Abstract
Purpose
To determine histopathologic findings in the cochlea of human temporal bones with serous labyrinthitis.
Materials and Methods
We compared human temporal bones with serous labyrinthitis (20 cases) associated with silent otitis media and without serous labyrinthitis (20 cases) to study location of serous labyrinthitis, the degree of endolymphatic hydrops, number of spiral ganglion cells and hair cells, loss of fibrocytes in the spiral ligament, and areas of the spiral ligament and stria vascularis
Results
The serous labyrinthitis caused significant loss of outer hair cells in the lower basal (P = 0.006), upper basal (P = 0.005), and lower middle (P = 0.011) cochlear turns, and significant increase in the degree of endolymphatic hydrops than the control group (P = 0.036). No significant difference was found in the loss of inner hair cells, in the number of spiral ganglion cells and FIBROCYTES IN THE SPIRAL LIGAMENT, and in areas of the stria vascularis and spiral ligament (P > 0.05).
Conclusions
Serous labyrinthitis resulted in significant loss of outer hair cells and significant increase in the degree of endolymphatic hydrops.
Keywords: Serous labyrinthitis, inner ear, cochlea, hair cells, endolymphatic hydrops, spiral ganglion cells, stria vascularis, spiral ligament, human temporal bones, histopathology
1. Introduction
Middle ear inflammation and interaction between the middle and inner ears can cause inner ear damage and hearing loss secondary to otitis media. Serous labyrinthitis (SL) is an inflammation of the labyrinth in the absence of bacteria, or viruses [1]. The underlying problems of serous labyrinthitis may include cochlear damage due to middle and inner ear interaction, perilymph contamination during surgery, the presence of inflammatory mediators (meningogenic/otogenic), perilymph fistula, or neoplasia [2-4]. SL and some other inner ear complications can occur due to otitis media when bacterial products, viruses, and inflammatory mediators enter the inner ear through the round window membrane [5,6]. In this study, we investigated histopathological changes in human temporal bones with SL. In contrast to widespread opinion [7,8], our hypothesis is that SL can cause permanent pathologic damage in the cochlea.
2. Materials and methods
We obtained samples from the human temporal bone collection at the University of Minnesota. All temporal bones had previously been removed at autopsy, fixed in formalin solution, decalcified, embedded in celloidin, and serially sectioned in the horizontal plane at a thickness of 20 μm. Every 10th section was stained with hematoxylin-eosin (H&E) and mounted on a glass slide for light-microscopic observation. This study was approved by the institutional review board of the University of Minnesota (0206M26181).
We specifically defined SL as the presence of serous fluid in the labyrinth, with fibrous or granular precipitate in the inner ear in the absence of bacteria, viruses, or inflammatory cells. Silent otitis media has been described as a chronic pathologic condition behind an intact tympanic membrane [9]. For our study, we selected only temporal bones from patients with SL associated with silent otitis media. We excluded temporal bones from patients who had a history of acoustic trauma, meningitis, systemic autoimmune disorders, otosclerosis, Meniere's disease, or metastatic tumors, because all of those conditions can contribute to changes in the inner ear.
The SL group included 20 temporal bones from 16 patients (12 men and 4 women) associated with silent otitis media; their age ranged from 5 months to 88 years (mean, 31.4 ± 27.6 years). THE control group included 20 temporal bones from 16 healthy patients (9 men and 7 women) who did not have SL; their age ranged from 4 days to 64 years (mean, 37.3 ± 20.5 years). We investigated the location of SL in the inner ear, the degree of endolymphatic hydrops, the number of spiral ganglion cells and hair cells, mean loss of fibrocytes in spiral ligament, and the areas of the stria vascularis and spiral ligament.
2.1. Spiral Ganglion Cells
We divided Rosenthal's canal into 4 segments as described previously: segment I (from base to 6 mm); II (6 to 15 mm); III (15 to 22 mm) and IV (22 mm to apex) [10]. All nuclei were counted in each section. The number of ganglion cells was determined for each segment and for the cochlea as a whole by multiplying their summed counts by 10 to account for the unmounted sections and by a factor of 0.9 to account for cells that would be counted because of their location at the interface between sections.
