Abstract
Although silicon (Si) has been widely reported to alleviate plant nutrient deficiency, the underlying mechanism in potassium (K) deficiency is poorly understood. In this study, sorghum seedlings were treated with Si under a K deficiency condition for 15 days. Under control conditions, plant growth was not affected by Si application. The growth and water status were reduced by K-deficient stress, but Si application significantly alleviated these decreases. The leaf gas exchanges, whole-plant hydraulic conductance (Kplant), and root hydraulic conductance (Lpr) were reduced by K deficiency, but Si application moderated the K-deficiency-induced reductions, suggesting that Si alleviated the plant hydraulic conductance. In addition, 29% of Si-alleviated transpiration was eliminated by HgCl2 treatment, suggesting that aquaporin was not the primary cause for the reversal of plant hydraulic conductance. Moreover, the K+ concentration in xylem sap was significantly increased and the xylem sap osmotic potential was decreased by Si application, suggesting that the major cause of Si-induced improvement in hydraulic conductance could be ascribed to the enhanced xylem sap K+ concentration, which increases the osmotic gradient and xylem hydraulic conductance. The results of this study show that Si mediates K+ accumulation in xylem, which ultimately alleviates the plant-water status under the K-deficient condition.
Potassium (K) is the most abundant cation in plants. It is involved in various important cellular processes, such as the stabilization of protein synthesis, activation of enzymes, and neutralization of negative charges on proteins1,2. In addition, it also plays a key role in osmotic processes that contribute to cellular turgor, photosynthesis, and transpiration3. K deficiency can disturb these activities directly, and inhibit plant growth and development2,4,5. K is also essential for high-yield crop production, and it can be a limiting factor for such crops under certain environmental conditions6,7. The uptake of silicon (Si), which is one of the most abundant elements in the Earth’s crust, is beneficial for increasing plant resistance to various stresses8,9,10. Si application has also been widely reported to alleviate plant nutrient deficiency, including alleviating K deficiency4,11,12,13.
Compared with the benefits of Si under other stresses, the performance and underlying mechanism of Si in alleviating K deficiency has rarely been investigated4. To date, only one study has reported that Si application alleviated K-deficiency-induced growth inhibition in soybean. Several studies also reported that Si can affect tissue K concentration under salt stress conditions14,15. These previous studies suggested that Si can influence the K absorption and/or distribution under certain conditons. K deficiency disturbs plant growth and development in various ways. It is not clear if there are other ways in which Si participates in regulating K deficiency. A better understanding of the interaction between Si application and K deficiency will provide important information about Si and K fertilizer use in crop production.
The Plant-water status involves the balance of the difference in water uptake through the roots and transpiration in leaves; maintaining water status is the basis of metabolic activities in tissues15,16. K+ is involved in regulating the plant-water status in various ways. First, K+ plays an important role in stomatal opening and closing, which controls leaf transpiration and plant-water status17,18. Second, at the whole-plant level, K+ accumulation in the root xylem vessels is also involved in osmotic water absorption19. Third, several recent reports have suggested that xylem K+ plays a critical role in modifying xylem hydraulic conductance and water relations in plants5,20. In contrast, it has been widely reported that K deficiency affects the plant-water status in different ways. Severe K deficiency generally induces stomatal closure and lowers transpiration rates21. As the main osmotic solute, K deficiency also affects the osmotic potential gradient and decreases water uptake20,22. K deficiency was found to reduce aquaporin activity, thereby suppressing root hydraulic conductance and water supply to the stem and leaf transpiration21. K fertilizer, however, was found to increase plant hydraulic conductance and transpiration20. In sum, K is involved in regulating the plant water status, and severe K deficiency causes tissue dehydration21.
There are no case studies showing that Si application affects the plant-water status under K deficiency stress, but Si was found to be involved in regulating the plant-water status in response to other stresses. Romero-Aranda et al.23 demonstrated that Si application enhanced the tomato leaf water content under salt stress. Recently, Liu et al.24,25 reported that enhanced root hydraulic conductance by aquaporin regulation is responsible for Si-alleviated plant-water status under drought and salt stresses. Under normal growth conditions, Si application was also found to affect the plant-water status15 and up-regulate aquaporin gene (PIPs) expression25. In addition, the potential effect of Si application on K+ absorption and distribution may be involved in regulating the plant water status. Therefore, the close coupling between K deficiency and Si application on the plant-water status suggests that Si may be involved in regulating the plant water-status under K-deficiency conditions.
