Abstract
The photoreceptor cell death associated with the various genetic forms of retinitis pigmentosa (RP) is currently untreatable and leads to partial or complete vision loss. Carnosic acid (CA) upregulates endogenous antioxidant enzymes and has proven neuroprotective in studies of neurodegenerative models affecting the brain. In this study, we examined the potential effect of CA on photoreceptor death in the Pde6brd10 mouse model of RP. Our data shows that CA provided morphological and functional preservation of photoreceptors. CA appears to exert its neuroprotective effects through inhibition of oxidative stress and endoplasmic reticulum stress.
Retinitis pigmentosa (RP) is a class of inherited diseases which are characterized by the gradual degeneration of rod photoreceptors followed by cone photoreceptor cell dysfunction and death1. RP is a significant cause of vision loss, and affects approximately 1 in 3,700 people2. The initial symptoms of RP impact the peripheral retina. RP in late stages will involve central vision and may result in legal blindness3. Although effective treatments for RP should start early in life, there are currently no effective medications available for controlling the development of RP due to the limited therapeutic benefits or potential side effects of currently available treatment options4,5. It is therefore important to search for novel therapeutics for RP treatment6.
Photoreceptors work in a very challenging environment characterized by high oxygen supply7, excessive light exposure8, dim ambient light and active metabolism9. These stressors induce oxidative damage of the biological macromolecules that comprise photoreceptors10. Increasing evidence obtained from animal models of RP suggests that oxidative stress11,12, as well as endoplasmic reticulum (ER) stress13, may be the critical mechanisms underlying photoreceptor damage and death14,15,16. Consistent with this hypothesis, a number of studies have demonstrated that early administration of agents that inhibit oxidative stress could significantly decrease the rate of photoreceptor cell death in animal models of RP11,17,18.
Carnosic acid (CA) is a potent antioxidant isolated from Rosmarinus officinalis. CA can readily cross the blood-brain barrier19 and exert its protective effects after conversion from its catechol form to an electrophilic quinone form. This conversion allows CA to bind to Kelch-like ECH-associated protein 1 (Keap1) in the cytoplasm and subsequently release protective transcription factors20,21. Unlike other antioxidants, CA does not deplete the endogenous antioxidant glutathione20.
The Pde6brd10 (rd10) mouse is a well-characterized model of RP22,23. The rd10 mouse carries a missense mutation in exon 13 of the beta subunit of the rod phosphodiesterase gene (Pde6b)22,23, mutations in which also cause human RP24,25. In rd10 mice, rod cell death begins around postnatal day (P) 1826, and is near complete by P3527. In this study, we demonstrate that CA slows rod degeneration in the rd10 mouse, by reducing oxidative stress and ER stress.
Results
Electroretinography
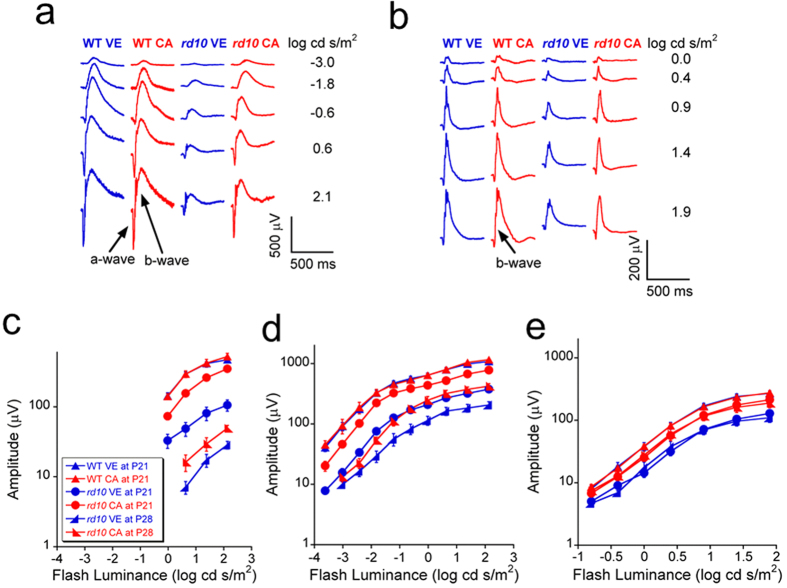
Electroretinography (ERG) was used to compare outer retinal function of mice (Fig. 1). Figure 1a,b compare representative ERG recordings made from wild-type (WT) and rd10 mice at post natal day (P) 21 under dark-adapted and light-adapted conditions, while Fig. 1c–e present summary luminance-response functions obtained across all animals studied at P21 and P28. Consistent with prior characterization of the rd10 retina28, the amplitude of the ERGs obtained from vehicle treated rd10 mice were markedly reduced in comparison to WT in both dark-adapted (1a,c,d) and light-adapted (1b,e) stimulus conditions at P21 and P28. CA treatment did not impact ERG amplitude in WT mice (all p > 0.05). In comparison, responses of rd10 mice treated with CA were much larger than those given vehicle at P21 (all p < 0.01) and P28 (all p < 0.05), respectively. Significant (all p < 0.05) improvement at P21 and P28 was noted for the a-wave, generated by rod photoreceptors, and the b-wave, which reflects rod (1d) or cone (1e) depolarizing bipolar cells. These results indicate that CA can delay the degeneration of rod and cone photoreceptors in rd10 mouse retina. While both CA and vehicle treated groups showed significantly lower ERG a- and b-wave amplitudes at P28 than at P21 in dark-adapted condition (a-wave: all p < 0.01; b-wave: all p < 0.05), the decrease of ERG b-wave amplitude was not significant in light-adapted condition (all p > 0.05). It is consistent with the finding that the extent of ERG amplitude reduction is more severe in dark-adapted condition compared to light-adapted condition at specific age in rd10 mice29.
