Abstract
Data from subjects in nine phase 1 (n = 153) and six phase 2/3 (n = 129) clinical trials were combined to identify factors contributing to interindividual variability in daptomycin pharmacokinetics (PK). Over 30 covariates were considered. A two-compartment model with first-order elimination provided the best fit for data on daptomycin concentrations in plasma over time. In the final population PK model, daptomycin plasma clearance (CL) was a function of renal function, body temperature, and sex. Of these factors, renal function contributed most significantly to interindividual variability. CL varied linearly with the estimated creatinine clearance. CL among dialysis subjects was approximately one-third that of healthy subjects (0.27 versus 0.81 liter/h). CL in females was 80% that in males; however, in clinical trials, the outcome was not affected by sex and therefore this effect is not considered clinically meaningful. The relationship with body temperature should be interpreted cautiously since the analysis included only a limited number of subjects who were hyperthermic. The volume of distribution of the peripheral compartment (V2) and intercompartmental clearance (Q) were linearly related to body weight. V2 increased approximately twofold in the presence of an acute infection. No factors were identified that significantly impacted V1. This analysis supports the dosing of daptomycin on a milligram-per-kilogram-of-body-weight basis and suggests that modified dosing regimens are indicated for patients with severe renal disease and for those undergoing dialysis.
Daptomycin (N-decanoyl-l-tryptophyl-l-asparaginyl-l-aspartyl-l-threonylglycyl-l-ornithyl-l-aspartyl-d-alanyl-l-aspartylglycyl-d-seryl-threo-3-methyl-l-glutamyl-3-anthraniloyl-l-alanineɛ1-lactone) is a novel cyclic lipopeptide antibiotic derived from the fermentation of Streptomyces roseosporus. Daptomycin was recently approved for the treatment of complicated skin and skin structure infections (cSSSI) caused by aerobic gram-positive bacteria, including those caused by methicillin-resistant Staphylococcus aureus and methicillin-susceptible S. aureus. In vitro, daptomycin demonstrates a rapid concentration-dependent bactericidal activity against most clinically relevant gram-positive pathogenic bacteria, including bacterial isolates that are resistant to methicillin, vancomycin, and linezolid (9). The MICs for daptomycin at which 90% of isolates tested are inhibited are typically ≤1 μg/ml for staphylococci and streptococci and 2 to 4 μg/ml for enterococci, including vancomycin-resistant isolates (7). Although the mechanism of action has not been fully defined, it is distinct from those of other antibiotics and appears to be mediated by the disruption of multiple aspects of membrane function (5, 17). In phase 3 trials for the treatment of cSSSI caused by susceptible gram-positive bacteria, clinical and microbiological outcomes of patients treated with daptomycin were comparable to those for patients receiving conventional antibiotic therapy, such as pencillinase-resistant penicillins or vancomycin (1, 18).
Studies of healthy human subjects have demonstrated linear pharmacokinetics after single (20) and multiple (8) intravenous daptomycin doses up to 6 mg/kg of body weight over 14 days. After once-daily doses of 4 mg/kg, the average steady-state trough daptomycin concentration in plasma was 5.89 μg/ml and varied by 27% among individuals (8). Daptomycin is >90% bound to plasma proteins and has a low steady-state volume of distribution, averaging 0.06 to 0.15 liter/kg (8, 19, 20), consistent with distribution into extracellular fluid. Elimination is primarily achieved by renal excretion of unchanged drug. In healthy adult subjects, the mean urinary recovery over a 24-h period is 50 to 60% of the administered dose (8, 19). In phase 1 studies involving healthy adult subjects and subjects with graded renal insufficiencies, including end-stage renal disease, daptomycin plasma clearance (CL) was significantly reduced among subjects with creatinine clearances (CLCR) of ≤40 ml/min or who were on dialysis (D. A. Sica, T. Gehr, and B. Dvorchik, Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. 2257, 2002).
For the present analysis, data from 15 clinical trials were combined and a population analysis approach was used to evaluate possible sources of interindividual variability in daptomycin pharmacokinetics. The specific objectives were to develop a model to describe the pharmacokinetics of daptomycin in both healthy volunteers and subjects with acute bacterial infections who were representative of the target patient population and to identify clinical characteristics that impact daptomycin pharmacokinetics. The factors examined included age, sex, weight, and the presence of bacterial infection, end-organ dysfunction, comorbidities, and concomitant medications.
MATERIALS AND METHODS
Study design.
The analysis included data collected from 282 subjects enrolled in 15 phase 1, 2, and 3 clinical trials of daptomycin (Table 1). Frequent blood samples were collected from each of 153 subjects in nine phase 1 clinical trials. The subjects received single or multiple doses of 4 to 8 mg of daptomycin/kg administered by a 30-min intravenous infusion every 24 h. Blood samples for pharmacokinetic analysis were collected from a subset of 129 subjects with gram-positive bacterial infections from six phase 2 and/or 3 clinical trials who received various regimens of intravenous daptomycin. All subjects included in the population pharmacokinetic analysis received at least one dose of daptomycin, had at least one measured daptomycin concentration from plasma, and had accurate documentation of the dates and times of the dose and concentration measurements. Subject and study design details are shown in Table 1.
TABLE 1.
