Antibiotic-associated diarrhea is an important clinical problem, associated with morbidity, mortality and healthcare costs. Our randomized, placebo controlled multicenter trial do not support the efficacy of Saccharomyces boulardii in the prevention of antibiotic-associated diarrhea.
Keywords: antibiotic-associated diarrhea, Clostridium difficile-associated diarrhea, probiotic, randomized controlled trial, Saccharomyces boulardii
Abstract
Background. Antibiotic-associated diarrhea (AAD) and Clostridium difficile-associated diarrhea (CDAD) are common complications of antibiotic use. Data on the efficacy of probiotics to prevent AAD and CDAD are unclear. We aimed to evaluate the efficacy of Saccharomyces boulardii to prevent AAD and CDAD in hospitalized adult patients.
Methods. We conducted a multicenter, phase III, double-masked, randomized, placebo-controlled trial in hospitalized patients who received systemic antibiotic treatment in 15 hospitals in Germany between July 2010 and October 2012. Participants received Perenterol forte 250 mg capsules or matching placebo twice per day within 24 hours of initiating antibiotic treatment, continued treatment for 7 days after antibiotic discontinuation, and were then observed for 6 weeks.
Results. Two thousand four hundred forty-four patients were screened. The trial was stopped early for futility after inclusion of 477 participants. Two hundred forty-six patients aged 60.1 ± 16.5 years and 231 patients aged 56.5 ± 17.8 were randomized to the S boulardii group and the placebo group, respectively, with 21 and 19 AADs in the respective groups (P = .87). The hazard ratio of AAD in the S boulardii group compared with the placebo group was 1.02 (95% confidence interval, .55–1.90; P = .94). Clostridium difficile-associated diarrhea occurred in 0.8% of participants (4 of 477). Nine serious adverse events were recorded in the S boulardii group, and 3 serious adverse events were recorded in the placebo group. None were related to study participation.
Conclusions. We found no evidence for an effect of S boulardii in preventing AAD or CDAD in a population of hospitalized patients without particular risk factors apart from systemic antibiotic treatment.
ClinicalTrials.gov Identifier. NCT01143272.
Antibiotic-associated diarrhea (AAD) refers to otherwise unexplained diarrhea that occurs in association with the administration of antibiotics. It has been reported to occur in up to one third of patients receiving antibiotics [1–3]. The most severe form is Clostridium difficile-associated diarrhea (CDAD). Consequences of AAD and CDAD include increased morbidity [4, 5] and mortality, extended hospital stays, an increased risk of developing complications such as colitis or toxic megacolon, discontinuation of the needed antibiotic, and higher healthcare cost.
Bacterial preparations and yeast are used widely for different indications. Sales grow globally by approximately 7% per year and are predicted to be $48 billion by 2017 [6]. Prevention of AAD is a common indication. Based on the hypothesized dysbiosis of the gut microbiome due to antibiotics [1], coadministering yeast or bacterial preparations for preventive purposes may help re-establishing the disrupted intestinal microflora, enhancing immune responses, lowering colonic pH favoring the growth of nonpathogenic bacteria, and clearing pathogens and their toxins from the host [7, 8]. The nonpathogenic, live yeast Saccharomyces cerevisiae var boulardii (S boulardii) has been favorably discussed because it has an excellent safety profile and is not subject to the effects of concurrently administered antibiotics. However, some cases of generalized fungal infections occurring under unfavorable circumstances have been described [9].
Despite some evidence suggesting that S boulardii may reduce the relative risk of AAD by approximately 60% [10, 11], the quality of the data is still weak. Heterogeneity, high risk of bias, and small sample sizes limit the interpretation of findings. Because of the increasing clinical importance of AAD and CDAD [12, 13] and encouraging yet inconclusive data, we tested the efficacy of Perenterol forte 250 mg capsules in addition to any antibiotic treatment for reducing the hazard of AAD in hospitalized patients in Germany. To address a broad patient group, we did not restrict the sample to a specific at-risk group such as the elderly. Because the effects of yeast and microbial preparations may be strain-specific [14], we did not administer a mix of strains.
METHODS
Study Overview and Ethics
Between June 2010 and October 2012, we conducted a multicenter, double-masked, randomized, placebo-controlled trial in hospitalized patients who received systemic antibiotic treatment. Seventeen academic hospitals across Germany participated in the trial, and 15 hospitals recruited 477 patients, whereas 2 hospitals recruited none. The trial was approved by the Ethics Committee of the Chamber of Physicians of Hamburg (reference: PVN 3440 on April 19, 2010) and the ethics committees of the participating clinical centers. The trial was conducted according to the Declaration of Helsinki.
