Abstract
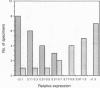
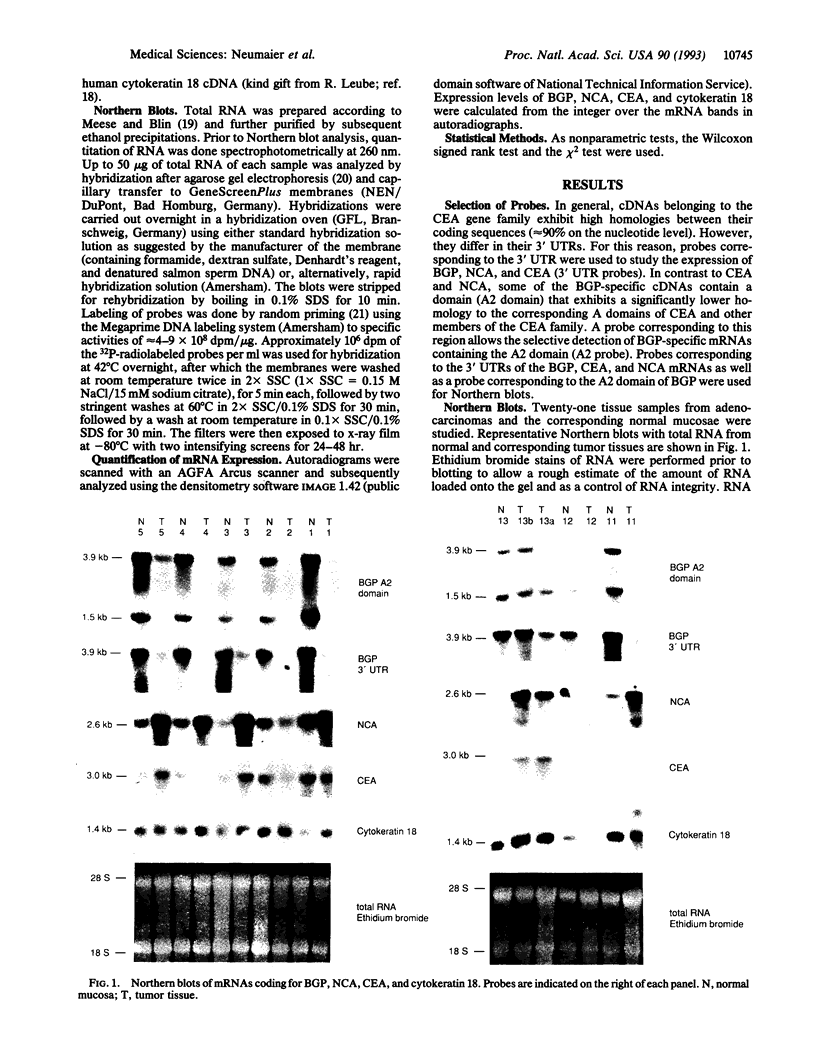
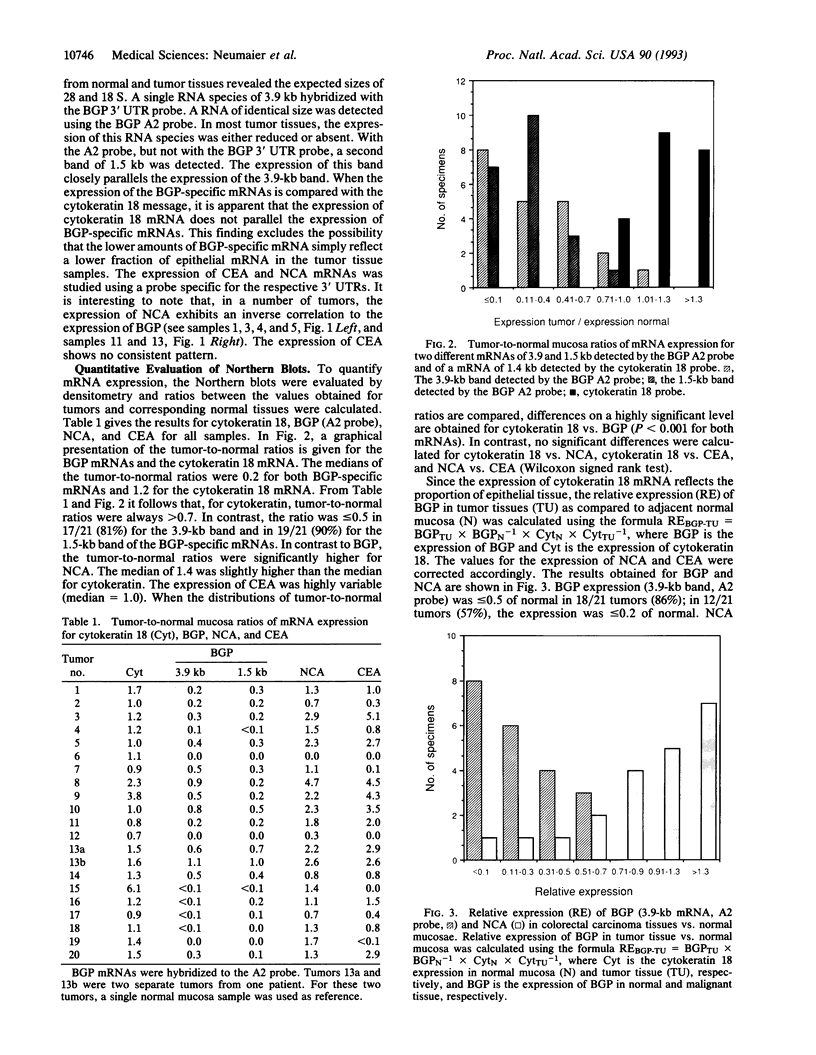
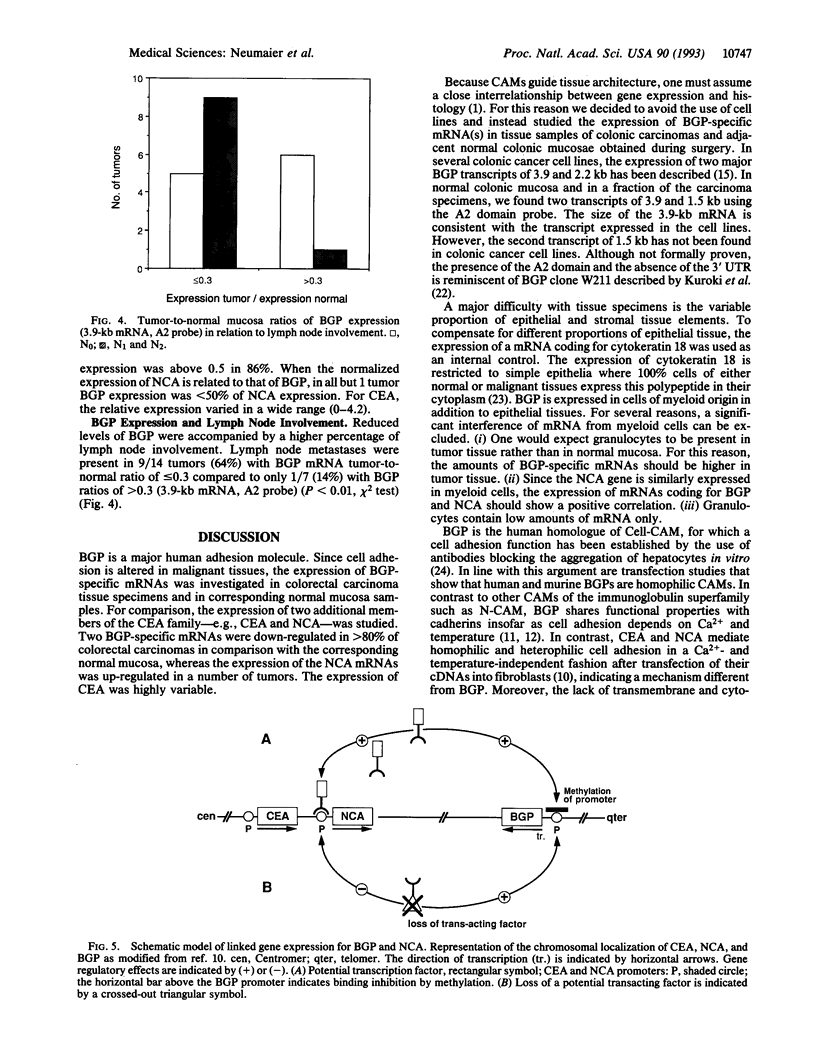
Biliary glycoprotein (BGP) is the human homologue of a cell adhesion molecule (CAM) of the rat designated Cell-CAM. The BGP gene is a member of the carcinoembryonic antigen gene family, which belongs to the immunoglobulin superfamily. BGP is expressed in cells of epithelial and myeloid origin. In granulocytes, BGP is a main antigen of the CD66 cluster of differentiation antigens that mediate the binding to endothelial E-selectin. Since BGP is a major human CAM, the expression of BGP was studied in 21 colorectal carcinoma tissue specimens and in the respective adjacent normal mucosae. As an internal control for epithelial mRNA, the expression of cytokeratin 18 was evaluated in parallel. In addition, the expression of carcinoembryonic antigen and nonspecific crossreacting antigen, which are highly homologous to BGP, was investigated. Two BGP mRNAs of 3.9 and 1.5 kilobases were detected in the normal colonic mucosa samples. The median of the tumor-to-normal ratios of mRNA expression was 0.2 for both BGP mRNAs. In contrast, the median was 1.2 for cytokeratin, 1.0 for carcinoembryonic antigen, and 1.4 for nonspecific crossreacting antigen. Relative to cytokeratin 18 expression, the expression of BGP was reduced to < or = 0.1 in half of the tumors and to < or = 0.4 in > 80% of the tumors. These findings indicate that the loss or reduced expression of the adhesion molecule BGP is a major event in colorectal carcinogenesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arch R., Wirth K., Hofmann M., Ponta H., Matzku S., Herrlich P., Zöller M. Participation in normal immune responses of a metastasis-inducing splice variant of CD44. Science. 1992 Jul 31;257(5070):682–685. doi: 10.1126/science.1496383. [DOI] [PubMed] [Google Scholar]
- Aurivillius M., Hansen O. C., Lazrek M. B., Bock E., Obrink B. The cell adhesion molecule Cell-CAM 105 is an ecto-ATPase and a member of the immunoglobulin superfamily. FEBS Lett. 1990 May 21;264(2):267–269. doi: 10.1016/0014-5793(90)80264-j. [DOI] [PubMed] [Google Scholar]