Study designs
| Clinical phase | Study no. | Subject characteristic(s)c | Dosing regimena | No. of subjects | No. of samples/subject | Sample collection period (h)b |
|---|---|---|---|---|---|---|
| 1 | 1 | Healthy | 4 mg/kg every 24 h for 7 days (n = 6); 6 mg/kg every 24 h for 7 days (n = 6), or 8 mg/kg every 24 h for 7 days (n = 6) | 18 | 27-31 | 24 (1st dose), 72 (last dose), trough samples |
| 1 | 2 | Healthy | 4 mg/kg | 7 | 12-14 | 24 |
| 1 | 3 | Healthy | 4 mg/kg ± i.v. aztreonam (crossover) | 17 | 15 | 24.5 |
| 1 | 4 | Healthy, 18-30 yr old (n = 12); or healthy, ≥75 yr old (n = 12) | 4 mg/kg | 23 | 13 | 24 |
| 1 | 5 | Healthy (BMI, <25 kg/m2) (n = 12), moderately obese (BMI, 25-39.9 kg/m2) (n = 6), or extremely obese (BMI, >40 kg/m2) (n = 7) | 4 mg/kg | 25 | 13 | 24 |
| 1 | 6 | Healthy (n = 10), or hepatic impairment, child pugh B (n = 9) | 6 mg/kg | 19 | 15 | 48 |
| 1 | 7 | Healthy (n = 5), mild renal failure (n = 6), moderate to severe renal failure (n = 7), on hemodialysis (n = 6), or on peritoneal dialysis (n = 5) | Healthy subjects, 4 mg/kg ± p.o. probenecid (crossover design); all other subjects, 4 mg/kg | 29 | 12-14 | 72-96 |
| 1 | 8 | Moderate renal impairment | 4 mg/kg every 24 h for 14 days (n = 4) or 6 mg/kg every 24 h for 14 days (n = 4) | 8 | 26 | 24 (1st and last doses), trough samples |
| 1 | 9 | On hemodialysis | 4 mg/kg, then six doses of 3 mg/kg every 48 h (n = 6), or 6 mg/kg, then six doses of 4 mg/kg every 48 h (n = 1) | 7 | 26 | 36 (1st dose), 48 (last dose), trough samples |
| 2 | 10 | Subjects with bacteremic infections due to gram-positive bacteria | 6 mg/kg every 24 h for 7 to 14 days (n = 13), 4 mg/kg every 24 h for 7 to 14 days (n = 14), 6 mg/kg followed by 3 mg/kg every 12 h for 7 to 14 days (n = 13), or other regimen (n = 1) | 41 | 5 | 6 (on 5th day of dosing) |
| 2 | 11 | Subjects with infections due to gram-positive bacteria resistant to vancomycin or otherwise refractory to or contraindicated for currently available therapy | 6 mg/kg every 24 h for 7 to 14 days | 27 | 5 | 6 (on 5th to 7th day of dosing) |
| 3 | 12 | Subjects with moderate to severe community-acquired acute bacterial pneumonia due to Streptococcus pneumoniae or other gram-positive cocci | 4 mg/kg every 24 h for 5 to 10 days | 6 | 4 | 12 (on 5th day of dosing) |
| 3 | 13 | Subjects with moderate to severe community-acquired acute bacterial pneumonia due to S. pneumoniae or other gram-positive cocci | 4 mg/kg every 24 h for 5 to 10 days | 12 | 4 | 12 (on 5th day of dosing) |
| 3 | 14 | Subjects with cSSSI due to gram-positive bacteria | 4 mg/kg every 24 h for 7 to 14 days | 16 | 5 | 6 (on at least 3rd day of dosing) |
| 3 | 15 | Subjects with cSSSI due to gram-positive bacteria | 4 mg/kg every 24 h for 7 to 14 days | 27 | 5 | 6 (on at least 3rd day of dosing) |
Administered by intravenous infusion for 30 min.
Period following dose administration.
BMI, body mass index.
A validated high-performance liquid chromatography (HPLC) method was used for an analysis of daptomycin concentrations in plasma for 13 studies (8). The lower limit of quantitation was 3 μg/ml and the interassay coefficient of variation was 3.51%. A validated LC/MS/MS method (Cubist Pharmaceutical, internal report) was used for the quantification of daptomycin concentrations in plasma for a study of the pharmacokinetics of daptomycin in healthy and renally impaired subjects. There was a close linear relationship (correlation coefficient, 0.965) between concentrations in plasma obtained by the LC/MS/MS and HPLC methods. However, the LC/MS/MS assay was more sensitive than the HPLC assay (lower limits of quantitation, 0.1 and 3 μg/ml, respectively). For one study of the use of daptomycin in healthy male volunteers, a microbiological assay was used to quantify daptomycin concentrations in plasma (19). The interassay coefficient of variation and the limit of quantitation were 6.3% and 2 μg/ml, respectively. The results of the assay correlated well with those of the HPLC assay, as evidenced by the fact that the pharmacokinetic parameters for daptomycin for this group of subjects were in excellent agreement with data obtained in other studies with healthy volunteers (8).
The covariates explored as possible sources of interindividual variability in daptomycin pharmacokinetics are listed in Table 2. Body surface area was calculated by the following formula (R. D. Mosteller, Letter, N. Engl. J. Med. 317:1098, 1987):
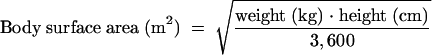 |
CLCR was estimated for nondialysis subjects by use of the Cockcroft-Gault equation (6):
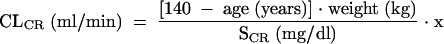 |
in which x = 1 for males and 0.85 for females. For single-dose studies with healthy subjects, hepatically impaired subjects, obese subjects, and healthy geriatric subjects, creatinine levels in serum and body weights were determined at screening, on the morning of dose administration, and prior to discharge from the clinical research unit. For each study, intraindividual differences in these parameters were within 10% and estimated CLCR values for these subjects were considered stable. For subjects in the single-dose graded renal study (excluding those undergoing dialysis), entry criteria required that CLCR be determined from two separately measured 24-hour CLCR values performed within 21 days of dosing. Subjects with CLCR values within 30% of each other were considered to have a stable CLCR and were enrolled in the study. Creatinine levels in serum and body weights were also obtained prior to dosing and prior to discharge from the clinical research unit. Intraindividual estimates of CLCR were within the protocol criteria for a stable CLCR. For subjects in multiple-dose renal studies (excluding those undergoing dialysis), creatinine levels in serum were determined at screening, predose, and every other day from day 3 to discharge (day 15) from the clinical research unit. No clinically significant change was observed in the intraindividual values over time. For phase 2 and/or 3 clinical studies, creatinine levels in serum and body weights were determined at various times throughout the duration of the study. Estimated CLCR values were calculated by using creatinine levels in serum obtained on the day of the pharmacokinetic blood draw and the day closest to the pharmacokinetic day and the mean of the highest and lowest creatinine levels in serum obtained over the course of the entire study. No clinically significant intraindividual differences were observed, regardless of the value chosen. To maintain consistency with single-dose studies, we calculated the estimated CLCR values used in the final analysis by using the creatinine level in serum and the actual body weight measured closest to the pharmacokinetic day. The weight used was the actual body weight on the day of the pharmacokinetic draw or the day closest to the pharmacokinetic day. Individual CLCR values were estimated to be >150 ml/min for 38 subjects and were all set to 150 ml/min. These subjects represented multiple different studies; their median body weight was 85.3 kg (range, 52 to 153 kg). Sixty-three percent had normal creatinine levels in serum, with actual body weights ranging from 72.6 to 152.8 kg; the remaining 37% had creatinine levels in serum ranging from 0.2 to 0.6 mg/dl, with actual body weights ranging from 56.3 to 130.6 kg. The lowest estimated CLCR for subjects was 14 ml/min. Body temperature was evaluated only for subjects in phase 2 and/or 3 studies. Concomitant medications examined for possible pharmacokinetic interactions with daptomycin included acidic drugs that are actively secreted in the renal tubule and drugs that are highly (>95%) bound to albumin (3, 13, 15). Comorbidities included diseases producing fluid accumulation (e.g., ascites and edema), the presence of infection, diabetes, hypertension, and congestive heart failure. All covariates were values recorded at baseline, with the exception of body temperature, which was the value recorded on the day of pharmacokinetic sampling. Missing continuous covariates were replaced with the median value of the covariate for subjects of the same sex in the same study.
