Abstract
Alzheimer's disease (AD) is an irreversible brain disorder characterized by progressive cognitive decline and neurodegeneration of brain regions that are crucial for learning and memory. Although intracellular neurofibrillary tangles and extracellular senile plaques, composed of insoluble amyloid-β (Aβ) peptides, have been the hallmarks of postmortem AD brains, memory impairment in early AD correlates better with pathological accumulation of soluble Aβ oligomers and persistent weakening of excitatory synaptic strength, which is demonstrated by inhibition of long-term potentiation, enhancement of long-term depression, and loss of synapses. However, current, approved interventions aiming to reduce Aβ levels have failed to retard disease progression; this has led to a pressing need to identify and target alternative pathogenic mechanisms of AD. Recently, it has been suggested that the disruption of Hebbian synaptic plasticity in AD is due to aberrant metaplasticity, which is a form of homeostatic plasticity that tunes the magnitude and direction of future synaptic plasticity based on previous neuronal or synaptic activity. This review examines emerging evidence for aberrant metaplasticity in AD. Putative mechanisms underlying aberrant metaplasticity in AD will also be discussed. We hope this review inspires future studies to test the extent to which these mechanisms contribute to the etiology of AD and offer therapeutic targets.
1. Introduction
Neurons communicate with each other at specialized intercellular junctions, called synapses. The strength of synaptic transmission can be dynamically and persistently altered in response to changes in neuronal activity. In the book The Organization of Behavior, Donald Hebb postulated that connections between neurons that are simultaneously active are strengthened [1]. Such “Hebbian plasticity” was first demonstrated at excitatory glutamatergic synapses in rabbit hippocampus by the seminal work of Bliss and Lomo [2]. High frequency stimulation of presynaptic axons in the perforant pathway induces stronger and long-lasting excitatory postsynaptic potentials (EPSPs) in neurons of the postsynaptic dentate gyrus [2]. This long-term potentiation (LTP) of excitatory synaptic strength lasts hours to months [2] and can be induced electrically in brain slices as well as in vivo in behaving animals [2, 3]. Hence, the associative and input-specific synaptic plasticity such as LTP and its counterpart long-term depression (LTD) is thought to underlie cellular correlates of learning and memory [4–7].
Hebbian plasticity also represents a positive feedback mechanism. Once LTP is induced, saturated synapses undergo further potentiation with greater ease than before the LTP induction, leading to unstable runaway excitation [8–10]. Similarly, continuous synaptic depression during LTD could result in unnecessary synaptic silencing and elimination [8–10]. In order to sense and counteract destabilizing effects of LTP and LTD, neurons employ negative feedback processes called homeostatic synaptic plasticity [11–14]. This adaptive plasticity offers a compensatory refinement of synaptic strength to maintain the stability of network activity within a physiologic limit [13–15]. For example, prolonged elevation of neuronal activity results in compensatory downscaling of synaptic strength to prevent hyperexcitation, whereas prolonged suppression of neuronal activity leads to compensatory upscaling of synaptic strength to prevent synapse silencing and elimination [13–15]. Without this homeostatic mechanism, the capacity of an active synapse would get saturated due to unconstrained potentiation, limiting its ability to store information (i.e., memory). Homeostatic synaptic plasticity is therefore a vital partner of Hebbian synaptic plasticity.
Defects in homeostatic synaptic plasticity could, in principle, cause abnormal Hebbian plasticity at synapses, leading to pathologic levels of synaptic potentiation or elimination in neurologic diseases. For example, Alzheimer's disease (AD) is characterized by progressive and irreversible memory impairment [16] and associated with inhibition of LTP and enhancement of LTD in the hippocampus [17–27]. While physiologic levels of soluble amyloid-β (Aβ) oligomers have been shown to enhance synaptic activity and LTP [28, 29], pathologic levels of soluble Aβ oligomers impair LTP and enhance LTD in acute hippocampal slices [30–33]. Such impairment in Hebbian synaptic plasticity correlates strongly with memory impairment in early AD when Aβ plaques and neuronal degeneration are minimal [34–36]. Recent studies suggest that this abnormal Hebbian plasticity is due to pathologic engagement or disruption of metaplasticity [27, 32, 37], a form of homeostatic synaptic plasticity that controls the induction threshold of LTP and LTD [38]. Interestingly, Aβ-induced aberrant hyperexcitability is found in cortical and hippocampal neuronal networks of human AD and mouse models of AD [39–45]. Further, epileptiform electrical seizures and neuronal activity stimulate Aβ synthesis and its release from the neurons in the hippocampus [46–48]. Indeed, a pathologic positive feedback loop between Aβ production and neuronal hyperexcitability would favor LTP inhibition and LTD induction.
These studies have provided a possible link between abnormal metaplasticity and cognitive dysfunction in AD pathogenesis, although our knowledge on the underlying mechanisms is limited. An understanding of the molecular mechanisms through which altered metaplasticity contributes to AD synaptopathology will be crucial in decoding the etiology of AD and may facilitate “correcting metaplasticity” as a putative novel therapy to restore Hebbian synaptic plasticity and treat cognitive dysfunction in early AD. In this paper, we review recent studies demonstrating aberrant metaplasticity in AD and discuss the possible underlying mechanisms focused on glutamate receptor regulation.
2. Abnormal Hebbian Synaptic Plasticity in AD
AD is a neurodegenerative disorder characterized by progressive and irreversible cognitive decline [16]. It is the 6th leading cause of death in the United States and the most common cause of dementia, which affects over 44 million people worldwide [49]. The molecular hallmarks of AD are amyloid plaques (extracellular deposits consisting of aggregated insoluble Aβ) and neurofibrillary tangles (intracellular filamentous aggregates of hyperphosphorylated tau) in the hippocampus and cortices [50–52], the brain regions critical for learning and memory. Interestingly, genetic suppression of endogenous tau blocks cognitive dysfunction in AD animal models, in which Aβ expression has been increased using a transgene [53–55], suggesting that tau acts downstream of Aβ in AD pathogenesis.
Importantly, soluble Aβ peptides rather than insoluble amyloid plaques have emerged to play critical roles in the early stages of AD pathogenesis. First, amyloid plaques are found at later stages after memory loss is already evident in humans and AD animal models with genetically elevated Aβ level [17–27]. Second, intracranial injection of soluble Aβ oligomers is sufficient to cause memory loss [29, 33, 56–59]. Third, rare early-onset autosomal dominant familial AD (FAD) is associated with increased levels of soluble Aβ due to mutations in genes whose protein products are involved in Aβ production and processing [60, 61]. Aβ peptides are generated by successive proteolysis of amyloid-β precursor protein (APP), a large transmembrane glycoprotein that is initially cleaved by the β-site APP-cleaving enzyme 1 (BACE1) and subsequently by γ-secretase in the transmembrane domain [62–64]. The FAD mutations are found in APP and presenilins [60, 61], which are catalytic components of γ-secretase [65]. Lastly, a major genetic risk factor for most AD (i.e., sporadic AD) is polymorphic ε4 allele of apolipoprotein E [66, 67]. The encoded ApoE4 is less efficient in clearing Aβ than the common ApoE3, suggesting a strong association between sporadic AD and increased levels of soluble Aβ [68].
How could pathologic levels of soluble Aβ oligomers cause cognitive dysfunction? The first clue came from the studies in AD mouse models with genetically elevated Aβ [17–27]. Before the development of amyloid plaques is evident, these AD mouse models display severe impairment of hippocampal LTP [17–27]. Furthermore, LTD is induced in these AD mouse hippocampi with subthreshold stimulations, which normally cannot induce LTD in wild-type control mice [17–27]. Subsequent studies have shown that direct application of soluble Aβ oligomers (synthetic, cell-culture secreted, or AD brain-derived) at pathologic levels inhibits LTP and enhances LTD in acute hippocampal slice [30–33]. A persistent and unchecked decrease in synaptic strength is expected to lead to the pathologic elimination of synapses [69–71]. Indeed, decreases in synapse density are evident in hippocampi of patients with early AD [72–75]. Therefore, abnormal Hebbian synaptic plasticity is thought to be the basis of memory loss in early AD when amyloid plaques and neuronal degeneration are minimal [34–36].
3. Is Abnormal Hebbian Synaptic Plasticity due to Defective Homeostatic Synaptic Plasticity in AD?
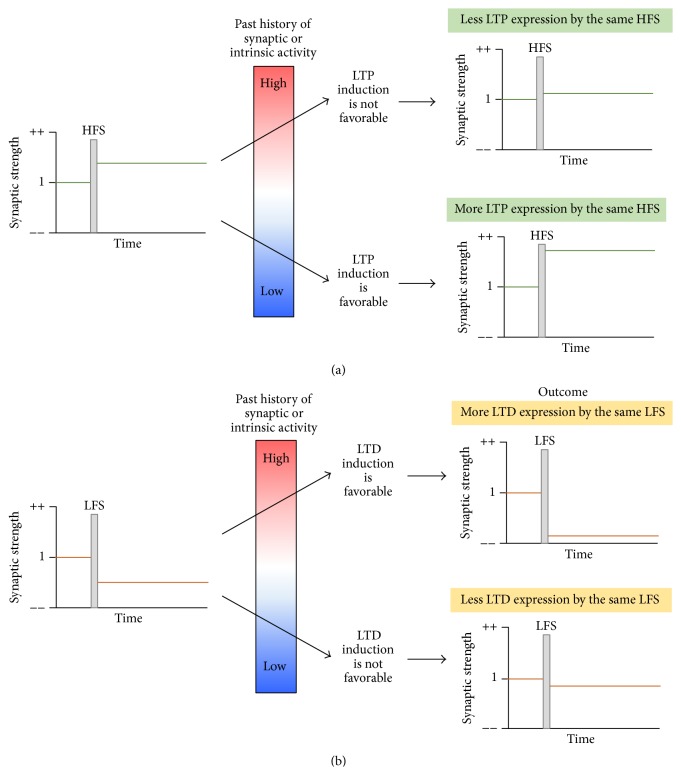
Decades of studies cited above have compared the magnitudes of LTP and LTD in AD transgenic mouse models to determine if pathologic levels of soluble Aβ oligomers affect Hebbian synaptic plasticity. However, the absolute changes in the LTP and LTD magnitudes vary with age and AD mouse model [147], suggesting age- and strain-dependent differences for the induction threshold of LTP and LTD in these animals. The induction thresholds of LTP and LTD can be modified as a consequence of previous postsynaptic neuronal activity (Figure 1) [10, 148, 149]. LTP induction is favorable in neurons whose previous synaptic and intrinsic activities were low, whereas LTD induction is preferred when the previous activities were high [10, 148, 149]. Such compensatory adjustment of the induction thresholds for LTP and LTD, called “metaplasticity,” occurs as a form of homeostatic synaptic plasticity and provides stability to neuronal networks and supports Hebbian synaptic plasticity [10].
Figure 1.
Metaplasticity. The induction threshold of LTP and LTD can be modified as a consequence of overall past synaptic or intrinsic activity of postsynaptic neurons. Such compensatory adjustment called “metaplasticity” provides stability to neuronal networks that support Hebbian synaptic plasticity. (a) LTP induction by conventional high frequency stimulation (HFS) is favorable in the neurons whose previous synaptic and intrinsic activities were low. (b) LTD induction by conventional low frequency stimulation (LFS) is favorable in the neurons whose previous synaptic and intrinsic activities were high.
Hence, it is possible that the abnormal Hebbian synaptic plasticity in AD could arise from the defects in metaplasticity. Several studies have provided supporting evidence for this hypothesis. Aberrant neuronal hyperexcitability has been observed in cortical and hippocampal neuronal networks of patients with early AD [150] and FAD AD mouse models with heightened levels of APP and Aβ [21, 41–44, 151]; this is also consistent with reports that patients with early AD and FAD animal models exhibit epileptic seizures [21, 45, 152–160]. Pharmacological inhibition of epileptic seizures inhibits memory loss in AD mouse models [156], implicating critical roles of aberrant neuronal hyperexcitability in cognitive dysfunction presented early in AD pathogenesis [39, 40]. Hence, Aβ-induced cognitive dysfunction in early AD may result from the inability of neurons to adapt to persistent increases in overall neural network activity rather than the absolute changes in LTP and LTD magnitudes.
Additional support for this hypothesis comes from the report that soluble Aβ oligomers result in excessive activation of N-methyl D-aspartate receptors (NMDARs) containing GluN2B subunits, causing LTP inhibition and LTD facilitation via ERK and CREB signaling pathways [161]. GluN2B-selective antagonists effectively prevent Aβ-induced LTP inhibition [161–163], suggesting that early activation of extrasynaptic NMDARs primes the synapse to inhibit LTP induction and facilitate LTD induction. Consistent with this notion, GluN2B-selective antagonists prevent priming-induced inhibition of LTP [164]. The beneficial effects of the partial NMDAR antagonist memantine in AD also support the possible role of metaplasticity in AD-associated synaptic dysfunction because memantine does not block LTP acutely but restores LTP induction impaired by tonic NMDAR activation [165, 166].