2.2. Hair Cells
We assessed the presence of outer and inner hair cells in each cochlear turn. The percentage loss of cochlear hair cells was counted to compare 2 groups.
2.3. Endolymphatic Hydrops
We subdivided our temporal bone samples according to the degree of endolymphatic hydrops, per the classification of Cureoglu et al. [11]; (1) slight hydrops, i.e., bulging of Reissner's membrane without contact with the bony wall of the scala vestibuli; (2) moderate hydrops, i.e., displacement of Reissner's membrane with contact with the wall of the scala vestibuli but with an angle with the osseous spiral lamina of less than 90 degrees; and (3) profound hydrops, i.e., displacement of Reissner's membrane with bony contact, with an angle with the osseous spiral lamina of more than 90 degrees.
2.4. Stria Vascularis
In all of the cochlear turns at the midmodiolar level, as well as on the adjacent 2 sections, we obtained morphometric measurements of the stria vascularis. We acquired each image with a digital camera at a magnification of ×200. Using a computer, we quantified the areas of the cut surfaces of the stria vascularis. For the measurements, we used image analyses software (SPOT Advanced, SPOT Imaging Solutions, MI, USA). Excluded from the area of the stria vascularis were any secondary changes, such as cystic-like structural areas or concretions.
2.5. Spiral Ligament
We subdivided the spiral ligament into 4 segments according to the appearance of different types of fibrocytes, per previous studies by Spicer and Schulte [12]: Type I fibrocytes lie circumferentially aligned between the stria vascularis and bone. Type II fibrocytes occupy the superficial inferior spiral ligament between the basilar crest and the stria. Type III fibrocytes are longitudinally located in the deepest part of the inferior spiral ligament. Type IV fibrocytes lie radially oriented, inferior to the basilar crest.
To estimate and evaluate the mean loss of fibrocytes in the spiral ligament, we used a rating scala: 0, within normal limits (missing less than 1/3 of the fibrocytes); 1, missing 1/3 of the fibrocytes; 2, missing 2/3 of the fibrocytes; and 3, severe or complete loss of the fibrocytes on sections at the midmodiolar level, per the methods of Hequembourg and Liberman [13]. The calibrated image was obtained at an original magnification of ×40.
Morphometric measurements of spiral ligament's area were made in all turns of the cochlea at the midmodiolar level and the adjacent two sections. The image was obtained with a charge-coupled device camera that was connected to a personal computer. The calibrated image was obtained at an original magnification of ×40. The areas of spiral ligament were quantified by determining the areas of their cut surfaces, with the aid of the computer. Measurements were made using commercially available image analysis software (SPOT Advanced, SPOT Imaging Solutions, MI, USA).
2.6. Statistical analysis
To analyze any differences between the temporal bones with the SL and the control groups, we used Student t test. Significance was defined as P < 0.05.
3. Results
We most often observed SL in the scala tympani of the basal turn of the cochlea; least often, in the superior semicircular canal of the inner ear. Of the 20 temporal bone samples in the SL group, 16 (80%) had serous effusion in the scala tympani of the basal turn (Table 1).
Table 1.