- Baker S. J., Fearon E. R., Nigro J. M., Hamilton S. R., Preisinger A. C., Jessup J. M., vanTuinen P., Ledbetter D. H., Barker D. F., Nakamura Y. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science. 1989 Apr 14;244(4901):217–221. doi: 10.1126/science.2649981. [DOI] [PubMed] [Google Scholar]
- Baker S. J., Preisinger A. C., Jessup J. M., Paraskeva C., Markowitz S., Willson J. K., Hamilton S., Vogelstein B. p53 gene mutations occur in combination with 17p allelic deletions as late events in colorectal tumorigenesis. Cancer Res. 1990 Dec 1;50(23):7717–7722. [PubMed] [Google Scholar]
- Barnett T. R., Kretschmer A., Austen D. A., Goebel S. J., Hart J. T., Elting J. J., Kamarck M. E. Carcinoembryonic antigens: alternative splicing accounts for the multiple mRNAs that code for novel members of the carcinoembryonic antigen family. J Cell Biol. 1989 Feb;108(2):267–276. doi: 10.1083/jcb.108.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos J. L., Fearon E. R., Hamilton S. R., Verlaan-de Vries M., van Boom J. H., van der Eb A. J., Vogelstein B. Prevalence of ras gene mutations in human colorectal cancers. 1987 May 28-Jun 3Nature. 327(6120):293–297. doi: 10.1038/327293a0. [DOI] [PubMed] [Google Scholar]
- Brandriff B. F., Gordon L. A., Tynan K. T., Olsen A. S., Mohrenweiser H. W., Fertitta A., Carrano A. V., Trask B. J. Order and genomic distances among members of the carcinoembryonic antigen (CEA) gene family determined by fluorescence in situ hybridization. Genomics. 1992 Apr;12(4):773–779. doi: 10.1016/0888-7543(92)90308-f. [DOI] [PubMed] [Google Scholar]
- Drzeniek Z., Lamerz R., Fenger U., Wagener C., Haubeck H. D. Identification of membrane antigens in granulocytes and colonic carcinoma cells by a monoclonal antibody specific for biliary glycoprotein, a member of the carcinoembryonic antigen family. Cancer Lett. 1991 Feb;56(2):173–179. doi: 10.1016/0304-3835(91)90093-w. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Crossin K. L. Cell adhesion molecules: implications for a molecular histology. Annu Rev Biochem. 1991;60:155–190. doi: 10.1146/annurev.bi.60.070191.001103. [DOI] [PubMed] [Google Scholar]