Study Population
Inclusion and exclusion criteria are reported in Table 1. Any eligible patient was informed in writing about the trial procedures, goals, and potential risks. If the patient was willing to participate in the trial, he/she signed the informed consent form.
Table 1.
Inclusion and exclusion criteria for the SacBo trial
| Inclusion criteria: |
|
| Exclusion criteria: |
|
Randomization, Concealment, and Masking
A blocked randomization stratified by center with an allocation ratio of 1:1 was performed using a computer-assisted method. The allocation sequence was generated by an independent statistician and concealed from any participant or member of the study team until the databases were locked. Allocation concealment was achieved by an internet-based central treatment allocation. For emergency purposes, a sealed, opaque envelope in each center contained allocation information for each participant in that center. Participants, study staff, and data analysts were masked to treatment assignment.
Antibiotic Treatment
The basic treatment in both trial arms was systemic antibiotics according to the predetermined treatment approach. The antibiotics were from the groups outlined in panel (See Supplementary Data). The treatment could include more than 1 antibiotic.
Trial Intervention
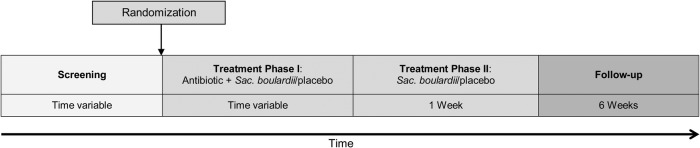
The trial medication was Perenterol forte 250 mg capsules (containing at least 1.8 × 1010 live cells/g lyophilisate) or a matching placebo administrated orally twice per day. In treatment phase I (Figure 1), which was of variable duration, the trial medication was given simultaneously with any systemic antibiotic. The trial medication was started immediately after the first-time administration of an antibiotic. The mean time until start of the study medication was half a day (range, 0–2 days; standard deviation = .5 days). When the antibiotic treatment was discontinued, the participant started treatment phase II and took the trial medication for 7 more days. However, the maximum treatment duration was 8 weeks. When, during treatment phase II, a new antibiotic was prescribed, the participant returned to treatment phase I. This happened in 44 participants, 23 (9.4%) in the S boulardii group and 21 (9.1%) in the placebo group. After treatment phase II, the participant was observed for 6 weeks. Drug accountability was done by counting capsules that were returned to the data coordinating center. The consistency of each bowel movement as well as the stool frequency was recorded by the participant herself/himself in the participant's diary. Outcome data were collected by weekly telephone interviews and review of the diary, the prespecified source document.
Figure 1.
Study flow. Abbreviation: Sac., Saccharomyces.
Study Outcomes
Previous trials suffered from inconsistent definitions for diarrhea. Therefore, we prespecified 3 definitions. The World Health Organization (WHO) defines diarrhea as the passage of 3 or more loose or liquid stools (mostly in larger amounts) within 24 hours (http://www.who.int/topics/diarrhoea/en/). Our main definition was more stringent. Participants had to fulfill the above definition for at least 2 days (“modified WHO definition”). Our third definition was the WHO definition lasting at least 5 days (“severe diarrhea definition”). Only 1 participant fulfilled this definition. Antibiotic-associated diarrhea was defined as diarrhea with onset not before the third day of antibiotic treatment. Clostridium difficile-associated diarrhea was defined as AAD plus detection of C difficile toxin A and/or B in the stool, or of toxin producing C difficile in the stool using polymerase chain reaction, or of typical membranes in colonoscopy or sigmoidoscopy.
The primary outcome was the risk of AAD expressed as hazard ratios. Secondary outcomes were as follows: risk of CDAD; risk of AAD without signs of CDAD; risk of CDAD among all AAD patients; association of AAD with increased initial leukocyte count and C-reactive protein (CRP); association of CDAD with increased initial leukocyte count and CRP; incidence density of AAD or CDAD; average duration of AAD or CDAD; average stool frequency in patients with AAD or CDAD; hazard of a discontinuation or change of the initially administered antibiotic, respectively.
Statistical Analysis
A 15% incidence of AAD was assumed for the placebo group while on antibiotic treatment. 686 patients would be needed in each group for 80% power to detect 5% difference in the cumulative incidence of AAD between treatment groups. We planned to include 1520 patients to account for losses to follow-up.