Locations of SL in the inner ear in 20 temporal bone samples
| Location | Number of temporal bone samples |
|---|---|
| Superior SC | 1 |
| Lateral SC | 10 |
| Posterior SC | 6 |
| Utricle | 13 |
| Saccule | 11 |
| Basal turn | |
| SV | 12 |
| SM | 8 |
| ST | 16 |
| Middle turn | |
| SV | 6 |
| SM | 5 |
| ST | 6 |
| Apical turn | |
| SV | 2 |
| SM | 2 |
| ST | 5 |
SC: semicircular canal; SL: serous labyrinthitis; SM: scala media; ST: scala tympani; SV: scala vestibuli
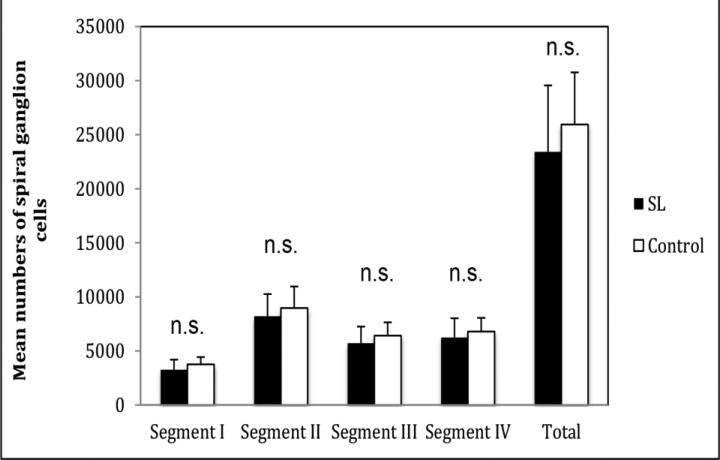
We found no significant difference in the total number of spiral ganglion cells between the SL group (23,412 ± 6,148) and the control group (25,949 ± 4,819) (P > 0.05). We found no significant differences in any segment between the 2 groups (P > 0.05) (Figure 1).
Figure 1.
Diagram showing mean number of spiral ganglion cells by segment. SL: serous labyrinthitis; n.s. = not significant
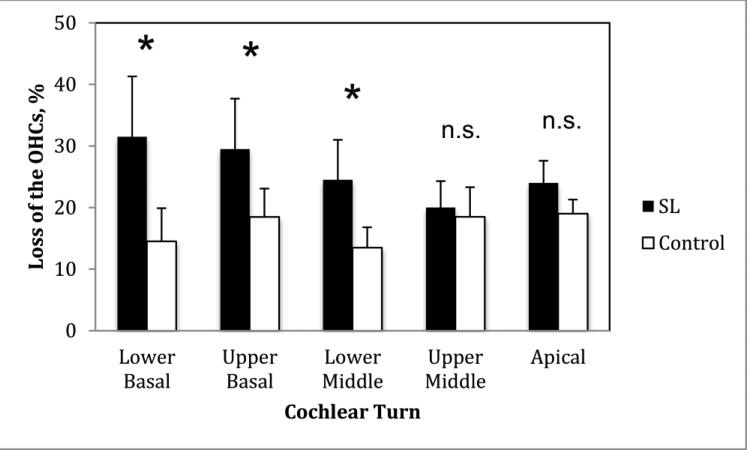
We found no significant differences between the SL and control groups in the number of outer hair cells in the upper middle and apical turns (P > 0.05). However, we did find significant differences in the lower basal (P = 0.006), upper basal (P = 0.005), and lower middle cochlear turns (P = 0.011) (Figure 2 and 3). We found no significant differences between the 2 groups in the loss of inner hair cells in any cochlear turn (P > 0.05).
Figure 2.