- Fantini J., Rognoni J. B., Culouscou J. M., Pommier G., Marvaldi J., Tirard A. Induction of polarized apical expression and vectorial release of carcinoembryonic antigen (CEA) during the process of differentiation of HT29-D4 cells. J Cell Physiol. 1989 Oct;141(1):126–134. doi: 10.1002/jcp.1041410119. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Cho K. R., Nigro J. M., Kern S. E., Simons J. W., Ruppert J. M., Hamilton S. R., Preisinger A. C., Thomas G., Kinzler K. W. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science. 1990 Jan 5;247(4938):49–56. doi: 10.1126/science.2294591. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Hedrick L., Cho K. R., Vogelstein B. Cell adhesion molecules as tumour suppressors. Trends Cell Biol. 1993 Feb;3(2):36–39. doi: 10.1016/0962-8924(93)90148-t. [DOI] [PubMed] [Google Scholar]
- Heider K. H., Hofmann M., Hors E., van den Berg F., Ponta H., Herrlich P., Pals S. T. A human homologue of the rat metastasis-associated variant of CD44 is expressed in colorectal carcinomas and adenomatous polyps. J Cell Biol. 1993 Jan;120(1):227–233. doi: 10.1083/jcb.120.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinoda Y., Neumaier M., Hefta S. A., Drzeniek Z., Wagener C., Shively L., Hefta L. J., Shively J. E., Paxton R. J. Molecular cloning of a cDNA coding biliary glycoprotein I: primary structure of a glycoprotein immunologically crossreactive with carcinoembryonic antigen. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6959–6963. doi: 10.1073/pnas.85.18.6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpers T. W., Hoogerwerf M., van der Laan L. J., Nagel G., van der Schoot C. E., Grunert F., Roos D. CD66 nonspecific cross-reacting antigens are involved in neutrophil adherence to cytokine-activated endothelial cells. J Cell Biol. 1992 Jul;118(2):457–466. doi: 10.1083/jcb.118.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki M., Arakawa F., Matsuo Y., Oikawa S., Nakazato H., Matsuoka Y. Three novel molecular forms of biliary glycoprotein deduced from cDNA clones from a human leukocyte library. Biochem Biophys Res Commun. 1991 Apr 30;176(2):578–585. doi: 10.1016/s0006-291x(05)80223-2. [DOI] [PubMed] [Google Scholar]