Due to slow recruitment and unexpectedly few events, an unplanned, masked interim analysis according to the Müller and Schäfer [15] procedure was carried out as suggested by the principal investigator (S. E.) jointly with the Data Safety and Monitoring Board. The conditional power was estimated based on a simulation of the conditional rejection error probability, assuming consistent recruitment of 300 additional participants and treatment effect. We prespecified that the study would only continue if the conditional power was at least 70%. When the conditional power was estimated to be approximately 10.5% in October 2012, the trial was stopped for futility after recruitment had been paused since September 2012. This analysis is based on data of 477 participants who were recruited and observed until trial termination.
Data were analyzed for each of the 3 diarrhea definitions and according to the intention-to-treat principle (ITT). The ITT population included all participants as randomized. Per-protocol (PP) analyses were performed as part of extensive sensitivity analyses (Supplementary Appendix). The PP population excluded participants with missing data and those who deviated from the protocol or were erroneously included. Safety parameters were evaluated “as-treated”, including all participants who have received at least 1 dose of trial medication.
Survival analysis was done to estimate the efficacy of S boulardii to reduce the hazard of AAD (primary endpoint) using all available data. A recurrent event Cox proportional hazards model (Prentice, Williams, and Peterson model) [16] was constructed to evaluate the overall hazard ratio (HR) of AAD comparing both groups. Participants were censored if they did not have AAD by the end of the observation period or were lost to follow-up. Log rank test was used to compare the number of AADs in the S boulardii and the placebo group. Finally, we calculated a covariate adjusted Cox regression model adjusting for center, age, sex, duration of antibiotic treatment, and readministration of antibiotics after completion of treatment phase I. All analyses using the WHO definition are presented in the Supplementary Appendix.
For secondary endpoints, a Cox proportional hazard model was used to evaluate the HR of CDAD; participants having AAD without signs of CDAD; participants having CDAD among all AAD patients; and discontinuation or change of the initial antibiotic comparing the 2 groups. The associations between AAD and leukocyte count and CRP were assessed in the Cox regression model.
The protocol prespecified that the primary and secondary endpoints be analyzed using a Cochran-Mantel-Haenszel χ2 test. Because approximately half of the study participants had some missing observations, the prespecified statistical approach would have resulted in an inefficient analysis of only participants with complete data. We present these results in the Supplementary Appendix. In short, the results of both analytic techniques are similar. Inverse probability weighting did not suggest selection bias by differential losses to follow-up. The trial is registered: ClinicalTrials.gov identifier: NCT01143272.
RESULTS
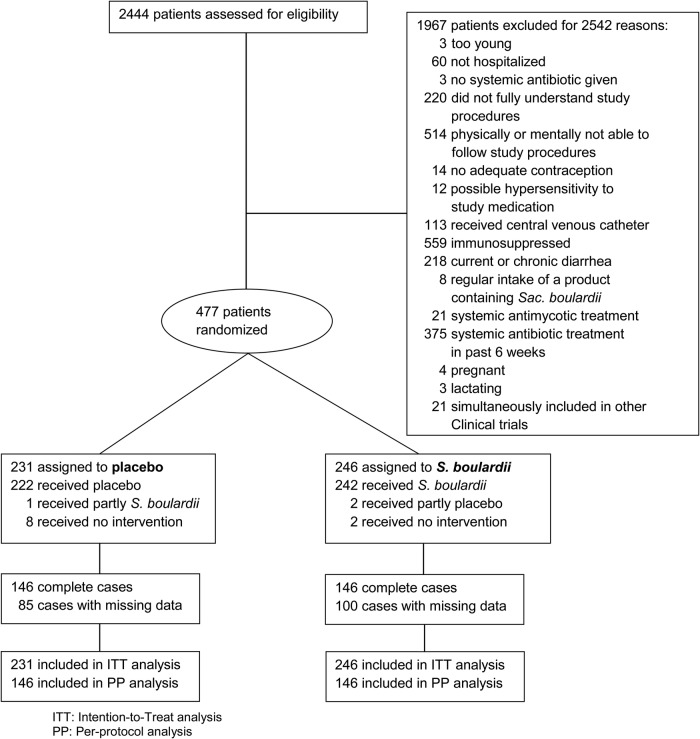
Two thousand four hundred forty-four patients were screened, and 1967 were ineligible or unwilling to participate (Figure 2). Two hundred forty-six and 231 patients were randomized to the active treatment and placebo groups, respectively. Three study participants (1 in the placebo and 2 in the S boulardii group) erroneously received the wrong medication. This was corrected within 2 days. One hundred eighty-five participants (100 in the S boulardii group and 85 in the placebo group) did not document their daily stools completely throughout the observation period. The data of all 477 patients were used for the ITT analysis, whereas 292 patients (61%) with complete observations were included in the PP analysis.