The aim of this study was to determine whether or not Si improves the plant-water status under K-deficiency conditions. We hypothesized that K deficiency induces a decrease in plant hydraulic conductance, which decreases the water supply for leaf transpiration and plant-water status. Si application increases plant hydraulic conductance, possibly favoring higher gas exchange rates and photosynthesis, thus alleviating the K deficiency. To accomplish the objective of this study, the effect of Si application on leaf relative water content (LRWC), plant transpiration, whole-plant hydraulic conductance (Kplant) and root hydraulic conductance (Lpr), and the effect of aquaporin on transpiration and xylem sap K+ concentration were measured under K-deficiency and Si application conditions. Moreover, the expression of aquaporin and K+-related genes were also investigated.
Results
Si application alleviated the K deficiency in sorghum and improved the plant-water status
Extreme K deficiency inhibits plant growth26. In the present study, Si application did not affect the sorghum seedling growth under control conditions. However, K deficiency significantly decreased plant growth after 15 days incubation and Si application alleviated the K-deficiency-induced growth inhibition (Fig. 1a, Supplementary Fig. S1). The dry weight of Si-treated plants was 29% (p < 0.05) higher than that of untreated plants under K deficiency conditions. At the same time, the root/shoot ratio was not affected by either K deficiency or Si application (Fig. 1b). These results showed that Si application enhanced plants’ K deficiency tolerance. Similar to the change in plant dry weight, the stomatal conductance, transpiration rate, and photosynthetic rate were also found to be decreased under K deficiency in various plant species21,27,28. In this study, the leaf gas exchange was not influenced by Si under control conditions, but it was significantly inhibited by K deficiency. Si application alleviated the K-deficiency-induced reduction in the photosynthetic rate, stomatal conductance and transpiration rate (Fig. 2). In Si-treated plants, the leaf photosynthetic rate, stomatal conductance and transpiration rate were 139% (p < 0.05), 49% (p < 0.05) and 94% (p < 0.05) higher than that of plants without Si application under K-deficiency conditions (Fig. 2).
Figure 1. Effects of silicon (Si, 1 mM) on the plant growth of sorghum plants grown under control (3 mM KCl) and low K (0.05 mM KCl) conditions.
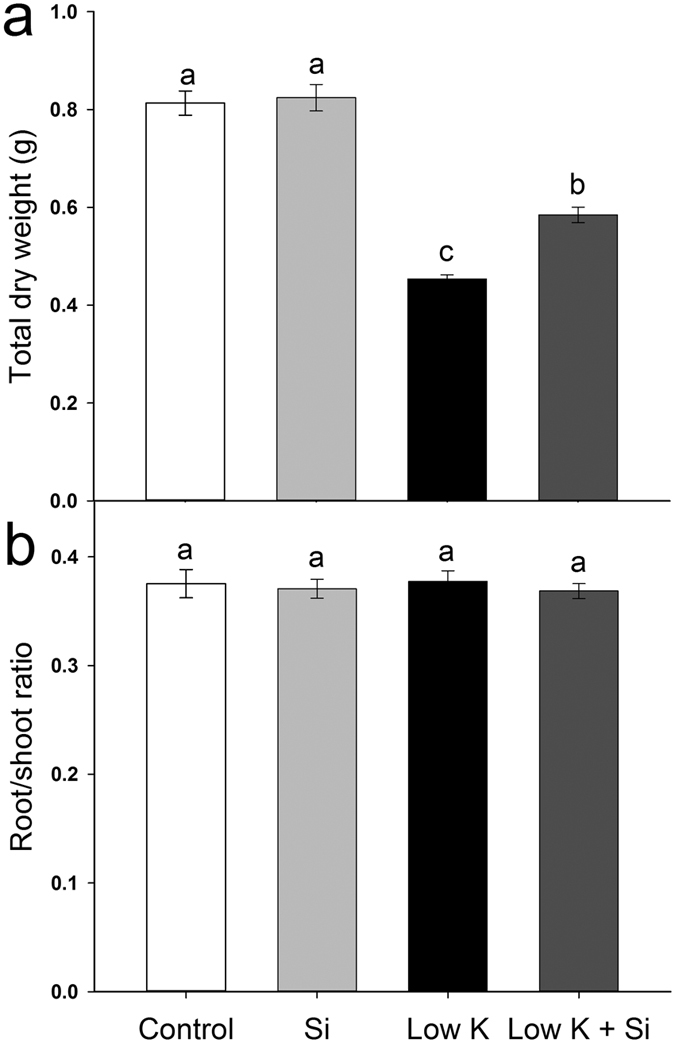
(a) Total dry weight, (b) root/shoot ratio. All parameters were measured after 15 days of treatment. Values are presented as the mean ± SE (n = 24). Different letters in one measure indicate statistically significant differences at P < 0.05.
