Abstract
The emergence in the United States of resistance to expanded-spectrum cephalosporin (e.g., ceftriaxone) within the salmonellae has been associated primarily with three large (>100-kb) plasmids (designated types A, B, and C) and one 10.1-kb plasmid (type D) that carry the blaCMY-2 gene. In the present study, the distribution of these four known blaCMY-2-carrying plasmids among 35 ceftriaxone-resistant Salmonella isolates obtained from 1998 to 2001 was examined. Twenty-three of these isolates were Salmonella enterica serotype Newport, 10 were Salmonella enterica serotype Typhimurium, 1 was Salmonella enterica serotype Agona, and 1 was Salmonella enterica serotype Reading. All 23 serotype Newport isolates carried a type C plasmid, and 5, 4, and 1 serovar Typhimurium isolate carried type B, A, and C plasmids, respectively. Both the serotype Agona and serotype Reading isolates carried type A plasmids. None of the isolates carried a type D plasmid. Hybridization data suggested that plasmid types A and C were highly related replicons. DNA sequencing revealed that the region surrounding blaCMY-2 was highly conserved in all three plasmid types analyzed (types B, C, and D) and was related to a region surrounding blaCMY-5 from the Klebsiella oxytoca plasmid pTKH11. These findings are consistent with a model in which blaCMY-2 has been disseminated primarily through plasmid transfer, and not by mobilization of the gene itself, to multiple Salmonella chromosomal backbones.
Salmonellae are a leading cause of food-borne illness throughout the world. Although the worldwide incidence is not known, in the United States alone there are an estimated 1.4 million cases of salmonellosis each year. Nearly all of these cases (95%) are thought to be due to transmission of the bacteria in contaminated foods such as eggs, dairy products, produce, and meats (33). Typically, salmonellae cause a self-limiting gastroenteritis that does not require treatment with antibiotics. Antimicrobial agents are usually not essential for the treatment of patients with Salmonella infections; for such patients, antimicrobial agents may not reduce the severity of the gastrointestinal symptoms and may prolong the duration of the carrier state (2). Antimicrobial agents may be life-saving, however, for patients with severe invasive infection. Septicemia occurs in approximately 6% of the 30,000 to 32,000 culture-confirmed cases of salmonellosis in the United States annually (19, 33; http://www.cdc.gov/foodnet; http://www.cdc.gov/ncidod/dbmd/phlisdata/default.htm). Invasive infections commonly occur in children, particularly in infants. In the United States, 10% of culture-confirmed infections in which salmonellae were isolated from the blood or central nervous system occurred in infants ≤1 year old (22, 32). Expanded-spectrum cephalosporins (e.g., ceftriaxone and cefotaxime) are the antimicrobial agents of choice for invasive Salmonella infections of pediatric patients. Ceftriaxone is the expanded-spectrum cephalosporin used most often for pediatric patients, because its long half-life allows for a single daily administration, whereas other antibiotics require two to three daily doses. The fluoroquinolones (e.g., ciprofloxacin) are often used for treatment of salmonellosis in adults but are not approved for use in pediatric patients.
Decreased susceptibility (MIC, 16 to 32 μg/ml) or resistance (MIC, ≥64 μg/ml) to ceftriaxone within the salmonellae is a growing public health concern. Since 1991, Salmonella serotypes resistant to expanded-spectrum cephalosporins have been reported worldwide (1, 4, 9, 14, 35). From these isolates, a wide variety of β-lactamases belonging to Bush groups 1 and 2be have been described (10). In the United States before 1996, all reported cases of infection with ceftriaxone-resistant salmonellae were known or believed to be acquired abroad (19). However, the prevalence of ceftriaxone resistance (or decreased susceptibility) among Salmonella isolates from humans increased more than sevenfold from 1996 to 1998, from 1 of 1,272 (0.1%) isolates in 1996 to 5 of 2,205 (0.2%) isolates in 1997 and 9 of 1,466 (0.6%) in 1998 (14). Year-2001 data from the National Antimicrobial Resistance Monitoring System (NARMS) demonstrated that 3% of non-serotype Typhi Salmonella isolates from humans exhibited decreased susceptibility to ceftriaxone while 2% were resistant to ceftriaxone (12). A similar study performed in the state of Nebraska in 2000 demonstrated that decreased susceptibility to ceftriaxone reached 6.5% within the salmonellae and 18% among all Salmonella enterica serotype Typhimurium isolates (P. D. Fey, S. L. Greenwood, A. R. Sambol, P. C. Iwen, M. E. Rupp, T. J. Safranek, and S. H. Hinrichs, Abstr. 101st Gen. Meet. Am. Soc. Microbiol., abstr. 203, 2001).
In the United States, decreased susceptibility to ceftriaxone in domestically acquired Salmonella infections is almost exclusively mediated through the production of the Citrobacter freundii-derived blaCMY-2 cephamycinase (14, 17, 29, 36). Previous restriction fragment length polymorphism (RFLP) and Southern blot experiments have demonstrated that blaCMY-2 is carried on at least three separate large (>100-kb) plasmid replicons termed types A, B, and C as well as on a 10-kb plasmid described by Winokur et al. (11, 38). From 1996 to 1999, these blaCMY-2-carrying plasmids were isolated most frequently from serotype Typhimurium (14). However, since 2000, blaCMY-2-carrying plasmids have been isolated at a substantial rate from isolates representing an apparently highly related strain of Salmonella enterica serotype Newport (6, 17, 29). Decreased susceptibility to ceftriaxone was noted in 25% of all serotype Newport isolates collected from humans in 2001 through the NARMS program (12).
The purpose of this study was to determine the extent to which the four known blaCMY-2-carrying plasmid replicons were found in 35 ceftriaxone-resistant Salmonella isolates obtained throughout the United States from 1999 to 2001. In addition, the DNA sequence that surrounded blaCMY-2, which was found to be highly conserved, was analyzed for three separate plasmid replicons.
MATERIALS AND METHODS
Strains used in the study.