Figure 2.
Enrollment, randomization, and follow-up. Abbreviations: Sac., Saccharomyces.
The baseline characteristics of study participants were comparable between groups in the ITT and PP populations (Table 2 and Supplementary Appendix Table 1). Table 3 shows antibiotic treatment by class and treatment assignment.
Table 2.
Baseline Characteristics of the Study Participants According to Treatment Assignment
| Characteristic | Total (n = 477) | Saccharomyces boulardii Group (n = 246) | Placebo Group (n = 231) |
|---|---|---|---|
| Age, years | 58.4 ± 17.2 | 60.1 ± 16.5 | 56.5 ± 17.8 |
| Male sex, no. (%) | 269 (56.4) | 140 (56.9) | 129 (55.8) |
| Body height, cm (mean ± SD) | 171.7 ± 9.7 | 171.8 ± 9.4 | 171.7 ± 10.0 |
| Body weight, kg (mean ± SD) | 83.0 ± 17.5 | 83.1 ± 17.0 | 83.0 ± 18.1 |
| Body mass index (mean ± SD) | 28.1 ± 5.4 | 28.1 ± 5.2 | 28.1 ± 5.6 |
| CRP (mg/L, mean ± SD) | 71.6 ± 93.9 | 71.0 ± 89.3 | 72.2 ± 98.9 |
| Leukocyte count (109/L, mean ± SD) | 10.8 ± 5.7 | 10.3 ± 4.6 | 11.4 ± 6.7 |
| Time under observation, days (mean ± SD) | 44.0 ± 22.4 | 44.1 ± 22.5 | 44.0 ± 22.3 |
| Duration of antibiotic treatment, days (mean ± SD) | 7.6 ± 6.5 | 7.9 ± 7.8 | 7.2 ± 4.9 |
Abbreviations: CRP, C-reactive protein; SD, standard deviation.
Table 3.
Antibiotic Therapy by Class and Treatment Group
| Antibiotic Class | Saccharomyces boulardii Group (n = 246) | Placebo Group (n = 231) |
|---|---|---|
| β-lactam antibiotics | 201 (81.7%) | 181 (78.4%) |
| Tetracycline | 0 | 1 (0.4%) |
| Aminoglycosides | 1 (0.4%) | 0 |
| Macrolides | 52 (21.4%) | 55 (23.8%) |
| Lincosamides | 4 (1.6%) | 3 (1.3%) |
| Gyrase inhibitors | 40 (16.3%) | 41 (17.8%) |
| Sulfonamide/trimethoprim | 3 (1.2%) | 2 (0.9%) |
| Glycopeptide antibiotics | 0 | 0 |
| Polypeptide antibiotics | 0 | 0 |
| Nitroimidazole derivatives | 38 (15.5%) | 30 (13.0%) |
| Combination antibiotic therapy | ||
| 1 class only | 153 (62.2%) | 149 (64.5%) |
| 2 classes | 93 (37.8%) | 82 (35.5%) |
Primary Endpoint
Forty cases of AAD occurred in 425 study participants, and the total time at risk was 19 165 days. Twenty-one and 19 AADs happened in the S boulardii group and the placebo group, respectively (P = .87). The mean number of episodes of AADs is shown in Table 4. The HR of AAD in the S boulardii group compared with the placebo group was 1.02 (95% CI, .55–1.90; P = .94). None of the prespecified factors (center, age, sex, duration of antibiotic treatment, and readministration of antibiotics after completion of treatment phase I) were associated with risk of AAD.
Table 4.
Number of Study Participants With 0, 1, or 2 Episodes of Antibiotic-Associated Diarrhea
| Number of Episodes of AAD | ITT Population |
PP Population |
||||
|---|---|---|---|---|---|---|
| S boulardii Group (n = 246) | Placebo Group (n = 231) | P Value | S boulardii Group (n = 146) | Placebo Group (n = 146) | P Value | |
| 0 | 225 (91.5%) | 214 (92.6%) | .25 | 130 (91.1%) | 133 (89.0%) | .26 |
| 1 | 21 (8.5%) | 15 (6.5%) | 16 (11.0%) | 11 (7.5%) | ||
| 2 | 0 | 2 (0.9%) | 0 | 2 (1.4%) | ||
Abbreviations: AAD, antibiotic-associated diarrhea; ITT, intention-to-treat; PP, per-protocol.