Figure 1. ERG results obtained from rd10 mice treated with vehicle (n = 12 at P21; n = 5 at P28) and CA (n = 10 at P21; n = 8 at P28), and from wild-type (WT) mice treated with vehicle (n = 4 at P21) and CA (n = 4 at P21).
(a) Typical dark-adapted ERG waveforms at P21. (b) Typical light-adapted ERG waveforms at P21. (c) Luminance-response curves of dark-adapted ERG a-wave at P21 and P28. (d) Luminance-response curves of dark-adapted ERG b-wave at P21 and P28. (e) Luminance-response curves of light-adapted ERG b-wave at P21 and P28. The error bars indicate standard errors. Under both dark-adapted and light-adapted conditions, the ERG a- and b-wave amplitudes were significantly higher in CA treated group than in the vehicle group at P21 (all p < 0.01) and P28 (all p < 0.05), while the difference was not significant in the WT groups (all p > 0.05).
Detection of cell death by terminal deoxynucleotidyl transferase dUTP nick end labeling
To better understand the impact of CA on the rd10 retina, we stained retinal sections at P21 using a terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay30,31,32. In the representative images shown in Fig. 2, TUNEL-positive cells appear as red spots. In the WT retina, TUNEL-positive cells were seen only rarely. In rd10 retinas, TUNEL-positive cells were mainly seen in the outer nuclear layer (ONL) and their frequency was significantly reduced by treatment with CA (p < 0.01). These TUNEL-positive cells may include apoptotic cells33 and all kinds of dying cells30.
Figure 2. CA treatment reduced retinal cell death in rd10 mice at P21.
(a) Representative images (20×) of TUNEL assay in rd10 mice and WT mice treated with vehicle or CA. The red spots indicate the TUNEL-positive cells. Scale bar indicates 50 μm. (b) Average number of dead cells in retinas of rd10 mice and WT mice. Data was expressed as mean ± SD (n = 3). **p < 0.01 vs. vehicle group.
Histological analysis
Figure 3 shows the representative images of histology of rd10 mice at P21 and P28. Because about 97% of photoreceptor nuclei in the ONL of mouse retina are rod cells34, the thickness ratio of ONL to inner nuclear layer (INL) was used as an index to assess the rod death. The reduction of the ONL thickness was significantly ameliorated after the treatment with CA in rd10 mice at P21 and P28 (all p < 0.01), while the ratio of ONL to INL is lower at P28 compared to P21 in vehicle treated mice as well as in CA-treated mice. These data prove the neuroprotective effect of CA against the degeneration of photoreceptors in this mouse model and confirms that the ONL degeneration develops with age.
Figure 3. CA treatment ameliorated the reduction of ONL thickness in rd10 mice.
(a) Representative images (40×) of retinal histology from rd10 mice treated with vehicle or CA. Scale bar indicates 30 μm. (b) Ratio of ONL to INL thickness. Data was expressed as mean ± SD in vehicle group (n = 4 at P21, n = 6 at P28) and CA group (n = 6 at P21 and n = 6 at P28). **p < 0.01 vs. vehicle group.
Analysis of protein pathway biomarkers
Upregulation of Nrf2 expression
Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) is a transcription factor that can respond to increased level of reactive oxygen species and promote the expression of phase II enzymes and endogenous antioxidants to restore the homeostasis of reactive oxygen species. Nrf2 has been recognized as a transcription factor that involves mechanisms of cellular defense response against oxidative stress. Nrf2 protein stays in the cytoplasm by binding to Keap1 and remains inoperative35. Upon activation, activators interact with a cysteine on Keap1 to release Nrf2 and resultantly Nrf2 moves into the cell nucleus, binds with antioxidant response element, and induces expression of cytoprotective target proteins, including phase II detoxifying enzymes, antioxidant proteins, and the molecular proteasome/chaperones36,37. Because CA was reported to play its role of antioxidant by activating Nrf2 pathway20, the expression of Nrf2 was determined by western blot in this study. The antibody only tested the Nrf2 protein which disassociated from Keap1 and relocated to nuclei. Our data shows that both total and nuclear Nrf2 protein expression was increased in the retina of the CA treated rd10 mice (Fig. 4), which confirms that CA treatment can activate Nrf2 pathway.
Figure 4. CA treatment enhanced Nrf2 expression in rd10 retina at P21.
a. Representative images of western blot for total Nrf2 and nuclear Nrf2. b. Average protein expression of total Nrf2 and nuclear Nrf2. n = 3 in each group. The error bars indicate standard deviations. *p < 0.05 vs. vehicle group.
Downregulation of expression of p-JNK and p-p38
There are three well-defined subgroups of MAPKs: the extracellular signal regulated kinases (ERKs), the c-Jun N-terminal kinases (JNKs), and p38 MAPKs (mitogen-activated protein kinases). Each of these subgroups is activated by a cascade of phosphorylation events and involved in the regulation of gene expression, differentiation, cell survival and death38. JNK and p38, subgroups of MAPKs, are widely in regulating apoptosis and cell survival, which can be activated by phosphorylation after a variety of different stresses and inflammation and cause excessive generation of MAPK-regulated genes, uncontrolled proliferation and cell death. According to our western blot data, p-JNK and p-p38 expressions were obviously decreased, while total JNK and total p38 expressions were not changed significantly after treatment with CA in rd10 mice (Fig. 5b,c). It may be related to the amelioration of oxidative stress by CA treatment. In order to further reveal this effect in different layers of retina, p-JNK was tested by immunohistochemistry (Fig. 5a). Our images show that there was strong expression of p-JNK in ONL and the inner segment (IS) of the vehicle group, which was significantly ameliorated after CA treatment (Fig. 5a–c). Our data further confirms that CA plays its role of antioxidant in the photoreceptors of rd10 mice.