Diagram showing mean loss (%) of outer hair cells (OHCs). SL: serous labyrinthitis; * P < 0.05; n.s. = not significant
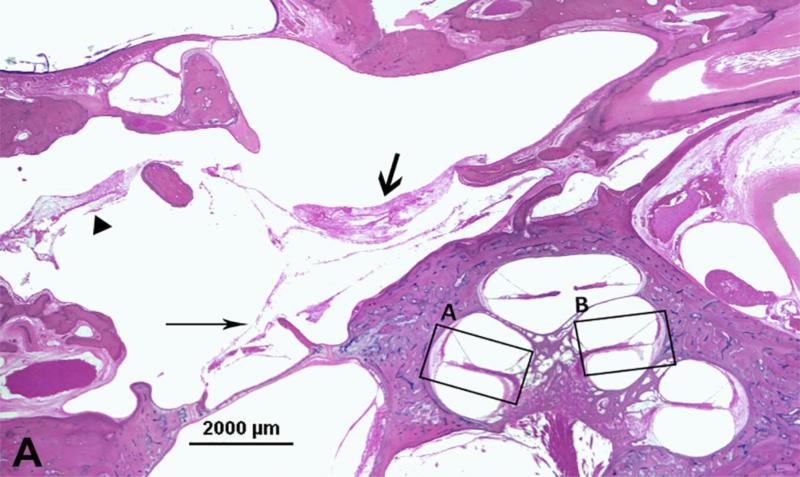
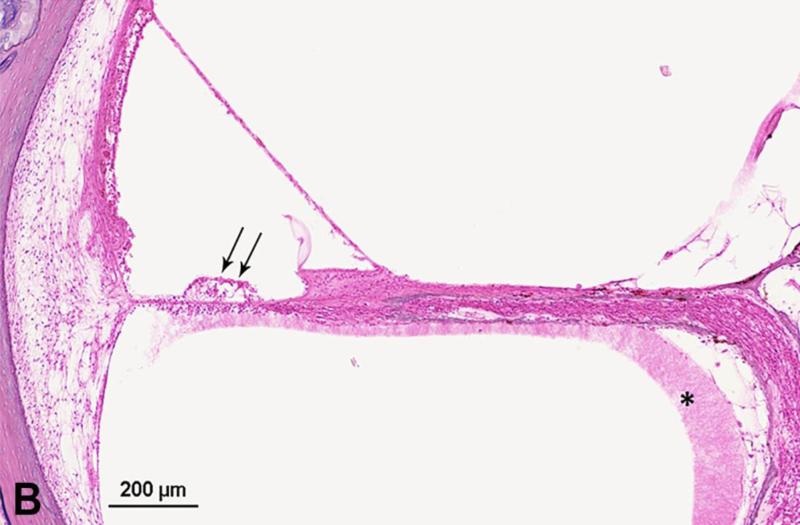
Figure 3.
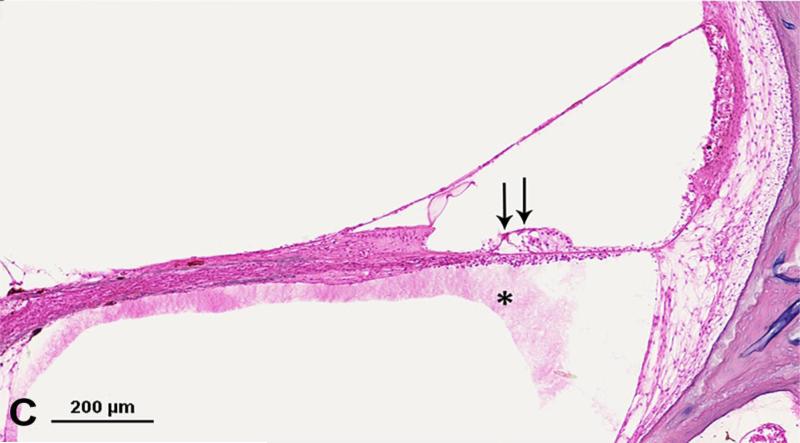
Photomicrograph showing loss of outer hair cells (OHCs) in the temporal bone from an 18-year-old man with serous labyrinthitis. A. Low-power image of upper basal turn and lower middle turn. Thick arrow = mucopurulent secretion; thin arrow = fibrous structure; arrowhead = fibrocystic structure. B. Enlarged view of box A. Arrows = loss of OHCs. * serous labyrinthitis. C. Enlarged view of box B. Arrows = loss of OHCs. * serous labyrinthitis. Staining with hematoxylin-eosin (H&E).
However, we did find a significant difference in the degree of endolymphatic hydrops between the 2 groups (P = 0.036) (Figure 4). Of the 20 temporal bone samples with SL group, 3 (15%) had slight, and 1(5%) had moderate hydrops in the basal and/or middle turns of the cochlea. The control group did not show any hydropic changes.
Figure 4.