Secondary Endpoints
Overall, 4 cases (0.8%) of CDAD were observed: two occurred in the S boulardii group and 2 in the placebo group. Due to the small number of CDAD cases, the planned analyses were not performed. Thirty-six cases of AAD without signs of CDAD occurred in 421 participants, and the total time at risk was 18 483 days. The HR of AAD without signs of CDAD in the S boulardii group compared with the placebo group was 0.92 (95% CI, .49–1.73; P = .80). In a multivariate Cox model adjusting for age, sex, CRP, leukocyte count, duration of antibiotic treatment, and administration of antibiotics, the HR of AAD without signs of CDAD in the S boulardii group compared with the placebo group was 1.03 (95% CI, .53–2.03; P = .93). Of AAD cases per year, 0.78 and 0.64 were observed in the S boulardii group and the placebo group, respectively (P = .33). The mean duration of an AAD was 4.48 days in the S boulardii group and 4.26 days in the placebo group (P = .79). The mean frequency of loose or liquid stools per day of AAD was slightly higher in the S boulardii group compared with the placebo group (4.48 vs 4.07, respectively; P = .18). The mean time to onset of AAD was 18.4 days in the S boulardii group and 18.9 days in the placebo group (P = .87). Fourteen participants in the S boulardii group and 7 in the placebo group changed initial antibiotics (6.4% vs 3.4%, P = .15). Antibiotic treatment had to be stopped in 2 participants in the S boulardii group and in 1 participant in the placebo group due to an AAD or CDAD (P = .67). Given these small numbers, we did not conduct further analyses.
Adverse Events
Ten participants did not take any study drug and were therefore excluded from the safety analysis. Eighteen of 245 (7.3%) participants in the S boulardii group and 12 of 222 (5.4%) participants in placebo group had at least 1 adverse event (AE) (P = .39). Overall, we recorded 36 AEs (24 in the S boulardii and 12 in the placebo group). Three and 5 AEs in the S boulardii and placebo group, respectively, were suspected to be causally related to the trial drug. The 3 AEs in the S boulardii group were as follows: 1 abdominal cramps, 1 flatulence, and 1 nausea, vomiting, and decreased appetite. The 5 AEs in the placebo group were as follows: 1 abdominal pain, 2 cases of nausea, 1 increased temperature, and 1 allergic exanthema. Nine serious AEs (SAEs) happened in the S boulardii group: 3 gastrointestinal disorders, 1 cardiac death, 1 cholecystitis, 2 cases of bacterial sepsis, 1 pulmonary empyema, and 1 renal failure. Three SAEs happened in the placebo group: 1 decompensated liver cirrhosis, 1 bronchial carcinoma, and 1 renal failure. None of the SAEs were related to the study drug.
DISCUSSION
Hospitalized patients exposed to antibiotics are at risk of AAD. However, estimates about the incidence vary widely [10]. Antibiotic-associated diarrhea, although mostly mild and transient, is responsible for increased morbidity, mortality, and healthcare costs. Several strategies have been put forward to prevent AAD and CDAD in healthcare settings, among which the coadministration of yeast or microbial preparations (“probiotics”) is thought to be promising.
We found no statistical difference between the risk of AAD in the S boulardii and the placebo group. The S boulardii group had a slightly higher but not statistically significant incidence density of AAD, longer duration of AAD, and higher frequency of loose stools during a diarrhea episode than the placebo group. The findings were robust in the PP as well as several sensitivity analyses (Supplementary Appendix). Age, sex, administration of additional antibiotics, duration of the antibiotic treatment, leukocyte count, and CRP levels were not associated with AAD risk. In this study, CDAD was rare.
The incidence of AAD and CDAD in our study was lower than suggested previously [17–19] but in line with the recently published large PLACIDE trial [20]. We calculated the number of episodes based on patient-reported stool frequency and consistency instead of asking directly about diarrhea, a concept with substantial interperson variability. Another reason for this low incidence may be that our study population was relatively young. We did not target a specific at-risk population. We may also have missed AAD and CDAD cases due to attrition, despite all our efforts to stay in touch with participants.
Some previous studies and systematic reviews indicated the efficacy of yeast or microbial preparations to prevent AAD or CDAD, reduce the duration of diarrhea, or reduce stool frequency during a diarrhea episode [3, 11, 21, 22]. However, studies varied greatly regarding study population (age, disease severity), type of probiotic, and duration of treatment, probiotic dose, the definition of the outcome, and follow-up duration. The large PLACIDE trial did not find a lower frequency of AAD or CDAD in elderly inpatients after the administration of Lactobacilli and Bifidobacteria [20]. However, caution is needed when extrapolating data from bacterial preparations to yeast or of one strain to another because effects may be strain-specific [23]. Two systematic reviews suggested a specific beneficial effect of S boulardii [24, 25]. Yet, the trials included in the systematic reviews were of substantial heterogeneity, and some had a high risk of bias. Some randomized controlled trials (RCTs) failed to find a beneficial effect of the yeast. Pozzoni et al [17] conducted a trial in 275 older inpatients in Italy and found the administration of S boulardii ineffective for reducing AAD and CDAD. We believe that the mechanism of action of “probiotic substances” needs to be understood much better before conducting further expensive RCTs.