Figure 5. CA treatment downregulated the expression of some protein markers of oxidative stress in rd10 retina at P21.
a. Representative images (20x) of retinal immunohistochemistry for p-JNK (red). Scale bar indicates 50 µm. b. Representative images of western blot of JNK, p-JNK, p38 and p-p38. c. Average protein expression of JNK, p-JNK, p38 and p-p38. n = 3 in each group. The error bars indicate standard deviations. *p < 0.05, **p < 0.01 vs. vehicle group.
Down regulation of expression of GRP78, ATF4, ATF6 and p-IRE1α
ER stress may result in cell death which causes photoreceptor degeneration. Four protein markers of ER stress (GRP78, ATF4, ATF6 and p-IRE1α) were detected in this study to understand whether CA treatment can reduce ER stress in rd10 retina. Western blot was performed to detect the change of these proteins after the CA treatment. Our data shows that all these proteins decreased significantly after treatment with CA in rd10 mice compared to the vehicle group (Fig. 6b). Because GRP78 is a master regulator of the unfolded protein response in ER, immunohistochemistry was performed to detect GRP78 in situ (Fig. 6a). Our images reveal that GRP78 exists in inner segment, inner nuclear layer, and ganglion cell layer, which is consistent with the finding of Nookala et al.39 and our previous finding40. After CA treatment, GRP78 was mainly downregulated in the inner segment of photoreceptors and inner nuclear layer (Fig. 6a,b), which implies that ER stress is reduced after CA treatment.
Figure 6. CA treatment downregulated the expression of some markers of ER stress in rd10 retina at P21. CA treatment inhibits the expression of some markers of ER stress.
a. Representative images (20x) of retinal immunohistochemistry for GRP78/BiP (red). Scale bar indicates 50 μm. b. Representative images of western blot for GRP78/BiP, p-IRE1α, ATF4 and ATF6. c. Average protein expression of GRP78/BiP, p-IRE1α, ATF4 and ATF6 tested by western blot. n = 3 in each group. The error bars indicate standard deviations. *p < 0.05, **p < 0.01 vs. vehicle group.
Upregulation of SIRT1 expression and down-regulation of p-p65 expression
Sirtuin type 1 (SIRT1) is a member of mammalian sirtuin family that generates enzyme activity in a NAD+-dependent way to deacetylase histones and prolong survival. In this study, the expression of SIRT1 increased after treatment with CA (Fig. 7b,c). Moreover, SIRT1 was mainly upregulated in the photoreceptor inner segment and outer plexiform layer in CA-treated retina of rd10 mice (Fig. 7a). It may provide neuroprotective effect to the photoreceptors.
Figure 7. CA treatment upregulated SIRT1 expression and downregulated p-p65 expression in rd10 retina at P21. CA treatment upregulated SIRT1 expression and downregulated p-p65 expression.
a. Representative images (20x) of retinal immunohistochemistry for SIRT1 (red). Scale bar indicates 50 μm. b. Representative images of western blot for SIRT1, p65 and p-p65. c. Average protein expression of SIRT1, p65 and p-p65 tested by western blot. n = 3 in each group. The error bars indicate standard deviations. *p < 0.05 vs. vehicle group.
NF-κB (nuclear factor-kappaB) is a heterodimeric protein consisting of p50, p52, p65, RelB, and Rel and is related to inflammatory reaction. The presence of inflammatory reaction was observed in the eyes of rd10 mice and patients with RP. The chronic inflammation may play a pathogenic role in RP41,42. Therefore, p-p65 was tested with western blot to study relationship of CA treatment and NF-κB pathway. Our data shows that CA treatment could inhibit the activation of p-p65 in rd10 mice (Fig. 7b,c), which means CA can control the inflammatory responses.
Discussion
RP is a type of hereditary retinal diseases with progressive degeneration of photoreceptors characterized, as the result of rod-specific gene mutations. About a hundred genes are known to be related to RP43,44. More than 50 genes are found to be relevant to non-syndromic RP and about 3100 mutations have been revealed in these genes. Mutations in 29 genes are found to cause syndromic RP, in which 12 genes are associated with Usher syndrome and 17 genes cause Bardet-Biedl syndrome. There are 1200 mutations in these 29 genes. Despite the genetic heterogeneity, RP seems to involve elevated oxidative stress and ER stress and the photoreceptors seem to die by a common apoptotic mechanism6,14,45,46. Retina is particularly sensitive to oxidative damage because of its high oxygen demand and high content of unsaturated lipids47. The redox balance is disrupted in RP, which is associated with photoreceptor apoptosis and degeneration6. Due to the imbalance between the intracellular oxidative and anti-oxidative defense system, supplement of external antioxidants is needed to eliminate excessive oxidative stress as a potential therapeutics in RP. This raises the idea that these processes could be impacted pharmacologically and therefore be applicable to many genetic forms of RP. This idea has been supported by other studies that demonstrated reducing oxidative stress slowed the rate of photoreceptor degeneration11,17,18,20,48. Here we have demonstrated that CA administration had a similar neuroprotective effects against photoreceptor death in the rd10 mouse model of RP. Our data showed that CA improved photoreceptor function, reduced photoreceptor cell death and degeneration. In addition, our study reveal the protein pathways of oxidative stress, ER stress and inflammatory response affected by CA treatment, which are the possible mechanisms underlie CA protective effect in rd10 retinas.
A lot of evidences have shown oxidative stress is one of the main pathogenic factors contributing to photoreceptor cell death in RP, which is probably related to the activation of Nrf2 pathway, MAPK pathway and NF-κB pathway48,49. Our data show that JNK and p38 subgroups of MAPKs, as well as NF-κB p65 were downregulated significantly in CA treated group than in the vehicle group, which implies that these pathways may be related to the photoreceptor degeneration in rd10 mice and CA takes effect through these cellular pathways. Previous study reported that p38 MAPK can activate NF-κB pathway50. Further study is required to clarify the role of MAPKs and NF-κB pathway in the retinal cell death.