TABLE 2.
Subject characteristics
| Characteristic | Median (continuous variables) or no. (categorical variables) | Range (continuous variables) or % (categorical variable) |
|---|---|---|
| Continuous variables | ||
| Body weight (kg) | 75.1 | 48.2-152.8 |
| Body surface area (m2) | 1.9 | 1.5-2.8 |
| Age (yr) | 51 | 18-93 |
| Body temp on day of pharmacokinetic study (°C)a | 37.2 | 36.1-40.1 |
| Baseline serum albumin (g/dl)b | 4.2 | 2.8-5.0 |
| Baseline alkaline phosphatase (IU/liter) | 81 | 27-730 |
| Baseline ALT (IU/liter) | 21 | 3-176 |
| Baseline AST (IU/liter) | 21 | 4-292 |
| Baseline total bilirubin (mg/dl) | 0.5 | 0.2-15.7 |
| Baseline BUN (mg/dl) | 15 | 4-115 |
| Baseline blood glucose (mg/dl) | 98 | 41-382 |
| Baseline serum creatinine (mg/dl)c | 1.0 | 0.2-18.2 |
| Baseline creatinine clearance (ml/min)d,e | 91.2 | 14.0-150.0 |
| Average serum creatinine (mg/dl)c,f | 1.0 | 0.2-15.5 |
| Average creatinine clearance (ml/min)d,g | 92.2 | 14.0-150.0 |
| Categorical variables | ||
| Study (phase 1, phase 2/3) | 153, 129 | 54, 46 |
| Sex (male, female) | 166, 116 | 59, 41 |
| Race (Caucasian, African-American, other) | 163, 51, 68 | 58, 18, 24 |
| Dialysis (yes, no) | 21, 26 | 7, 93 |
| Renal function 5 categories (≥80 ml/min, ≥50 to <80 ml/min, ≥30 to <50 ml/min, <30 ml/min, on dialysis) | 165, 64, 24, 8, 21 | 59, 23, 9, 3, 7 |
| Renal function 4 categories (≥80 mL/min, >40 to <80 ml/min, ≤40 ml/min, on dialysis) | 165, 80, 16, 21 | 59, 28, 6, 7 |
| Elevated baseline BUN (>ULN = 25 mg/dl) | 52 | 18 |
| Elevated baseline serum creatinine (>ULN = 1.4 mg/dl) | 40 | 14 |
| Elevated baseline blood glucose (>ULN = 109 mg/dl) | 55 | 20 |
| Hyperthermic (≥38°C) | 14 | 14 |
| Has diabetes | 47 | 17 |
| Has hypertension | 52 | 18 |
| Has congestive heart failure | 19 | 7 |
| Has fluid accumulation (edema or ascites) | 22 | 8 |
| Has gram-positive bacterial infection | 129 | 46 |
| Taking concomitant aztreonam | 17 | 6 |
| Taking concomitant metronidazole | 0 | 0 |
| Taking concomitant medication that is secreted in the renal tubule (including probenecid) | 93 | 33 |
| Taking concomitant medication that is >95% bound to albumin | 64 | 23 |
On day of blood samples obtained for pharmacokinetics (n = 100). Data are missing for all phase 1 studies and for 29 subjects in phase 2/3 studies.
n = 164 (data are missing for five phase 2/3 studies).
n = 282.
n = 261 (excludes subjects on dialysis).
Includes 38 subjects (14.6%) for whom the estimated creatinine clearance was set to 150 ml/min.
Average of minimum and maximum values recorded over the period of the pharmacokinetic study.
Includes 34 subjects (13.0%) for whom the estimated creatinine clearance was set to 150 ml/min.
Pharmacokinetic analysis.
Population pharmacokinetic models were built by a nonlinear mixed-effects modeling approach and first-order conditional maximum likelihood estimation with eta-epsilon interaction in the NONMEM program (double precision, version V, level 1.1) (2).
(i) Base model selection.
One-, two-, and three-compartment structural models were fit to the data for concentrations in plasma over time; graphical displays of the data were also evaluated. Hypothesis testing to discriminate among alternative hierarchical structural models was performed by using the likelihood ratio test (16). For comparisons of alternative models, the difference in the NONMEM objective function was approximately chi-square distributed, with n degrees of freedom, where n was the difference in the number of parameters between the hierarchical models. A decrease of ≥3.84 in the value of the NONMEM objective function, which is less than twice the maximum logarithm of the likelihood of the data, is significant in the likelihood ratio test (n = 1; P < 0.05). The goodness of fit was evaluated by using diagnostic scatter plots (not shown).
The duration of infusion (D1) was estimated for a subset of 108 subjects included in the phase 3 clinical trials for whom the date and time of the start of daptomycin infusion and the time of the first pharmacokinetic blood draw for concentration measurements, but not the time of cessation of the infusion, were recorded. All estimates were consistent with the protocols, which specified an infusion time of 30 min. Interindividual variability in D1 could not be estimated and was not included in the model.
All pharmacokinetic parameters were assumed to be logarithmically normally distributed, and exponential interindividual variability terms were included in the pharmacokinetic parameters in the model. Various residual error models were tested, including an evaluation of possible systematic differences between phase 1 and phase 2/3 studies and among studies that used different assay methods.
(ii) Population pharmacokinetic model building.
Exploratory analyses were used to guide the model building process. Relationships between individual covariates and Bayesian estimates of the pharmacokinetic parameters were explored graphically. Generalized additive models were used to evaluate both linear and nonlinear relationships between parameters and covariates (14). In addition, measures of body size and renal function markers were tested as possible sources of interindividual variability for each pharmacokinetic parameter. All possible covariate-parameter relationships thus selected were tested, with the exception that possible drug interactions and the effect of the daptomycin dose were examined only for CL and the volume of the central compartment (V1), as appropriate.