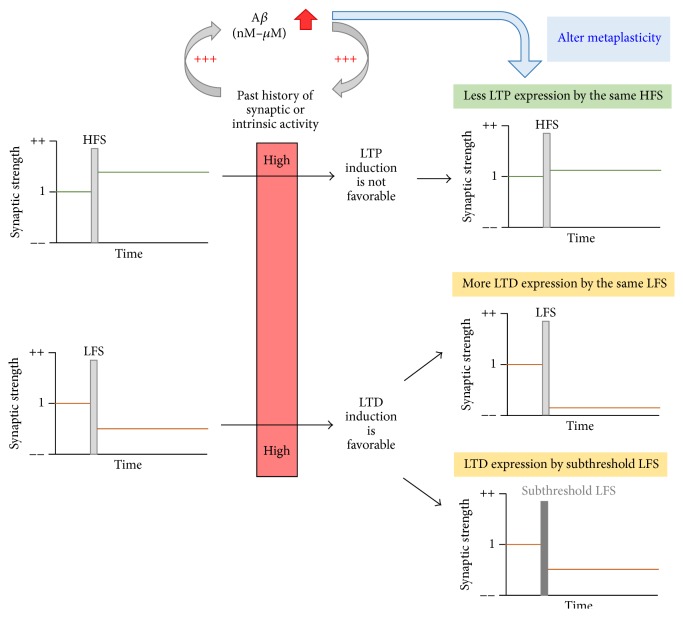
A recent study by Megill et al. has provided direct evidence for impaired metaplasticity in an AD transgenic mouse model [27]. This study examined frequency- and age-dependent synaptic plasticity in the APP/PS1 AD mouse model [27], which has two FAD-linked mutations (a Swedish mutation in APP and a deletion FAD mutation in exon 9 of presenilin-1) [167]. These mutations increase total Aβ production, resulting in a higher level of aggregation-prone Aβ42 peptides, and accelerated AD pathology [168] and age-dependent cognitive deficits [169, 170]. While the wild-type mice show a shift of the induction threshold to favor LTP and suppress LTD at the hippocampal CA1 Schaffer collateral synapses with age, the APP/PS1 transgenic mice fail to undergo this normal developmental metaplasticity [27]. As a result, the magnitudes of LTP and LTD remained the same in the APP/PS1 transgenic mice from when they were young (1 month of age) until they were adult (6 months of age). When the absolute magnitudes of LTP and LTD were compared, the adult APP/PS1 mice display LTP inhibition and LTD facilitation compared to age-matched wild-type mice [27]. Although electrophysiological characterization of other AD mouse models with elevated Aβ levels should be performed to see if impaired developmental metaplasticity is a general phenomenon for AD, these findings suggest that the Hebbian synaptic plasticity defects in AD could be due to the inability of neurons to undergo developmental metaplasticity (Figure 2).
Figure 2.
Aberrant metaplasticity in AD. Aβ increases the activity of excitatory neurons, which in turn stimulates synthesis and release of Aβ in a positive feedback loop, leading to pathologic accumulation of Aβ. Neuronal hyperexcitability or early activation of GluN2B-containing NMDAR by heightened Aβ expression induces aberrant metaplasticity, leading to inhibition of LTP by HFS and enhancement of LTD in the hippocampus by LFS or normal LTD induction by subthreshold LFS.
4. Putative Mechanisms Underlying Defective Homeostatic Synaptic Plasticity in AD
How can pathologic levels of soluble Aβ oligomers cause aberrant metaplasticity in AD? One way to mediate metaplasticity is to alter the induction mechanisms of LTP and LTD by regulating the function of NMDARs [10, 171–174] because calcium (Ca2+) influx through NMDARs at the postsynaptic density (PSD) is critical for inductions of NMDAR-dependent LTP and LTD [175–177]. An effective means to alter Ca2+ current per unit charge through NMDAR is to change subunit composition of NMDAR [177]. Such a change influences Ca2+/calmodulin-dependent protein kinase II (CaMKII) interaction with NMDARs and has been shown to control Hebbian synaptic plasticity [178]. For example, GluN2B-containing NMDARs bind to CaMKII with high affinity whereas those containing GluN2A interact with CaMKII with low affinity. Consistent with their decreased affinity for CaMKII, altering synaptic NMDARs from GluN2B-containing receptors to GluN2A-containing ones markedly reduces LTP induction [178]. Developmental metaplasticity in the visual cortex has also been suggested to involve experience-dependent changes in the GluN2 subunit composition of NMDARs that influence the induction thresholds of LTP and LTD [173, 179].
Soluble Aβ oligomers have been shown to decrease glutamate reuptake and subsequently increase extracellular glutamate levels [32, 180]. Such glutamate spillover would activate extracellular NMDARs, which are mostly composed of GluN2B-containing NMDARs at mature synapses [175, 176]. Indeed, soluble Aβ oligomers enhance activation of GluN2B-containing NMDARs more rapidly than synaptic depression and such actions would prime excitatory synapses to inhibit LTP induction and favor LTD induction [161]. However, NMDAR subunit composition and current are similar between wild-type mice and APP/PS1 mice at all ages [27], suggesting that developmental metaplasticity defect in the APP/PS1 mice is not due to altered NMDAR function during the induction of LTP and LTD.
Another way to induce metaplasticity is to alter the expression mechanisms of LTP and LTD by regulating α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) [181], which mediate the majority of excitatory synaptic current upon glutamate binding [182]. A major postsynaptic expression mechanism for LTP is the synaptic recruitment of AMPARs from a perisynaptic reserve pool and their subsequent stabilization at excitatory synapses, whereas that for LTD is the removal and internalization of synaptic AMPARs [175, 182]. Insertion and removal of synaptic AMPARs during the expression of LTP and LTD, respectively, are tightly regulated processes by phosphorylation of AMPAR subunit GluA1 at Ser-845 and Ser-831 [183–185]. Phosphorylation of GluA1 at Ser-845 by protein kinase A (PKA) is necessary for synaptic targeting of GluA1 driven by CaMKII [186], whereas dephosphorylation at Ser-845 mediates GluA1 internalization [183, 187, 188] and NMDAR-dependent LTD [185]. In addition, Ser845 phosphorylation of GluA1 mediates synaptic insertion of Ca2+-permeable GluA1-containing AMPARs during synaptic scaling in cultured dissociated cortical neurons upon chronic activity deprivation [92] and homeostatic synaptic scaling in the visual cortex upon sensory deprivation [189, 190]. Phosphorylation of GluA1 at Ser831 by protein kinase C (PKC) [191] and CaMKII [192, 193] increases following LTP induction [184, 194] and supports LTP expression [183–185]. Although GluA1 phosphorylation at Ser-845 and Ser-831 has been shown to reduce the induction threshold for LTP [195, 196], adult APP/PS1 mice display normal levels of GluA1 phosphorylation and perisynaptic AMPARs compared to those of wild-type mice [27]. Hence, the developmental metaplasticity defect in APP/PS1 mice is not due to insufficient AMPAR availability for synaptic insertion; rather, it is due to regulation of AMPAR trafficking by means other than GluA1 phosphorylation.
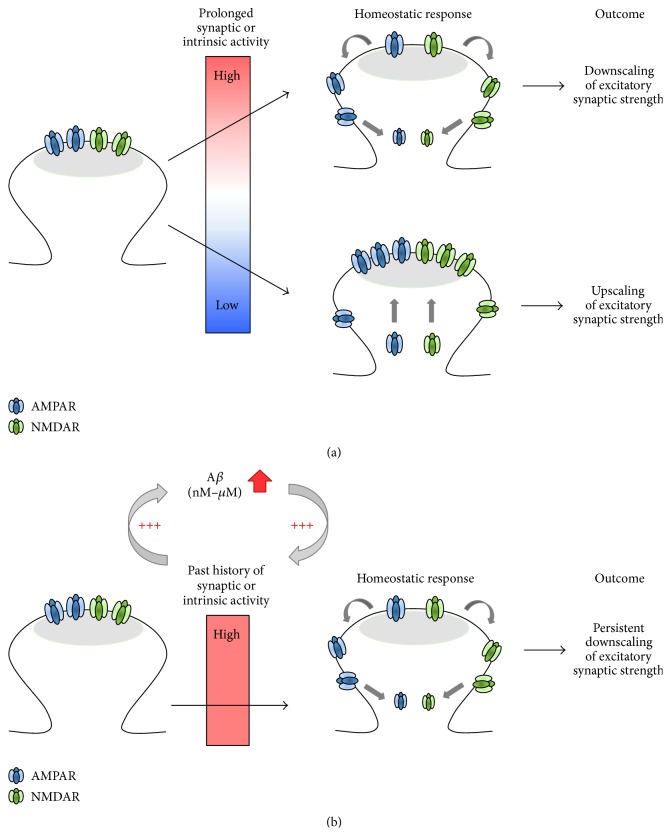
Metaplasticity is a form of homeostatic synaptic plasticity in which the magnitude and polarity of synaptic plasticity are adjusted accordingly based on the past history of synaptic and neural activity [38]. Since metaplasticity can occur at a single synapse [197, 198], it is tempting to speculate that pathologic levels of Aβ may impair developmental metaplasticity by altering postsynaptic expression mechanisms of homeostatic synaptic plasticity (Figure 3). Homeostatic synaptic plasticity has been extensively investigated using primary dissociated culture of neocortical and hippocampal neurons (Table 1). In these studies, prolonged blockade of network activity for 48 hours (h) with the sodium channel blocker tetrodotoxin (TTX) induces a significant increase in AMPAR-mediated miniature excitatory postsynaptic current (mEPSC) amplitude and synaptic AMPAR density, indicating the postsynaptic expression of homeostatic synaptic scaling [78, 80, 94, 138, 199–201]. Conversely, mEPSC amplitudes are scaled down in dissociated neuronal culture after prolonged enhancement of network activity by KCl depolarization or blocking inhibitory neurotransmission with antagonists for A-type gamma-aminobutyric acid (GABAA) receptors, such as bicuculline [78, 80, 90, 199, 202, 203]. Interestingly, many of the crucial mediators of homeostatic synaptic plasticity have also been implicated in AMPAR regulation during LTP and LTD expression and AD pathology (Table 1). Taken together, these correlated functional roles raise an intriguing possibility that pathologic accumulation of Aβ may impair molecular mechanisms involved in homeostatic synaptic plasticity, which manifests as disruption of Hebbian synaptic plasticity in AD (Figure 3).
Figure 3.
Postsynaptic expression mechanisms in normal and AD synapses. (a) In normal synapses, chronic activity blockade leads to synaptic scaling expressed by a compensatory increase in synaptic AMPAR density and current, whereas chronic activity elevation leads to synaptic downscaling expressed by a compensatory decrease in synaptic AMPAR density and current. (b) In AD, Aβ increases neuronal excitability and/or synaptic activity, leading to induction of synaptic downscaling. Because pathologic feedback loop continues to produce and release Aβ, synaptic downscaling becomes persistent and opposes the postsynaptic expression mechanisms for LTP.
Table 1.
Molecular mechanisms and players involved in AD and the expression of homeostatic synaptic plasticity.
| Roles in synaptic scaling | Involvement in AD | References | |
|---|---|---|---|
| AMPAR scaffolding proteins | |||
| GRIP1 | Synaptic accumulation and removal of GRIP1 mediate synaptic scaling and downscaling, respectively, by regulating synaptic AMPAR targeting. | [76, 77] | |
| PICK1 | PICK1 degradation mediates synaptic scaling. | PICK1 interaction with GluA2 mediates Aβ-induced synaptic depression. | [78, 79] |
|
| |||
| Regulators of AMPAR trafficking | |||
| Arc/Arg3.1 | Downregulation of Arc/Arg3.1 mediates synaptic scaling by increasing surface AMPAR density. Upregulation of Arc/Arg3.1 mediates synaptic downscaling by promoting AMPAR endocytosis. | Arc/Arg3.1 expression is elevated in AD and mediates activity-dependent generation of Aβ by binding to presinilin-1 and regulating γ-secretase trafficking. | [80–82] |
| Homer1a | Downregulation of Homer1a mediates synaptic scaling, whereas upregulation of Homer1a mediates synaptic downscaling by regulating surface AMPAR density and Tyr-phosphorylation. | [83] | |
|
| |||
| Regulators of synaptic AMPAR density | |||
| PSD-95 | Synaptic accumulation of PSD-95 mediates synaptic scaling, whereas its interaction with TARP mediates synaptic downscaling. | Pathological level of Aβ leads to PSD-95 degradation. | [84–89] |
| PSD-93 | PSD-93 mediates synapticscaling. | [84] | |
| GKAP | Synaptic accumulation and removal of GKAP mediate synaptic scaling and downscaling, respectively, by regulating surface AMPAR density. | Pathological level of Aβ leads to GKAP degradation. | [90, 91] |
|
| |||
| Posttranslation modification of AMPAR | |||
| Calcineurin | Reduced calcineurin activity mediates synaptic scaling via GluA1-Ser845 dephosphorylation and subsequent synaptic trafficking of Ca2+-permeable AMPARs. | In AD mouse model, increased activity of calcineurin induces dephosphorylation and synaptic removal of the GluR1 subunit of AMPAR. | [92, 93] |
| STEP61 | Downregulation of STEP61 mediates synaptic scaling, whereas enhanced STEP61 upon chronic activity induces dephosphorylation of GluN2B and GluA2. | STEP61 expression is elevated in AD and mediates Aβ-induced dephosphorylation and internalization of NMDARs and AMPARs, whereas inhibition of STEP61 prevents cognitive deficits and impaired hippocampal LTP in AD mouse models. | [94–98] |
| PP1 | Downregulation of PP1 inhibitor-2 (I-2) mediates synaptic downscaling by reducing surface AMPARs. | Inhibition of PP1 blocks Aβ-induced impairment in hippocampal LTP. | [99, 100] |
| DHHC2 | Translocation of DHHC2 to PSD mediates synaptic scaling by enhancing synaptic targeting of PSD95 and AMPAR. | [86] | |
| Nedd4-1 | Upregulation of Nedd4-1 mediates synaptic downscaling by reducing surface AMPAR density. | Nedd4-1 expression is elevated in AD. | [101, 102] |
| SUMO-1 and Ubc9 | SUMOylation of Arc/Arg3.1 mediates synaptic scaling. | SUMO-conjugating enzyme, Ubc9, enhances SUMOylation and rescues Aβ-induced deficits in hippocampal LTP and learning and memory. | [103, 104] |
|
| |||
| Local dendritic translation of AMPAR | |||
| eEF2 | Increased eEF2 activity mediates synaptic scaling by stimulating local dendritic synthesis. | [105] | |
| miRNA-92a | Inhibition of miRNA-92A mediates synaptic scaling by stimulating local dendritic synthesis of GluA1. | [106] | |
| Retinoic acid (RA) | Increased RA activity mediates synaptic scaling by stimulating local dendritic synthesis of GluA1 through RA receptor. | RA regulates the expression of APP processing genes, attenuates Aβ deposition, and rescues memory deficits in AD mouse model. | [107–114] |
|
| |||
| Secreted factors | |||
| BDNF | Downregulation of BDNF mediates synaptic scaling. | Downregulation of BDNF levels is associated with the degree of synaptic and cognitive deficits during the progression of AD. | [81, 115–117] |
| TNFα | TNFα mediates synaptic scaling in primary neuronal culture and visual cortex upon activity deprivation. | TNFα contributes to AD-related brain neuroinflammation and amyloidogenesis via β-secretase regulation. | [118–127] |
|
| |||
| Cell adhesion molecules | |||
| β3 integrin | Enhanced surface expression of β3 integrin inhibits the small GTPase Rap1 and mediates synaptic scaling by stabilizing synaptic. | [128, 129] | |
| MHC-1 | MHC-1 mediates TTX-induced synaptic scaling in hippocampal cultured neurons. | [130] | |
| N-Cadherin | N-Cadherin interaction with β-catenin mediates synaptic scaling and downscaling by regulating GluA1-containing AMPARs. | Inhibition of N-Cadherin interaction with β-catenin accelerates Aβ-induced synaptic impairments. | [131–135] |
| EphA4 | Increased Eph4 activity mediates synaptic downscaling by stimulating ubiquitin-dependent proteasome degradation of GluA1. | Soluble Aβ oligomers upregulate EphA4 whereas genetic ablation or inhibition of EphA4 prevents hippocampal LTP impairment in AD transgenic model mice. | [136, 137] |
|
| |||
| Transcriptional regulation | |||
| CaMKK-CaMK4 | Reduced activity of the CaMKK/CaMK4 signaling pathway mediates synaptic scaling, whereas its stimulation mediates synaptic downscaling. | [138–140] | |
| MSK1 | MSK1 mediates TTX-induced synaptic scaling in hippocampal neurons by increasing surface AMPAR density. | MSK1 activity is elevated in AD. | [141, 142] |
| MeCP2 | MeCP2 mediates synaptic scaling in visual cortex upon visual deprivation in vivo. | [143] | |
|
| |||
| Other proteins | |||
| Plk2 | Increase in Plk2 activity mediates synaptic downscaling. | [144, 145] | |
| Cdk5 | Increase in Cdk5 activity mediates synaptic downscaling. | Enhanced Cdk5 activity in AD contributes to Tau phosphorylation and toxicity. | [144, 146] |
4.1. AMPAR Scaffolding Proteins
Glutamate receptor-interacting protein 1 (GRIP1) and PICK1 (protein interacting with C-kinase 1) are PDZ (postsynaptic density 95/discs large/zona occludens) domain-containing proteins that regulate AMPAR trafficking by binding to the same intracellular C-terminus of GluA2 [204, 205]. GRIP1 binding to the unphosphorylated GluA2 C-terminus promotes synaptic targeting of AMPARs [206] whereas PICK1 can bind to both phosphorylated and unphosphorylated GluA2 [206] and mediates activity-dependent endocytosis of GluA2-containing AMPARs and stabilizes them in intracellular pools [207–209]. Recent studies have reported that chronic activity deprivation increases GRIP1 abundance at excitatory synapses and its interaction with GluA2, leading to synaptic targeting of AMPARs in cortical cultured neurons [76, 77]. In contrast, chronic enhancement of neuronal activity removes GRIP1 from excitatory synapses, which decreases surface AMPARs at synapses [76]. Compared to bidirectional modulation of synaptic GRIP1 expression in homeostatic synaptic plasticity, PICK1 expression is only altered by chronic activity blockade [78]. The TTX-induced synaptic scaling accompanies lysosome-mediated PICK1 degradation and can be occluded by genetic knock-out or shRNA knock-down of PICK1 [78]. Interestingly, pathologic levels of Aβ oligomers fail to reduce surface GluA2 expression and excitatory synaptic transmission in PICK knock-out neurons [79], indicating that GluA2 interaction with PICK1 mediates Aβ-induced synaptic depression. Thus, Aβ-dependent modulation of PICK1 and GRIP1 levels may likely contribute to aberrant developmental metaplasticity in AD.