Our data shows ER stress was reduced by CA treatment in rd10 mice according to the downregulation of GRP78, ATF4, ATF6 and p-IRE1α. Protein misfolding in the ER leads to the pathogenesis of many diseases. Activation of the unfolded protein response (UPR) causes oxidative stress and induces apoptosis through generating reactive oxygen species51. Chemical intervention, such as antioxidant, to reduce reactive oxygen species could improve protein folding and cell survival, providing an effective route to treat diseases caused by ER stress52,53. ER stress has been established as a pathogenic factor contributing to photoreceptor cell death54,55,56. Pathological ER stress will activate the apoptotic process leading to cell death and degeneration, and chronic ER stress has been shown to promote inflammatory response through activation NF-κB57,58. In addition, ER stress can also activate MAPKs pathway to induce autophagy59,60,61, and trigger apoptosis62. Therefore, CA treatment in rd10 mice may play its role of neuroprotection partially through the inhibition of ER stress. It was reported that ER stress can be reduced by antioxidants51 through the elimination of reactive oxygen species, and oxidative stress and ER stress are closely linked together63. Further study is necessary to understand the underlying mechanism of CA in regulation of ER stress.
SIRT1 was reported to be pivotal in the regulation of cell fate in the response of cellular stress in mammalian cells and protect cells from death caused by oxidative stress64. Inhibiting SIRT1 by pharmacological inhibitor or SIRT1 siRNA significantly promotes apoptotic neuron death65. The deficiency of SIRT1 also causes retinal damage. In SIRT1-deficient mice, multiple retinal cell layers were significantly thinner than in normal eyes and the ONL was disorganized66. In rd10 mice, a previous study showed that SIRT1 expression is strong at P15 and gradually decreases after that age in ONL67. Our data of SIRT1 immunostaining in rd10 mice of vehicle group is similar to their data at P24 while the rd10 CA-treated group showed upregulation of SIRT1 in the inner segment layer and outer plexiform layer. Numerous protein targets can be deacetylased by SIRT1, thereby regulating multiple cellular pathways related to stress responses, apoptosis and inflammation68. SIRT1 deacetylates the DNA repair factor Ku70 to reduce the disruption of Ku70-Bax interaction, keeping Bax away from mitochondria, thereby inhibiting apoptosis69. It was also reported that SIRT1 releases ER stress to protect cell against dysfunction70. Moreover, SIRT1 played its antioxidant role through the FOXO family71 and down-regulated the pro-inflammatory factor NF-κB directly by deacetylating the p65 subunit of NF-κB complex72. In the present study, SIRT1 was upregulated after treatment with CA in rd10 mice, followed by the inhibition of pro-inflammatory factor NF-κB. Reactive oxygen species is a possible factor which links oxidative stress to SIRT1 pathway as a SIRT1 inhibitor73. CA may reduce the level of reactive oxygen species so that SIRT1 can be upregulated.
Apoptosis was considered as the main pathway of cell death in hereditary retinal degeneration in previous studies74,75. However, recent reports revealed the involvement of alternative cell death pathways in neuronal degeneration, featured by the activation of histone deacetylase, poly-ADP-ribose-polymerase (PARP), calpain, as well as accumulation of cyclic guanosine monophosphate and poly-ADP-ribose, and calcium overload76,77,78. In this alternative pathway, the activities of calpain and PARP co-localize to a large extent with the TUNEL assay79,80. In our study, the photoreceptor cell death may include apoptotic and non-apoptotic death33 and all kinds of dying cells30. Further study is necessary to explore the exact death mechanism of photoreceptor cells in rd10 mice.
In summary, our results imply that CA plays the role of anti-oxidation, anti-ER stress and anti-inflammation through the regulation of multiple cellular pathways in rd10 mice. For further studies of CA in the control of RP, more RP models with different mutations in different genes can be studied. The combination of CA with other antioxidants can also be explored. While genetic based treatments are developed, this type of anti-stress treatment may be used to slow RP progression with long-term administration. Further study is necessary to observe the long-term effect and toxicity of CA and decide whether multiple antioxidants should be used alternatively to reduce side-effects and extend the treatment effect for longer period. Our study provides scientific rationale for further study of CA as a potential supplementary treatment of RP in the future.
Materials and Methods
Animals and treatment
All animal procedures were approved by the Institutional Animal Care and Use Committee of the Cleveland Clinic Foundation and were conducted in accordance with the regulation of the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The breeding pairs of C57BL/6J (WT mouse, Stock Number: 000664) and rd10 mice (Stock Number: 004297) were purchased from the Jackson Laboratory (Bar Harbor, Maine). Carnosic acid (CA, 15 mg/kg, Enzo Life Sciences, Farmingdale, NY) dissolved in canola oil (0.91 mg/mil, Sigma-Aldrich, St. Louis, MO) or equal volume of canola oil were administered by intraperitoneal injection to rd10 mice daily from P6 to P20 or P27 because rd10 mice were reported to start retinal abnormality from P781. At P21 or P28, a series of electroretinograms (ERGs) were recorded. Eyes were then harvested for anatomical and protein studies.
ERG
After overnight dark adaptation, mice were anesthetized with a mixture of ketamine (80 mg/kg) and xylazine (16 mg/kg) diluted in saline. The pupils were dilated with eye drops (2.5% phenylephrine HCl, 1% cyclopentolate HCl, 1% mydriacyl) and the corneal surface was anesthetized with 0.5% proparacaine HCl eye drops. Mice were placed on a temperature-regulated heating pad during the ERG recording session. The tester was double-blinded to the treating status of the mice during the ERG test. To analyze the retinal function, we used the published protocol40,82,83 for dark-adapted and light adapted ERG tests. In dark-adapted session, the flash luminance ranged from 3.6 to 2.1 log cd s/m2. In light-adapted session, the flash luminance ranged from −0.8 to 1.9 log cd s/m2. The time for light-adaptation was 7 minutes before the first light-adapted ERG was recorded.