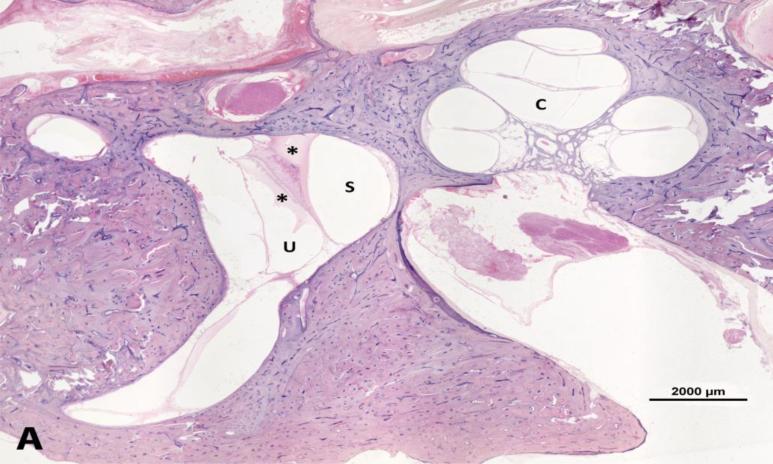
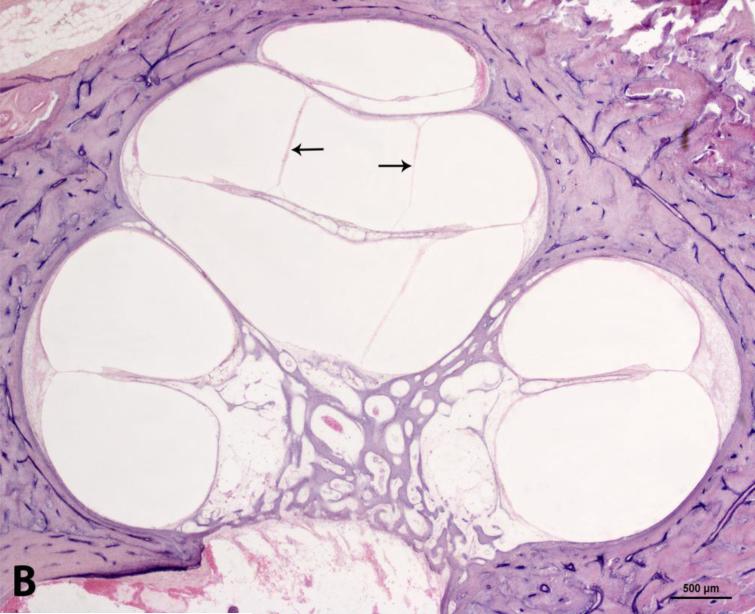
Photomicrograph showing moderate endolymphatic hydrops in the temporal bone from a 71-years-old man. A. Lower magnification image shows serous labyrinthitis in vestibule. B. Higher magnification of the cochlea. C: cochlea; S: saccule; U: utricle. Arrows = endolymphatic hydrops. * serous labyrinthitis. Staining with hematoxylin-eosin (H&E).
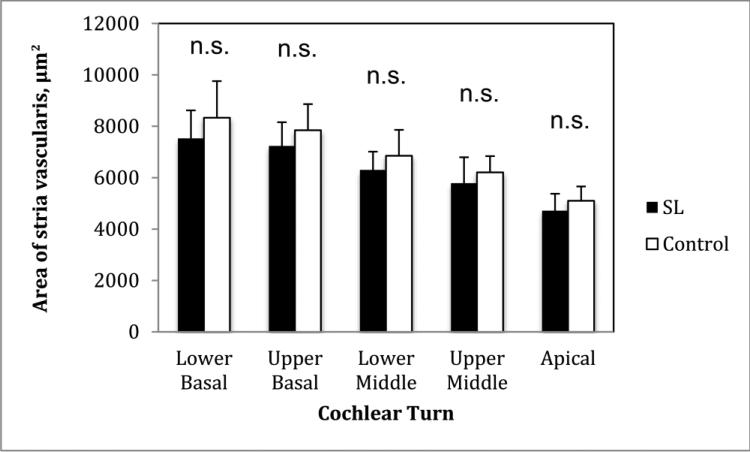
We found no significant differences between the SL and control groups in atrophy of the stria vascularis in any cochlear turn (P > 0.05) (Figure 5).