AMPARs at excitatory synapses are also regulated by scaffolding proteins of the membrane associated guanylate kinase (MAGUK) family, which includes PSD-95, PSD-93, and SAP102 [210]. Chronic activity blockade increases synaptic accumulation of PSD95 and SAP102, whereas chronic activity enhancement decreases synaptic accumulation of PSD95 alone [84–86]. Double knock-down of PSD95/PSD93 or triple knock-down of PSD95/PSD93/SAP102 completely blocks chronic inactivity-induced increase in mEPSC amplitude [84], suggesting that PSD95 and PSD93 mediate synaptic scaling. In contrast, synaptic downscaling requires the PDZ1/2 domains of PSD-95 [84], which interact with transmembrane AMPAR regulatory proteins [TARPs] [211, 212]. Since TARPs link PSD-95 to AMPARs and promote synaptic insertion and stabilization of AMPARs [211, 212], these findings raise the possibility that reduced PSD95-TARP interaction may contribute to synaptic downscaling. Importantly, decreased PSD-95 expression is evident in AD mouse models [87] and Aβ application in cortical neuronal culture leads to downregulation of PSD-95 expression and dispersal of Shank1 [88, 89], another scaffolding protein enriched in excitatory glutamatergic synapses [213]. Interestingly, synaptic accumulation of guanylate kinase-associated protein (GKAP), which links Shank1 to PSD95 [214, 215], is increased upon chronic inhibition of neural activity and decreased by chronic excitation [90]. Such regulation of synaptic GKAP targeting contributes to bidirectional homeostatic scaling of excitatory synaptic strength [90]. Consistent with reports that pathological levels of Aβ increase degradation of PSD-95 and GKAP [89, 91], diminished interactions between PSD-95, TARP, and GKAP could dysregulate homeostatic synaptic plasticity in AD.
4.2. AMPAR Trafficking Regulators
Multiple proteins regulate synaptic AMPAR density by controlling their trafficking. One of them is Arc/Arg3.1, which is an immediate early gene. Arc/Arg3.1 mRNAs accumulate at excitatory synapses, where they are locally translated following synaptic simulation [216, 217]. Arc/Arg3.1 protein facilitates AMPAR internalization from the postsynaptic membrane by interacting with endocytosis mediators, endophilin2/3 and dynamin [218]. Chronic activity blockade of hippocampal or cortical cultured neurons has been shown to decrease mRNA and protein expression of Arc/Arg3.1 [80, 81]. Further, genetic ablation of Arc/Arg3.1 increases basal mEPSC amplitude and surface density of GluA1 and occludes TTX-induced increase in synaptic scaling [80]. Conversely, chronic elevation of neuronal activity increases Arc/Arg3.1 levels and decreases surface density of GluA1, whereas this regulation is absent in Arc/Arg3.1 knock-out neurons [80]. In addition to the critical roles of Arc/Arg3.1 in homeostatic synaptic plasticity, Arc/Arg3.1 expression is elevated in the medial prefrontal cortex of human AD patients [82], suggesting that elevated Arc/Arg3.1 expression may likely lead to AMPAR internalization during AD pathogenesis. In support of this notion, Arc/Arg3.1 is required for metabotropic glutamate receptor- (mGluR-) dependent LTD [219], a form of LTD that is also induced by application of Aβ oligomers [33]. Furthermore, Arc/Arg3.1 has been shown to mediate activity-dependent generation of Aβ by binding to presinilin-1 and regulating γ-secretase trafficking [82]. Based on these reports, persistent elevated Arc/Arg3.1 expression may act in multiple ways to disrupt synaptic homeostasis in AD by enhancing Aβ production and reducing synaptic AMPAR density.
Another immediate early gene, Homer1a, also contributes to homeostatic synaptic plasticity in Arc/Arg3.1-independent pathway [83]. Homer1a interrupts crosslinking action of constitutively expressed forms of Homer [220], thereby activating group I mGluRs in the absence of glutamate [221]. Chronic elevation of activity enhances Homer1a mRNA and protein expression, whereas chronic inactivity reduces Homer1a expression in cortical cultured neurons [83]. Importantly, mGluR inhibition or genetic ablation of Homer1a blocks bidirectional scaling of mEPSC amplitude and surface AMPAR density [83], implicating mGluR signaling and Homer1a in homeostatic synaptic plasticity. Interestingly, elevated tyrosine phosphorylation of GluA2 has been observed in Homer1a knock-out neurons [83] whereas tyrosine phosphorylation of GluA2 is decreased following group 1 mGluR stimulation through striatal enriched protein phosphatase (STEP61) [95]. Although the specific Tyr residues on GluA2 regulated by STEP61 are unknown, the downregulation of GluA2 tyrosine phosphorylation decreases surface expression of GluA2-containing AMPARs [222, 223]. Our recent study has demonstrated that chronic activity deprivation decreases protein and mRNA expression of STEP61 and increases Tyr-phosphorylation of its substrates, including the NMDAR subunit GluN2B and the AMPAR subunit GluA2 in hippocampal cultured neurons [94]. Increasing STEP61 activity blocks the increases in mEPSC amplitude and Tyr-phosphorylation of GluN2B and GluA2 induced by chronic activity blockade [94], suggesting that downregulation of STEP61 is crucial for mediating homeostatic synaptic scaling. Conversely, chronic activity enhancement increases STEP61 expression and decreases Tyr-phosphorylation of GluN2B and GluA2 [94]. Interestingly, elevated STEP61 expression is observed in cortices of human AD patients and causes dephosphorylation and internalization of AMPARs in AD mouse models [95–97]. Further, genetic ablation or pharmacologic inhibition of STEP61 prevents cognitive deficits and impaired hippocampal LTP in AD mouse models [96–98]. Given that STEP61 may also participate in metaplasticity [224], persistent elevation of STEP61 and Homer1a may disrupt developmental metaplasticity in AD.
In addition, alterations in Ca2+ influx modulates Ca2+-dependent activation of kinases such as Polo-like kinase 2 (Plk2) and Cyclin D kinase 5 (Cdk5) as well as protein phosphatases including calcineurin and protein phosphatase-1 (PP1) during homeostatic synaptic plasticity [93, 144, 145]. The increases in Plk2 and Cdk5 activity are thought to contribute to synaptic downscaling [144, 145] and AD pathogenesis [146]. Calcineurin-induced dephosphorylation of GluA1 at Ser845 has also been implicated in homeostatic synaptic plasticity [92] and AD [93]. Since PP1 activity downstream of calcineurin stimulation is required for LTD [225, 226], calcineurin may contribute to metaplasticity by regulating phosphorylation status of proteins which alters synaptic AMPAR density and function. In addition, Ca2+ influx through L-type voltage-gated Ca2+ channels (VGCCs) has been shown to increase PP1 activity via Ser43-phosphorylation of PP1 inhibitor-2 (I-2) following chronic activity elevation in hippocampal cultured neurons [99]. Furthermore, selective inhibition of PP1 blocks downscaling of surface AMPAR expression and mEPSC amplitude induced by chronic activity [99] as well as Aβ-induced impairment in hippocampal LTP [100], providing PP1 as another candidate signaling protein that may contribute to aberrant metaplasticity in AD.
4.3. Posttranslational Modification of AMPAR
Recent studies have revealed posttranslational modifications in addition to phosphorylation as important regulatory mechanisms of AMPAR expression during homeostatic synaptic plasticity. One such modification is palmitoylation, which mediates covalent attachment of palmitic acid [227]. The TTX-induced chronic silencing of network activity causes palmitoylation enzyme DHHC2 to be translocated from the dendrite to the postsynaptic density, resulting in homeostatic accumulation of PSD-95 and AMPARs at excitatory synapses [86]. Given that AMPAR trafficking is dynamically regulated by subunit-selective palmitoylation [228–230], these studies implicate palmitoylation of AMPAR subunits in the mechanism of synaptic scaling. Synaptic scaling also involves SUMOylation [103], which mediates covalent attachment of small ubiquitin-like modifiers (SUMO) [231]. Although there is no direct evidence for SUMOylation of AMPAR subunits [232], the TTX-induced elevation of surface AMPAR expression requires SUMOylation of Arc/Arg3.1 [103], a known regulator of AMPAR endocytosis [218]. Interestingly, reduced SUMOylation is observed in adult AD model mice [104]. While inhibition of SUMOylation blocks hippocampal LTP and hippocampal-dependent learning and memory in wild-type mice, the upregulation of SUMOylation by supplying its conjugating enzyme, Ubc9, rescues Aβ-induced deficits in hippocampal LTP and learning and memory [104]. Hence, reduced SUMOylation of Arc/Arg3.1 may contribute to defective developmental metaplasticity in AD.
Lastly, AMPARs are subjected to activity-dependent ubiquitination by the E3 ubiquitin ligase Nedd4-1, leading to their internalization and degradation in lysosomes [233–235]. Chronic elevation of neuronal activity increases Nedd4-1 protein levels, whereas shRNA-mediated knock-down of Nedd4-1 blocks the homeostatic reduction of surface AMPAR expression and mEPSC amplitudes induced by chronic activity [101], indicating that Nedd4-1 is required for homeostatic downscaling of excitatory synaptic strength. Given that elevated Nedd4-1 expression is found in human AD brain tissues [102], dysregulation of Nedd4-1 levels and subsequent impairment in homeostatic synaptic plasticity may play a role in AD etiology.
4.4. Regulation of Transcription and Translation
Ca2+ influx through NMDARs or L-type VGCCs activates signaling pathways that regulate transcriptions of genes important for neural development and plasticity. Consistent with this assertion, downscaling of excitatory synaptic strength induced by prolonged excitation of hippocampal CA1 neurons requires Ca2+ influx through L-type VGCCs and transcription activated downstream of CaMKK/CaMK4 signaling pathways [139]. TTX-induced synaptic scaling also requires transcription and translation; however, the mechanism involves a decrease in somatic Ca2+ influx through L-type VGCCs and subsequent reduction in CaMKK/CaMK4 signaling pathways [138, 140]. Recently, chronic inactivity was shown to increase transcription of genes encoding AMPARs and proteins that regulate AMPAR trafficking by decreasing cytosine methylation of genes [236]. Consistently, inhibition of DNA methylation alone induces synaptic scaling [236]. Furthermore, loss of methyl-CpG-binding protein-2 (MeCP2) prevents synaptic scaling in the visual cortex upon visual deprivation in vivo [143]. Taken together, these studies suggest that bidirectional homeostatic synaptic plasticity involves epigenetic modulation of genes whose protein products regulate excitatory synaptic transmission.