Preparation of frozen sections
Mouse eyes were enucleated, fixed in 4% paraformaldehyde for 20 min at 4 °C, punched a hole at corneal limbus for prevention of the shrinkage of the eyeball and further fixed in 4% paraformaldehyde for 4 h at 4 °C. Eyes were then processed through a graded series of sucrose in phosphate-buffered saline (PBS) solutions (10% for 1 h, 20% for 1 h and 30% for overnight) at 4 °C, and embedded in optimum temperature cutting compound. Samples were frozen on dry ice and stored at −80 °C. Frozen sections (10 μm) were cut sagittally passing through the optic nerve head and placed onto slides.
TUNEL
Apoptosis and necrosis was detected using the In Situ Cell Death Detection Kit (Roche Applied Science, Indianapolis, IN). 10 μm retinal sections were prepared as described above and incubated with freshly prepared 0.1% Triton X-100/0.1% sodium citrate permeabilization solution for 2 min on ice. After rinsing with PBS 3 times, sections were incubated with the TUNEL reaction mixture for 60 min at 37 °C in the dark and then rinsed with PBS 3 times. Sections were mounted with VECTASHIELD mounting medium with 4′,6-diamidino-2-phenylindole (DAPI) (Burlingame, CA), and visualized with the fluorescence microscope.
Histology
After treatment, intact mouse eyes were enucleated, fixed in Karnovsky’s fixative (2% paraformaldehyde and 2.5% glutaraldehyde in 0.1 M phosphate buffer) for 60 min, removed the cornea and further fixed in Karnovsky’s fixative overnight. Then the eyes were fixed in 1% osmium tetroxide, dehydrated in graded ethanol and then propylene oxide. Next, eyes were transferred to a plastic resin mixture containing Polybed 812 and Araldite 502 (Polysciences) with polymerizer. After polymerization, five to ten sections of 1 μm-thickness were sectioned sagittally, passing through the optic nerve head. Slides were placed 1–2 drops of 1% toluidine blue in 1% sodium borate on a hot plate for 20 s and then rinsed with a gentle stream of distilled water to wash off the excess stain. Slides were covered by coverslips and visualized under the microscope. Pictures were taken on either side of the optic nerve head using a microscope, and the thickness of the ONL and INL was measured at 200 μm from the edge of the optic disc using ImageJ 1.48v software (National Institutes of Health, MD).
Immunohistochemistry
Protein expression and location was examined by in situ immunofluorescence staining. Sections were incubated in PBS containing 5% normal goat serum, 1% bovine serum albumin (BSA) and 0.5% Triton X-100 for 1 h to block non-specific binding, followed by incubation with primary antibodies (anti-GRP78/BiP, 1:400, ab21685; anti-p-JNK, 1:100, ab124956; anti-SIRT1, 1:50, ab12193, Abcam, Cambrigde, MA) overnight at 4 °C. After three washes with PBS, sections were incubated with goat anti-rabbit IgG H&L Alexa Fluo® 555 (1:600, ab150086, Abcam, Cambrigde, MA) for 2 h at room temperature. After washing with PBS, sections were mounted with VECTASHIELD mounting medium with DAPI (VECTOR LABORATORIES, Burlingame, CA) and examined under a fluorescent microscope.
Western blot
Total cellular and nuclear protein was extracted from retinas and quantified using bicinchoninic acid assay kit. Homogenate in 2 × sodium dodecyl sulfate (SDS) sample buffer was boiled for 5 min, and then equal amounts of protein (40 μg) from each sample were subjected to electrophoresis on a 10% (v/v) SDS-polyacrylamide gel. After proteins were electroblotted to a polyvinylidene difluoride membrane, the membrane was blocked with Phosphate-buffered saline containing 5% dried non-fat milk or 3% BSA at room temperature for 1 h, and incubated with indicated primary antibodies (anti-ATF4, 1:1000, SC-200, Santa Cruz Biotechnology, Dallas, TX; anti-ATF6, 1:1500, ab37149; anti-GRP78/BiP, 1:2000, ab21685; anti-pIRE1α, 1:1000, ab48187, Abcam, Cambrigde, MA; anti-Nrf2, 1:1000, SC-722; Santa Cruz Biotechnology, Dallas, TX; anti-p38, 1:500, ab27986; anti-p-p38, 1:1000, ab4822; anti-JNK, 1:1000, ab59227; anti-p-JNK, 1:2000, ab124956; anti-p65, 1:600, ab7970; anti-p-p65, 1:2000, ab86299; anti-SIRT1, 1:2000, ab12193, Abcam, Cambrigde, MA) at 4 °C overnight, followed by incubating with the goat-anti-rabbit horseradish peroxidase-conjugated secondary antibody for 2 h. After incubation, membrane was washed three times, and the antigen-antibody complexes were visualized by the enhanced chemiluminescence system (PerkinElmer, Akron, OH).
Statistical analysis
For the analysis of ERG data, two-way repeated measure ANOVA was used. The power analysis was conducted by the F-test of one-way ANOVA, where we considered numbers as outcome and groups as the factor. All other comparisons were made by one-way ANOVA. p < 0.05 was considered significant.
Additional Information
How to cite this article: Kang, K. et al. Carnosic acid slows photoreceptor degeneration in the Pde6brd10 mouse model of retinitis pigmentosa. Sci. Rep. 6, 22632; doi: 10.1038/srep22632 (2016).