Continuous covariates were entered into the population pharmacokinetic model according to the following equation:
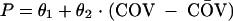 |
where P is the individual estimate of the parameter, COV is the value of the covariate, and CŌV is the median value of the covariate in the study population. θ1 is the typical value of the parameter (when COV = CŌV) and θ2 is the slope of the effect of the covariate on the parameter.
Categorical covariates were included in the model by using indicator variables, as shown in the following equation:
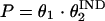 |
where P is the individual estimate of the parameter, θ1 is the typical value of the parameter when the covariate is not present (IND = 0), and θ2 is the fractional change in the value of P when the covariate is present (IND = 1).
The statistical significance of each covariate-parameter relationship was screened individually in NONMEM, and the model was built by stepwise additions to obtain a full model. Stepwise deletions were used to obtain the final (reduced) model. The likelihood ratio test was used for hypothesis testing to discriminate among alternative hierarchical models. A strict inclusion criterion (P < 0.001) corresponding to a change in the value of the NONMEM objective function of 10.83 (n = 1 degrees of freedom) was used to account for multiple hypothesis testing. At each stage of the analysis, the goodness of fit was evaluated by using diagnostic scatterplots.
(iii) Pharmacokinetic parameter calculations.
The terminal half-life (t1/2), volume of distribution at a steady state (Vss), and area under the curve from time zero to infinity [AUC(0-∞)] were calculated from individual pharmacokinetic parameter estimates obtained by Bayesian estimation from the final population pharmacokinetic model. For a two-compartment model (10), the following equations were used:
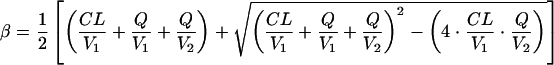 |
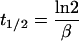 |
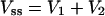 |
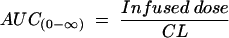 |
where β is the terminal phase rate constant (per hour), CL is the daptomycin plasma clearance (liters per hour), Q is the intercompartmental clearance (liters per hour), V1 is the volume of the central compartment (liters), V2 is the volume of the peripheral compartment (liters), and t1/2 is the terminal phase half-life (hours). All calculations were performed in NONMEM.
(iv) Statistics.
Individual Bayes estimates and calculated pharmacokinetic parameter values were grouped according to four renal function categories. Three categories were defined by using the estimated CLCR values: the groups were values of ≥80 ml/min, >40 to <80 ml/min, and ≤40 ml/min. These ranges were chosen based on an analysis of phase 1 studies with renally impaired subjects (Sica et al., 42nd ICAAC). Subjects on dialysis comprised a fourth category (thus, the categories were CLCR values of ≥80 ml/min, <80 to >40 ml/min, and ≤40 ml/min and subjects on dialysis). Differences between groups were evaluated by analysis of variance with Scheffe's test (S-PLUS Professional; Insightful Corp., Seattle, Wash.). P values of <0.05 were considered significant.
RESULTS
Data.
Measurements of daptomycin concentrations over time were available for 3,325 plasma specimens collected from 282 adult subjects. Two outlying concentrations, one for a specimen reported as a trough plasma with a daptomycin concentration of 106 μg/ml (sixfold higher than the average trough value) and one for a specimen reported as obtained 0.58 h after dose administration with a daptomycin concentration of 309 μg/ml (fivefold higher than the average plasma concentration at 0.58 h), were excluded from the analysis.
Imputed covariate values were generated for a total of 37 subjects; laboratory tests of hepatic function were the most frequently missing values. Height, used in the calculation of body surface area, was imputed for 3 subjects; baseline creatinine values in serum were imputed for 13 subjects. Albumin levels in serum were not recorded in one phase 1 study and three phase 2/3 studies and were considered to be missing for all subjects in those studies. Missing body temperature values on the day of pharmacokinetic sampling were not imputed for 29 subjects from the phase 2/3 studies. The population pharmacokinetic model was coded to remove the effect of missing covariates in the model.
Descriptive statistics for all covariates are presented in Table 2.
Pharmacokinetic analysis. (i) Base model.
Plots of data for concentrations in plasma versus time (not shown) showed a biphasic disposition of daptomycin. A review of the minimum objective function and diagnostic plots showed that the data for daptomycin concentrations in plasma over time were best described by using a two-compartment open model with first-order elimination. The structural pharmacokinetic model used the following parameters: clearance (CL), the volume of the central compartment (V1), intercompartmental clearance (Q), and the volume of the peripheral compartment (V2). In addition, the duration of infusion (D1) was estimated for several phase 3 subjects. Estimates of D1 were consistent with the duration of infusion specified in the clinical protocols.
The median daptomycin clearance for the study population was estimated to be 0.688 liter/h (11.5 ml/min) and the volume of the central compartment was 4.8 liters. Median estimates for the intercompartmental clearance and volume of distribution of the peripheral compartment were 3.6 liters/h and 3.6 liters, respectively. All pharmacokinetic parameters were precisely estimated, with relative standard errors (RSEs) of <3%. The estimated median duration of infusion for 108 subjects enrolled in phase 2/3 trials was 0.402 h (24 min), with an RSE of 23.8%.
Interindividual variabilities were estimated to be 52.1% for CL, 60.6% for the volume of the central compartment, 31.9% for the volume of the peripheral compartment, and 74.4% for intercompartmental clearance. A simple additive residual error model based on diagnostic plots provided the best fit for the data. A further evaluation of diagnostic plots indicated that there was a larger degree of misfit of model predictions for observations collected in phase 2/3 studies; therefore, different error structures were evaluated for phase 1 versus phase 2/3 studies. On the basis of the likelihood ratio test, the final residual error model was described by a combination of additive errors, reflecting the different assay methods used to determine daptomycin concentrations in plasma. The residual error was slightly lower for the study in which daptomycin concentrations in plasma were assayed by LC/MS/MS than for studies assayed by HPLC (2.08 versus 4.72 μg/ml, respectively), consistent with the higher sensitivity of the former method.
(ii) Population model.
Exploratory graphical analyses revealed a direct correlation between daptomycin clearance and various markers of renal function, including estimated creatinine clearance, renal function category, and laboratory markers of renal function. Intercompartmental clearance (in liters per hour) and the volume of the peripheral compartment (in liters) were correlated with body weight. There were no obvious relationships between V1 and any of the tested covariates and no significant association between daptomycin pharmacokinetics and either the concomitant medications or the concomitant diseases evaluated in this patient population.