Our laboratory recently identified genes regulated by chronic alterations of neuronal activity in hippocampal neurons using unbiased gene expression analysis [81]. We identified several immediate early genes as well as genes associated with gene ontology terms “synaptic transmission” and “regulation of synaptic plasticity” [81]. One of the immediate early genes encodes brain-derived neurotrophic factor (BDNF) [81]. BDNF, which is secreted in an activity-dependent manner [237], regulates synaptic transmission and plasticity and promotes neuronal survival and transcription [238, 239]. We showed that BDNF mRNA expression is decreased in cultured hippocampal neurons upon chronic activity blockade using TTX treatment [81]. Importantly, inhibition of TrkB receptor signaling alone causes synaptic scaling in a similar extent as prolonged TTX treatment [115] whereas exogenous BDNF application prevents TTX-induced synaptic scaling [115] presumably through activation of mitogen- and stress-activated kinase 1 (MSK1) [141]. Interestingly, downregulation of BDNF is associated with the degree of synaptic and cognitive deficits during AD progression [116, 117, 240] and MSK1 activity is also elevated in AD [142]. These studies raise a possibility that aberrant BDNF-TrkB-MSK1 signaling pathway may disrupt synaptic homeostasis in AD.
In addition to the importance of transcriptional regulation, dendritic protein synthesis may serve as a mechanism to locally maintain the stability of synaptic strength. Chronic silencing of excitatory synaptic inputs stimulates dendritic protein synthesis by increasing the activity of eukaryotic elongation factor-2 (eEF2) [105]. Furthermore, simultaneous treatment of hippocampal neurons with TTX (to block action potentials) and APV (to block NMDAR-mediated miniature synaptic transmission) increases the expression of GluA1 homomers by stimulating local dendritic translation of GluA1 mRNAs [107, 241–243]. This synaptic scaling is mediated by microRNA-92a, which is a small noncoding RNA that inhibits translation of GluA1 mRNAs by binding to their 3′ untranslated region (UTR) [106]. Other studies have also reported that retinoic acid (RA) signaling via RA receptor-α (RARα) interaction with the 5′ UTR of GluA1 mRNA contributes to synaptic scaling following prolonged cotreatment with TTX and APV by stimulating local dendritic synthesis of GluA1 [107–109]. Importantly, RAR signaling has been shown to regulate the expression of genes related to APP processing [110–113], attenuate Aβ deposition, and rescue memory deficits in AD mouse models [114], suggesting that alteration of RA signaling pathways may contribute to impaired metaplasticity in AD.
4.5. Cell Adhesion Molecules (CAMs)
β3 integrin is a cell adhesion molecule (CAM) enriched in excitatory synapses [244, 245] and controls synaptic currents mediated by GluA2-containing AMPARs [128]. Synaptic scaling induced by chronic activity blockade is associated with enhanced surface expression of β3 integrin in hippocampal neurons and is absent in β3 integrin knock-out neurons [128, 129]. Pharmacological perturbation of β3 integrin enhances GluA2 internalization and reduces synaptic AMPAR currents by activating the small GTPase Rap1 [128], which has been implicated in homeostatic downscaling of excitatory synapses [144, 246]. In addition to β3 integrin, class I major histocompatibility complex (MHC-1) proteins, which are found postsynaptically at excitatory synapses, also contribute to synaptic scaling following chronic activity blockade [130]. Although the role of β3 integrin and MHC-1 in AD pathogenesis remains unknown, their neuronal expression is regulated by glia-derived tumor necrosis factor α (TNFα) [128, 247, 248], which is involved in AD pathology in humans and AD mouse models [118–124]. TNFα elevates AMPAR-mediated mEPSC amplitude through activation of TNFα receptor during synaptic scaling [125, 126] whereas TNFα knock-out mice lack synaptic scaling in their visual cortex [127] but display normal LTP [127, 249, 250]. Hence, TNFα may influence metaplasticity through β3 integrin and MHC-1, and such a signaling pathway may be disrupted in AD.
N-Cadherin is another CAM that is enriched at excitatory synapses and has been implicated in AD as well as homeostatic synaptic plasticity. N-Cadherin promotes APP dimerization, modulates Aβ secretion, and reduces surface expression of presinilin-1 [131, 132]. N-Cadherin also binds to the extracellular domains of GluA1 in a Ca2+-dependent manner and regulates GluA1 surface expression [251, 252]. Although N-Cadherin interaction with the actin cytoskeleton [133, 253] contributes to dendritic spine enlargement during LTP expression [253–257], the interaction between N-Cadherin and β-catenin mediates bidirectional homeostatic synaptic plasticity by regulating GluA1-containing AMPARs [133, 134]. Given that inhibition of N-Cadherin interaction with β-catenin accelerates Aβ-induced synaptic impairments [135], dysregulation of N-Cadherin may likely impair homeostatic synaptic plasticity in AD. Ephrin receptor tyrosine kinase subfamily EphA4 is another CAM implicated in homeostatic synaptic plasticity. Increased activity of EphA4 mediates homeostatic downscaling by stimulating ubiquitin-dependent proteasome degradation of GluA1 [136]. Interestingly, soluble Aβ oligomers induce EphA4 activation, whereas genetic ablation or inhibition of EphA4 prevents hippocampal LTP impairment in AD transgenic model mice [137], raising an interesting possibility that Aβ-induced enhancement in EphA4 activity may impair metaplasticity in AD by regulating AMPAR degradation.
5. Conclusions
Recent studies have uncovered an exciting link between pathologic accumulation of Aβ and aberrant metaplasticity, a form of homeostatic synaptic plasticity that controls the induction threshold for LTP and LTD. Specifically, these studies have suggested a novel hypothesis that aberrant metaplasticity may contribute to LTP inhibition and LTD enhancement in AD. However, the molecular mechanism underlying Aβ-dependent alteration of metaplasticity remains largely unknown. Since many molecular players involved in homeostatic synaptic plasticity have been shown to regulate synaptic AMPAR density in Hebbian synaptic plasticity, it is tempting to speculate that pathologic levels of Aβ mediate their effect via a common mechanism shared between Hebbian and homeostatic plasticity at excitatory synapses. Challenges lie ahead in understanding how the molecular players and pathways reviewed here work together to express homeostatic plasticity at excitatory synapses and how Aβ disrupts homeostatic synaptic plasticity in AD. Future studies designed to tackle these challenges should offer substantial insights into the homeostatic control of excitatory synaptic strength in normal brain and AD brain. These studies may also facilitate the search for targeted therapeutic interventions to correct aberrant metaplasticity in AD, thus reversing persistent synaptic weakening and cognitive dysfunction in AD.
Acknowledgments
The authors thank Dr. Eric Bolton at the University of Illinois at Urbana Champaign for his insightful comments on the paper. This work is supported by ICR start-up funding from the University of Illinois at Urbana Champaign and National Institute of Health funding (NS083402) to Hee Jung Chung.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Hebb D. O. The Organization of Behavior: A Neuropsychological Theory. New York, NY, USA: John Wiley & Sons; 1949. [Google Scholar]
- 2.Bliss T. V. P., Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. The Journal of Physiology. 1973;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitlock J. R., Heynen A. J., Shuler M. G., Bear M. F. Learning induces long-term potentiation in the hippocampus. Science. 2006;313(5790):1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- 4.Lynch M. A. Long-term potentiation and memory. Physiological Reviews. 2004;84(1):87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- 5.Malenka R. C., Bear M. F. LTP and LTD: an embarrassment of riches. Neuron. 2004;44(1):5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Neves G., Cooke S. F., Bliss T. V. P. Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nature Reviews Neuroscience. 2008;9(1):65–75. doi: 10.1038/nrn2303. [DOI] [PubMed] [Google Scholar]
- 7.Feldman D. E. Synaptic mechanisms for plasticity in neocortex. Annual Review of Neuroscience. 2009;32:33–55. doi: 10.1146/annurev.neuro.051508.135516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abbott L. F., Nelson S. B. Synaptic plasticity: taming the beast. Nature Neuroscience. 2000;3:1178–1183. doi: 10.1038/81453. [DOI] [PubMed] [Google Scholar]
- 9.Turrigiano G. G., Nelson S. B. Hebb and homeostasis in neuronal plasticity. Current Opinion in Neurobiology. 2000;10(3):358–364. doi: 10.1016/S0959-4388(00)00091-X. [DOI] [PubMed] [Google Scholar]
- 10.Cooper L. N., Bear M. F. The BCM theory of synapse modification at 30: interaction of theory with experiment. Nature Reviews Neuroscience. 2012;13(11):798–810. doi: 10.1038/nrn3353. [DOI] [PubMed] [Google Scholar]
- 11.Marder E., Prinz A. A. Current compensation in neuronal homeostasis. Neuron. 2003;37(1):2–4. doi: 10.1016/S0896-6273(02)01173-X. [DOI] [PubMed] [Google Scholar]
- 12.Davis G. W. Homeostatic control of neural activity: from phenomenology to molecular design. Annual Review of Neuroscience. 2006;29:307–323. doi: 10.1146/annurev.neuro.28.061604.135751. [DOI] [PubMed] [Google Scholar]
- 13.Turrigiano G. G. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135(3):422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pozo K., Goda Y. Unraveling mechanisms of homeostatic synaptic plasticity. Neuron. 2010;66(3):337–351. doi: 10.1016/j.neuron.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turrigiano G. Homeostatic synaptic plasticity: local and global mechanisms for stabilizing neuronal function. Cold Spring Harbor Perspectives in Biology. 2012;4(1) doi: 10.1101/cshperspect.a005736.a005736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKhann G., Drachman D., Folstein M. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 17.Chapman P. F., White G. L., Jones M. W., et al. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nature Neuroscience. 1999;2(3):271–276. doi: 10.1038/6374. [DOI] [PubMed] [Google Scholar]
- 18.Fitzjohn S. M., Morton R. A., Kuenzi F., et al. Age-related impairment of synaptic transmission but normal long-term potentiation in transgenic mice that overexpress the human APP695SWE mutant form of amyloid precursor protein. The Journal of Neuroscience. 2001;21(13):4691–4698. doi: 10.1523/JNEUROSCI.21-13-04691.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobsen J. S., Wu C.-C., Redwine J. M., et al. Early-onset behavioral and synaptic deficits in a mouse model of Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(13):5161–5166. doi: 10.1073/pnas.0600948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsia A. Y., Masliah E., McConlogue L., et al. Plaque-independent disruption of neural circuits in Alzheimer's disease mouse models. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(6):3228–3233. doi: 10.1073/pnas.96.6.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palop J. J., Chin J., Roberson E. D., et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron. 2007;55(5):697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larson J., Lynch G., Games D., Seubert P. Alterations in synaptic transmission and long-term potentiation in hippocampal slices from young and aged PDAPP mice. Brain Research. 1999;840(1-2):23–35. doi: 10.1016/s0006-8993(99)01698-4. [DOI] [PubMed] [Google Scholar]
- 23.Roder S., Danober L., Pozza M. F., Lingenhoehl K., Wiederhold K.-H., Olpe H.-R. Electrophysiological studies on the hippocampus and prefrontal cortex assessing the effects of amyloidosis in amyloid precursor protein 23 transgenic mice. Neuroscience. 2003;120(3):705–720. doi: 10.1016/S0306-4522(03)00381-6. [DOI] [PubMed] [Google Scholar]
- 24.Dewachter I., Ris L., Croes S., et al. Modulation of synaptic plasticity and Tau phosphorylation by wild-type and mutant presenilin1. Neurobiology of Aging. 2008;29(5):639–652. doi: 10.1016/j.neurobiolaging.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 25.Gureviciene I., Ikonen S., Gurevicius K., et al. Normal induction but accelerated decay of LTP in APP + PS1 transgenic mice. Neurobiology of Disease. 2004;15(2):188–195. doi: 10.1016/j.nbd.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Trinchese F., Liu S., Battaglia F., Walter S., Mathews P. M., Arancio O. Progressive age-related development of Alzheimer-like pathology in APP/PS1 mice. Annals of Neurology. 2004;55(6):801–814. doi: 10.1002/ana.20101. [DOI] [PubMed] [Google Scholar]
- 27.Megill A., Tran T., Eldred K., et al. Defective age-dependent metaplasticity in a mouse model of Alzheimer's disease. The Journal of Neuroscience. 2015;35(32):11346–11357. doi: 10.1523/jneurosci.5289-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puzzo D., Privitera L., Leznik E., et al. Picomolar amyloid-β positively modulates synaptic plasticity and memory in hippocampus. The Journal of Neuroscience. 2008;28(53):14537–14545. doi: 10.1523/jneurosci.2692-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puzzo D., Privitera L., Palmeri A. Hormetic effect of amyloid-beta peptide in synaptic plasticity and memory. Neurobiology of Aging. 2012;33(7):1484–e24. doi: 10.1016/j.neurobiolaging.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 30.Hsieh H., Boehm J., Sato C., et al. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52(5):831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shankar G. M., Bloodgood B. L., Townsend M., Walsh D. M., Selkoe D. J., Sabatini B. L. Natural oligomers of the Alzheimer amyloid-β protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. The Journal of Neuroscience. 2007;27(11):2866–2875. doi: 10.1523/jneurosci.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li S., Hong S., Shepardson N. E., Walsh D. M., Shankar G. M., Selkoe D. Soluble oligomers of amyloid β protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62(6):788–801. doi: 10.1016/j.neuron.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shankar G. M., Li S., Mehta T. H., et al. Amyloid-β protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nature Medicine. 2008;14(8):837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shankar G. M., Walsh D. M. Alzheimer's disease: synaptic dysfunction and Aβ . Molecular Neurodegeneration. 2009;4, article 48 doi: 10.1186/1750-1326-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parihar M. S., Brewer G. J. Amyloid-β as a modulator of synaptic plasticity. Journal of Alzheimer's Disease. 2010;22(3):741–763. doi: 10.3233/jad-2010-101020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klyubin I., Cullen W. K., Hu N.-W., Rowan M. J. Alzheimer's disease Aβ assemblies mediating rapid disruption of synaptic plasticity and memory. Molecular Brain. 2012;5(1, article 25) doi: 10.1186/1756-6606-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zorumski C. F., Izumi Y. NMDA receptors and metaplasticity: mechanisms and possible roles in neuropsychiatric disorders. Neuroscience and Biobehavioral Reviews. 2012;36(3):989–1000. doi: 10.1016/j.neubiorev.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abraham W. C. Metaplasticity: tuning synapses and networks for plasticity. Nature Reviews Neuroscience. 2008;9(5):387–399. doi: 10.1038/nrn2356. [DOI] [PubMed] [Google Scholar]