Acknowledgments
This study was supported by Knights Templar Eye Foundation Pediatric Ophthalmology Grant, Eversight Eye and Vision Research Grant, Challenge Grant from Research to Prevent Blindness to the Department of Ophthalmology of the Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, and a Center Grant from the Foundation Fighting Blindness. We thank Mei Yin of Research Core Services, Lerner Institute of Research, Cleveland Clinic Foundation for the preparation of part of the histological slides, and thank Dr. Neal Peachey of Department of Ophthalmic Research, Cleveland Clinic Foundation for reviewing the manuscript.
Footnotes
The authors declare no competing financial interests.
Author Contributions M.Z.Y. conceived and designed the study and oversaw the whole project. K.K., M.Y., M.J.T., X.Y., C.B. and P.B. performed the experiments. K.K. and M.Y. analyzed the data. K.K. and M.Y. wrote and edited the manuscript. All authors reviewed and approved the manuscript.
References
- Daiger S. P., Bowne S. J. & Sullivan L. S. Perspective on genes and mutations causing retinitis pigmentosa. Arch. Ophthalmol. 125, 151–158 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boughman J. A., Conneally P. M. & Nance W. E. Population genetic studies of retinitis pigmentosa. Am. J. Hum. Genet. 32, 223–235 (1980). [PMC free article] [PubMed] [Google Scholar]
- Hartong D. T., Berson E. L. & Dryja T. P. Retinitis pigmentosa. Lancet 368, 1795–1809 (2006). [DOI] [PubMed] [Google Scholar]
- Berson E. L. et al. Clinical trial of lutein in patients with retinitis pigmentosa receiving vitamin a. Arch. Ophthalmol. 128, 403–411 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemson C. M. et al. Therapeutic potential of valproic acid for retinitis pigmentosa. Br. J. Ophthalmol. 95, 89–93 (2011). [DOI] [PubMed] [Google Scholar]
- Campochiaro P. A. et al. Is there excess oxidative stress and damage in eyes of patients with retinitis pigmentosa? Antioxid Redox Signal 643–648 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D. Y. & Cringle S. J. Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Prog. Retin. Eye Res. 20, 175–208 (2001). [DOI] [PubMed] [Google Scholar]
- Albarracin R., Eells J. & Valter K. Photobiomodulation protects the retina from light-induced photoreceptor degeneration. Invest. Ophthalmol. Vis. Sci. 52, 3582–3592 (2011). [DOI] [PubMed] [Google Scholar]
- Okawa H., Sampath A. P., Laughlin S. B. & Fain G. L. ATP consumption by mammalian rod photoreceptors in darkness and in light. Curr. Biol. 18, 1917–1921 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y. et al. Mutt homolog-1 attenuates oxidative DNA damage and delays photoreceptor cell death in inherited retinal degeneration. Am. J. Pathol. 181, 1378–1386 (2012). [DOI] [PubMed] [Google Scholar]
- Komeima K., Rogers B. S., Lu L. & Campochiaro P. A. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc. Natl. Acad. Sci. USA 103, 11300–11305 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Fernandez de la Camara C. et al. Altered antioxidant-oxidant status in the aqueous humor and peripheral blood of patients with retinitis pigmentosa. PLoS One 8, e74223 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. et al. Secretory defect and cytotoxicity: The potential disease mechanisms for the retinitis pigmentosa (rp)-associated interphotoreceptor retinoid-binding protein (irbp). J. Biol. Chem. 288, 11395–11406 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J. et al. Oxidative damage is a potential cause of cone cell death in retinitis pigmentosa. J. Cell. Physiol. 203, 457–464 (2005). [DOI] [PubMed] [Google Scholar]
- Doonan F., Donovan M. & Cotter T. G. Activation of multiple pathways during photoreceptor apoptosis in the rd mouse. Invest. Ophthalmol. Vis. Sci. 46, 3530–3538 (2005). [DOI] [PubMed] [Google Scholar]
- Yang L. P., Wu L. M., Guo X. J. & Tso M. O. Activation of endoplasmic reticulum stress in degenerating photoreceptors of the rd1 mouse. Invest. Ophthalmol. Vis. Sci. 48, 5191–5198 (2007). [DOI] [PubMed] [Google Scholar]
- Komeima K., Rogers B. S. & Campochiaro P. A. Antioxidants slow photoreceptor cell death in mouse models of retinitis pigmentosa. J Cell Physiol. 213, 809–815 (2007). [DOI] [PubMed] [Google Scholar]
- Emoto Y. et al. Green tea extract suppresses n-methyl-n-nitrosourea-induced photoreceptor apoptosis in sprague-dawley rats. Graefes Arch. Clin. Exp. Ophthalmol. 252, 1377–1384 (2014). [DOI] [PubMed] [Google Scholar]
- Satoh T. et al. Carnosic acid, a catechol-type electrophilic compound, protects neurons both in vitro and in vivo through activation of the Keap/Nrf2 pathway via s-alkylation of targeted cysteines on Keap1. J. Neurochem. 104, 1116–1131 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaie T. et al. Protective effect of carnosic acid, a pro-electrophilic compound, in models of oxidative stress and light-induced retinal degeneration. Invest. Ophthalmol. Vis. Sci. 53, 7847–7854 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D. et al. Protection from cyanide-induced brain injury by the Nrf2 transcriptional activator carnosic acid. J. Neurochem. 133, 898–908 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang B. et al. Two mouse retinal degenerations caused by missense mutations in the beta-subunit of rod cgmp phosphodiesterase gene. Vision Res. 47, 624–633 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang B. et al. Retinal degeneration mutants in the mouse. Vision Res. 42, 517–525 (2002). [DOI] [PubMed] [Google Scholar]
- McLaughlin M. E., Sandberg M. A., Berson E. L. & Dryja T. P. Recessive mutations in the gene encoding the beta-subunit of rod phosphodiesterase in patients with retinitis pigmentosa. Nat. Genet. 4, 130–134 (1993). [DOI] [PubMed] [Google Scholar]