Figure 5.
Diagram showing mean area (μm2) of the stria vascularis. SL: serous labyrinthitis; n.s. = not significant
Finally, we found no significant differences between the SL and control groups in the numbers of fibrocytes (for any of the types) and in atrophy of the spiral ligament in any cochlear turn (P > 0.05).
4. Discussion
In our study, we found that SL was primarily located in the basal turn of the cochlea, in addition to the vestibular labyrinth. Merchant and Gopen [14] observed SL mainly in the vestibular labyrinth in patients with acute bacterial meningitis; however, in our study, we only included SL associated with silent otitis media.
We found that the degree of endolymphatic hydrops was significantly higher in the SL group than in the control group. This finding consistent with previously reported data [4,8]. Hydrops may be due to the cellular changes in Reissner's membrane that cause ionic imbalance in endolymph [15-18]. The increased protein content in labyrinthine fluid, and the changes resulting from inflammation, may cause structural damage to Reissner's membrane [18]. Endolymphatic hydrops may play an important role in sensorineural hearing loss. Histopathological analysis by Yoon et al. [19] revealed endolymphatic hydrops in some patients with sensorineural hearing loss, although the relationship between hydrops and sensorineural hearing loss was not clear.
Sensorineural hearing loss is an important complication of silent otitis media [20-23]. A number of substances —such as proteins, antibiotics, and bacterial products— can pass from the middle ear into the inner ear through the round window membrane [24-26]. The only soft tissue barrier between the middle ear and the inner ear, the round window membrane is a conceivable route for diffusing bacterial toxins and inflammatory mediators from the middle ear into the inner ear [27,28]. In our study, we found the loss of outer hair cells in the basal, and lower middle turns of cochlea in the SL group. The mechanism for the loss of outer hair cells may involve the ion and/or protein imbalance in the inner ear due to the inflammatory response.
Merchant and Gopen found severe loss of spiral ganglion cells in temporal bone samples from patients with acute bacterial meningitis although the stria vascularis and spiral ligament were normal in addition to normal sensory and neural structures in their cases [14]. Joglekar et al. found significant loss of outer and inner hair cells, as well as a significant decrease in areas of the stria vascularis in the basal turn of the cochlea in human temporal bones of patients with chronic active otitis media [29]. In contrast, our temporal bone samples from patients with silent otitis media, showed no significant differences in the number of spiral ganglion cells, in the areas of stria vascularis and spiral ligament, and in the distribution of fibrocytes in the spiral ligament.
Most clinicians assume that SL leads to reversible sensorineural hearing loss, with recovery of cochlear functions if SL is diagnosed and properly treated [1,8]. However, Merchant and Gopen [14] suggested that the traditional assumption of SL causing only temporary effects should be questioned: in 1 of the 14 temporal bones with SL that they analyzed (from patients with acute bacterial meningitis caused by Pseudomonas aeruginosa), loss of organ of Corti was significant, in all cochlear turns. Moreover, in chinchilla animal model, Paparella et al. [20] found significant cochlear hair cell loss with SL caused by purulent otitis media. This study clearly showed that silent otitis media can be associated with SL and can result in loss of outer hair cells in the lower cochlear turns.
5. Conclusions
Histopathologic analysis of temporal bone samples with SL revealed significant loss of outer hair cells in the basal, and lower middle cochlear turns, as well as a significant increase in the degree of endolymphatic hydrops. Physicians should keep in mind that serous labyrinthitis can be associated with silent otitis media and cause permanent changes such as outer hair cell damage and hydrops in the cochlea.