The final model for daptomycin clearance was determined to be the following:
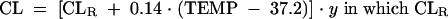 |
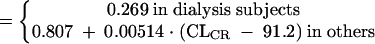 |
where CL = daptomycin clearance (liters per hour), CLR = daptomycin clearance as a function of renal function only (liters per hour), CLCR = estimated creatinine clearance (milliliters per minute), TEMP = body temperature (oC), and y = 0.8 for females and 1 for males.
Both intercompartmental clearance and the volume of the peripheral compartment were determined to be functions of body weight, as follows:
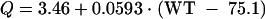 |
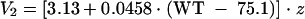 |
where WT = body weight (kilograms) and z = 1.93 for subjects with a bacterial infection and 1 for noninfected subjects.
The median value for V1 was estimated to be 4.80 liters. Although the interindividual variability in V1 was 57%, none of the covariates investigated, including body weight, was identified as a significant source of variability in V1.
Additional exploratory analyses were performed to evaluate whether any other covariate could explain the effect of sex on daptomycin clearance or the effect of the presence of infection on V2. A review of the covariate graphics indicated that body weight, body surface area, and race differed by sex. Each of these covariates was substituted into the clearance model to determine if it could be substituted for sex in the model, but none produced a significant change in the objective function value. Consequently, the clearance model including sex represented the final model.
Infections were only present in subjects in the phase 2 and/or 3 clinical trials. Therefore, in the model the presence of infection could be a marker for an unmonitored covariate that differed between the phase 1 and phase 2/3 clinical trials. In a graphical evaluation, age and serum albumin were determined to differ between the two groups. Each of these covariates, as well as body temperature, was substituted into the V2 model, and none produced a significant change in the objective function value. The V2 model including infection represented the final model.
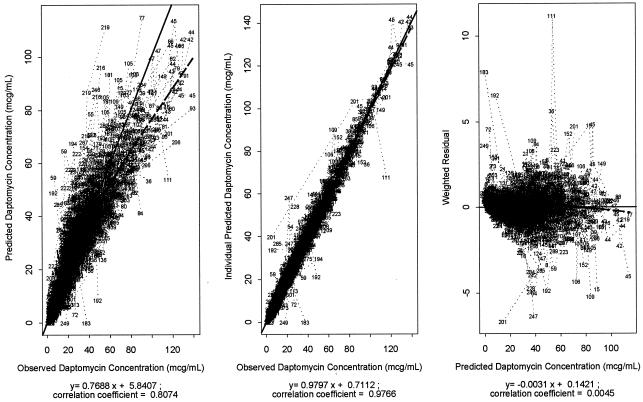
Parameter estimates for the final population pharmacokinetic model are presented in Table 3. Pharmacokinetic parameters were precisely estimated and diagnostic plots showed a good fit of the final model to the observed daptomycin concentrations in plasma (Fig. 1).
TABLE 3.
Population pharmacokinetic parameter estimates for daptomycinb
| Structural model parameter | Median value (RSE [%]) | % Interindividual CV (RSE [%]) |
|---|---|---|
| CL (liter/h) for male subject with median creatinine clearance (91.2 ml/min) | 0.807 (2.9) | 30.6 (10.5) |
| Change in CL (liter/h) for each 10 ml/min that creatinine clearance differs from the median value | 0.0514 (10.9) | |
| CL (liter/h) for subject on dialysis | 0.269 (6.1) | |
| Fractional change in CL for female subject | 0.801 (4.0) | |
| Change in CL (liter/h) for each °C that temp differs from the median value (37.2°) | 0.14 (32.1) | |
| V1 (liters) | 4.80 (4.2) | 56.7 (26.8) |
| Q (liter/h) for subject with median body weight (75 kg) | 3.46 (6.3) | 65.2 (39.5) |
| Change in Q (liter/h) for each 10 kg that body weight differs from the median value | 0.593 (20.4) | |
| V2 (liters) for subject with median body weight (75 kg) | 3.13 (2.7) | 19.1 (27.4) |
| Change in V2 (liters) for each 10 kg that body weight differs from the median value | 0.458 (12.0) | |
| Fractional change in V2 for subject with infection | 1.93 (9.5) | |
| Duration of infusion (h) | 0.384 (27.3) | NEa |
NE, not estimated.
For the residual error parameter σ2add for studies that used the LC/MS/MS assay, the estimated value was 4.28, with an RSE of 23.1% and an intraindividual error SD of 2.07 μg/ml. For the parameter σ2add for studies that used the HPLC assay, the estimated value was 22.4, with an RSE of 20.3% and an intraindividual error SD of 4.73 μg/ml.
FIG. 1.
Diagnostic plots for daptomycin population pharmacokinetic model. Observed versus predicted daptomycin concentrations in plasma (left), observed versus individual predicted daptomycin concentrations in plasma (middle), and weighted residuals versus predicted daptomycin concentrations in plasma (right panel) are shown. Circles represent individual data points. Dashed lines represent regression lines. Solid lines represent unity.
Based on the final population pharmacokinetic model, the apparent Vss for a typical healthy subject with a median body weight of 75 kg was estimated to be 7.9 liters. In comparison, the Vss was increased 37%, to 10.8 liters, if the subject had an acute bacterial infection. The median terminal elimination t1/2 of daptomycin was determined to be 7.07 h in a typical normothermic male with normal renal function. The median terminal elimination t1/2 for a male subject with a creatinine clearance of 40 ml/min was 10.36 h, and for a male subject receiving dialysis, the median terminal elimination t1/2 was 20.68 h.
Individual estimates of CL and V1 were obtained from the final population pharmacokinetic model by Bayesian estimation. These were used to calculate individual estimates of the t1/2, Vss, and AUC(0-∞) for a single 4-mg/kg intravenous dose from the individual parameter estimates and were summarized by renal function category. Summary statistics for these estimates are presented for all subjects (Table 4) and separately for phase 1 and phase 2/3 subjects (Table 5).
TABLE 4.