This trial was stopped early for futility. Among the 2444 patients that we screened, 1967 (80.5%) were ineligible or not willing to participate. The most common reasons for ineligibility were immunosuppression, inability to follow study procedures, current or chronic diarrhea, and systemic antibiotic treatment in the past 6 weeks. It is clear that recruiting in large University Hospitals with a high proportion of very sick patients was a prime reason for the recruitment difficulties. Moreover, the patients who were eligible may have been relatively healthy resulting in few events. These practical difficulties need to be considered when designing trials in future.
This is the largest RCT to investigate the efficacy of S boulardii for the prevention of AAD. Nevertheless, this study has limitations. First, 1967 of 2444 screened patients were ineligible for participation. This may limit the external validity of the trial. Yet, approximately one third of these noninclusions were due to contraindications listed by the manufacturer. Others were due to ethical or operational constraints. Only 15% of screened patients declined to participate. Second, a large proportion of participants did not completely document stool frequencies and were thus excluded from the prespecified analysis (Supplementary Appendix). However, the survival analysis reported here used all available data, and multiple sensitivity analyses as well as the prespecified analysis showed consistent, robust results (Supplementary Appendix). In short, we compared the characteristics of participants who had complete data and those who had not, and there were no significant differences between the 2 groups except for CRP levels. We did several sensitivity analyses imputing different, extreme scenarios. We imputed all patients with missing data as (1) having the primary outcome (AAD) and (2) not having the primary outcome. We also performed inverse probability weighting to assess the impact of missing data. All sensitivity analyses revealed consistent results and did not suggest an effect of S boulardii (Supplementary Appendix). We performed all analyses using the modified WHO definition as well as the WHO definition of diarrhea as outcome, and we did not find a difference between both groups. Third, we did not record previous hospital admission and previous gastrointestinal surgery, which may be risk factors for AAD [2]. However, randomization should distribute measured and unmeasured confounders evenly between the 2 groups. In addition, this is one of the few studies that rigorously administered S boulardii while taking antibiotics and 1 week thereafter and then observed participants over 6 weeks. This tedious regimen may have contributed to the relatively high proportion of participants with some missing data. Finally, we may have missed treatment effects due to a type-2 error. We reached only one third of the projected sample size with respective consequences for the power of the trial. However, based on our observed incidence of AAD in the 2 groups, if we had reached the targeted sample size, we still would not have found a difference between the groups.
CONCLUSIONS
In conclusion, we found no evidence for the efficacy of the tested S boulardii regimen to prevent AAD in a population of hospitalized patients who received systemic antibiotic treatment.
Supplementary Data
Supplementary material is available online at Open Forum Infectious Diseases online (http://OpenForumInfectiousDiseases.oxfordjournals.org/).
Acknowledgments
We thank the following members of the Data Safety and Monitoring Board: Professor Thomas Eschenhagen (University Medical Center Hamburg-Eppendorf, Hamburg), Professor Heiner Greten (Hanseatisches Herzzentrum, Hamburg), and Professor Alexander Kraemer (School of Public Health, Bielefeld).