The 35 ceftriaxone-resistant isolates used in the study are listed in Table 1. The isolates were collected from 1998 to 2001 through either NARMS or the Nebraska Public Health Laboratory. Representative Escherichia coli transformants carrying type A, B, and C plasmids harboring blaCMY-2 were as follows: C6/pNF34 (type A), DH/pNF4656 (type C), and C6/pNF1358 (type B) (11). DH/pIW759 is a type D plasmid isolated from a Salmonella enterica serotype Heidelberg strain (porcine origin) in Iowa (37, 38).
TABLE 1.
Ceftriaxone-resistant Salmonella strains used in the study
| Strain | Serotype (PFGE type) | Antibiotic resistance phenotypea | E. coli backgroundb | Antibiotic resistance phenotype of E. colic | Plasmid type | State of origin | Yr of isolation |
|---|---|---|---|---|---|---|---|
| 1041 | Newport (A) | ACSSuTCroFx | D10 | Same as wild type | C | Nebr. | 1999 |
| 5313 | Newport (A) | ACSSuTCroFx | C | Minn. | 1999 | ||
| 5501 | Newport (A) | ACSSuTCroFx | DH | Same as wild type | C | N.J. | 1999 |
| 5653 | Newport (A) | ACSSuTCroFx | C | Fla. | 1999 | ||
| 7073 | Newport (A) | ACSSuTCroFx | D10 | Same as wild type | C | Mass. | 1999 |
| 7075 | Newport (A) | ACSSuTCroFx | C | Mass. | 1999 | ||
| 7079 | Newport (A) | ACSSuTCroFx | C | Mass. | 1999 | ||
| 7467 | Newport (A) | ACSSuTCroFx | C | Oreg. | 1999 | ||
| 8611 | Newport (A) | ACSSuTCroFx | D10 | Same as wild type | C | Tenn. | 2000 |
| 8683 | Newport (A) | ACSSuTCroFx | D10 | Same as wild type | C | Wash. | 2000 |
| 6163 | Newport (B) | ACSSuSxtTCroFx | C | Ga. | 1999 | ||
| 7040 | Newport (B) | ACSSuSxtTCroFx | D10 | All except Sxt | C | Mass. | 1999 |
| 7041 | Newport (B) | ACSSuSxtTCroFx | C | Mass. | 1999 | ||
| 5561 | Newport (C) | ACSSuTCroFx | C6 | Same as wild type | C | N.Y. | 1999 |
| 6100 | Newport (C) | ACSSuTCroFx | C | Calif. | 1999 | ||
| 712 | Newport (D) | ACSSuSxtTCroFx | D10 | All except Sxt | C | Nebr. | 1999 |
| 2231 | Newport (D) | ACSSuSxtTCroFx | D10 | Same as wild type | C | Nebr. | 2000 |
| 4962 | Newport (E) | ACSSuTCroFx | D10 | Same as wild type | C | Colo. | 1999 |
| 5299 | Newport (E) | ACSSuTCroFx | C | Colo. | 1999 | ||
| 5924 | Newport (F) | ACSSuTKCroFx | D10 | Same as wild type | C | Kans. | 1999 |
| 6980 | Newport (G) | ACSSuTCroFx | DH | Same as wild type | C | N.J. | 1999 |
| 10091 | Newport (H) | ACSSuTCroFx | D10 | Same as wild type | C | Md. | 2001 |
| 2050 | Newport (I) | ACSSuTCroFx | D10 | Same as wild type | C | Nebr. | 2000 |
| 5436 | Typhimurium | ACroFx | D10B | Same as wild type | B | Ga. | 1999 |
| 5655 | Typhimurium | ACSSuTCroFx | D10 | Same as wild type | C | Fla. | 1999 |
| 5678 | Typhimurium | ACSSuTCroFx | C6 | Same as wild type | A | Kans. | 1999 |
| 5927 | Typhimurium | ACSSuTCroFx | C6 | All except SSuT | B | Kans. | 1999 |
| 5965 | Typhimurium | ACroFx | C6 | Same as wild type | B | Conn. | 1999 |
| 6191 | Typhimurium | ACroFx | C6 | Same as wild type | B | Ga. | 1999 |
| 6516 | Typhimurium | ACroFx | C6 | Same as wild type | B | N.J. | 1999 |
| 1568 | Typhimurium | ACSSuTCroFx | D10 | Same as wild type | A | Nebr. | 2000 |
| 2000 | Typhimurium | ACSSuTKCroFx | D10 | Same as wild type | A | Nebr. | 2000 |
| 2232 | Typhimurium | ACSSuTCroFx | D10 | Same as wild type | A | Nebr. | 2000 |
| 2042 | Agona | ACSSuSxtTCroFx | D10 | Same as wild type | A | Nebr. | 2000 |
| 2151 | Reading | ACSSuTKCroFx | D10 | All except K | A | Nebr. | 2000 |
Abbreviations: A, ampicillin; C, chloramphenicol; S, streptomycin; Su, sulfisoxazole; Sxt, trimethoprim-sulfamethoxazole; T, tetracycline; K, kanamycin; Cro, ceftriaxone; Fx, cefoxitin.
Background to which plasmid was transferred via conjugation (C6), transformation (DH), or electroporation (D10).
“All except” indicates resistance to the same antimicrobial agents as the wild-type Salmonella strain, except for the particular antimicrobials noted. “Same as wild type” indicates resistance to the same antimicrobial agents as the wild-type Salmonella strain.
Plasmid transfer.
Plasmids carrying blaCMY-2 were transferred to E. coli through either transformation, conjugation, or electroporation. Transformation was performed with E. coli DH5α (Invitrogen, Carlsbad, Calif.) according to the manufacturer's recommendations, while E. coli DH10B (Invitrogen) was electroporated by using the method described by Sheng et al. (31). Conjugation was performed by using E. coli C600N (ampicillin susceptible, nalidixic acid resistant) as previously described (3, 27). Transformants and electroporants were selected on Luria-Bertani agar (Difco, Detroit, Mich.) containing 50 μg of ampicillin (Sigma, St. Louis, Mo.)/ml. All blaCMY-2 C600N, DH5α, and DH10B transconjugants, transformants, or electroporants were named either C6, DH, or D10 (standing for C600N, DH5α, or DH10B, respectively) followed by the appropriate wild-type Salmonella strain designation. Antimicrobial susceptibility testing of Salmonella and E. coli, as well as E. coli ATCC 25922 (quality control strain), was performed by using disk diffusion according to NCCLS standards (25). The following antimicrobial agents were tested: ampicillin, cefoxitin, ciprofloxacin, tetracycline, chloramphenicol, kanamycin, streptomycin, gentamicin, sulfamethoxazole, trimethoprim-sulfamethoxazole, and nalidixic acid. Susceptibility to ceftriaxone was determined by using the E-test according to the manufacturer's recommendations (AB-Biodisk, Solna, Sweden).
