acknowledgments
The authors are grateful to the technician staffs of the Physiology Department, Faculty of Medicine, Assiut University, for their help and valuable technical assistance for carrying out this study and also the staff of the animal housing at the Faculty of Medicine, Assiut University. The authors would also like to thank Dr. Suzan Kamel ElSayed, Associate Professor at the Biomedical Sciences Department, Oakland University, William Beaumont School of Medicine, USA, for her valuable comments on the paper. This project was funded by Assiut University, Assiut, Egypt.
Ethical Approval
Ethical approval was obtained from the Research Ethics Committee of the Faculty of Medicine, Assiut University, Assiut, Egypt (REC/AU, 28/06/2014). The procedure followed the instructions of Good Laboratory Practice (GLP).
Conflict of Interests
The authors declare no conflict of interests.
References
- 1.Kelly M. A., Rayner M. L., Mijovic C. H., Barnett A. H. Molecular aspects of type 1 diabetes. Journal of Clinical Pathology. 2003;56(1):1–10. doi: 10.1136/mp.56.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel D. K., Kumar R., Laloo D., Hemalatha S. Diabetes mellitus: an overview on its pharmacological aspects and reported medicinal plants having antidiabetic activity. Asian Pacific Journal of Tropical Biomedicine. 2012;2(5):411–420. doi: 10.1016/S2221-1691(12)60067-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan T., Xiang Y., Chang C., Zhou Z. Alteration of regulatory T cells in type 1 diabetes mellitus: a comprehensive review. Clinical Reviews in Allergy & Immunology. 2014;47(2):234–243. doi: 10.1007/s12016-014-8440-0. [DOI] [PubMed] [Google Scholar]
- 4.Erejuwa O. O., Sulaiman S. A., Wahab M. S. Honey—a novel antidiabetic agent. International Journal of Biological Sciences. 2012;8(6):913–934. doi: 10.7150/ijbs.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw J. E., Sicree R. A., Zimmet P. Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Research and Clinical Practice. 2010;87(1):4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Gad M. Z., El-Sawalhi M. M., Ismail M. F., El-Tanbouly N. D. Biochemical study of the anti-diabetic action of the Egyptian plants fenugreek and balanites. Molecular and Cellular Biochemistry. 2006;281(1-2):173–183. doi: 10.1007/s11010-006-0996-4. [DOI] [PubMed] [Google Scholar]
- 7.Eddouks M., Chattopadhyay D., Zeggwagh N. A. Animal models as tools to investigate antidiabetic and anti-inflammatory plants. Evidence-Based Complementary and Alternative Medicine. 2012;2012:14. doi: 10.1155/2012/142087.142087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008;51(2):216–226. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- 9.Gnoula C., Megalizzi V., De Neve N., et al. Balanitin-6 and -7: diosgenyl saponins isolated from Balanites aegyptiaca Del. display significant anti-tumor activity in vitro and in vivo. International Journal of Oncology. 2008;32(1):5–15. [PubMed] [Google Scholar]
- 10.Kamel M. Study of Balanites aegyptiaca Del. and Salvadora persica l. Reputed to Have Hypoglycemic Effects. Assiut, Egypt: Department of Pharmacognosy, Faculty of Pharmacy; 1991. [Google Scholar]
- 11.Lugasi A., Hóvári J. Flavonoid aglycons in foods of plant origin I. vegetables. Acta Alimentaria. 2000;29(4):345–352. doi: 10.1556/AAlim.29.2000.4.4. [DOI] [Google Scholar]
- 12.Ahmed E. S., Twaty N. H., Fakiha K. G., Bibars M. A. Mutageneic and antimutagenic effects of some plant extracts in Drosophilla melanogaster . Nature and Science. 2010;8(4):77–82. [Google Scholar]
- 13.Fazeli S. A., Gharravi A. M., Jahanshahi M., Ghafari S., Behnampour N., Golalipour M. J. Resistance of CA1 pyramidal cells to STZ-induced diabetes in young rats. International Journal of Morphology. 2009;27(4):997–1001. [Google Scholar]
- 14.Sener G., Saçan Ö., Yanardag R., Ayanoglu-Dülger G. Effects of parsley (Petroselinum crispum) on the aorta and heart of STZ induced diabetic rats. Plant Foods for Human Nutrition. 2003;58(3):1–7. [Google Scholar]