- McLaughlin M. E., Ehrhart T. L., Berson E. L. & Dryja T. P. Mutation spectrum of the gene encoding the beta subunit of rod phosphodiesterase among patients with autosomal recessive retinitis pigmentosa. Proc. Natl. Acad. Sci. USA 92, 3249–3253 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargini C., Terzibasi E., Mazzoni F. & Strettoi E. Retinal organization in the retinal degeneration 10 (rd10) mutant mouse: A morphological and ERG study. J. Comp. Neurol. 500, 222–238 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jae S. A. et al. Electrophysiological and histologic evaluation of the time course of retinal degeneration in the rd10 mouse model of retinitis pigmentosa. Korean J. Physiol. Pharmacol. 17, 229–235 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosch S., Johnen S., Muller F., Pfarrer C. & Walter P. Correlations between ERG, OCT, and anatomical findings in the rd10 mouse. J Ophthalmol 2014, 874751 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piano I. et al. Cone survival and preservation of visual acuity in an animal model of retinal degeneration. Eur. J. Neurosci. 37, 1853–1862 (2013). [DOI] [PubMed] [Google Scholar]
- Grasl-Kraupp B. et al. In situ detection of fragmented DNA (tunel assay) fails to discriminate among apoptosis, necrosis, and autolytic cell death: A cautionary note. Hepatology 21, 1465–1468 (1995). [DOI] [PubMed] [Google Scholar]
- Murakami Y. et al. Receptor interacting protein kinase mediates necrotic cone but not rod cell death in a mouse model of inherited degeneration. Proc. Natl. Acad. Sci. USA 109, 14598–14603 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trichonas G. et al. Receptor interacting protein kinases mediate retinal detachment-induced photoreceptor necrosis and compensate for inhibition of apoptosis. Proc. Natl. Acad. Sci. USA 107, 21695–21700 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y. & Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol. 119, 493–501 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solovei I. et al. Nuclear architecture of rod photoreceptor cells adapts to vision in mammalian evolution. Cell 137, 356–368 (2009). [DOI] [PubMed] [Google Scholar]
- Itoh K. et al. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 13, 76–86 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushmore T. H., Morton M. R. & Pickett C. B. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J. Biol. Chem. 266, 11632–11639 (1991). [PubMed] [Google Scholar]
- Kobayashi M. & Yamamoto M. Nrf2-keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv. Enzyme Regul. 46, 113–140 (2006). [DOI] [PubMed] [Google Scholar]
- Pearson G. et al. Mitogen-activated protein (map) kinase pathways: Regulation and physiological functions. Endocr. Rev. 22, 153–183 (2001). [DOI] [PubMed] [Google Scholar]
- Nookala S. et al. In search of the identity of the XAP-1 antigen: A protein localized to cone outer segments. Invest. Ophthalmol. Vis. Sci. 51, 2736–2743 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M. et al. Deficiency of cc chemokine ligand 2 and decay-accelerating factor causes retinal degeneration in mice. Exp. Eye Res. 138, 126–133 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida N. et al. Laboratory evidence of sustained chronic inflammatory reaction in retinitis pigmentosa. Ophthalmology 120, e5–12 (2013). [DOI] [PubMed] [Google Scholar]
- Yoshida N. et al. Clinical evidence of sustained chronic inflammatory reaction in retinitis pigmentosa. Ophthalmology 120, 100–105 (2013). [DOI] [PubMed] [Google Scholar]
- Daiger S. P., Sullivan L. S. & Bowne S. J. Genes and mutations causing retinitis pigmentosa. Clin. Genet. 84, 132–141 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger W., Kloeckener-Gruissem B. & Neidhardt J. The molecular basis of human retinal and vitreoretinal diseases. Prog. Retin. Eye Res. 29, 335–375 (2010). [DOI] [PubMed] [Google Scholar]
- Sahaboglu A. et al. Retinitis pigmentosa: Rapid neurodegeneration is governed by slow cell death mechanisms. Cell Death Dis. 4, e488 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griciuc A., Aron L. & Ueffing M. ER stress in retinal degeneration: A target for rational therapy? Trends Mol. Med. 17, 442–451 (2011). [DOI] [PubMed] [Google Scholar]
- Cingolani C. et al. Retinal degeneration from oxidative damage. Free Radic. Biol. Med. 40, 660–669 (2006). [DOI] [PubMed] [Google Scholar]
- Wang K. et al. Retinal structure and function preservation by polysaccharides of wolfberry in a mouse model of retinal degeneration. Sci. Rep. 4, 7601 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klettner A. Oxidative stress induced cellular signaling in RPE cells. Front Biosci (Schol Ed) 4, 392–411 (2012). [DOI] [PubMed] [Google Scholar]
- Baeza-Raja B. & Munoz-Canoves P. p38 MAPK-induced nuclear factor-kappaB activity is required for skeletal muscle differentiation: Role of interleukin-6. Mol. Biol. Cell 15, 2013–2026 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra J. D. et al. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc. Natl. Acad. Sci. USA 105, 18525–18530 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki H. et al. The antioxidant edaravone attenuates ER-stress-mediated cardiac apoptosis and dysfunction in rats with autoimmune myocarditis. Free Radic. Res. 44, 1082–1090 (2010). [DOI] [PubMed] [Google Scholar]