DNA sequencing.
The plasmid-harbored blaCMY-2 regions from pNF4656 and pNF1358 were cloned and sequenced by first preparing plasmid DNA from E. coli DH/pNF4656 and E. coli C6/pNF1358. DNA was extracted by using a QIAGEN (Valencia, Calif.) large-construct kit. The DNA was air sheared into 1.0- to 1.5-kb fragments according to the manufacturer's recommendations (Invitrogen) and cloned into the pCR 4 blunt-TOPO vector (Invitrogen). The genomic library was transformed into E. coli Top10 cells (Invitrogen), and random clones (approximately 4× coverage) were sequenced on a LiCor (Lincoln, Nebr.) 4000 sequencer by using IR800-labeled T3 and T7 primers (13, 21). Contigs were generated by using Vector NTI Contig Express software (Informax, North Bethesda, Md.).
Molecular methods.
All restriction enzymes used in the study were purchased from Invitrogen. Southern blot hybridizations were performed by standard methods (30) with a blaCMY-2-specific DNA probe labeled with digoxigenin ddUTP (Roche, Indianapolis, Ind.). The primers used to amplify a blaCMY-specific probe, or to detect the gene, have been described previously (11). The primers shown in Table 2 were used to amplify the junction regions between the plasmid sequence and ISEcp1 upstream of blaCMY-2 and between the plasmid sequence and sugE (type B) or ecnR (types C and D) downstream of blaCMY-2 in pNF1358 (type B), pNF4656 (type C), and pIW759 (type D).
TABLE 2.
Primers used to amplify junction region between each plasmid replicon and the blaCMY-2 region
| Region | Primer sequencea | Amplicon size (bp) |
|---|---|---|
| pNF1358 upstream junction (type B) | For, GTCATCAGACCTGTGCG | 505 |
| Rev, CGGAAATATCAAACTCGT | ||
| pNF1358 downstream junction (type B) | For, GTGTATTTCAGGCCAATCGC | 659 |
| Rev, CTGGGTATTCTCTGGTGCA | ||
| pNF4656 upstream junction (type C) | For, GACACCTTGCCGTTAATC | 523 |
| Rev, CGGAAATATCAAACTCGT | ||
| pNF4656 downstream junction (type C) | For, GCGAGACGCACTCCAGTC | 1,378 |
| Rev, CTGTTGCAAATAGTCGGTG | ||
| pIW759 upstream junction (type D) | For, CGCTGAACATGAAAGGA | 706 |
| Rev, CGGAAATATCAAACTCGT | ||
| pIW759 downstream junction (type D) | For, GCGAGACGCACTCCAGTC | 641 |
| Rev, GCTGTATACGCAGTGCTTT |
Abbreviations: For; forward primer. Rev; reverse primer.
PFGE.
Genomic DNA suitable for pulsed-field gel electrophoresis (PFGE) was prepared according to standard methods outlined by PulseNet (15, 34). Salmonella enterica serotype Braenderup H9812 was used as a standard. The DNA embedded in agarose was digested with XbaI and electrophoresed on a CHEF DR-III instrument (Bio-Rad, Richmond, Calif.) by using the following conditions: initial switching time, 2.2 s; final switching time, 63.8 s; total time, 19 h. Salmonella plasmid DNA was digested with PstI and electrophoresed by using the following conditions: initial switching time, 0.1 s; final switching time, 12 s; total time, 6 h. The RFLP patterns were compared by using Bionumerics software (Applied Maths, Kortrijk, Belgium) with a 0.75% molecular weight position tolerance.
Nucleotide sequence accession numbers.
The GenBank accession numbers for ISEcp1 and the C. freundii ampR and ampC region are AY125469 and AY125469, respectively. The accession numbers for the DNA sequences of the blaCMY-2 regions from pIW759, pNF1358, and pNF4656 are AY581205, AY581206, and AY581207, respectively.
RESULTS
Plasmid typing.
In a previously published study, blaCMY-2-carrying plasmids isolated from three Salmonella serotypes were placed into three categories (A, B, and C) based on RFLP and Southern blot analyses (11). Thirty-five additional ceftriaxone-resistant isolates were obtained from the NARMS program (n = 26) and the Nebraska Public Health Laboratory (n = 9) from 1999 to 2001 (Table 1). Twenty-three of these isolates belonged to serotype Newport, 10 belonged to serotype Typhimurium, and 1 each belonged to serotypes Agona and Reading. All 35 of the isolates were resistant to cefoxitin and ampicillin as well as ceftriaxone (Table 1). Thirty-one (88.5%) of 35 isolates were also resistant to chloramphenicol, streptomycin, sulfisoxazole, and tetracycline. Three (8.6%) and six (17.1%) were resistant to kanamycin and trimethoprim-sulfamethoxazole, respectively. None of the isolates were resistant to ciprofloxacin, gentamicin, or nalidixic acid. PCR experiments demonstrated that all Salmonella isolates encoded a blaCMY-2-like β-lactamase, as predicted (data not shown) (11, 14).