- 39.Palop J. J., Mucke L. Amyloid-beta-induced neuronal dysfunction in Alzheimer's disease: from synapses toward neural networks. Nature Neuroscience. 2010;13(7):812–818. doi: 10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palop J. J., Mucke L. Synaptic depression and aberrant excitatory network activity in Alzheimer's disease: two faces of the same coin? NeuroMolecular Medicine. 2010;12(1):48–55. doi: 10.1007/s12017-009-8097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Busche M. A., Chen X., Henning H. A., et al. Critical role of soluble amyloid-β for early hippocampal hyperactivity in a mouse model of Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(22):8740–8745. doi: 10.1073/pnas.1206171109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lalonde R., Fukuchi K.-I., Strazielle C. Neurologic and motor dysfunctions in APP transgenic mice. Reviews in the Neurosciences. 2012;23(4):363–379. doi: 10.1515/revneuro-2012-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jankowsky J. L., Slunt H. H., Gonzales V., et al. Persistent amyloidosis following suppression of Aβ production in a transgenic model of Alzheimer disease. PLoS Medicine. 2005;2(12, article e355) doi: 10.1371/journal.pmed.0020355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vogt D. L., Thomas D., Galvan V., Bredesen D. E., Lamb B. T., Pimplikar S. W. Abnormal neuronal networks and seizure susceptibility in mice overexpressing the APP intracellular domain. Neurobiology of Aging. 2011;32(9):1725–1729. doi: 10.1016/j.neurobiolaging.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amatniek J. C., Hauser W. A., DelCastillo-Castaneda C., et al. Incidence and predictors of seizures in patients with Alzheimer's disease. Epilepsia. 2006;47(5):867–872. doi: 10.1111/j.1528-1167.2006.00554.x. [DOI] [PubMed] [Google Scholar]
- 46.Cirrito J. R., Yamada K. A., Finn M. B., et al. Synaptic activity regulates interstitial fluid amyloid-β levels in vivo. Neuron. 2005;48(6):913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 47.Cirrito J. R., Kang J.-E., Lee J., et al. Endocytosis is required for synaptic activity-dependent release of amyloid-β in vivo. Neuron. 2008;58(1):42–51. doi: 10.1016/j.neuron.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kamenetz F., Tomita T., Hsieh H., et al. APP processing and synaptic function. Neuron. 2003;37(6):925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 49.Wilson R. S., Segawa E., Boyle P. A., Anagnos S. E., Hizel L. P., Bennett D. A. The natural history of cognitive decline in Alzheimer's disease. Psychology and Aging. 2012;27(4):1008–1017. doi: 10.1037/a0029857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blennow K., de Leon M. J., Zetterberg H. Alzheimer's disease. The Lancet. 2006;368(9533):387–403. doi: 10.1016/s0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 51.Hardy J., Selkoe D. J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 52.Tanzi R. E., Bertram L. Twenty years of the Alzheimer's disease amyloid hypothesis: a genetic perspective. Cell. 2005;120(4):545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 53.Roberson E. D., Scearce-Levie K., Palop J. J., et al. Reducing endogenous tau ameliorates amyloid β-induced deficits in an Alzheimer's disease mouse model. Science. 2007;316(5825):750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- 54.Roberson E. D., Halabisky B., Yoo J. W., et al. Amyloid-β/fyn-induced synaptic, network, and cognitive impairments depend on tau levels in multiple mouse models of Alzheimer's disease. The Journal of Neuroscience. 2011;31(2):700–711. doi: 10.1523/jneurosci.4152-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ittner L. M., Ke Y. D., Delerue F., et al. Dendritic function of tau mediates amyloid-β toxicity in Alzheimer's disease mouse models. Cell. 2010;142(3):387–397. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 56.Cleary J. P., Walsh D. M., Hofmeister J. J., et al. Natural oligomers of the amyloid-β protein specifically disrupt cognitive function. Nature Neuroscience. 2005;8(1):79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- 57.Nitta A., Itoh A., Hasegawa T., Nabeshima T. β-amyloid protein-induced Alzheimer's disease animal model. Neuroscience Letters. 1994;170(1):63–66. doi: 10.1016/0304-3940(94)90239-9. [DOI] [PubMed] [Google Scholar]
- 58.Maurice T., Lockhart B. P., Privat A. Amnesia induced in mice by centrally administered β-amyloid peptides involves cholinergic dysfunction. Brain Research. 1996;706(2):181–193. doi: 10.1016/0006-8993(95)01032-7. [DOI] [PubMed] [Google Scholar]
- 59.Cheng Y.-F., Wang C., Lin H.-B., et al. Inhibition of phosphodiesterase-4 reverses memory deficits produced by Aβ25-35 or Aβ1-40 peptide in rats. Psychopharmacology. 2010;212(2):181–191. doi: 10.1007/s00213-010-1943-3. [DOI] [PubMed] [Google Scholar]
- 60.Webster S. J., Bachstetter A. D., Nelson P. T., Schmitt F. A., Van Eldik L. J. Using mice to model Alzheimer's dementia: an overview of the clinical disease and the preclinical behavioral changes in 10 mouse models. Frontiers in Genetics. 2014;5, artcile 88 doi: 10.3389/fgene.2014.00088.Article 88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Puzzo D., Lee L., Palmeri A., Calabrese G., Arancio O. Behavioral assays with mouse models of Alzheimer's disease: practical considerations and guidelines. Biochemical Pharmacology. 2014;88(4):450–467. doi: 10.1016/j.bcp.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.De Strooper B., Saftig P., Craessaerts K., et al. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature. 1998;391(6665):387–390. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- 63.Vassar R., Bennett B. D., Babu-Khan S., et al. β-Secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286(5440):735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 64.Edbauer D., Winkler E., Regula J. T., Pesold B., Steiner H., Haass C. Reconstitution of γ-secretase activity. Nature Cell Biology. 2003;5(5):486–488. doi: 10.1038/ncb960. [DOI] [PubMed] [Google Scholar]
- 65.Passer B., Pellegrini L., Russo C., et al. Generation of an apoptotic intracellular peptide by α-secretase cleavage of Alzheimer's amyloid β protein precursor. Journal of Alzheimer's Disease. 2000;2(3-4):289–301. doi: 10.3233/jad-2000-23-408. [DOI] [PubMed] [Google Scholar]
- 66.Corder E. H., Saunders A. M., Strittmatter W. J., et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 67.Slooter A. J. C., Cruts M., Kalmijn S., et al. Risk estimates of dementia by apolipoprotein E genotypes from a population-based incidence study: the Rotterdam study. Archives of Neurology. 1998;55(7):964–968. doi: 10.1001/archneur.55.7.964. [DOI] [PubMed] [Google Scholar]
- 68.Castellano J. M., Kim J., Stewart F. R., et al. Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Science Translational Medicine. 2011;3(89) doi: 10.1126/scitranslmed.3002156.89ra57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou Q., Homma K. J., Poo M.-M. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 2004;44(5):749–757. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 70.Bastrikova N., Gardner G. A., Reece J. M., Jeromin A., Dudek S. M. Synapse elimination accompanies functional plasticity in hippocampal neurons. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(8):3123–3127. doi: 10.1073/pnas.0800027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lamprecht R., LeDoux J. Structural plasticity and memory. Nature Reviews Neuroscience. 2004;5(1):45–54. doi: 10.1038/nrn1301. [DOI] [PubMed] [Google Scholar]
- 72.Masliah E., Mallory M., Alford M., et al. Altered expression of synaptic proteins occurs early during progression of Alzheimer's disease. Neurology. 2001;56(1):127–129. doi: 10.1212/wnl.56.1.127. [DOI] [PubMed] [Google Scholar]
- 73.Scheff S. W., Price D. A., Schmitt F. A., Dekosky S. T., Mufson E. J. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology. 2007;68(18):1501–1508. doi: 10.1212/01.wnl.0000260698.46517.8f. [DOI] [PubMed] [Google Scholar]
- 74.Scheff S. W., Price D. A. Alzheimer's disease-related alterations in synaptic density: neocortex and hippocampus. Journal of Alzheimer's Disease. 2006;9(supplement 3):101–115. doi: 10.3233/jad-2006-9s312. [DOI] [PubMed] [Google Scholar]
- 75.Scheff S. W., Price D. A., Schmitt F. A., Mufson E. J. Hippocampal synaptic loss in early Alzheimer's disease and mild cognitive impairment. Neurobiology of Aging. 2006;27(10):1372–1384. doi: 10.1016/j.neurobiolaging.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 76.Tan H. L., Queenan B. N., Huganir R. L. GRIP1 is required for homeostatic regulation of AMPAR trafficking. Proceedings of the National Academy of Sciences. 2015;112(32):10026–10031. doi: 10.1073/pnas.1512786112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gainey M. A., Tatavarty V., Nahmani M., Lin H., Turrigiano G. G. Activity-dependent synaptic GRIP1 accumulation drives synaptic scaling up in response to action potential blockade. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(27):E3590–E3599. doi: 10.1073/pnas.1510754112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Anggono V., Clem R. L., Huganir R. L. PICK1 loss of function occludes homeostatic synaptic scaling. The Journal of Neuroscience. 2011;31(6):2188–2196. doi: 10.1523/jneurosci.5633-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alfonso S., Kessels H. W., Banos C. C., et al. Synapto-depressive effects of amyloid beta require PICK1. The European Journal of Neuroscience. 2014;39(7):1225–1233. doi: 10.1111/ejn.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shepherd J. D., Rumbaugh G., Wu J., et al. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron. 2006;52(3):475–484. doi: 10.1016/j.neuron.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee K., Royston S. E., Vest M. O., et al. N-methyl-D-aspartate receptors mediate activity-dependent down-regulation of potassium channel genes during the expression of homeostatic intrinsic plasticity. Molecular Brain. 2015;8, article 4 doi: 10.1186/s13041-015-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu J., Petralia R. S., Kurushima H., et al. Arc/Arg3.1 regulates an endosomal pathway essential for activity-dependent beta-amyloid generation. Cell. 2011;147(3):615–628. doi: 10.1016/j.cell.2011.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hu J.-H., Park J. M., Park S., et al. Homeostatic scaling requires group I mGluR activation mediated by Homer1a. Neuron. 2010;68(6):1128–1142. doi: 10.1016/j.neuron.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sun Q., Turrigiano G. G. PSD-95 and PSD-93 play critical but distinct roles in synaptic scaling up and down. The Journal of Neuroscience. 2011;31(18):6800–6808. doi: 10.1523/jneurosci.5616-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim M. J., Futai K., Jo J., Hayashi Y., Cho K., Sheng M. Synaptic accumulation of PSD-95 and synaptic function regulated by phosphorylation of serine-295 of PSD-95. Neuron. 2007;56(3):488–502. doi: 10.1016/j.neuron.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 86.Noritake J., Fukata Y., Iwanaga T., et al. Mobile DHHC palmitoylating enzyme mediates activity-sensitive synaptic targeting of PSD-95. The Journal of Cell Biology. 2009;186(1):147–160. doi: 10.1083/jcb.200903101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Simon A.-M., Schiapparelli L., Salazar-Colocho P., et al. Overexpression of wild-type human APP in mice causes cognitive deficits and pathological features unrelated to Aβ levels. Neurobiology of Disease. 2009;33(3):369–378. doi: 10.1016/j.nbd.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 88.Roselli F., Tirard M., Lu J., et al. Soluble β-amyloid1-40 induces NMDA-dependent degradation of postsynaptic density-95 at glutamatergic synapses. The Journal of Neuroscience. 2005;25(48):11061–11070. doi: 10.1523/jneurosci.3034-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roselli F., Hutzler P., Wegerich Y., Livrea P., Almeida O. F. X. Disassembly of shank and homer synaptic clusters is driven by soluble beta-amyloid1–40 through divergent NMDAR-dependent signalling pathways. PLoS ONE. 2009;4(6) doi: 10.1371/journal.pone.0006011.e6011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shin S. M., Zhang N., Hansen J., et al. GKAP orchestrates activity-dependent postsynaptic protein remodeling and homeostatic scaling. Nature Neuroscience. 2012;15(12):1655–1666. doi: 10.1038/nn.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Roselli F., Livrea P., Almeida O. F. X. CDK5 is essential for soluble amyloid β-induced degradation of GKAP and remodeling of the synaptic actin cytoskeleton. PLoS ONE. 2011;6(7) doi: 10.1371/journal.pone.0023097.e23097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim S., Ziff E. B. Calcineurin mediates synaptic scaling via synaptic trafficking of Ca2+-permeable AMPA receptors. PLoS Biology. 2014;12(7):1–15. doi: 10.1371/journal.pbio.1001900.e1001900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.D'Amelio M., Cavallucci V., Middei S., et al. Caspase-3 triggers early synaptic dysfunction in a mouse model of Alzheimer's disease. Nature Neuroscience. 2011;14(1):69–76. doi: 10.1038/nn.2709. [DOI] [PubMed] [Google Scholar]