The SacBo Study Group comprises the following individuals: Dr. Bernhard Ruf (Klinikum St. Georg, Klinik für Infektiologie, Tropenmedizin und Nephrologie, Leipzig, Germany); Dr. Rainer Porschen (Klinikum Bremen Ost, Klinik für Innere Medizin, Bremen, Germany); Dr. Guido Trenn (Knappschaftskrankenhaus Bottrop, Medizinische Klinik, Bottrop, Germany); Drs. Trude Butterfaß-Bahloul and Gudrun Wuerthwein and Marc Urban (Zentrum für Klinische Studien Münster, Westfälische Wilhelms-Universität Münster, Münster, Germany); Drs. Frank Oeder, Andreas Runge, Esther Klauss, and Nina Hansen-Rosenblatt (Agaplesion Diakonieklinikum Hamburg, Department of Internal Medicine, Hamburg, Germany); Drs. Tobias Werner, Kornelius Schulze, Benno Kreuels, Guido Schäfer, Peter Hübener, Annette Hennigs, Claudia Beisel, Dorothee Fischer-Brügge, Katharina Zimmermann-Fraedrich, and Claudia Röder and Nadine Grigo (Department of Medicine I, University Medical Center Hamburg-Eppendorf, Hamburg, Germany); Drs. Armin Riecke and Helmut Schreckenbauer (Federal Armed Forces Hospital Ulm, Department of Internal Medicine, Ulm, Germany); Drs. Christoph Hemmer, Sebastian Klammt, Hilte Geerdes-Fenge, and Silvius Frimmel (Rostock University Medical Center, Department of Internal Medicine, Division for Tropical Medicine and Infectious Diseases, Rostock, Germany); Drs. Jens M. Kittner, Johannes W. Rey, Joern M. Schattenberg, and Florian Thieringer (Johannes-Gutenberg-Universität Mainz, University Medical Centre, I. Department of Internal Medicine, Mainz, Germany); Drs. Rudolf Schmits, Daniel Grandt, Philipp Martin Büch, Sybille Lehnen, Daniel Tiefengraber, Klaus Radecke and Alexander Klebert and Marc Andreas Mittag (Hospital of Saarbrücken, Saarbrücken, Germany); Dr. Iris Hering (Diakoniekrankenhaus Rotenburg [Wümme] GmbH, Zentrum fuer Pneumologie, Rothenburg [Wümme], Germany): Drs. Wolfgang Zeller, Lisa Rundt, Peter Baltes, Dani Dajani, and Niehls Kurniawan and Lars Brandt and Carola Pflüger (Bethesda Krankenhaus Bergedorf, Klinik für Innere Medizin, Hamburg, Germany); Nassim Behjat, Ulrike Engel, and Martina Unger (Clinical Research Unit, Bernhard Nocht Institute for Tropical Medicine, Hamburg, Germany).
Disclaimer. Neither the funding organization nor the sponsor had access to the data or any role in the design, implementation, or analysis of the trial or the reporting of the data.
Author contributions. S. E., J. M., A. W. L., G. D. B., R. H., and S. Scho. conceived the study, wrote the protocol, oversaw data collection and wrote the report. N. G., S. E., S. L., M. E., and R. M. H. analyzed the data and contributed to writing the report. M. R., M. P. S., S. Schm., M. K., E. C. R., A. L., A. d. W., M. S., and T. S. oversaw and contributed to data collection, did safety analyses, reviewed the analytic strategy, wrote sections of and critically reviewed the manuscript. All authors have approved the manuscript.
Financial support. This investigator-initiated trial was funded by the German Federal Ministry of Education and Research (Bundesministerium fur Bildung und Forschung; reference 01KG0902) and received institutional funding by the Bernhard Nocht Institute for Tropical Medicine and the University Medical Center Hamburg-Eppendorf. The sponsor and data coordinating center of the trial was the Bernhard Nocht Institute for Tropical Medicine in Hamburg, Germany. The study drug (Perenterol forte) was purchased via the Pharmacy of the University Hospital Carl Gustav Carus, Dresden, Germany, which also produced matching placebos.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Contributor Information
Collaborators: for the SacBo Study Group, Bernhard Ruf, Rainer Porschen, Guido Trenn, Trude Butterfaß-Bahloul, Gudrun Wuerthwein, Frank Oeder, Andreas Runge, Esther Klauss, Nina Hansen-Rosenblatt, Tobias Werner, Kornelius Schulze, Benno Kreuels, Guido Schäfer, Peter Hübener, Annette Hennigs, Claudia Beisel, Dorothee Fischer-Brügge, Katharina Zimmermann-Fraedrich, Claudia Röder, Nadine Grigo, Armin Riecke, Helmut Schreckenbauer, Christoph Hemmer, Sebastian Klammt, Hilte Geerdes-Fenge, Silvius Frimmel, Jens M. Kittner, Johannes W. Rey, Joern M. Schattenberg, Florian Thieringer, Rudolf Schmits, Daniel Grandt, Philipp Martin Büch, Alexander Klebert, Marc Andreas Mittag, Sybille Lehnen, Daniel Tiefengraber, Klaus Radecke, Iris Hering, Wolfgang Zeller, Lisa Rundt, Lars Brandt, Peter Baltes, Dani Dajani, Niehls Kurniawan, Carola Pflüger, Nassim Behjat, Ulrike Engel, and Martina Unger