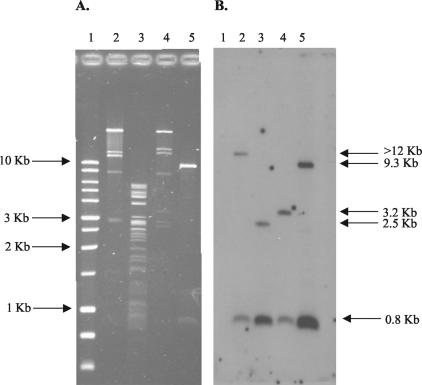
Plasmid transfer experiments were performed for all 10 serotype Typhimurium isolates, isolate 2042 (serotype Agona), isolate 2151 (serotype Reading), and 14 serotype Newport isolates (representing the nine PFGE groups delineated; see below) by using either conjugation, transformation, or electroporation (Table 1). For all 26 Salmonella isolates, we were able to transfer plasmid DNA resulting in decreased ceftriaxone susceptibility to E. coli (either C600N, DH5α, or DH10B) by one of the three methods of transfer. PFGE demonstrated that all E. coli transconjugants, transformants, and electroporants (referred to collectively as transformants below) were of the appropriate genomic lineage and were not contaminants (data not shown). Plasmid DNA was isolated from the E. coli transformants, digested with PstI, electrophoresed by PFGE, and analyzed by Southern hybridization using blaCMY-2 as a probe. The resulting RFLP patterns from all transformants were consistent with either a type A, B, or C plasmid as previously described by Carattoli et al. (11). None of the isolates carried a type D plasmid. Figure 1 shows a PstI digest and a subsequent Southern blot after hybridization with blaCMY-2 of the four known plasmid types (A to D). The blaCMY-2 probe hybridizes to an 800-bp fragment in all four plasmid types, whereas it hybridizes to a band of >12 kb in type A plasmids, a 2.5-kb band in type B plasmids, a 3.2-kb band in type C plasmids, and a 9.3-kb band in type D plasmids. Four of the 10 serotype Typhimurium E. coli transformants carried type A plasmids, 5 carried type B plasmids, and 1 carried a type C plasmid. Both D10/2151 (serotype Reading) and D10/2042 (serotype Agona) carried type A plasmids, whereas all serotype Newport E. coli transformants carried type C plasmids. All E. coli transformants that carried type A or type C plasmids were resistant to multiple classes of antibiotics, whereas those that carried type B plasmids were resistant only to β-lactam antibiotics (ceftriaxone, cefoxitin, and ampicillin) (Table 1). In 22 of 26 isolates, the E. coli transformants were resistant to all of the same antibiotics as wild-type Salmonella.
FIG. 1.
(A) PstI digest of the four known blaCMY-2-containing plasmid types. Lanes: 1, 1-kb ladder (Promega, Madison, Wis.); 2, C6/pNF34 (type A); 3, C6/pNF1358 (type B); 4, DH/pNF4656 (type C); 5, DH/pIW759 (type D). Sizes of the DNA ladder markers are given on the left. (B) Subsequent Southern hybridization of the gel in panel A probed with full-length blaCMY-2. Sizes of bands hybridizing to the blaCMY-2 DNA probe, as explained in the text, are given on the right.
PFGE.
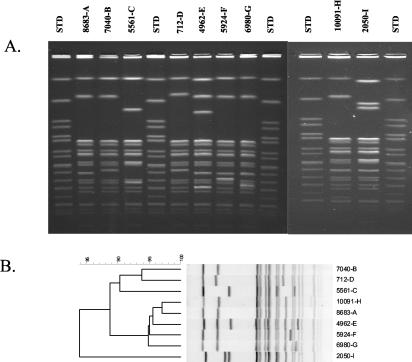
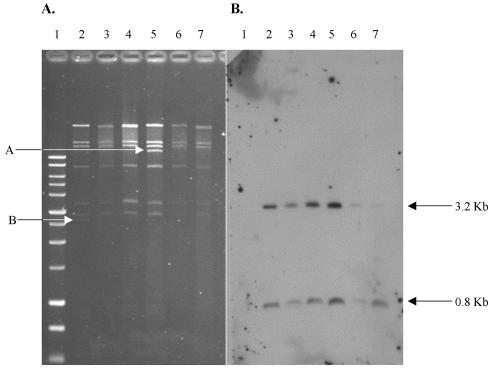
PFGE was performed on all ceftriaxone-resistant serotype Typhimurium and serotype Newport isolates. All serotype Typhimurium isolates had divergent PFGE patterns, as previously demonstrated with the 1996-to-1998 ceftriaxone-resistant isolates (data not shown) (14). In contrast, PFGE patterns were highly related for all serotype Newport isolates, as has been reported previously (Fig. 2) (6, 17, 29). Among the 23 serotype Newport isolates, nine distinguishable but similar RFLP patterns were identified (A to I) (Table 1). The most highly represented PFGE group was group A, which consisted of 10 isolates; group B consisted of 3 isolates, and groups C, D, and E consisted of 2 isolates each. The remaining isolates (n = 4) had unique PFGE patterns (F to I). Plasmid typing demonstrated that, regardless of PFGE type, the blaCMY-2-carrying type C plasmids found in all serotype Newport isolates were very similar. Figure 3 shows plasmid DNA following digestion with PstI and a Southern blot using blaCMY-2 as a probe for five serotype Newport transformants (representing four of nine PFGE groups). Note that the only detectable difference between these plasmids is a ∼11- to 12-kb band in C6/5561 (Fig. 3A, lane 5) and a 2.7-kb band in DH/pNF4656 (Fig. 3A, lane 2). For those isolates of serotype Newport for which no attempt was made to isolate a transformant with decreased susceptibility to ceftriaxone, wild-type plasmid DNA was isolated and digested with PstI. In all cases, Southern blot analysis with a blaCMY-2 probe demonstrated that each serotype Newport isolate carried a type C plasmid.
FIG. 2.
(A) PFGE RFLP patterns of serotype Newport isolates resistant to expanded-spectrum cephalosporin. The strain number, followed by the PFGE group designation, is given above each lane. STD, standard. (B) Dendrogram of RFLP patterns shown in panel A following normalization and analysis with Bionumerics software. Strain numbers, followed by PFGE group designations, are given on the right.
FIG. 3.
(A) PstI digest of serotype Newport type C plasmids. Lanes: 1, 1-kb ladder (Promega); 2, DH/pNF4656; 3, D10/4962 (PFGE group E); 4, DH/5501 (PFGE group A); 5, C6/5561 (PFGE group C); 6, D10/7040 (PFGE group B); 7, D10/7073 (PFGE group A). Arrow A indicates a ∼11- to 12-kb band in C6/5561, and arrow B indicates a 2.7-kb band in DH/pNF4656; these bands constitute the only differences in the plasmid RFLP patterns, as discussed in the text. (B) Subsequent Southern hybridization of the gel in panel A probed with full-length blaCMY-2. Sizes of bands hybridizing to the blaCMY-2 probe, as explained in the text, are given on the right.