- Leube R. E., Bosch F. X., Romano V., Zimbelmann R., Höfler H., Franke W. W. Cytokeratin expression in simple epithelia. III. Detection of mRNAs encoding human cytokeratins nos. 8 and 18 in normal and tumor cells by hybridization with cDNA sequences in vitro and in situ. Differentiation. 1986;33(1):69–85. doi: 10.1111/j.1432-0436.1986.tb00412.x. [DOI] [PubMed] [Google Scholar]
- Lin S. H., Guidotti G. Cloning and expression of a cDNA coding for a rat liver plasma membrane ecto-ATPase. The primary structure of the ecto-ATPase is similar to that of the human biliary glycoprotein I. J Biol Chem. 1989 Aug 25;264(24):14408–14414. [PubMed] [Google Scholar]
- Meese E., Blin N. Simultaneous isolation of high molecular weight RNA and DNA from limited amounts of tissues and cells. Gene Anal Tech. 1987 May-Jun;4(3):45–49. doi: 10.1016/0735-0651(87)90017-3. [DOI] [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Neumaier M., Zimmermann W., Shively L., Hinoda Y., Riggs A. D., Shively J. E. Characterization of a cDNA clone for the nonspecific cross-reacting antigen (NCA) and a comparison of NCA and carcinoembryonic antigen. J Biol Chem. 1988 Mar 5;263(7):3202–3207. [PubMed] [Google Scholar]
- Ocklind C., Obrink B. Intercellular adhesion of rat hepatocytes. Identification of a cell surface glycoprotein involved in the initial adhesion process. J Biol Chem. 1982 Jun 25;257(12):6788–6795. [PubMed] [Google Scholar]
- Oikawa S., Nakazato H., Kosaki G. Primary structure of human carcinoembryonic antigen (CEA) deduced from cDNA sequence. Biochem Biophys Res Commun. 1987 Jan 30;142(2):511–518. doi: 10.1016/0006-291x(87)90304-4. [DOI] [PubMed] [Google Scholar]
- Powell S. M., Zilz N., Beazer-Barclay Y., Bryan T. M., Hamilton S. R., Thibodeau S. N., Vogelstein B., Kinzler K. W. APC mutations occur early during colorectal tumorigenesis. Nature. 1992 Sep 17;359(6392):235–237. doi: 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- Rojas M., Fuks A., Stanners C. P. Biliary glycoprotein, a member of the immunoglobulin supergene family, functions in vitro as a Ca2(+)-dependent intercellular adhesion molecule. Cell Growth Differ. 1990 Nov;1(11):527–533. [PubMed] [Google Scholar]