References
- 1.Bartlett JG, Clinical practice. Antibiotic-associated diarrhea. N Engl J Med 2002; 346:334–9. [DOI] [PubMed] [Google Scholar]
- 2.McFarland LV. Epidemiology, risk factors and treatments for antibiotic-associated diarrhea. Dig Dis 1998; 16:292–307. [DOI] [PubMed] [Google Scholar]
- 3.Johnston BC, Ma SS, Goldenberg JZ, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Ann Intern Med 2012; 157:878–88. [DOI] [PubMed] [Google Scholar]
- 4.Bergogne-Berezin E. Treatment and prevention of antibiotic associated diarrhea. Int J Antimicrob Agents 2000; 16:521–6. [DOI] [PubMed] [Google Scholar]
- 5.Kyne L, Hamel MB, Polavaram R, Kelly CP et al. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile . Clin Infect Dis 2002; 34:346–53. [DOI] [PubMed] [Google Scholar]
- 6.McFarland LV. From yaks to yogurt: the history, development, and current use of probiotics. Clin Infect Dis 2015; 60:S85–90. [DOI] [PubMed] [Google Scholar]
- 7.Elmer GW. Probiotics: “living drugs”. Am J Health Syst Pharm 2001; 58:1101–9. [DOI] [PubMed] [Google Scholar]
- 8.Antunes LC, Han J, Ferreira RB et al. Effect of antibiotic treatment on the intestinal metabolome. Antimicrob Agents Chemother 2011; 55:1494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whelan K, Myers CE. Safety of probiotics in patients receiving nutritional support: a systematic review of case reports, randomized controlled trials, and nonrandomized trials. Am J Clin Nutr 2010; 91:687–703. [DOI] [PubMed] [Google Scholar]
- 10.Szajewska H, Mrukowicz J. Meta-analysis: non-pathogenic yeast Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment Pharmacol Ther 2005; 22:365–72. [DOI] [PubMed] [Google Scholar]
- 11.McFarland LV. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am J Gastroenterol 2006; 101:812–22. [DOI] [PubMed] [Google Scholar]
- 12.Lynen Jansen P, Stallmach A, Lohse AW, Lerch MM et al. [Development of gastrointestinal infectious diseases between 2000 and 2012]. Z Gastroenterol 2014; 52:549–57. [DOI] [PubMed] [Google Scholar]
- 13.Lessa FC, Mu Y, Bamberg WM et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015; 372:825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sullivan A, Nord CE. Probiotics and gastrointestinal diseases. J Intern Med 2005; 257:78–92. [DOI] [PubMed] [Google Scholar]
- 15.Müller HH, Schäfer H. A general statistical principle for changing a design any time during the course of a trial. Stat Med 2004; 23:2497–508. [DOI] [PubMed] [Google Scholar]
- 16.Prentice RL, Williams BJ, Peterson AV. On the regression analysis of multivariate failure time data. Biometrika 1981; 68:373–9. [Google Scholar]
- 17.Pozzoni P, Riva A, Bellatorre AG et al. Saccharomyces boulardii for the prevention of antibiotic-associated diarrhea in adult hospitalized patients: a single-center, randomized, double-blind, placebo-controlled trial. Am J Gastroenterol 2012; 107:922–31. [DOI] [PubMed] [Google Scholar]
- 18.Hickson M, D'Souza AL, Muthu N et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ 2007; 335:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao XW, Mubasher M, Fang CY et al. Dose-response efficacy of a proprietary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for antibiotic-associated diarrhea and Clostridium difficile-associated diarrhea prophylaxis in adult patients. Am J Gastroenterol 2010; 105:1636–41. [DOI] [PubMed] [Google Scholar]
- 20.Allen SJ, Wareham K, Wang D et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2013; 382:1249–57. [DOI] [PubMed] [Google Scholar]
- 21.Hempel S, Newberry SJ, Maher AR et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA 2012; 307:1959–69. [DOI] [PubMed] [Google Scholar]
- 22.Sazawal S, Hiremath G, Dhingra U et al. Efficacy of probiotics in prevention of acute diarrhoea: a meta-analysis of masked, randomised, placebo-controlled trials. Lancet Infect Dis 2006; 6:374–82. [DOI] [PubMed] [Google Scholar]
- 23.Guandalini S. Probiotics for prevention and treatment of diarrhea. J Clin Gastroenterol 2011; 45:S149–53. [DOI] [PubMed] [Google Scholar]
- 24.Goldenberg JZ, Ma SS, Saxton JD et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev 2013; 5:CD006095. [DOI] [PubMed] [Google Scholar]
- 25.Videlock EJ, Cremonini F. Meta-analysis: probiotics in antibiotic-associated diarrhoea. Aliment Pharmacol Ther 2012; 35:1355–69. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.