Summary of pharmacokinetic parameters sorted by estimated CLCR and obtained by Bayesian estimation from the final model
| Pharmacokinetic parameter | Value for subjects in CLCR group
|
|||
|---|---|---|---|---|
| ≥80 ml/min (n = 165) | <80 to >40 ml/min (n = 80) | ≤40 ml/min (n = 16) | On dialysis (n = 21) | |
| CL (liter/h) | ||||
| Median | 0.86 | 0.64b | 0.37b,c | 0.24b,c |
| Minimum | 0.41 | 0.23 | 0.21 | 0.17 |
| Maximum | 2.19 | 1.64 | 0.70 | 0.58 |
| V1 (liters) | ||||
| Median | 4.44 | 4.41 | 6.05c | 6.60b,c |
| Minimum | 0.63 | 0.20 | 3.27 | 3.76 |
| Maximum | 24.93 | 14.95 | 29.93 | 13.15 |
| Vss (liters) | ||||
| Median | 9.73 | 8.75 | 10.36 | 10.44 |
| Minimum | 5.10 | 3.77 | 6.19 | 6.84 |
| Maximum | 32.77 | 19.24 | 34.89 | 17.63 |
| AUC(0−∞) (μg · h/ml)a | ||||
| Median | 400.77 | 436.54b | 716.24b,c | 1,205.60b,c,d |
| Minimum | 160.60 | 151.68 | 297.72 | 367.01 |
| Maximum | 1,143.70 | 1,181.00 | 1,677.00 | 1,906.00 |
| t1/2 (h) | ||||
| Median | 8.28 | 9.07b | 18.96b,c | 29.32b,c,d |
| Minimum | 4.64 | 5.17 | 8.84 | 14.69 |
| Maximum | 48.01 | 71.16 | 58.83 | 41.80 |
Calculated for a single 4-mg/kg dose.
Significantly different from ≥80-ml/min group.
Significantly different from <80- to >40-ml/min group.
Significantly different from ≤40-ml/min group.
TABLE 5.
Summary of pharmacokinetic parameters by study phase and estimated CLCR of individual parameters obtained by Bayesian estimation from the final model
| Pharmacokinetic parameter | Value for phase 1 subjects in CLCR group
|
Value for phase 2/3 subjects in CLCR group
|
||||||
|---|---|---|---|---|---|---|---|---|
| ≥80 ml/min (n = 79) | <80 to >40 ml/min (n = 48) | ≤40 ml/min (n = 8) | On dialysis (n = 18) | ≥80 ml/min (n = 86) | <80 to >40 ml/min (n = 32) | ≤40 ml/min (n = 8) | On dialysis (n = 3) | |
| CL (liter/h) | ||||||||
| Median | 0.78 | 0.63 | 0.35 | 0.23 | 0.98 | 0.64 | 0.57 | 0.31 |
| Minimum | 0.48 | 0.29 | 0.21 | 0.17 | 0.41 | 0.23 | 0.22 | 0.29 |
| Maximum | 1.33 | 1.1 | 0.58 | 0.43 | 2.19 | 1.64 | 0.7 | 0.58 |
| V1 (liters) | ||||||||
| Median | 4.02 | 3.87 | 6.05 | 6.66 | 5.33 | 5.26 | 6.77 | 6.20 |
| Minimum | 2.14 | 1.89 | 3.27 | 3.76 | 0.63 | 0.20 | 3.51 | 6.14 |
| Maximum | 8.51 | 9.90 | 7.27 | 13.15 | 24.93 | 14.95 | 29.93 | 7.72 |
| Vss (liters) | ||||||||
| Median | 7.52 | 6.88 | 10.01 | 9.98 | 12.38 | 10.81 | 11.66 | 11.42 |
| Minimum | 5.10 | 3.77 | 6.19 | 6.84 | 6.10 | 5.91 | 9.86 | 11.32 |
| Maximum | 13.12 | 13.94 | 11.04 | 17.63 | 32.77 | 19.24 | 34.89 | 12.00 |
| AUC(0-∞) (μg · h/ml)a | ||||||||
| Median | 443.79 | 453.18 | 987.02 | 1,310.05 | 338.84 | 394.28 | 527.05 | 849.95 |
| Minimum | 247.12 | 206.12 | 477.08 | 772.29 | 160.60 | 151.68 | 297.72 | 367.01 |
| Maximum | 802.04 | 1,181.00 | 1,677.00 | 1,906.00 | 1,143.70 | 1,151.1 | 1,139.5 | 866.09 |
| t1/2 (h) | ||||||||
| Median | 7.08 | 8.08 | 20.71 | 30.51 | 9.89 | 12.45 | 14.61 | 25.64 |
| Minimum | 4.64 | 5.17 | 8.84 | 22.25 | 5.03 | 7.49 | 12.74 | 14.69 |
| Maximum | 12.30 | 26.11 | 34.17 | 41.80 | 48.01 | 71.16 | 58.83 | 27.25 |
Calculated for a single 4-mg/kg i.v. dose.
These analyses indicated that the daptomycin CL, t1/2, and AUC(0-∞) for a single 4-mg/kg intravenous dose were dependent on renal function. An analysis of variance indicated that compared with subjects with CLCR values of >40 ml/min, subjects whose CLCR values were ≤40 ml/min or who were on dialysis had significantly larger volumes of the central compartment. This factor was not significant in the population pharmacokinetic analysis, most likely because of the large variation in V1 and the relatively small number of subjects on dialysis. Vss was not dependent on renal function.
Relative to that in subjects with normal renal function (CLCR values of ≥80 ml/min), the daptomycin half-life was increased 2.3-fold in subjects with CLCR values of ≤40 ml/min and 3.5-fold in subjects who were on dialysis; changes in dose-normalized AUC(0-∞) values were 1.8-fold and 3-fold, respectively (Table 5). In comparison, median half-life and dose-normalized AUC(0-∞) values in subjects with CLCR values of ≥80 ml/min and in subjects with CLCR values of <80 and >40 ml/min differed <10%. These differences, although statistically significant, were not considered clinically meaningful.
DISCUSSION
Daptomycin is a novel lipopeptide antibiotic which was recently approved for the treatment of cSSSI caused by susceptible gram-positive microorganisms. It has a unique mechanism of action, and in vitro it demonstrates rapid, concentration-dependent bactericidal activity against drug-resistant clinical isolates of gram-positive microorganisms, a long postantibiotic effect, and a low rate of spontaneous resistance (4, 9, 11, 17). In vivo, daptomycin exhibits linear pharmacokinetics after both single and multiple once-daily doses (8, 20). Pharmacodynamic studies using a model of S. aureus thigh infections in neutropenic mice indicated that bacterial eradication best correlates with the ratio of AUC24 h to the MIC (12).