- 15.Bolkent S., Yanardag R., Ozsoy-Sacan O., Karabulut-Bulan O. Effects of parsley (Petroselinum crispum) on the liver of diabetic rats: a morphological and biochemical study. Phytotherapy Research. 2004;18(12):996–999. doi: 10.1002/ptr.1598. [DOI] [PubMed] [Google Scholar]
- 16.Erejuwa O. O., Sulaiman S. A., Ab Wahab M. S., Salam S. K. N., md Salleh M. S., Gurtu S. Comparison of antioxidant effects of honey, glibenclamide, metformin, and their combinations in the kidneys of streptozotocin-induced diabetic rats. International Journal of Molecular Sciences. 2011;12(1):829–843. doi: 10.3390/ijms12010829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris H. F. On the rapid conversion of haematoxylin into haematin in staining reactions. Journal of Applied Microscopy and Laboratory Methods. 1900;3, article 777 [Google Scholar]
- 18.Bancroft J. D., Steven A. Theory and Practice of Histological Techniques. 4th. New York, NY, USA: Churchill Livingstone; 1996. [Google Scholar]
- 19.Yousefi F., Mahjoub S., Pouramir M., Khadir F. Hypoglycemic activity of Pyrus biossieriana Buhse leaf extract and arbutin: inhibitory effects on alpha amylase and alpha glucosidase. Caspian Journal of Internal Medicine. 2013;4(4):763–767. [PMC free article] [PubMed] [Google Scholar]
- 20.Jung H. W., Jung J. K., Ramalingam M., Yoon C.-H., Bae H., Park Y.-K. Anti-diabetic effect of Wen-pi-tang-Hab-Wu-ling-san extract in streptozotocin-induced diabetic rats. Indian Journal of Pharmacology. 2012;44(1):97–102. doi: 10.4103/0253-7613.91877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azadbakhta M., Safapour S., Ahmadi A., Ghasemi M., Shokrzadeh M. Anti-diabetic effects of aqueous fruits extract of Diospyros lotus L. on streptozotocin-induced diabetic rats and the possible morphologic changes in the liver, kidney and heart. Journal of Pharmacognosy and Phytotherapy. 2010;2(2):10–16. [Google Scholar]
- 22.Ene A. C., Nwankwo E. A., Samdi L. M. Alloxan-induced diabetes in rats and the effects of Black caraway (Carum carvi L.) oil on their body weights. Journal of Pharmacology and Toxicology. 2008;3(2):141–146. doi: 10.3923/jpt.2008.141.146. [DOI] [Google Scholar]
- 23.Akbarzadeh A., Norouzian D., Mehrabi M. R., et al. Induction of diabetes by streptozotocin in rats. Indian Journal of Clinical Biochemistry. 2007;22(2):60–64. doi: 10.1007/bf02913315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yanardağ R., Bolkent Ş., Tabakoğlu-Oğuz A., Özsoy-Saçan Ö. Effects of Petroselinum crispum extract on pancreatic B cells and blood glucose of streptozotocin-induced diabetic rats. Biological and Pharmaceutical Bulletin. 2003;26(8):1206–1210. doi: 10.1248/bpb.26.1206. [DOI] [PubMed] [Google Scholar]
- 25.Eidi A., Eidi M., Darzi R. Antidiabetic effect of Olea europaea L. in normal and diabetic rats. Phytotherapy Research. 2009;23(3):347–350. doi: 10.1002/ptr.2629. [DOI] [PubMed] [Google Scholar]
- 26.Eidi A., Eidi M., Sokhteh M. Effect of fenugreek (Trigonella foenum-graecum L) seeds on serum parameters in normal and streptozotocin-induced diabetic rats. Nutrition Research. 2007;27(11):728–733. doi: 10.1016/j.nutres.2007.09.006. [DOI] [Google Scholar]
- 27.Mahjoub S., Davari S., Moazezi Z., Qujeq D. Effect of Teucrium polium flower extract on the activities of nucleoside diphosphate kinase and acetyl-CoA carboxylase in normal and diabetic rats. African Journal of Pharmacy and Pharmacology. 2012;6(15):1106–1110. doi: 10.5897/AJPP12.240. [DOI] [Google Scholar]