- 94.Jang S. S., Royston S. E., Xu J., et al. Regulation of STEP61 and tyrosine-phosphorylation of NMDA and AMPA receptors during homeostatic synaptic plasticity. Molecular Brain. 2015;8(1, article 55) doi: 10.1186/s13041-015-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang Y., Venkitaramani D. V., Gladding C. M., et al. The tyrosine phosphatase STEP mediates AMPA receptor endocytosis after metabotropic glutamate receptor stimulation. The Journal of Neuroscience. 2008;28(42):10561–10566. doi: 10.1523/jneurosci.2666-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kurup P., Zhang Y., Xu J., et al. Aβ-mediated NMDA receptor endocytosis in alzheimer's disease involves ubiquitination of the tyrosine phosphatase STEP61. The Journal of Neuroscience. 2010;30(17):5948–5957. doi: 10.1523/jneurosci.0157-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang Y., Kurup P., Xu J., et al. Genetic reduction of striatal-enriched tyrosine phosphatase (STEP) reverses cognitive and cellular deficits in an Alzheimer's disease mouse model. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(44):19014–19019. doi: 10.1073/pnas.1013543107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xu J., Chatterjee M., Baguley T. D., et al. Inhibitor of the tyrosine phosphatase STEP reverses cognitive deficits in a mouse model of Alzheimer's disease. PLoS Biology. 2014;12(8) doi: 10.1371/journal.pbio.1001923.e1001923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Siddoway B. A., Altimimi H. F., Hou H., et al. An essential role for inhibitor-2 regulation of protein phosphatase-1 in synaptic scaling. The Journal of Neuroscience. 2013;33(27):11206–11211. doi: 10.1523/jneurosci.5241-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Knobloch M., Farinelli M., Konietzko U., Nitsch R. M., Mansuy I. M. Aβ oligomer-mediated long-term potentiation impairment involves protein phosphatase 1-dependent mechanisms. The Journal of Neuroscience. 2007;27(29):7648–7653. doi: 10.1523/jneurosci.0395-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Scudder S. L., Goo M. S., Cartier A. E., et al. Synaptic strength is bidirectionally controlled by opposing activity-dependent regulation of Nedd4-1 and USP8. The Journal of Neuroscience. 2014;34(50):16637–16649. doi: 10.1523/jneurosci.2452-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kwak Y.-D., Wang B., Li J. J., et al. Upregulation of the E3 ligase NEDD4-1 by oxidative stress degrades IGF-1 receptor protein in neurodegeneration. The Journal of Neuroscience. 2012;32(32):10971–10981. doi: 10.1523/jneurosci.1836-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Craig T. J., Jaafari N., Petrovic M. M., Rubin P. P., Mellor J. R., Henley J. M. Homeostatic synaptic scaling is regulated by protein SUMOylation. The Journal of Biological Chemistry. 2012;287(27):22781–22788. doi: 10.1074/jbc.m112.356337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee L., Dale E., Staniszewski A., et al. Regulation of synaptic plasticity and cognition by SUMO in normal physiology and Alzheimer's disease. Scientific Reports. 2014;4, article 7190 doi: 10.1038/srep07190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sutton M. A., Taylor A. M., Ito H. T., Pham A., Schuman E. M. Postsynaptic decoding of neural activity: eEF2 as a biochemical sensor coupling miniature synaptic transmission to local protein synthesis. Neuron. 2007;55(4):648–661. doi: 10.1016/j.neuron.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 106.Letellier M., Elramah S., Mondin M., et al. MiR-92a regulates expression of synaptic GluA1-containing AMPA receptors during homeostatic scaling. Nature Neuroscience. 2014;17(8):1040–1042. doi: 10.1038/nn.3762. [DOI] [PubMed] [Google Scholar]
- 107.Aoto J., Nam C. I., Poon M. M., Ting P., Chen L. Synaptic signaling by all-trans retinoic acid in homeostatic synaptic plasticity. Neuron. 2008;60(2):308–320. doi: 10.1016/j.neuron.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Poon M. M., Chen L. Retinoic acid-gated sequence-specific translational control by RARα . Proceedings of the National Academy of Sciences of the United States of America. 2008;105(51):20303–20308. doi: 10.1073/pnas.0807740105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Maghsoodi B., Poon M. M., Nam C. I., Aoto J., Ting P., Chen L. Retinoic acid regulates RARα-mediated control of translation in dendritic RNA granules during homeostatic synaptic plasticity. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(41):16015–16020. doi: 10.1073/pnas.0804801105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lahiri D. K., Nall C. Promoter activity of the gene encoding the beta-amyloid precursor protein is up-regulated by growth factors, phorbol ester, retinoic acid and interleukin-1. Molecular Brain Research. 1995;32(2):233–240. doi: 10.1016/0169-328X(95)00078-7. [DOI] [PubMed] [Google Scholar]
- 111.Yang Y., Quitschke W. W., Brewer G. J. Upregulation of amyloid precursor protein gene promoter in rat primary hippocampal neurons by phorbol ester, IL-1 and retinoic acid, but not by reactive oxygen species. Molecular Brain Research. 1998;60(1):40–49. doi: 10.1016/S0169-328X(98)00164-8. [DOI] [PubMed] [Google Scholar]
- 112.Culvenor J. G., Evin G., Cooney M. A., et al. Presenilin 2 expression in neuronal cells: induction during differentiation of embryonic carcinoma cells. Experimental Cell Research. 2000;255(2):192–206. doi: 10.1006/excr.1999.4791. [DOI] [PubMed] [Google Scholar]
- 113.Satoh J.-I., Kuroda Y. Amyloid precursor protein β-secretase (BACE) mRNA expression in human neural cell lines following induction of neuronal differentiation and exposure to cytokines and growth factors. Neuropathology. 2000;20(4):289–296. doi: 10.1046/j.1440-1789.2000.00349.x. [DOI] [PubMed] [Google Scholar]
- 114.Ding Y., Qiao A., Wang Z., et al. Retinoic acid attenuates β-amyloid deposition and rescues memory deficits in an Alzheimer's disease transgenic mouse model. The Journal of Neuroscience. 2008;28(45):11622–11634. doi: 10.1523/jneurosci.3153-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rutherford L. C., Nelson S. B., Turrigiano G. G. BDNF has opposite effects on the quantal amplitude of pyramidal neuron and interneuron excitatory synapses. Neuron. 1998;21(3):521–530. doi: 10.1016/s0896-6273(00)80563-2. [DOI] [PubMed] [Google Scholar]
- 116.Phillips H. S., Hains J. M., Armanini M., Laramee G. R., Johnson S. A., Winslow J. W. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer's disease. Neuron. 1991;7(5):695–702. doi: 10.1016/0896-6273(91)90273-3. [DOI] [PubMed] [Google Scholar]
- 117.Connor B., Young D., Yan Q., Faull R. L. M., Synek B., Dragunow M. Brain-derived neurotrophic factor is reduced in Alzheimer's disease. Molecular Brain Research. 1997;49(1-2):71–81. doi: 10.1016/s0169-328x(97)00125-3. [DOI] [PubMed] [Google Scholar]
- 118.Alvarez A., Cacabelos R., Sanpedro C., Garcia-Fantini M., Aleixandre M. Serum TNF-alpha levels are increased and correlate negatively with free IGF-I in Alzheimer disease. Neurobiology of Aging. 2007;28(4):533–536. doi: 10.1016/j.neurobiolaging.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 119.Collins J. S., Perry R. T., Watson B., Jr., et al. Association of a haplotype for tumor necrosis factor in siblings with late-onset Alzheimer disease: the NIMH Alzheimer disease genetics initiative. American Journal of Medical Genetics. 2000;96(6):823–830. doi: 10.1002/1096-8628(20001204)96:6<823::aid-ajmg26>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 120.Fillit H., Ding W., Buee L., et al. Elevated circulating tumor necrosis factor levels in Alzheimer's disease. Neuroscience Letters. 1991;129(2):318–320. doi: 10.1016/0304-3940(91)90490-k. [DOI] [PubMed] [Google Scholar]
- 121.Ma S. L., Tang N. L. S., Lam L. C. W., Chiu H. F. K. Association between tumor necrosis factor-α promoter polymorphism and Alzheimer's disease. Neurology. 2004;62(2):307–309. doi: 10.1212/01.wnl.0000103288.49441.e4. [DOI] [PubMed] [Google Scholar]
- 122.Paganelli R., Di Iorio A., Patricelli L., et al. Proinflammatory cytokines in sera of elderly patients with dementia: levels in vascular injury are higher than those of mild-moderate Alzheimer's disease patients. Experimental Gerontology. 2002;37(2-3):257–263. doi: 10.1016/s0531-5565(01)00191-7. [DOI] [PubMed] [Google Scholar]
- 123.Tan Z. S., Beiser A. S., Vasan R. S., et al. Inflammatory markers and the risk of Alzheimer disease: the Framingham study. Neurology. 2007;68(22):1902–1908. doi: 10.1212/01.wnl.0000263217.36439.da. [DOI] [PubMed] [Google Scholar]
- 124.Tobinick E., Gross H., Weinberger A., Cohen H. TNF-alpha modulation for treatment of Alzheimer's disease: a 6-month pilot study. MedGenMed—Medscape General Medicine. 2006;8(2, article 25) [PMC free article] [PubMed] [Google Scholar]
- 125.Stellwagen D., Malenka R. C. Synaptic scaling mediated by glial TNF-α . Nature. 2006;440(7087):1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 126.Steinmetz C. C., Turrigiano G. G. Tumor necrosis factor-α signaling maintains the ability of cortical synapses to express synaptic scaling. The Journal of Neuroscience. 2010;30(44):14685–14690. doi: 10.1523/jneurosci.2210-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kaneko M., Stellwagen D., Malenka R. C., Stryker M. P. Tumor necrosis factor-α mediates one component of competitive, experience-dependent plasticity in developing visual cortex. Neuron. 2008;58(5):673–680. doi: 10.1016/j.neuron.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cingolani L. A., Thalhammer A., Yu L. M. Y., et al. Activity-dependent regulation of synaptic AMPA receptor composition and abundance by beta3 integrins. Neuron. 2008;58(5):749–762. doi: 10.1016/j.neuron.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cingolani L. A., Goda Y. Differential involvement of β3 integrin in pre- and postsynaptic forms of adaptation to chronic activity deprivation. Neuron Glia Biology. 2008;4(3):179–187. doi: 10.1017/S1740925X0999024X. [DOI] [PubMed] [Google Scholar]
- 130.Goddard C. A., Butts D. A., Shatz C. J. Regulation of CNS synapses by neuronal MHC class I. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(16):6828–6833. doi: 10.1073/pnas.0702023104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Uemura K., Lill C. M., Banks M., et al. N-cadherin-based adhesion enhances Aβ release and decreases Aβ 42/40 ratio. Journal of Neurochemistry. 2009;108(2):350–360. doi: 10.1111/j.1471-4159.2008.05760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Asada-Utsugi M., Uemura K., Noda Y., et al. N-cadherin enhances APP dimerization at the extracellular domain and modulates Aβ production. Journal of Neurochemistry. 2011;119(2):354–363. doi: 10.1111/j.1471-4159.2011.07364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Okuda T., Yu L. M. Y., Cingolani L. A., Kemler R., Goda Y. β-catenin regulates excitatory postsynaptic strength at hippocampal synapses. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(33):13479–13484. doi: 10.1073/pnas.0702334104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vitureira N., Letellier M., White I. J., Goda Y. Differential control of presynaptic efficacy by postsynaptic N-cadherin and β-catenin. Nature Neuroscience. 2012;15(1):81–89. doi: 10.1038/nn.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Andreyeva A., Nieweg K., Horstmann K., et al. C-terminal fragment of N-cadherin accelerates synapse destabilization by amyloid-β . Brain. 2012;135(7):2140–2154. doi: 10.1093/brain/aws120. [DOI] [PubMed] [Google Scholar]
- 136.Fu A. K. Y., Hung K.-W., Fu W.-Y., et al. APC(Cdh1) mediates EphA4-dependent downregulation of AMPA receptors in homeostatic plasticity. Nature Neuroscience. 2011;14(2):181–191. doi: 10.1038/nn.2715. [DOI] [PubMed] [Google Scholar]
- 137.Fu A. K. Y., Hung K.-W., Huang H., et al. Blockade of EphA4 signaling ameliorates hippocampal synaptic dysfunctions in mouse models of Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(27):9959–9964. doi: 10.1073/pnas.1405803111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ibata K., Sun Q., Turrigiano G. G. Rapid synaptic scaling induced by changes in postsynaptic firing. Neuron. 2008;57(6):819–826. doi: 10.1016/j.neuron.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 139.Goold C. P., Nicoll R. A. Single-cell optogenetic excitation drives homeostatic synaptic depression. Neuron. 2010;68(3):512–528. doi: 10.1016/j.neuron.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gainey M. A., Hurvitz-Wolff J. R., Lambo M. E., Turrigiano G. G. Synaptic scaling requires the GluR2 subunit of the AMPA receptor. The Journal of Neuroscience. 2009;29(20):6479–6489. doi: 10.1523/jneurosci.3753-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Corrêa S. A. L., Hunter C. J., Palygin O., et al. MSK1 regulates homeostatic and experience-dependent synaptic plasticity. The Journal of Neuroscience. 2012;32(38):13039–13051. doi: 10.1523/jneurosci.0930-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Webber K. M., Smith M. A., Lee H.-G., et al. Mitogen- and stress-activated protein kinase 1: convergence of the ERK and p38 pathways in Alzheimer's disease. Journal of Neuroscience Research. 2005;79(4):554–560. doi: 10.1002/jnr.20380. [DOI] [PubMed] [Google Scholar]
- 143.Blackman M. P., Djukic B., Nelson S. B., Turrigiano G. G. A critical and cell-autonomous role for MeCP2 in synaptic scaling up. The Journal of Neuroscience. 2012;32(39):13529–13536. doi: 10.1523/jneurosci.3077-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Seeburg D. P., Feliu-Mojer M., Gaiottino J., Pak D. T. S., Sheng M. Critical role of CDK5 and Polo-like kinase 2 in homeostatic synaptic plasticity during elevated activity. Neuron. 2008;58(4):571–583. doi: 10.1016/j.neuron.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Seeburg D. P., Sheng M. Activity-induced polo-like kinase 2 is required for homeostatic plasticity of hippocampal neurons during epileptiform activity. The Journal of Neuroscience. 2008;28(26):6583–6591. doi: 10.1523/jneurosci.1853-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wilkaniec A., Czapski G. A., Adamczyk A. Cdk5 at crossroads of protein oligomerization in neurodegenerative diseases: facts and hypotheses. Journal of Neurochemistry. 2016;136(2):222–233. doi: 10.1111/jnc.13365. [DOI] [PubMed] [Google Scholar]