- Schipper J. H., Frixen U. H., Behrens J., Unger A., Jahnke K., Birchmeier W. E-cadherin expression in squamous cell carcinomas of head and neck: inverse correlation with tumor dedifferentiation and lymph node metastasis. Cancer Res. 1991 Dec 1;51(23 Pt 1):6328–6337. [PubMed] [Google Scholar]
- Stoffel A., Neumaier M., Gaida F. J., Fenger U., Drzeniek Z., Haubeck H. D., Wagener C. Monoclonal, anti-domain and anti-peptide antibodies assign the molecular weight 160,000 granulocyte membrane antigen of the CD66 cluster to a mRNA species encoded by the biliary glycoprotein gene, a member of the carcinoembryonic antigen gene family. J Immunol. 1993 Jun 1;150(11):4978–4984. [PubMed] [Google Scholar]
- Svenberg T. Carcinoembryonic antigen-like substances of human bile. Isolation and partial characterization. Int J Cancer. 1976 May 15;17(5):588–596. doi: 10.1002/ijc.2910170506. [DOI] [PubMed] [Google Scholar]
- Svenberg T., Hammarström S., Hedin A. Purification and properties of biliary glycoprotein I (BGP I). Immunochemical relationship to carcinoembryonic antigen. Mol Immunol. 1979 Apr;16(4):245–252. doi: 10.1016/0161-5890(79)90063-4. [DOI] [PubMed] [Google Scholar]
- Thompson J. A., Grunert F., Zimmermann W. Carcinoembryonic antigen gene family: molecular biology and clinical perspectives. J Clin Lab Anal. 1991;5(5):344–366. doi: 10.1002/jcla.1860050510. [DOI] [PubMed] [Google Scholar]
- Turbide C., Rojas M., Stanners C. P., Beauchemin N. A mouse carcinoembryonic antigen gene family member is a calcium-dependent cell adhesion molecule. J Biol Chem. 1991 Jan 5;266(1):309–315. [PubMed] [Google Scholar]
- Umbas R., Schalken J. A., Aalders T. W., Carter B. S., Karthaus H. F., Schaafsma H. E., Debruyne F. M., Isaacs W. B. Expression of the cellular adhesion molecule E-cadherin is reduced or absent in high-grade prostate cancer. Cancer Res. 1992 Sep 15;52(18):5104–5109. [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Kern S. E., Hamilton S. R., Preisinger A. C., Nakamura Y., White R. Allelotype of colorectal carcinomas. Science. 1989 Apr 14;244(4901):207–211. doi: 10.1126/science.2565047. [DOI] [PubMed] [Google Scholar]