- 28.Snell-Bergeon J. K., Wadwa R. P. Hypoglycemia, diabetes, and cardiovascular disease. Diabetes Technology and Therapeutics. 2012;14(supplement 1):S51–S58. doi: 10.1089/dia.2012.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmed D., Kumar V., Verma A., Shukla G. S., Sharma M. Antidiabetic, antioxidant, antihyperlipidemic effect of extract of Euryale ferox salisb. with enhanced histopathology of pancreas, liver and kidney in streptozotocin induced diabetic rats. SpringerPlus. 2015;4, article 315 doi: 10.1186/s40064-015-1059-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Baragob A. E., Almalki W., Shahid I., Bakhdhar F., Bafhaid H., Eldeen O. M. The hypoglycemic effect of the aqueous extract of the fruits of Balanites aegypticea in Alloxan-induced diabetic rats. Pharmacognosy Research. 2014;6(1):1–5. doi: 10.4103/0974-8490.122909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morsy A. M. A., Ahmad I. A., Kamel A. M. Some biomedical applications of Balanites aegyptiaca grown naturally in radioactive area, Southeastern Desert, Egypt. Journal of Hazardous Materials. 2010;178(1–3):725–728. doi: 10.1016/j.jhazmat.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 32.Motaal A. A., Shaker S., Haddad P. S. Antidiabetic activity of standardized extracts of Balanites aegyptiaca fruits using cell-based bioassays. Pharmacognosy Journal. 2012;4(30):20–24. doi: 10.5530/pj.2012.30.4. [DOI] [Google Scholar]
- 33.Abdel-Moneim A. Effect of some medicinal plants and gliciazide on insulin release in vitro. Journal of the Egyptian German Society of Zoology. 1998;25:423–445. [Google Scholar]
- 34.El-Bayomy M. M., El-Mously M., El-Stoohy F., Mehanna S. S. Effect of oral administration of the aqueous extract of Balanites aegyptiaca dates on blood glucose, serum insulin and lipids in streptozotocin-induced diabetes in rats. Journal of Biomedical and Therapeutic Sciences. 1992;8(8):82–89. [Google Scholar]
- 35.Hassan M. Physiological and Herpbiochemical Studies on the Effects of Balanites Aegyptiaca in Albino Ratápdez. Asyut, Egypt: Department of Botany, Faculty of Science, Assiut University; 2000. [Google Scholar]
- 36.Khan A. H., Pessin J. E. Insulin regulation of glucose uptake: a complex interplay of intracellular signalling pathways. Diabetologia. 2002;45(11):1475–1483. doi: 10.1007/s00125-002-0974-7. [DOI] [PubMed] [Google Scholar]
- 37.Sellamuthu P. S., Muniappan B. P., Perumal S. M., Kandasamy M. Antihyperglycemic effect of mangiferin in streptozotocin induced diabetic rats. Journal of Health Science. 2009;55(2):206–214. doi: 10.1248/jhs.55.206. [DOI] [Google Scholar]
- 38.Anand P., Murali K. Y., Tandon V., Murthy P. S., Chandra R. Insulinotropic effect of cinnamaldehyde on transcriptional regulation of pyruvate kinase, phosphoenolpyruvate carboxykinase, and GLUT4 translocation in experimental diabetic rats. Chemico-Biological Interactions. 2010;186(1):72–81. doi: 10.1016/j.cbi.2010.03.044. [DOI] [PubMed] [Google Scholar]
- 39.Clark C., Newgard C. B. Hepatic regulation of fuel metabolism. In: Saltiel A. R., Pessin J. E., editors. Mechanisms of Insulin Action. Austin, Tex, USA: Landes Bioscience; 2007. pp. 90–109. [Google Scholar]
- 40.Heidarian E., Soofiniya Y. Hypolipidemic and hypoglycemic effects of aerial part of Cynara scolymus in streptozotocin-induced diabetic rats. Journal of Medicinal Plants Research. 2011;5(13):2717–2723. [Google Scholar]
- 41.Jiang N., Zhang S., Zhu J., Shang J., Gao X. Hypoglycemic, hypolipidemic and antioxidant effects of peptides from red deer antlers in streptozotocin-induced diabetic mice. The Tohoku Journal of Experimental Medicine. 2015;236(1):71–79. doi: 10.1620/tjem.236.71. [DOI] [PubMed] [Google Scholar]
- 42.Winiarska K., Fraczyk T., Malinska D., Drozak J., Bryla J. Melatonin attenuates diabetes-induced oxidative stress in rabbits. Journal of Pineal Research. 2006;40(2):168–176. doi: 10.1111/j.1600-079x.2005.00295.x. [DOI] [PubMed] [Google Scholar]
- 43.Kroncke K.-D., Fehsel K., Sommer A., Rodriguez M.-L., Kolb-Bachofen V. Nitric oxide generation during cellular metabolization of the diabetogenic N-methyl-N-nitroso-urea streptozotozin contributes to islet cell DNA damage. Biological Chemistry. 1995;376(3):179–185. doi: 10.1515/bchm3.1995.376.3.179. [DOI] [PubMed] [Google Scholar]