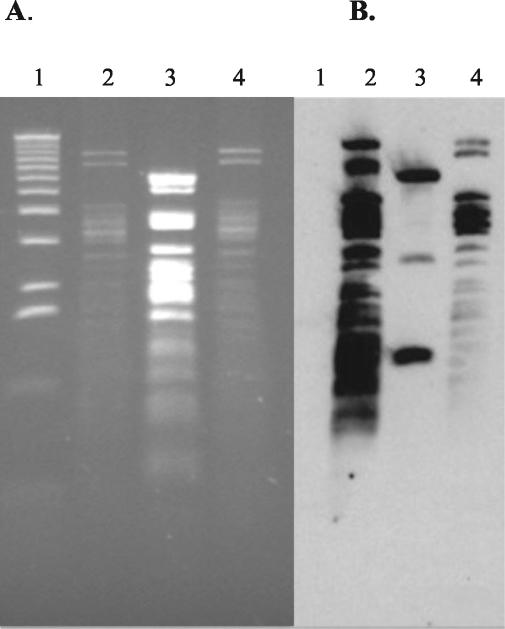
Relatedness of plasmid types A and C.
Due to the highly similar PstI RFLP patterns of the type A and C plasmids, it was hypothesized that they were related replicons. To address this question, plasmid DNA was prepared from C6/pNF34 (type A) and DH/pNF4656 (type C) and digested with HincII. HincII-digested C6/pNF1358 was used as a negative control. As predicted, the HincII RFLP patterns were similar for type A and C plasmids, whereas that for type B was divergent (Fig. 4). Southern blot analysis, using pNF4656 as a probe, demonstrated that pNF4656 hybridized to each distinguishable HincII band in pNF34. In contrast, pNF4656 hybridized to only three HincII bands from pNF1358.
FIG. 4.
(A) HincII digest of plasmid types A, B, and C. Lanes; 1, 1-kb ladder (BRL, Bethesda, Md.); 2, C6/pNF34 (type A); 3, C6/pNF1358 (type B); 4, DH/pNF4656. (B) Subsequent Southern hybridization of gel in panel A probed with whole pNF4656 DNA.
DNA sequence of region surrounding blaCMY-2.
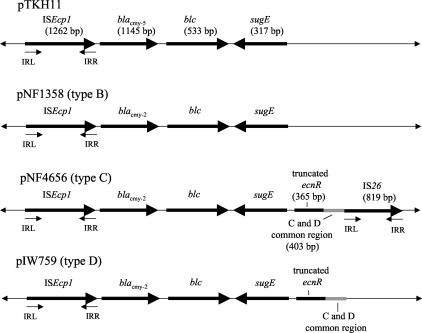
Plasmid libraries from DH/pNF4656 and C6/pNF1358 were generated by using the pCR4 blunt-TOPO vector, and ∼500 clones were sequenced for both the DH/pNF4656 and C6/pNF1358 libraries (∼4× coverage). In contrast, the DNA sequence of the blaCMY-2 region from pIW759 was generated by using primers within blaCMY-2, and sequencing was carried out from the native plasmid in both directions. Subsequent DNA sequencing primers were then constructed through the DNA sequence generated. After the blaCMY-2 gene and the regions surrounding blaCMY-2 were identified, it became apparent that these regions were highly conserved in all three plasmid types and had the same general organization as that already described for pTKH11, a plasmid carrying blaCMY-5 isolated from Klebsiella oxytoca (Fig. 5) (39). Including ISEcp1 (see below) upstream of blaCMY-2 to the 5′ end of the blc gene, there was 100% sequence identity among all three plasmid types and 99.3% sequence identity to the same region in pTKH11.
FIG. 5.
Map of blaCMY-2 regions from plasmid types B, C, and D in comparison with that from pTKH11. IRL, left inverted repeat; IRR, right inverted repeat. blc, sugE, and ecnR encode bacterial lipocalin, a small multidrug resistance protein, and the response regulator for the enterocidin gene, respectively.
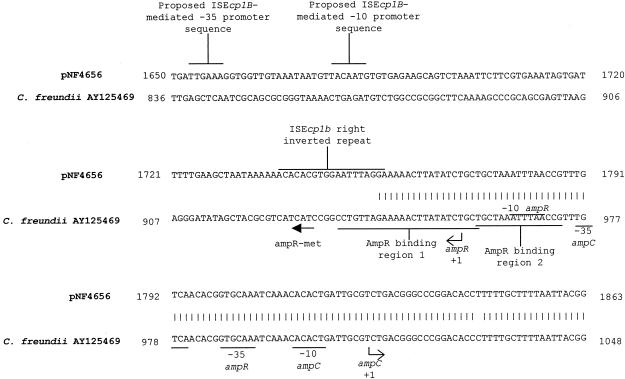
The sequences of all three plasmids immediately downstream of blaCMY-2 contained open reading frames (ORFs) that had 96.3 and 96.9% sequence identity, respectively, to blc and sugE, two genes found just downstream of the C. freundii chromosomal blaampC gene. In C. freundii, blc encodes an outer membrane lipoprotein whereas sugE is hypothesized to be a member of the small multidrug resistance (SMR) family of multidrug efflux systems (8, 16, 26). Therefore, neither of these genes is thought to be involved in blaCMY-2 regulation (39). Upstream and adjacent to sugE in the type C and D plasmids is partial sequence (364 bp) of an ORF that has 96.7% sequence identity to ecnR (599 bp), a gene which is found just upstream of sugE in the C. freundii chromosome (7). ecnR encodes the response regulator for ecnAB, which acts as an antidote-toxin gene pair involved in programmed cell death. Adjacent to and downstream of the truncated ecnR, there is a 405-bp region that is shared by type C and D plasmids. No significant identity to any known bacterial DNA sequence was found in this region. Adjacent to this common region, an IS26 insertion sequence was found in the type C plasmid but not in the type D plasmid (23). BLAST searches demonstrated that DNA sequence generated from pIW759 outside of the blaCMY-2 region was not found in the DNA sequence from pNF4656 or pNF1358, suggesting that the type D plasmid is an independent replicon and was not once part of a composite plasmid with either a type B or a type C plasmid. Located just upstream of blaCMY-2 is an ORF with 99.8% sequence identity with ISEcp1 (20). ISEcp1 is inserted at bp 942 of the C. freundii ampR-ampC promoter region. The region into which ISEcp1 inserts is known to be an AmpR binding region (Fig. 6). The DNA sequence is divergent in all three plasmid replicons immediately adjacent to the left inverted repeat of ISEcp1.