- 147.Marchetti M., Marie H. Hippocampal synaptic plasticity in Alzheimer's disease: what have we learned so far from transgenic models? Reviews in the Neurosciences. 2011;22(4):373–402. doi: 10.1515/rns.2011.035. [DOI] [PubMed] [Google Scholar]
- 148.Bienenstock E. L., Cooper L. N., Munro P. W. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. The Journal of Neuroscience. 1982;2(1):32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bear M. F. Mechanism for a sliding synaptic modification threshold. Neuron. 1995;15(1):1–4. doi: 10.1016/0896-6273(95)90056-X. [DOI] [PubMed] [Google Scholar]
- 150.Sperling R. A., Dickerson B. C., Pihlajamaki M., et al. Functional alterations in memory networks in early Alzheimer's disease. NeuroMolecular Medicine. 2010;12(1):27–43. doi: 10.1007/s12017-009-8109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Busche M. A., Eichhoff G., Adelsberger H., et al. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer's disease. Science. 2008;321(5896):1686–1689. doi: 10.1126/science.1162844. [DOI] [PubMed] [Google Scholar]
- 152.Larner A. J., Doran M. Reply to Dr Raux et al.: molecular diagnosis of autosomal dominant early onset Alzheimer's disease: an update (J Med Genet 2005;42:793–5) Journal of Medical Genetics. 2006;43(8, article e44) doi: 10.1136/jmg.2005.040121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jayadev S., Leverenz J. B., Steinbart E., et al. Alzheimer's disease phenotypes and genotypes associated with mutations in presenilin 2. Brain. 2010;133(4):1143–1154. doi: 10.1093/brain/awq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Westmark C. J., Westmark P. R., Beard A. M., Hildebrandt S. M., Malter J. S. Seizure susceptibility and mortality in mice that over-express amyloid precursor protein. International Journal of Clinical and Experimental Pathology. 2008;1(2):157–168. [PMC free article] [PubMed] [Google Scholar]
- 155.Lalonde R., Dumont M., Staufenbiel M., Strazielle C. Neurobehavioral characterization of APP23 transgenic mice with the SHIRPA primary screen. Behavioural Brain Research. 2005;157(1):91–98. doi: 10.1016/j.bbr.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 156.Sanchez P. E., Zhu L., Verret L., et al. Levetiracetam suppresses neuronal network dysfunction and reverses synaptic and cognitive deficits in an Alzheimer's disease model. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(42):E2895–E2903. doi: 10.1073/pnas.1121081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Verret L., Mann E. O., Hang G. B., et al. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell. 2012;149(3):708–721. doi: 10.1016/j.cell.2012.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Corbett B. F., Leiser S. C., Ling H.-P., et al. Sodium channel cleavage is associated with aberrant neuronal activity and cognitive deficits in a mouse model of Alzheimer's disease. The Journal of Neuroscience. 2013;33(16):7020–7026. doi: 10.1523/jneurosci.2325-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Minkeviciene R., Rheims S., Dobszay M. B., et al. Amyloid β-induced neuronal hyperexcitability triggers progressive epilepsy. The Journal of Neuroscience. 2009;29(11):3453–3462. doi: 10.1523/jneurosci.5215-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ziyatdinova S., Gurevicius K., Kutchiashvili N., et al. Spontaneous epileptiform discharges in a mouse model of Alzheimer's disease are suppressed by antiepileptic drugs that block sodium channels. Epilepsy Research. 2011;94(1-2):75–85. doi: 10.1016/j.eplepsyres.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 161.Li S., Jin M., Koeglsperger T., Shepardson N. E., Shankar G. M., Selkoe D. J. Soluble Aβ oligomers inhibit long-term potentiation through a mechanism involving excessive activation of extrasynaptic NR2B-containing NMDA receptors. The Journal of Neuroscience. 2011;31(18):6627–6638. doi: 10.1523/jneurosci.0203-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hu N.-W., Klyubin I., Anwy R., Rowan M. J. GluN2B subunit-containing NMDA receptor antagonists prevent Aβ-mediated synaptic plasticity disruption in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(48):20504–20509. doi: 10.1073/pnas.0908083106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rönicke R., Mikhaylova M., Rönicke S., et al. Early neuronal dysfunction by amyloid β oligomers depends on activation of NR2B-containing NMDA receptors. Neurobiology of Aging. 2011;32(12):2219–2228. doi: 10.1016/j.neurobiolaging.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 164.Yang Q., Liao Z.-H., Xiao Y.-X., Lin Q.-S., Zhu Y.-S., Li S.-T. Hippocampal synaptic metaplasticity requires the activation of NR2B-containing NMDA receptors. Brain Research Bulletin. 2011;84(2):137–143. doi: 10.1016/j.brainresbull.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 165.Frankiewicz T., Parsons C. G. Memantine restores long term potentiation impaired by tonic N-methyl-d-aspartate (NMDA) receptor activation following reduction of Mg2+ In hippocampal slices. Neuropharmacology. 1999;38(9):1253–1259. doi: 10.1016/s0028-3908(99)00060-x. [DOI] [PubMed] [Google Scholar]
- 166.Zajaczkowski W., Frankiewicz T., Parsons C. G., Danysz W. Uncompetitive NMDA receptor antagoists attenuate NMDA-induced impairment of passive avoidance learning and LTP. Neuropharmacology. 1997;36(7):961–971. doi: 10.1016/S0028-3908(97)00070-1. [DOI] [PubMed] [Google Scholar]
- 167.Jankowsky J. L., Slunt H. H., Ratovitski T., Jenkins N. A., Copeland N. G., Borchelt D. R. Co-expression of multiple transgenes in mouse CNS: a comparison of strategies. Biomolecular Engineering. 2001;17(6):157–165. doi: 10.1016/s1389-0344(01)00067-3. [DOI] [PubMed] [Google Scholar]
- 168.Jankowsky J. L., Fadale D. J., Anderson J., et al. Mutant presenilins specifically elevate the levels of the 42 residue β-amyloid peptide in vivo: evidence for augmentation of a 42-specific γ secretase. Human Molecular Genetics. 2004;13(2):159–170. doi: 10.1093/hmg/ddh019. [DOI] [PubMed] [Google Scholar]
- 169.Savonenko A., Xu G. M., Melnikova T., et al. Episodic-like memory deficits in the APPswe/PS1dE9 mouse model of Alzheimer's disease: relationships to beta-amyloid deposition and neurotransmitter abnormalities. Neurobiology of Disease. 2005;18(3):602–617. doi: 10.1016/j.nbd.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 170.Volianskis A., Køstner R., Mølgaard M., Hass S., Jensen M. S. Episodic memory deficits are not related to altered glutamatergic synaptic transmission and plasticity in the CA1 hippocampus of the APPswe/PS1ΔE9-deleted transgenic mice model of β-amyloidosis. Neurobiology of Aging. 2010;31(7):1173–1187. doi: 10.1016/j.neurobiolaging.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 171.Quinlan E. M., Olstein D. H., Bear M. F. Bidirectional, experience-dependent regulation of N-methyl-D-aspartate receptor subunit composition in the rat visual cortex during postnatal development. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(22):12876–12880. doi: 10.1073/pnas.96.22.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Quinlan E. M., Lebel D., Brosh I., Barkai E. A molecular mechanism for stabilization of learning-induced synaptic modifications. Neuron. 2004;41(2):185–192. doi: 10.1016/s0896-6273(03)00874-2. [DOI] [PubMed] [Google Scholar]
- 173.Philpot B. D., Sekhar A. K., Shouval H. Z., Bear M. F. Visual experience and deprivation bidirectionally modify the composition and function of NMDA receptors in visual cortex. Neuron. 2001;29(1):157–169. doi: 10.1016/S0896-6273(01)00187-8. [DOI] [PubMed] [Google Scholar]
- 174.Philpot B. D., Espinosa J. S., Bear M. F. Evidence for altered NMDA receptor function as a basis for metaplasticity in visual cortex. The Journal of Neuroscience. 2003;23(13):5583–5588. doi: 10.1523/JNEUROSCI.23-13-05583.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Citri A., Malenka R. C. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology. 2008;33(1):18–41. doi: 10.1038/sj.npp.1301559. [DOI] [PubMed] [Google Scholar]
- 176.Shipton O. A., Paulsen O. GluN2A and GluN2B subunit-containing NMDA receptors in hippocampal plasticity. Philosophical Transactions of the Royal Society B: Biological Sciences. 2014;369(1633) doi: 10.1098/rstb.2013.0163.20130163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lau C. G., Zukin R. S. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nature Reviews Neuroscience. 2007;8(6):413–426. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- 178.Barria A., Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48(2):289–301. doi: 10.1016/j.neuron.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 179.Quinlan E. M., Philpot B. D., Huganir R. L., Bear M. F. Rapid, experience-dependent expression of synaptic NMDA receptors in visual cortex in vivo. Nature Neuroscience. 1999;2(4):352–357. doi: 10.1038/7263. [DOI] [PubMed] [Google Scholar]
- 180.Matos M., Augusto E., Oliveira C. R., Agostinho P. Amyloid-beta peptide decreases glutamate uptake in cultured astrocytes: involvement of oxidative stress and mitogen-activated protein kinase cascades. Neuroscience. 2008;156(4):898–910. doi: 10.1016/j.neuroscience.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 181.Huang S., Trevino M., He K., et al. Pull-push neuromodulation of LTP and LTD enables bidirectional experience-induced synaptic scaling in visual cortex. Neuron. 2012;73(3):497–510. doi: 10.1016/j.neuron.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Anggono V., Huganir R. L. Regulation of AMPA receptor trafficking and synaptic plasticity. Current Opinion in Neurobiology. 2012;22(3):461–469. doi: 10.1016/j.conb.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lee H.-K., Takamiya K., Han J.-S., et al. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell. 2003;112(5):631–643. doi: 10.1016/s0092-8674(03)00122-3. [DOI] [PubMed] [Google Scholar]
- 184.Lee H.-K., Barbarosie M., Kameyama K., Bear M. F., Huganir R. L. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature. 2000;405(6789):955–959. doi: 10.1038/35016089. [DOI] [PubMed] [Google Scholar]
- 185.Lee H.-K., Takamiya K., He K., Song L., Huganir R. L. Specific roles of AMPA receptor subunit GluR1 (GluA1) phosphorylation sites in regulating synaptic plasticity in the CA1 region of hippocampus. Journal of Neurophysiology. 2010;103(1):479–489. doi: 10.1152/jn.00835.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Esteban J. A., Shi S.-H., Wilson C., Nuriya M., Huganir R. L., Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nature Neuroscience. 2003;6(2):136–143. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- 187.Man H.-Y., Sekine-Aizawa Y., Huganir R. L. Regulation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking through PKA phosphorylation of the Glu receptor 1 subunit. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(9):3579–3584. doi: 10.1073/pnas.0611698104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Holman D., Feligioni M., Henley J. M. Differential redistribution of native AMPA receptor complexes following LTD induction in acute hippocampal slices. Neuropharmacology. 2007;52(1):92–99. doi: 10.1016/j.neuropharm.2006.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Goel A., Xu L. W., Snyder K. P., et al. Phosphorylation of AMPA receptors is required for sensory deprivation-induced homeostatic synaptic plasticity. PLoS ONE. 2011;6(3) doi: 10.1371/journal.pone.0018264.e18264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.He K., Song L., Cummings L. W., Goldman J., Huganir R. L., Lee H.-K. Stabilization of Ca2+-permeable AMPA receptors at perisynaptic sites by GluR1-S845 phosphorylation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(47):20033–20038. doi: 10.1073/pnas.0910338106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Roche K. W., O'Brien R. J., Mammen A. L., Bernhardt J., Huganir R. L. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16(6):1179–1188. doi: 10.1016/S0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- 192.Barria A., Derkach V., Soderling T. Identification of the Ca2+/calmodulin-dependent protein kinase II regulatory phosphorylation site in the α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate-type glutamate receptor. The Journal of Biological Chemistry. 1997;272(52):32727–32730. doi: 10.1074/jbc.272.52.32727. [DOI] [PubMed] [Google Scholar]
- 193.Mammen A. L., Kameyama K., Roche K. W., Huganir R. L. Phosphorylation of the alpha-amino-3-hydroxy-5-methylisoxazole4-propionic acid receptor GluR1 subunit by calcium/calmodulin-dependent kinase II. The Journal of Biological Chemistry. 1997;272(51):32528–32533. doi: 10.1074/jbc.272.51.32528. [DOI] [PubMed] [Google Scholar]
- 194.Barria A., Muller D., Derkach V., Griffith L. C., Soderling T. R. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997;276(5321):2042–2045. doi: 10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- 195.Hu X.-D., Huang Q., Yang X., Xia H. Differential regulation of AMPA receptor trafficking by neurabin-targeted synaptic protein phosphatase-1 in synaptic transmission and long-term depression in hippocampus. The Journal of Neuroscience. 2007;27(17):4674–4686. doi: 10.1523/jneurosci.5365-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Makino Y., Johnson R. C., Yu Y., Takamiya K., Huganir R. L. Enhanced synaptic plasticity in mice with phosphomimetic mutation of the GluA1 AMPA receptor. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(20):8450–8455. doi: 10.1073/pnas.1105261108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lee M.-C., Yasuda R., Ehlers M. D. Metaplasticity at single glutamatergic synapses. Neuron. 2010;66(6):859–870. doi: 10.1016/j.neuron.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Philpot B. D., Zukin R. S. Synapse-specific metaplasticity: to be silenced is not to silence 2B. Neuron. 2010;66(6):814–816. doi: 10.1016/j.neuron.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Turrigiano G. G., Leslie K. R., Desai N. S., Rutherford L. C., Nelson S. B. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391(6670):892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- 200.Watt A. J., Van Rossum M. C. W., MacLeod K. M., Nelson S. B., Turrigiano G. G. Activity coregulates quantal AMPA and NMDA currents at neocortical synapses. Neuron. 2000;26(3):659–670. doi: 10.1016/S0896-6273(00)81202-7. [DOI] [PubMed] [Google Scholar]