Acknowledgements
This study was supported by the National Institute on Deafness and Other Communication Disorders (NIDCD), grant number U24 DC011968-01; the International Hearing Foundation; the Starkey Hearing Foundation; the 5M Lions International; and the Scientific and Technological Research Council of Turkey (TUBITAK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None
References
- 1.Cureoglu S, Schachern PA, Rinaldo A, Tsuprun V, Ferlito A, Paparella MM. Round window membrane and labyrinthine pathological changes: an overview. Acta Otolaryngol. 2005;125:9–15. doi: 10.1080/00016480410022534. [DOI] [PubMed] [Google Scholar]
- 2.Merchant SN, Nadol JB. Schuknecht's Pathology of the Ear. 3rd ed. People's Medical Publishing House-USA; Shelton, CT: 2010. [Google Scholar]
- 3.Goldstein NA, Casselbrant ML, Bluestone CD, Kurs-Lasky M. Intratemporal complications of acute otitis media in infants and children. Otolaryngol Head Neck Surg. 1998;119:444–54. doi: 10.1016/S0194-5998(98)70100-7. [DOI] [PubMed] [Google Scholar]
- 4.Arts HA. Sensorineural hearing loss in adults. In: Flint PW, Haughey BH, Lund VJ, et al., editors. Cummings Otolaryngology—Head and Neck Surgery. Saunders-Elsevier; Philadelphia: 2014. pp. 2319–35. [Google Scholar]
- 5.Hellström S, Eriksson PO, Yoon YJ, Johansson U. Interactions between the middle ear and the inner ear: bacterial products. Ann N Y Acad Sci. 1997;830:110–9. doi: 10.1111/j.1749-6632.1997.tb51883.x. [DOI] [PubMed] [Google Scholar]
- 6.Goycoolea MV. Clinical aspects of round window membrane permeability under normal and pathological conditions. Acta Otolaryngol. 2001;121:437–47. doi: 10.1080/000164801300366552. [DOI] [PubMed] [Google Scholar]
- 7.Arts HA, Adams ME. Intratemporal and intracranial complications of otitis media. In: Johnson JT, Rosen CA, editors. Bailey's Head and Neck Surgery—Otolaryngology. Lippincott Williams & Wilkins; Philadelphia: 2014. pp. 2399–409. [Google Scholar]
- 8.Paparella MM, Sugiura S. The pathology of suppurative labyrinthitis. Ann Otol Rhinol Laryngol. 1967;76:554–86. doi: 10.1177/000348946707600303. [DOI] [PubMed] [Google Scholar]
- 9.Paparella MM, Goycoolea MV, Meyerhoff WL, Shea D. Endolymphatic hydrops and otitis media. Laryngoscope. 1979;89:43–58. doi: 10.1288/00005537-197901000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Otte J, Schuknecht HF, Kerr AG. Ganglion cell populations in normal and pathological human cochleae. Implications for cochlear implantation. Laryngoscope. 1978;88:1231–46. doi: 10.1288/00005537-197808000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Cureoglu S, Schachern PA, Paul S, Paparella MM, Singh RK. Cellular changes of Reissner's membrane in Meniere's disease: human temporal bone study. Otolaryngol Head Neck Surg. 2004;130:113–9. doi: 10.1016/j.otohns.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Spicer SS, Schulte BA. Differentiation of inner ear fibrocytes according to their ion transport related activity. Hear Res. 1991;56:53–64. doi: 10.1016/0378-5955(91)90153-z. [DOI] [PubMed] [Google Scholar]
- 13.Hequembourg S, Liberman MC. Spiral ligament pathology: a major aspect of age-related cochlear degeneration in C57BL/6 Mice. JARO. 2001;2:118–29. doi: 10.1007/s101620010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merchant SN, Gopen Q. A human temporal bone study of acute bacterial meningogenic labyrinthitis. Am J Otol. 1996;17:375–85. [PubMed] [Google Scholar]