FIG. 6.
Comparison of region upstream of blaCMY-2 in pNF4656 and the ampR-ampC promoter region from C. freundii.
blaCMY-2 region junction.
By using known sequence upstream and downstream of the blaCMY-2-carrying region (Fig. 5), primers were generated (Table 2) to amplify the junction regions between the plasmid sequence and ISEcp1 on one end (left end) and sugE (type B) or ecnR (types C and D) and the plasmid sequence on the other (right end). Because sequence downstream of IS26 was not available (sequencing of pNF4656 is not complete), the right-end junction primer for the type C plasmid was designed from sequence within IS26. Specific left-end primers designed from pNF4656 (type C) generated the expected products in all 23 type C plasmids and all 6 type A plasmids (data not shown). In contrast, the right-end primers designed from pNF4656 did not amplify an appropriate product in any of the 23 type C plasmids or in any of the 6 type A plasmids. Both right-end and left-end primers designed from pNF1358 amplified the expected products in all five type B plasmids. Left-end and right-end primers specific for pIW759 (type D) did not amplify a product in any type A, B, or C plasmid (data not shown).
DISCUSSION
The purpose of this study was to determine the extent to which the four characterized plasmid replicons that carry blaCMY-2 were found within 35 expanded-spectrum cephalosporin-resistant salmonellae collected from 1999 to 2001. These four plasmid types (types A to D) have been characterized previously in two separate studies (11, 38). In the present study, transfer of blaCMY-2 through conjugation to E. coli C600N was predictably successful only when a type B plasmid was being transferred. Conjugal transfer of type A and C plasmids was less successful, and transfer frequencies were extremely low (10−8). It is not known whether the majority of type A and C plasmids were defective in conjugal transfer or whether these observations are an experimental artifact. It was determined, however, that electroporation using the method of Sheng et al. (31) was an extremely reliable method of transferring blaCMY-2-containing plasmids from salmonellae to E. coli DH10B.
The observation that similar blaCMY-2-carrying plasmids were observed in two temporally distinct strain sets (the 1996-to-1998 set and the present strain set of 1999 to 2001) suggests that blaCMY-2 has moved to separate genomic backgrounds through plasmid transfer, as opposed to transfer of the element itself to separate plasmids or the chromosome (11). Within serotype Typhimurium, the most prevalent serotype isolated from humans, three plasmid types (types A, B, and C) were found in multiple genomic backgrounds. In contrast, only one blaCMY-2-containing plasmid type, type C, was found within the population of highly related ceftriaxone-resistant serotype Newport isolates. No isolates were found to contain a type D plasmid. Preliminary data suggest that the smaller pIW759-like plasmids are more common within E. coli populations than within salmonellae (P.L. Winokur, unpublished data). Since our Southern hybridization data suggest that plasmid types A and C are highly related, it appears that two variants of the same plasmid are mostly responsible for the dissemination of blaCMY-2 within ceftriaxone-resistant salmonellae in the United States (30 of 35 isolates belong to either type A or type C). Primers designed to identify the junction regions between the blaCMY-2 region and plasmid sequence demonstrate that the junction near ISEcp1 is conserved in type A and C plasmids. However, downstream, near ecnR, all 30 type A or C plasmids diverge from the representative type C plasmid, pNF4656. This result is not unexpected, since one of the primers used to amplify the right-end junction from pNF4656 was designed within a mobile element, IS26. Further DNA sequencing is needed to determine whether the right-end junction sequence within type A and C plasmids is conserved. One potential reason for the RFLP differences seen between type A and C plasmids is the fact that type A plasmids contain at least twice as many IS26 elements as type C plasmids (data not shown).
Recently, Poirel et al. reported that ISEcp1B, an insertion sequence element highly related to ISEcp1, was found upstream of blaCTX-M-19 in a Klebsiella pneumoniae isolate obtained in Vietnam (28). It was hypothesized, but not experimentally demonstrated, that ISEcp1B was responsible for the movement of blaCTX-M-19, because a separate inverted repeat with consistent nucleotide identity to the right inverted repeat of ISEcp1B was found downstream of blaCTX-M-19. In addition, primer extension studies demonstrated the presence of a promoter element just upstream of the right inverted repeat that directed the transcription of blaCTX-M-19. Therefore, ISEcp1B was hypothesized to be responsible not only for the movement of blaCTX-M-19 but also for its expression.
The DNA sequence comparison between the ampR-ampC intergenic promoter region in C. freundii and the region upstream of blaCMY-2 in pNF4656 is shown in Fig. 6. This analysis demonstrated that ISEcp1 inserted into AmpR binding region 1 in pNF4656. DNA binding studies have demonstrated that AmpR binds to region 1 but binds to region 2 only in the presence of region 1 (5, 18). These data strongly suggest that AmpR would not be able to activate or induce transcription from blaCMY-2 in pNF4656 if present. The proposed promoter from C. freundii blaampC is present in pNF4656, but it is not known what effect ISEcp1 has on its function. The promoter sequence found within ISEcp1B, which has been shown to drive the transcription of blaCTX-M-19, is also found in ISEcp1 upstream of blaCMY-2. The effect of this putative promoter sequence on blaCMY-2 transcription is unknown and warrants further study. In contrast to what was found for the relationship of ISEcp1B and blaCTX-M-19, no separate inverted repeat was found downstream of the blaCMY-2 region in any of the three plasmids sequenced. Multiple experiments attempting to demonstrate mobility of the blaCMY-2 region from pNF1358 to other plasmids were not successful (data not shown).