- 201.Thiagarajan T. C., Lindskog M., Tsien R. W. Adaptation to synaptic inactivity in hippocampal neurons. Neuron. 2005;47(5):725–737. doi: 10.1016/j.neuron.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 202.Lissin D. V., Gomperts S. N., Carroll R. C., et al. Activity differentially regulates the surface expression of synaptic AMPA and NMDA glutamate receptors. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(12):7097–7102. doi: 10.1073/pnas.95.12.7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Qiu Z., Sylwestrak E. L., Lieberman D. N., Zhang Y., Liu X.-Y., Ghosh A. The Rett syndrome protein MeCP2 regulates synaptic scaling. The Journal of Neuroscience. 2012;32(3):989–994. doi: 10.1523/jneurosci.0175-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Dong H., Zhang P., Song I., Petralia R. S., Liao D., Huganir R. L. Characterization of the glutamate receptor-interacting proteins GRIP1 and GRIP2. The Journal of Neuroscience. 1999;19(16):6930–6941. doi: 10.1523/JNEUROSCI.19-16-06930.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Xia J., Zhang X., Staudinger J., Huganir R. L. Clustering of AMPA receptors by the synaptic PDZ domain-containing protein PICK1. Neuron. 1999;22(1):179–187. doi: 10.1016/S0896-6273(00)80689-3. [DOI] [PubMed] [Google Scholar]
- 206.Chung H. J., Xia J., Scannevin R. H., Zhang X., Huganir R. L. Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins. The Journal of Neuroscience. 2000;20(19):7258–7267. doi: 10.1523/JNEUROSCI.20-19-07258.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Perez J. L., Khatri L., Chang C., Srivastava S., Osten P., Ziff E. B. PICK1 targets activated protein kinase Cα to AMPA receptor clusters in spines of hippocampal neurons and reduces surface levels of the AMPA-type glutamate receptor subunit 2. The Journal of Neuroscience. 2001;21(15):5417–5428. doi: 10.1523/JNEUROSCI.21-15-05417.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Terashima A., Cotton L., Dev K. K., et al. Regulation of synaptic strength and AMPA receptor subunit composition by PICK1. The Journal of Neuroscience. 2004;24(23):5381–5390. doi: 10.1523/jneurosci.4378-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lin D.-T., Huganir R. L. PICK1 and phosphorylation of the glutamate receptor 2 (GluR2) AMPA receptor subunit regulates GluR2 recycling after NMDA receptor-induced internalization. The Journal of Neuroscience. 2007;27(50):13903–13908. doi: 10.1523/jneurosci.1750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kim E., Sheng M. PDZ domain proteins of synapses. Nature Reviews Neuroscience. 2004;5(10):771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- 211.Chen L., Chetkovich D. M., Petralia R. S., et al. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 2000;408(6815):936–943. doi: 10.1038/35050030. [DOI] [PubMed] [Google Scholar]
- 212.Nicoll R. A., Tomita S., Bredt D. S. Auxiliary subunits assist AMPA-type glutamate receptors. Science. 2006;311(5765):1253–1256. doi: 10.1126/science.1123339. [DOI] [PubMed] [Google Scholar]
- 213.Jiang Y.-H., Ehlers M. D. Modeling autism by SHANK gene mutations in mice. Neuron. 2013;78(1):8–27. doi: 10.1016/j.neuron.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kim E., Naisbitt S., Hsueh Y.-P., et al. GKAP, a novel synaptic protein that interacts with the guanylate kinase- like domain of the PSD-95/SAP90 family of channel clustering molecules. The Journal of Cell Biology. 1997;136(3):669–678. doi: 10.1083/jcb.136.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Naisbitt S., Kim E., Tu J. C., et al. Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin. Neuron. 1999;23(3):569–582. doi: 10.1016/s0896-6273(00)80809-0. [DOI] [PubMed] [Google Scholar]
- 216.Guzowski J. F., Timlin J. A., Roysam B., McNaughton B. L., Worley P. F., Barnes C. A. Mapping behaviorally relevant neural circuits with immediate-early gene expression. Current Opinion in Neurobiology. 2005;15(5):599–606. doi: 10.1016/j.conb.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 217.Steward O., Wallace C. S., Lyford G. L., Worley P. F. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron. 1998;21(4):741–751. doi: 10.1016/s0896-6273(00)80591-7. [DOI] [PubMed] [Google Scholar]
- 218.Chowdhury S., Shepherd J. D., Okuno H., et al. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron. 2006;52(3):445–459. doi: 10.1016/j.neuron.2006.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Park S., Park J. M., Kim S., et al. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron. 2008;59(1):70–83. doi: 10.1016/j.neuron.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Brakeman P. R., Lanahan A. A., O'Brien R., et al. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature. 1997;386(6622):284–288. doi: 10.1038/386284a0. [DOI] [PubMed] [Google Scholar]
- 221.Ango F., Prézeau L., Muller T., et al. Agonist-independent activation of metabotropic glutamate receptors by the intracellular protein Homer. Nature. 2001;411(6840):962–965. doi: 10.1038/35082096. [DOI] [PubMed] [Google Scholar]
- 222.Hayashi T., Huganir R. L. Tyrosine phosphorylation and regulation of the AMPA receptor by SRC family tyrosine kinases. The Journal of Neuroscience. 2004;24(27):6152–6160. doi: 10.1523/jneurosci.0799-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ahmadian G., Ju W., Liu L., et al. Tyrosine phosphorylation of GluR2 is required for insulin-stimulated AMPA receptor endocytosis and LTD. The EMBO Journal. 2004;23(5):1040–1050. doi: 10.1038/sj.emboj.7600126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Pelkey K. A., Askalan R., Paul S., et al. Tyrosine phosphatase STEP is a tonic brake on induction of long-term potentiation. Neuron. 2002;34(1):127–138. doi: 10.1016/s0896-6273(02)00633-5. [DOI] [PubMed] [Google Scholar]
- 225.Mulkey R. M., Endo S., Shenolikar S., Malenka R. C. Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature. 1994;369(6480):486–488. doi: 10.1038/369486a0. [DOI] [PubMed] [Google Scholar]
- 226.Morishita W., Connor J. H., Xia H., Quinlan E. M., Shenolikar S., Malenka R. C. Regulation of synaptic strength by protein phosphatase 1. Neuron. 2001;32(6):1133–1148. doi: 10.1016/S0896-6273(01)00554-2. [DOI] [PubMed] [Google Scholar]
- 227.Thomas G. M., Huganir R. L. Palmitoylation-dependent regulation of glutamate receptors and their PDZ domain-containing partners. Biochemical Society Transactions. 2013;41(1):72–78. doi: 10.1042/bst20120223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Yang G., Xiong W., Kojic L., Cynader M. S. Subunit-selective palmitoylation regulates the intracellular trafficking of AMPA receptor. The European Journal of Neuroscience. 2009;30(1):35–46. doi: 10.1111/j.1460-9568.2009.06788.x. [DOI] [PubMed] [Google Scholar]
- 229.Hayashi T., Rumbaugh G., Huganir R. L. Differential regulation of AMPA receptor subunit trafficking by palmitoylation of two distinct sites. Neuron. 2005;47(5):709–723. doi: 10.1016/j.neuron.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 230.Lin D.-T., Makino Y., Sharma K., et al. Regulation of AMPA receptor extrasynaptic insertion by 4.1N, phosphorylation and palmitoylation. Nature Neuroscience. 2009;12(7):879–887. doi: 10.1038/nn.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Hoppe J. B., Salbego C. G., Cimarosti H. SUMOylation: novel neuroprotective approach for Alzheimer's disease? Aging and Disease. 2015;6(5):322–330. doi: 10.14336/ad.2014.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Martin S., Nishimune A., Mellor J. R., Henley J. M. SUMOylation regulates kainate-receptor-mediated synaptic transmission. Nature. 2007;447(7142):321–325. doi: 10.1038/nature05736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Schwarz L. A., Hall B. J., Patrick G. N. Activity-dependent ubiquitination of GluA1 mediates a distinct AMPA receptor endocytosis and sorting pathway. The Journal of Neuroscience. 2010;30(49):16718–16729. doi: 10.1523/jneurosci.3686-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Lin A., Hou Q., Jarzylo L., et al. Nedd4-mediated AMPA receptor ubiquitination regulates receptor turnover and trafficking. Journal of Neurochemistry. 2011;119(1):27–39. doi: 10.1111/j.1471-4159.2011.07221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Lussier M. P., Nasu-Nishimura Y., Roche K. W. Activity-dependent ubiquitination of the AMPA receptor subunit GluA2. The Journal of Neuroscience. 2011;31(8):3077–3081. doi: 10.1523/jneurosci.5944-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Meadows J. P., Guzman-Karlsson M. C., Phillips S., et al. DNA methylation regulates neuronal glutamatergic synaptic scaling. Science Signaling. 2015;8(382):p. ra61. doi: 10.1126/scisignal.aab0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Balkowiec A., Katz D. M. Cellular mechanisms regulating activity-dependent release of native brain-derived neurotrophic factor from hippocampal neurons. The Journal of Neuroscience. 2002;22(23):10399–10407. doi: 10.1523/JNEUROSCI.22-23-10399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Budni J., Bellettini-Santos T., Mina F., Lima Garcez M., Ioppi Zugno A. The involvement of BDNF, NGF and GDNF in aging and Alzheimer's disease. Aging and Disease. 2015;6(5):331–341. doi: 10.14336/ad.2015.0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Leal G., Afonso P. M., Salazar I. L., Duarte C. B. Regulation of hippocampal synaptic plasticity by BDNF. Brain Research. 2015;1621:82–101. doi: 10.1016/j.brainres.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 240.Peng S., Garzon D. J., Marchese M., et al. Decreased brain-derived neurotrophic factor depends on amyloid aggregation state in transgenic mouse models of Alzheimer's disease. The Journal of Neuroscience. 2009;29(29):9321–9329. doi: 10.1523/jneurosci.4736-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Sutton M. A., Ito H. T., Cressy P., Kempf C., Woo J. C., Schuman E. M. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell. 2006;125(4):785–799. doi: 10.1016/j.cell.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 242.Cajigas I. J., Tushev G., Will T. J., Tom Dieck S., Fuerst N., Schuman E. M. The local transcriptome in the synaptic neuropil revealed by deep sequencing and high-resolution imaging. Neuron. 2012;74(3):453–466. doi: 10.1016/j.neuron.2012.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Ju W., Morishita W., Tsui J., et al. Activity-dependent regulation of dendritic synthesis and trafficking of AMPA receptors. Nature Neuroscience. 2004;7(3):244–253. doi: 10.1038/nn1189. [DOI] [PubMed] [Google Scholar]
- 244.McGeachie A. B., Cingolani L. A., Goda Y. A stabilising influence: integrins in regulation of synaptic plasticity. Neuroscience Research. 2011;70(1):24–29. doi: 10.1016/j.neures.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Mortillo S., Elste A., Ge Y., et al. Compensatory redistribution of neuroligins and N-cadherin following deletion of synaptic β1-integrin. Journal of Comparative Neurology. 2012;520(9):2041–2052. doi: 10.1002/cne.23027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Pak D. T. S., Yang S., Rudolph-Correia S., Kim E., Sheng M. Regulation of dendritic spine morphology by SPAR, a PSD-95-associated RapGAP. Neuron. 2001;31(2):289–303. doi: 10.1016/s0896-6273(01)00355-5. [DOI] [PubMed] [Google Scholar]
- 247.Kubota K., Inoue K., Hashimoto R., et al. Tumor necrosis factor receptor-associated protein 1 regulates cell adhesion and synaptic morphology via modulation of N-cadherin expression. Journal of Neurochemistry. 2009;110(2):496–508. doi: 10.1111/j.1471-4159.2009.06099.x. [DOI] [PubMed] [Google Scholar]
- 248.Drew P. D., Lonergan M., Goldstein M. E., Lampson L. A., Ozato K., McFarlin D. E. Regulation of MHC class I and β2-microglobulin gene expression in human neuronal cells. Factor binding to conserved cis-acting regulatory sequences correlates with expression of the genes. The Journal of Immunology. 1993;150(8, part 1):3300–3310. [PubMed] [Google Scholar]
- 249.Albensi B. C., Mattson M. P. Evidence for the involvement of TNF and NF-κB in hippocampal synaptic plasticity. Synapse. 2000;35(2):151–159. doi: 10.1002/(SICI)1098-2396(200002)35:2<151::AID-SYN8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 250.Mattson M. P., Cheng B., Davis D., Bryant K., Lieberburg I., Rydel R. E. β-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. The Journal of Neuroscience. 1992;12(2):376–389. doi: 10.1523/JNEUROSCI.12-02-00376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Nuriya M., Huganir R. L. Regulation of AMPA receptor trafficking by N-cadherin. Journal of Neurochemistry. 2006;97(3):652–661. doi: 10.1111/j.1471-4159.2006.03740.x. [DOI] [PubMed] [Google Scholar]
- 252.Saglietti L., Dequidt C., Kamieniarz K., et al. Extracellular interactions between GluR2 and N-cadherin in spine regulation. Neuron. 2007;54(3):461–477. doi: 10.1016/j.neuron.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 253.Togashi H., Abe K., Mizoguchi A., Takaoka K., Chisaka O., Takeichi M. Cadherin regulates dendritic spine morphogenesis. Neuron. 2002;35(1):77–89. doi: 10.1016/s0896-6273(02)00748-1. [DOI] [PubMed] [Google Scholar]
- 254.Tang L., Hung C. P., Schuman E. M. A role for the cadherin family of cell adhesion molecules in hippocampal long-term potentiation. Neuron. 1998;20(6):1165–1175. doi: 10.1016/S0896-6273(00)80497-3. [DOI] [PubMed] [Google Scholar]
- 255.Bozdagi O., Shan W., Tanaka H., Benson D. L., Huntley G. W. Increasing numbers of synaptic puncta during late-phase LTP: N-cadherin is synthesized, recruited to synaptic sites, and required for potentiation. Neuron. 2000;28(1):245–259. doi: 10.1016/S0896-6273(00)00100-8. [DOI] [PubMed] [Google Scholar]
- 256.Bozdagi O., Wang X.-B., Nikitczuk J. S., et al. Persistence of coordinated long-term potentiation and dendritic spine enlargement at mature hippocampal CA1 synapses requires N-cadherin. The Journal of Neuroscience. 2010;30(30):9984–9989. doi: 10.1523/jneurosci.1223-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Mendez P., De Roo M., Poglia L., Klauser P., Muller D. N-cadherin mediates plasticity-induced long-term spine stabilization. The Journal of Cell Biology. 2010;189(3):589–600. doi: 10.1083/jcb.201003007. [DOI] [PMC free article] [PubMed] [Google Scholar]