- 15.Sakagami M, Fukozawa K, Kitamura K, Doi K, Mori N, Matsunaga T. Transport of HRP through Reissner's membrane in experimental endolymphatic hydrops. Acta Otolaryngol. 1991;111:872–8. doi: 10.3109/00016489109138424. [DOI] [PubMed] [Google Scholar]
- 16.Nakai Y, Hilding D. Adenosine triphosphatase distribution in the organ of Corti: histochemical study by light and electron microscopy. Acta Otolaryngol. 1967;64:477–91. doi: 10.3109/00016486709139133. [DOI] [PubMed] [Google Scholar]
- 17.Nakai Y, Hilding D. Cochlear development: some electron microscopic observations of maturation of hair cells, spiral ganglion and Reissner's membrane. Acta Otolaryngol. 1968;66:369–85. doi: 10.3109/00016486809126303. [DOI] [PubMed] [Google Scholar]
- 18.Yoon TH, Paparella MM, Schachern PA, Le CT. Cellular changes in Reissner's membrane in endolymphatic hydrops. Ann Otol Rhinol Laryngol. 1991;100:288–93. doi: 10.1177/000348949110000405. [DOI] [PubMed] [Google Scholar]
- 19.Yoon TH, Paparella MM, Schachern PA, Alleva M. Histopathology of sudden hearing loss. Laryngoscope. 1990;100:707–15. doi: 10.1288/00005537-199007000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Paparella MM, Morizono T, Le C, Mancini F, Sipilä P, Choo YB, et al. Sensorineural hearing loss in otitis media. Ann Otol Rhinol Laryngol. 1984;93:623–9. doi: 10.1177/000348948409300616. [DOI] [PubMed] [Google Scholar]
- 21.English GM, Northern JL, Fria TJ. Chronic otitis media as a cause of sensorineural hearing loss. Arch Otolaryngol. 1973;98:18–22. doi: 10.1001/archotol.1973.00780020022006. [DOI] [PubMed] [Google Scholar]
- 22.Hunter LL, Margolis RH, Rykken JR, Le CT, Daly KA, Giebink GS. High frequency hearing loss associated with otitis media. Ear Hear. 1996;17:1–11. doi: 10.1097/00003446-199602000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Paparella MM. Quiet labyrinthine complications from otitis media. J Laryngol Otol Suppl. 1983;8:53–8. [PubMed] [Google Scholar]
- 24.Goldberg B, Goycoolea MV, Schleivert PM, Shea D, Schachern P, Paparella MM, et al. Passage of albumin from the middle ear to the inner ear in otitis media in the chinchilla. Am J Otolaryngol. 1981;2:210–4. doi: 10.1016/s0196-0709(81)80017-8. [DOI] [PubMed] [Google Scholar]
- 25.Becvarovski Z, Bojrab DI, Michaelides EM, Kartush JM, Zappia JJ, LaRouere MJ. Round window gentamicin absorption: an in vivo human model. Laryngoscope. 2002;112:1610–3. doi: 10.1097/00005537-200209000-00015. [DOI] [PubMed] [Google Scholar]
- 26.Schachern PA, Paparella MM, Hyberston R, Sano S, Duvall AJ., III Bacterial tympanogenic labyrinthitis, meningitis and sensorineural damage. Arch Otolaryngol Head Neck Surg. 1992;118:53–7. doi: 10.1001/archotol.1992.01880010057016. [DOI] [PubMed] [Google Scholar]
- 27.Paparella MM. Insidious labyrinthine changes in otitis media. Acta Otolaryngol. 1981;92:513–20. doi: 10.3109/00016488109133290. [DOI] [PubMed] [Google Scholar]
- 28.Goycoolea MV, Paparella MM, Goldberg B, Schlievert PM, Carpenter AM. Permeability of the middle ear to Staphylococcal pyrogenic exotoxin in otitis media. Int J Pediatr Otorhinolaryngol. 1980;1:301–8. doi: 10.1016/0165-5876(80)90004-x. [DOI] [PubMed] [Google Scholar]
- 29.Joglekar S, Morita N, Cureoglu S, Schachern PA, Deroee AF, Tsuprun V, et al. Cochlear pathology in human temporal bones with otitis media. Acta Otolaryngol. 2010;130:472–6. doi: 10.3109/00016480903311252. [DOI] [PMC free article] [PubMed] [Google Scholar]