- Bhandary B., Marahatta A., Kim H. R. & Chae H. J. An involvement of oxidative stress in endoplasmic reticulum stress and its associated diseases. Int. J. Mol. Sci. 14, 434–456 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby R. T. & Gould D. B. Endoplasmic reticulum stress as a primary pathogenic mechanism leading to age-related macular degeneration. Adv. Exp. Med. Biol. 664, 403–409 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A., Kauppinen A., Hyttinen J. M., Toropainen E. & Kaarniranta K. Endoplasmic reticulum stress in age-related macular degeneration: Trigger for neovascularization. Mol. Med. 16, 535–542 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing G., Wang J. J. & Zhang S. X. ER stress and apoptosis: A new mechanism for retinal cell death. Exp. Diabetes Res. 2012, 589589 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam A. B., Mercado E. L., Hoffmann A. & Niwa M. ER stress activates NF-kappaB by integrating functions of basal IKK activity, IRE1 and perk. PLoS One 7, e45078 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil G. S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140, 900–917 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling N. J. & Cook S. J. The role of mapk signalling pathways in the response to endoplasmic reticulum stress. Biochim. Biophys. Acta 1843, 2150–2163 (2014). [DOI] [PubMed] [Google Scholar]
- Kim D. S. et al. p38 mitogen-activated protein kinase is involved in endoplasmic reticulum stress-induced cell death and autophagy in human gingival fibroblasts. Biol. Pharm. Bull. 33, 545–549 (2010). [DOI] [PubMed] [Google Scholar]
- Shimada Y. et al. Endoplasmic reticulum stress induces autophagy through activation of p38 MAPK in fibroblasts from Pompe disease patients carrying c.546g>T mutation. Mol. Genet. Metab. 104, 566–573 (2011). [DOI] [PubMed] [Google Scholar]
- Mishra R. & Karande A. A. Endoplasmic reticulum stress-mediated activation of p38 MAPK, Caspase-2 and Caspase-8 leads to abrin-induced apoptosis. PLoS One 9, e92586 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra J. D. & Kaufman R. J. Endoplasmic reticulum stress and oxidative stress: A vicious cycle or a double-edged sword? Antioxid Redox Signal 9, 2277–2293 (2007). [DOI] [PubMed] [Google Scholar]
- Li L. et al. SIRT1 and STAT3 protect retinal pigmented epithelium cells against oxidative stress. Mol Med Rep 12, 2231–2238 (2015). [DOI] [PubMed] [Google Scholar]
- Zhao Y. et al. Interactions between SIRT1 and MAPK/ERK regulate neuronal apoptosis induced by traumatic brain injury in vitro and in vivo. Exp. Neurol. 237, 489–498 (2012). [DOI] [PubMed] [Google Scholar]
- Mimura T., Kaji Y., Noma H., Funatsu H. & Okamoto S. The role of SIRT1 in ocular aging. Exp. Eye Res. 116, 17–26 (2013). [DOI] [PubMed] [Google Scholar]
- Jaliffa C. et al. Sirt1 involvement in rd10 mouse retinal degeneration. Invest. Ophthalmol. Vis. Sci. 50, 3562–3572 (2009). [DOI] [PubMed] [Google Scholar]
- Cattelan A. et al. NAD(+)-dependent SIRT1 deactivation has a key role on ischemia-reperfusion-induced apoptosis. Vascul. Pharmacol. 70, 35–44 (2015). [DOI] [PubMed] [Google Scholar]
- Azmi A. S. et al. Reactivation of p53 by novel MDM2 inhibitors: Implications for pancreatic cancer therapy. Curr. Cancer Drug Targets 10, 319–331 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. et al. Hepatic overexpression of SIRT1 in mice attenuates endoplasmic reticulum stress and insulin resistance in the liver. FASEB J. 25, 1664–1679 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A. et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303, 2011–2015 (2004). [DOI] [PubMed] [Google Scholar]
- Salminen A., Kauppinen A., Suuronen T. & Kaarniranta K. SIRT1 longevity factor suppresses NF-kappaB -driven immune responses: Regulation of aging via NF-kappaB ‘acetylation? Bioessays 30, 939–942 (2008). [DOI] [PubMed] [Google Scholar]
- Salminen A., Kaarniranta K. & Kauppinen A. Crosstalk between oxidative stress and sirt1: Impact on the aging process. Int. J. Mol. Sci. 14, 3834–3859 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portera-Cailliau C., Sung C. H., Nathans J. & Adler R. Apoptotic photoreceptor cell death in mouse models of retinitis pigmentosa. Proc. Natl. Acad. Sci. USA 91, 974–978 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marigo V. Programmed cell death in retinal degeneration: Targeting apoptosis in photoreceptors as potential therapy for retinal degeneration. Cell Cycle 6, 652–655 (2007). [DOI] [PubMed] [Google Scholar]
- Arango-Gonzalez B. et al. Identification of a common non-apoptotic cell death mechanism in hereditary retinal degeneration. PLoS One 9, e112142 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho-Pelluz J. et al. Photoreceptor cell death mechanisms in inherited retinal degeneration. Mol. Neurobiol. 38, 253–269 (2008). [DOI] [PubMed] [Google Scholar]
- Rodriguez-Muela N. et al. Lysosomal membrane permeabilization and autophagy blockade contribute to photoreceptor cell death in a mouse model of retinitis pigmentosa. Cell Death Differ. 22, 476–487 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquet-Durand F. et al. Calpain is activated in degenerating photoreceptors in the rd1 mouse. J. Neurochem. 96, 802–814 (2006). [DOI] [PubMed] [Google Scholar]
- Paquet-Durand F. et al. Excessive activation of poly(adp-ribose) polymerase contributes to inherited photoreceptor degeneration in the retinal degeneration 1 mouse. J. Neurosci. 27, 10311–10319 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasheff S. F., Shankar M. & Andrews M. P. Developmental time course distinguishes changes in spontaneous and light-evoked retinal ganglion cell activity in rd1 and rd10 mice. J. Neurophysiol. 105, 3002–3009 (2011). [DOI] [PubMed] [Google Scholar]
- Yu M. et al. Age-related changes in visual function in cystathionine-beta-synthase mutant mice, a model of hyperhomocysteinemia. Exp. Eye Res. 96, 124–131 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M. et al. Visual abnormalities associated with enhanced optic nerve myelination. Brain Res. 1374, 36–42 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]