This report represents the first population pharmacokinetic analysis of daptomycin and includes subjects from all three phases of the clinical development program. The increased use of population pharmacokinetic analysis has generated a number of newer software programs that, like NONMEM, have advantages and disadvantages. One topic of discussion has been the ability and ease of use of NONMEM to detect the presence of a nonnormal distribution, especially within a subpopulation. The intelligent use of any pharmacokinetic program is a prerequisite for a meaningful analysis. NONMEM, when used by trained personnel, does allow one to detect a distribution that is substantially nonnormal.
Among healthy subjects, the estimated pharmacokinetics were consistent with those previously reported for a phase 1 study in which single doses of 0.5 to 6 mg of daptomycin/kg were administered intravenously to healthy volunteers (20). The population analysis defined quantitatively the decrease in daptomycin CL associated with reduced renal function, a relationship that was suggested by earlier phase 1 studies. New findings included the increase in the volume of the peripheral compartment (V2) in subjects with bacterial infections relative to healthy subjects as well as the associations between weight and both intercompartmental clearance (Q) and V2.
Renal function, sex, and body temperature accounted for 21.5% of the interindividual variability in daptomycin clearance, with renal function being the single most significant explanatory variable. During the screening of covariates, adding just renal function (i.e., CLCR in nondialysis subjects and a flag for subjects on dialysis) to the clearance model reduced the interindividual variability by 18.9%, from 52.1% in the base model (no covariates) to 33.2% (with the addition of renal function markers) (data not shown). This finding is consistent with the fact that daptomycin, like other hydrophilic antibiotics, is cleared primarily by renal excretion (20).
The median estimated daptomycin clearance for a normothermic male with an estimated CLCR of 91.2 ml/min was 0.807 liter/h (13.5 ml/min). Among subjects on dialysis, the median daptomycin clearance was estimated to be 0.269 liter/h (4.5 ml/min), or approximately one-third that of nondialysis subjects. Among subjects who were not on dialysis, daptomycin clearance was a linear function of CLCR. For example, for an increase or decrease in the estimated CLCR of 10 ml/min, the daptomycin clearance increased or decreased by 0.05 liter/h (0.8 ml/min).
The dose of daptomycin recommended for the treatment of cSSSI is 4 mg/kg administered by intravenous infusion once every 24 h for subjects with CLCR of ≥30 ml/min and once every 48 h for subjects with lower CLCR values, including those who are on dialysis. These recommendations are based on several observations in addition to the data presented in this report. Sica et al. (42nd ICAAC) determined mean Cmax and AUCss values among 44 subjects with graded renal impairment or who were undergoing dialysis. The Cmax was consistent for all subjects and the AUCss was similar in all subjects who had an estimated CLCR of >40 ml/min. For subjects with an estimated CLCR of ≤40 ml/min, the AUC was increased 2.33-fold compared to that for subjects with a CLCR of >80 ml/min. The two phase 3 trials for the treatment of cSSSI included a limited number of subjects with estimated CLCR values between 30 and 40 ml/min. There was no increase in adverse events attributed to daptomycin among these subjects; none participated in the pharmacokinetic studies reported here. Additional studies of the pharmacokinetics and safety of daptomycin in renally impaired subjects and in those undergoing dialysis are in progress.
Daptomycin clearance was influenced to a lesser extent by sex and body temperature. Clearance in females was estimated to be approximately 80% that of male subjects with similar renal function. Among subjects with cSSSI treated with daptomycin in two recent large phase 3 trials, there were no clinically or statistically significant differences between the success rates for males (n = 230) and females (n = 192) (74.8 versus 77.1%, respectively; 95% confidence intervals, −7.2 and 8.3) (data on file, Cubist Pharmaceuticals). Thus, although the difference in daptomycin clearance related to sex was statistically significant, it does not appear to be clinically meaningful.
The observation that daptomycin clearance increased with elevated body temperatures (>37.2°C) should be interpreted cautiously since the analysis was limited to data obtained from 100 subjects in the phase 2/3 clinical studies, of whom only 14% were hyperthermic (body temperature of ≥38°C).
Comorbidities, including diseases producing fluid accumulation (e.g., ascites and edema), diabetes, hypertension, and congestive heart failure, were not significantly correlated with daptomycin clearance. Medications that were tested for possible pharmacokinetic interactions with daptomycin included acidic drugs that are actively secreted in the renal tubule and drugs that are highly (>95%) bound to albumin (3, 13, 15). These had no effect on daptomycin pharmacokinetics.
The estimated increases in Q and V2 for daptomycin with increased body weights were consistent with the physicochemical properties of daptomycin and the physiologic effects of weight. Daptomycin appears to be restricted to the extracellular space which increases with body weight (15). Similarly, the extravascular distribution of daptomycin occurs via diffusion (15), which would also be facilitated by the increased fluid (water) associated with an increased body weight.
V2 was estimated to be approximately twofold larger in subjects with acute bacterial infections than in uninfected subjects. This is consistent with the pathophysiology of acute bacterial infections, which is characterized by an inflammatory response associated with increased vascular permeability and the collection of extracellular fluid at the site of infection. However, since bacterial infections were only present among subjects in the phase 2/3 clinical trials, it is also possible that this factor was a surrogate for another, possibly unmonitored, covariate or an unidentified systematic difference between the phase 1 and phase 2/3 clinical trials. Currently, there is no recommendation regarding increased doses of daptomycin for patients with exceptionally severe infections or impaired host defenses. A trial of daptomycin at 6 mg/kg intravenously once a day for the treatment of infective endocarditis due to S. aureus is in progress.
Although there was an appreciable variability in the estimates of V1, none of the covariates investigated was identified as a significant source of this variability. In a recently reported phase 1 study of daptomycin pharmacokinetics using subjects with graded renal insufficiencies and end-stage renal disease (Sica et al., 42nd ICAAC), the total volume of distribution for daptomycin was increased among subjects on hemodialysis. In the present larger study, this relationship was extended and further defined as an increase in V1 in subjects with CLCR values of ≤40 ml/min as well as in subjects on dialysis, but only in a supplemental analysis of variance (Table 5). This may be because of the relatively small proportion of subjects who were undergoing dialysis or perhaps because the effect represents another, possibly unmonitored, covariate. Additional studies of daptomycin pharmacokinetics in subjects on hemodialysis are in progress.