In conclusion, this work demonstrates that blaCMY-2 is spread primarily through plasmid transfer and not through the mobilization of a transposon or integron to multiple plasmid replicons and chromosomes. These findings were unexpected, because Morosini and colleagues have demonstrated that salmonellae carrying a plasmid-encoded, Enterobacter cloacae-derived AmpC β-lactamase had a reduction in growth rate and were less invasive by an in vitro cell invasion assay (24). The pathogenesis of Salmonella spp. is dependent on the organism's ability to adhere to and invade intestinal epithelial cells. Therefore, one would predict that the prevalence of AmpC-producing salmonellae would be very low, because these strains would be less physiologically fit. Alternatively, the predicted decreased fitness may be counterbalanced by the selective pressure of antimicrobial usage, compensatory mutations within certain genomic backgrounds of Salmonella, varying effects on invasiveness and/or fitness associated with different species-derived AmpC β-lactamases, and/or regulation of the blaampC gene itself (i.e., promoter strength).
ADDENDUM IN PROOF
Hossain et al. have recently described an S. enterica serotype Typhimurium strain that carried a blaCMY-7 gene (A. Hossain, M. D. Reisbig, and N. D. Hanson, J. Antimicrob. Chemother. 53: 964-970, 2004). A promoter within an ISEcp1-like element, which was located just upstream of blaCMY-7 at the identical nucleotide position as described in the present study, was shown to drive transcription of blaCMY-7.
Acknowledgments
W.P.G. is supported through a USDA National Needs Fellowship.
REFERENCES
- 1.Allen, K. J., and C. Poppe. 2002. Occurrence and characterization of resistance to extended-spectrum cephalosporins mediated by β-lactamase CMY-2 in Salmonella isolated from food-producing animals in Canada. Can. J. Vet. Res. 66:137-144. [PMC free article] [PubMed] [Google Scholar]
- 2.Aserkoff, B., and J. V. Bennett. 1969. Effect of antibiotic therapy in acute salmonellosis on the fecal excretion of salmonellae. N. Engl. J. Med. 281:636-640. [DOI] [PubMed] [Google Scholar]
- 3.Bachman, B. 1987. Derivations and genotypes of some mutant derivatives of Escherichia coli K-12, p. 1190-1219. In F. C. Neidhardt, J. L. Ingraham, K. B. Low, B. Magasanik, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology, vol. 1. American Society for Microbiology, Washington, D.C.
- 4.Barnaud, G., G. Arlet, C. Verdet, O. Gaillot, P. H. Lagrange, and A. Philippon. 1997. Salmonella enteritidis: AmpC plasmid-mediated inducible β-lactamase (DHA-1) with an ampR gene from Morganella morganii. Antimicrob. Agents Chemother. 42:2352-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartowsky, E., and S. Normark. 1993. Interactions of wild-type and mutant AmpR of Citrobacter freundii with target DNA. Mol. Microbiol. 10:555-565. [DOI] [PubMed] [Google Scholar]
- 6.Berge, A. C., J. M. Adaska, and W. M. Sischo. 2004. Use of antibiotic susceptibility patterns and pulsed-field gel electrophoresis to compare historic and contemporary isolates of multidrug-resistant Salmonella enterica subsp. enterica serovar Newport. Appl. Environ. Microbiol. 70:318-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bishop, R. E., B. K. Leskiw, R. S. Hodges, C. M. Kay, and J. H. Weiner. 1998. The entericidin locus of Escherichia coli and its implications for programmed bacterial cell death. J. Mol. Biol. 280:583-596. [DOI] [PubMed] [Google Scholar]
- 8.Bishop, R. E., S. S. Penfold, L. S. Frost, J. V. Holtje, and J. H. Weiner. 1995. Stationary phase expression of a novel Escherichia coli outer membrane lipoprotein and its relationship with mammalian apolipoprotein D. Implications for the origin of lipocalins. J. Biol. Chem. 270:23097-23103. [DOI] [PubMed] [Google Scholar]
- 9.Bradford, P. A., Y. Yang, D. Sahm, I. Grope, D. Gardovska, and G. Storch. 1998. CTX-M-5, a novel cefotaxime-hydrolyzing β-lactamase from an outbreak of Salmonella typhimurium in Latvia. Antimicrob. Agents Chemother. 42:1980-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bush, K., G. A. Jacoby, and A. A. Medeiros. 1995. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob. Agents Chemother. 39:1211-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carattoli, A., F. Tosini, W. P. Giles, M. E. Rupp, S. H. Hinrichs, F. J. Angulo, T. J. Barrett, and P. D. Fey. 2002. Characterization of plasmids carrying CMY-2 from expanded-spectrum cephalosporin-resistant Salmonella strains isolated in the United States between 1996 and 1998. Antimicrob. Agents Chemother. 46:1269-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. 2003. National Antimicrobial Resistance Monitoring System: enteric bacteria. Human Isolates Final Report, 2001. Centers for Disease Control and Prevention, Atlanta, Ga.