- 44.Meenu J., Sunil S., Manoj K. Evaluation of antihyperglycemic activity of Dodonaea viscosa leaves in normal and STZ-diabetic rats. International Journal of Pharmacy and Pharmaceutical Sciences. 2011;3(3):69–74. [Google Scholar]
- 45.Nermien Z. A. Biochemical Studies on Some Antioxidant as Free Radical Scavengers. Cairo, Egypt: Department of Agricultural Sciences, Faculty of Agriculture; 2008. [Google Scholar]
- 46.Al-Mamary M. A. Antioxidant activity of commonly consumed vegetables in Yemen. Malaysian Journal of Nutrition. 2002;8(2):179–189. [PubMed] [Google Scholar]
- 47.Ayoola A. O., Akinloye O., Oguntibeju O. O., Oke J. M., Odetola A. A. Antioxidant activities of Parquetina nigrescens . African Journal of Biotechnology. 2011;10(24):4920–4925. [Google Scholar]
- 48.Bangar A. V., Saralaya M. G. Anti-hyperglycaemic activity of ethanol extract and chloroform extract of Indigofera tinctoria leaves in streptozotocin induced diabetic mice (family-Papilionaceae) Research Journal of Pharmaceutical, Biological and Chemical Sciences. 2011;2(1):445–455. [Google Scholar]
- 49.Kade I. J., Barbosa N. B. V., Ibukun E. O., Igbakin A. P., Nogueira C. W., Rocha J. B. T. Aqueous extracts of Sphagneticola trilobata attenuates streptozotocin-induced hyperglycaemia in rat models by modulating oxidative stress parameters. Biology and Medicine. 2010;2(3):1–13. [Google Scholar]
- 50.Velraj M., Singh M., Ravichandiran V., Nirmala S., Ragala S. Antidiabetic activity of ethyl acetate and ethanolic extract of Scindapsus officinalis fruit in alloxan induced diabetic rats. International Journal of PharmTech Research. 2011;3(3):1305–1310. [Google Scholar]
- 51.Heidari Z., Mahmoudzadeh-Sagheb H., Moudi B. A quantitative study of sodium tungstate protective effect on pancreatic beta cells in streptozotocin-induced diabetic rats. Micron. 2008;39(8):1300–1305. doi: 10.1016/j.micron.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 52.Hemalatha C., Dhamotharan R., Murugesan S. Effect of soy bean lectin on streptozotocin induced diabetic rats. Asian Journal of Experimental Biological Sciences. 2011;2(2):231–236. [Google Scholar]
- 53.Meier J. J., Bhushan A., Butler A. E., Rizza R. A., Butler P. C. Sustained beta cell apoptosis in patients with long-standing type 1 diabetes: indirect evidence for islet regeneration? Diabetologia. 2005;48(11):2221–2228. doi: 10.1007/s00125-005-1949-2. [DOI] [PubMed] [Google Scholar]
- 54.Maksoud S. A., El Hadidi M. N. The flavonoids of Balanites aegyptiaca (Balanitaceae) from Egypt. Plant Systematics and Evolution. 1988;160(3-4):153–158. doi: 10.1007/bf00936042. [DOI] [Google Scholar]
- 55.Fu Z., Gilbert E. R., Pfeiffer L., Zhang Y., Fu Y., Liu D. Genistein ameliorates hyperglycemia in a mouse model of nongenetic type 2 diabetes. Applied Physiology, Nutrition, and Metabolism. 2012;37(3):480–488. doi: 10.1139/h2012-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duvivier-Kali V. F., Omer A., Parent R. J., O'Neil J. J., Weir G. C. Complete protection of islets against allorejection and autoimmunity by a simple barium-alginate membrane. Diabetes. 2001;50(8):1698–1705. doi: 10.2337/diabetes.50.8.1698. [DOI] [PubMed] [Google Scholar]
- 57.Bonner-Weir S., Guo L., Li W.-C., et al. Islet neogenesis: a possible pathway for beta-cell replenishment. Review of Diabetic Studies. 2012;9(4):407–416. doi: 10.1900/rds.2012.9.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Back W. D., Zimm R., Brusch L. Transdifferentiation of pancreatic cells by loss of contact-mediated signaling. BMC Systems Biology. 2013;7, article 77 doi: 10.1186/1752-0509-7-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ansarullah, Bharucha B., Umarani M., et al. Oreocnide integrifolia flavonoids augment reprogramming for islet neogenesis and β-cell regeneration in pancreatectomized BALB/c mice. Evidence-Based Complementary and Alternative Medicine. 2012;2012:13. doi: 10.1155/2012/260467.260467 [DOI] [PMC free article] [PubMed] [Google Scholar]