In conclusion, this population analysis of daptomycin pharmacokinetics indicates that renal function is the single most significant factor contributing to interindividual variabilities in daptomycin clearance. Because of their reduced daptomycin clearance, patients on dialysis and those with severe renal disease (CLCR of <30 ml/min) will require adjusted dosage regimens to achieve systemic exposures that are clinically and pharmacologically comparable to those seen in subjects with higher levels of renal function. Daptomycin clearance was also impacted by sex and body temperature. However, an analysis of clinical outcomes suggested that the variation associated with sex is not clinically meaningful. The relationship with body temperature should be interpreted cautiously since the analysis was limited to the subset of subjects from phase 2/3 clinical studies, of which only 14% were hyperthermic. The relationships between body weight and the rate and extent of extravascular distribution support the dosing of daptomycin on the basis of milligrams per kilogram of body weight.
Acknowledgments
This work was supported by and conducted under the auspices of Cubist Pharmaceuticals, Inc.
We acknowledge the cooperation and assistance of the subjects, investigators, and study personnel who participated in these trials and the support of our colleagues in the Cubist Clinical Department.
REFERENCES
- 1.Arbeit, R. D., D. Maki, F. P. Tally, E. Campanaro, B. I. Eisenstein, and the Daptomycin 98-01 and 99-01 Investigators. 2004. The safety and efficacy of daptomycin in the treatment of complicated skin and skin structure infections. Clin. Infect. Dis. 1673-1681. [DOI] [PubMed]
- 2.Beal, S. L., and L. B. Sheiner (ed.). 1988-1998. NONMEM users guides. Parts I to VIII. GloboMax LLC, Hanover, Md.
- 3.Brater, D. C., and P. Chennavasin. 1984. Effects of renal disease: pharmacokinetic considerations, p. 119-148. In L. Z. Benet, N. Massoud, and J. G. Gambertoglio (ed.), Pharmacokinetic basis for drug treatment. Raven Press, New York, N.Y.
- 4.Bush, L. M., J. A. Boscia, M. Wendeler, P. G. Pitsakis, and D. Kaye. 1989. In vitro postantibiotic effect of daptomycin (L146032) against Enterococcus faecalis and methicillin-susceptible and methicillin-resistant Staphylococcus aureus strains. Antimicrob. Agents Chemother. 33:1198-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canepari, P., M. Boaretti, M. Marlleo, and G. Satta. 1990. Lipoteichoic acid as a new target for activity of antibiotics. Antimicrob. Agents Chemother. 34:1220-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cockcroft, D. W., and M. H. Gault. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31-41. [DOI] [PubMed] [Google Scholar]
- 7.Critchley, I. A., D. C. Draghi, D. F. Sahm, C. Thornsberry, M. E. Jones, and J. A. Karlowsky. 2003. Activity of daptomycin against susceptible and multi-drug resistant gram-positive pathogens collected in the SECURE study (Europe) during 2000-2001. J. Antimicrob. Chemother. 51:639-649. [DOI] [PubMed] [Google Scholar]
- 8.Dvorchik, B. H., D. Brazier, M. F. DeBruin, and R. D. Arbeit. 2003. Daptomycin pharmacokinetics and safety following administration of escalating doses once daily to healthy subjects. Antimicrob. Agents Chemother. 47:1318-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuchs, P. C., A. L. Barry, and S. D. Brown. 2002. In vitro bactericidal activity of daptomycin against staphylococci. J. Antimicrob. Chemother. 49:467-470. [DOI] [PubMed] [Google Scholar]
- 10.Gibaldi, M., and D. Perrier. 1982. Pharmacokinetics, 2nd ed. Marcel Dekker, Inc., New York, N.Y.
- 11.Hanbeger, H., L. E. Nilsson, R. Mailer, and B. Isaksson. 1991. Pharmacodynamics of daptomycin and vancomycin on Enterococcus faecalis and Staphylococcus aureus demonstrated by studies of initial killing and postantibiotic effect and influence of Ca2+ and albumin on these drugs. Antimicrob. Agents Chemother. 35:1710-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Louie, A., P. Kaw, W. Liu, N. Jumbe, M. H. Miller, and G. L. Drusano. 2001. Pharmacodynamics of daptomycin in a murine thigh model of Staphylococcus aureus infection. Antimicrob. Agents Chemother. 45:845-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mackichan, J. J. 1992. Influence of protein binding and use of unbound free drug concentrations, p. 5-48. In W. E. Evans, J. J. Schentag, and W. J. Jusko (ed.), Applied pharmacokinetics—principles of therapeutic drug monitoring, 3rd ed. Applied Therapeutics Inc., Vancouver, Canada.
- 14.Mandema, J. W., D. Verotta, and L. B. Sheiner. 1992. Building population pharmacokinetic-pharmacodynamic models. I. Models for covariate effects. J. Pharmacokinet. Biopharm. 20:511-528. [DOI] [PubMed] [Google Scholar]
- 15.Rowland, M., and T. N. Tozer. 1995. Clinical pharmacokinetics: concepts and applications, 3rd ed. Williams and Wilkins, Baltimore, Md.
- 16.Sheiner, L. B., B. Rosenberg, and V. V. Marathe. 1977. Estimation of population characteristics of pharmacokinetic parameters from routine clinical data. J. Pharmacokinet. Biopharm. 5:445-479. [DOI] [PubMed] [Google Scholar]
- 17.Silverman, J. A., N. Oliver, T. Andrew, and T. Li. 2001. Resistance studies with daptomycin. Antimicrob. Agents Chemother. 45:1799-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wesson, K. M., D. S. Lerner, N. B. Silverberg, and J. M. Weinberg. 2003. Linezolid, quinupristin/dalfopristin and daptomycin in dermatology. Clin. Dermatol. 21:64-69. [DOI] [PubMed] [Google Scholar]
- 19.Wise, R., T. Gee, J. M. Andrews, B. Dvorchik, and G. Marshall. 2002. Pharmacokinetic and inflammatory fluid penetration of intravenous daptomycin in volunteers. Antimicrob. Agents Chemother. 46:31-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woodworth, J. R., E. H. Nyhart, G. L. Brier, J. D. Wolny, and H. R. Black. 1992. Single-dose pharmacokinetics and antibacterial activity of daptomycin, a new lipopeptide antibiotic, in healthy subjects. Antimicrob. Agents Chemother. 36:318-325. [DOI] [PMC free article] [PubMed] [Google Scholar]