- 13.Dunn, J. J., and F. W. Studier. 1983. Complete nucleotide sequence of bacteriophage T7 DNA and the locations of T7 genetic elements. J. Mol. Biol. 166:477-535. [DOI] [PubMed] [Google Scholar]
- 14.Dunne, E. F., P. D. Fey, P. Kludt, R. Reporter, F. Mostashari, P. Shillam, J. Wicklund, C. Miller, B. Holland, K. Stamey, T. J. Barrett, J. K. Rasheed, F. C. Tenover, E. M. Ribot, and F. J. Angulo. 2000. Emergence of domestically acquired ceftriaxone-resistant Salmonella infections associated with AmpC β-lactamase. JAMA 284:3151-3156. [DOI] [PubMed] [Google Scholar]
- 15.Gautom, R. 1997. Rapid pulsed-field gel electrophoresis protocol for typing of Escherichia coli O157:H7 and other gram-negative organisms in 1 day. J. Clin. Microbiol. 35:2977-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greener, T., D. Govezensky, and A. Zamir. 1993. A novel multicopy suppressor of a groEL mutation includes two nested open reading frames transcribed from different promoters. EMBO J. 12:889-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta, A., J. Fontana, C. Crowe, B. Bolstorff, A. Stout, S. Van Duyne, M. P. Hoekstra, J. M. Whichard, T. J. Barrett, and F. J. Angulo. 2003. Emergence of multidrug-resistant Salmonella enterica serotype Newport infections resistant to expanded-spectrum cephalosporins in the United States. J. Infect. Dis. 188:1707-1716. [DOI] [PubMed] [Google Scholar]
- 18.Hanson, N. D., and C. C. Sanders. 1999. Regulation of inducible AmpC β-lactamase expression among Enterobacteriaceae. Curr. Pharm. Des. 5:881-894. [PubMed] [Google Scholar]
- 19.Herikstad, H., P. S. Hayes, J. Hogan, P. Floyd, L. Snyder, and F. J. Angulo. 1997. Ceftriaxone-resistant Salmonella in the United States. Pediatr. Infect. Dis. J. 9:904-905. [DOI] [PubMed] [Google Scholar]
- 20.Karim, A., L. Poirel, S. Nagarajan, and P. Nordmann. 2001. Plasmid-mediated extended-spectrum β-lactamase (CTX-M-3 like) from India and gene association with insertion sequence ISEcp1. FEMS Microbiol. Lett. 201:237-241. [DOI] [PubMed] [Google Scholar]
- 21.McGraw, N. J., J. N. Bailey, G. R. Cleaves, D. R. Dembinski, C. R. Gocke, L. K. Joliffe, R. S. MacWright, and W. T. McAllister. 1985. Sequence and analysis of the gene for bacteriophage T3 RNA polymerase. Nucleic Acids Res. 13:6753-6766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mollet, B., S. Iida, J. Shepherd, and W. Arber. 1983. Nucleotide sequence of IS26, a new prokaryotic mobile genetic element. Nucleic Acids Res. 11:6319-6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morosini, M. I., J. A. Ayala, F. Baquero, J. L. Martinez, and J. Blazquez. 2000. Biological cost of AmpC production for Salmonella enterica serotype Typhimurium. Antimicrob. Agents Chemother. 44:3137-3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.NCCLS. 2001. Performance standards for antimicrobial disk and dilution susceptibility tests. Approved standard M2-A7. NCCLS, Wayne, Pa.
- 26.Paulsen, I. T., R. A. Skurray, R. Tam, M. H. Saier, Jr., R. J. Turner, J. H. Weiner, E. B. Goldberg, and L. L. Grinius. 1996. The SMR family: a novel family of multidrug efflux proteins involved with the efflux of lipophilic drugs. Mol. Microbiol. 19:1167-1175. [DOI] [PubMed] [Google Scholar]
- 27.Pitout, J., K. Thomson, N. Hanson, A. Ehrhardt, E. Moland, and C. C. Sanders. 1998. β-Lactamases responsible for resistance to expanded-spectrum cephalosporins in Klebsiella pneumoniae, Escherichia coli, and Proteus mirabilis isolates recovered in South Africa. Antimicrob. Agents Chemother. 42:1350-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poirel, L., J. W. Decousser, and P. Nordmann. 2003. Insertion sequence ISEcp1B is involved in expression and mobilization of a blaCTX-M β-lactamase gene. Antimicrob. Agents Chemother. 47:2938-2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rankin, S. C., H. Aceto, J. Cassidy, J. Holt, S. Young, B. Love, D. Tewari, D. S. Munro, and C. E. Benson. 2002. Molecular characterization of cephalosporin-resistant Salmonella enterica serotype Newport isolates from animals in Pennsylvania. J. Clin. Microbiol. 40:4679-4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 31.Sheng, Y., V. Mancino, and B. Birren. 1995. Transformation of Escherichia coli with large DNA molecules by electroporation. Nucleic Acids Res. 23:1990-1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sirinavin, S., P. Jayanetra, and A. Thakkinstian. 1999. Clinical and prognostic categorization of extraintestinal nontyphoidal Salmonella infections in infants and children. Clin. Infect. Dis. 29:1151-1156. [DOI] [PubMed] [Google Scholar]
- 33.Stutman, H. 1994. Salmonella, Shigella, and Campylobacter: common bacterial causes of infectious diarrhea. Pediatr. Ann. 23:538-543. [DOI] [PubMed] [Google Scholar]
- 34.Swaminathan, B., T. J. Barrett, S. B. Hunter, and R. V. Tauxe. 2001. PulseNet: the molecular subtyping network for foodborne bacterial disease surveillance, United States. Emerg Infect. Dis. 7:382-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Threlfall, E. J., J. A. Skinner, A. Graham, L. R. Ward, and H. R. Smith. 2000. Resistance to ceftriaxone and cefotaxime in non-typhoidal Salmonella enterica in England and Wales, 1998-99. J. Antimicrob. Chemother. 46:860-862. [DOI] [PubMed] [Google Scholar]
- 36.White, D. G., S. Zhao, R. Sudler, S. Ayers, S. Friedman, S. Chen, P. F. McDermott, S. McDermott, D. D. Wagner, and J. Meng. 2001. The isolation of antibiotic-resistant salmonella from retail ground meats. N. Engl. J. Med. 345:1147-1154. [DOI] [PubMed] [Google Scholar]
- 37.Winokur, P. L., A. Brueggemann, D. L. DeSalvo, L. Hoffmann, M. D. Apley, E. K. Uhlenhopp, M. A. Pfaller, and G. V. Doern. 2000. Animal and human multidrug-resistant, cephalosporin-resistant Salmonella isolates expressing a plasmid-mediated CMY-2 AmpC β-lactamase. Antimicrob. Agents Chemother. 44:2777-2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winokur, P. L., D. L. Vonstein, L. J. Hoffman, E. K. Uhlenhopp, and G. V. Doern. 2001. Evidence for transfer of CMY-2 AmpC β-lactamase plasmids between Escherichia coli and Salmonella isolates from food animals and humans. Antimicrob. Agents Chemother. 45:2716-2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu, S. W., K. Dornbusch, G. Kronvall, and M. Norgren. 1999. Characterization and nucleotide sequence of a Klebsiella oxytoca cryptic plasmid encoding a CMY-type β-lactamase: confirmation that the plasmid-mediated cephamycinase originated from the Citrobacter freundii AmpC β-lactamase. Antimicrob. Agents Chemother. 43:1350-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]