Figure 2.
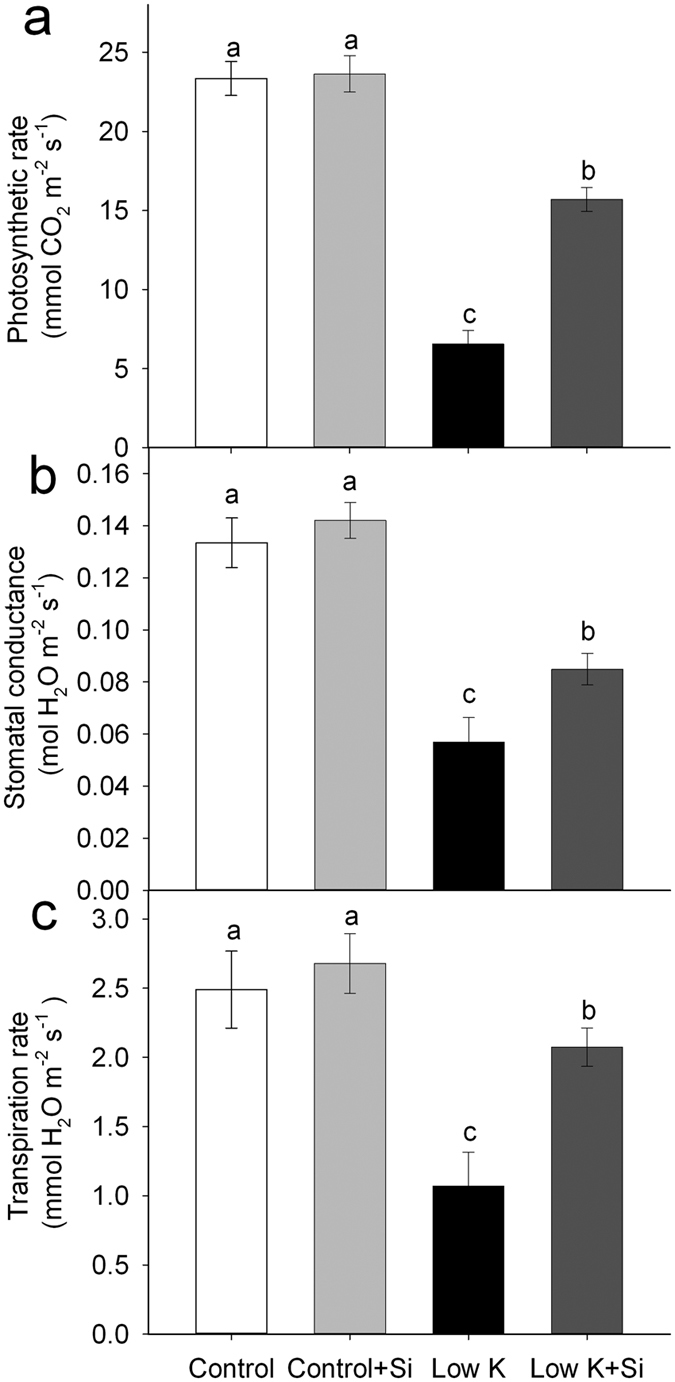
Effects of silicon (Si, 1 mM) on photosynthetic rate (a), stomatal conductance (b) and transpiration rate (c) of sorghum plants grown under control (3 mM KCl) and low K (0.05 mM KCl) conditions. All parameters were measured after 15 days of treatment. Values are presented as the mean ± SE (n = 6). Different letters in one measure indicate statistically significant differences at P < 0.05.
Water status is reflected by the LRWC15,16. K deficiency increases the plant’s susceptibility to water stress, and plants with an adequate K supply have better hydrated tissues than those with K deficiency15,16. In this study, the LRWC was decreased after 15 days of K deficiency and Si significantly reduced the K-deficiency-induced decrease in LRWC (Fig. 3a). Leaf water potential also decreased after the 15-day K-deficiency treatment, but Si application maintained the leaf water potential at the same level as that of control (Fig. 3b). These results indicated that Si application improved the plant water-status under K-deficiency conditions. Osmotic potential was significantly enhanced after a 15-day K-deficiency treatment, but Si application eliminated the K-deficiency-induced increase in osmotic potential (Fig. 3c). The lower osmotic potential in Si-treated plants suggested that Si could improve the plant-water status by decreasing the osmotic potential.
Figure 3.
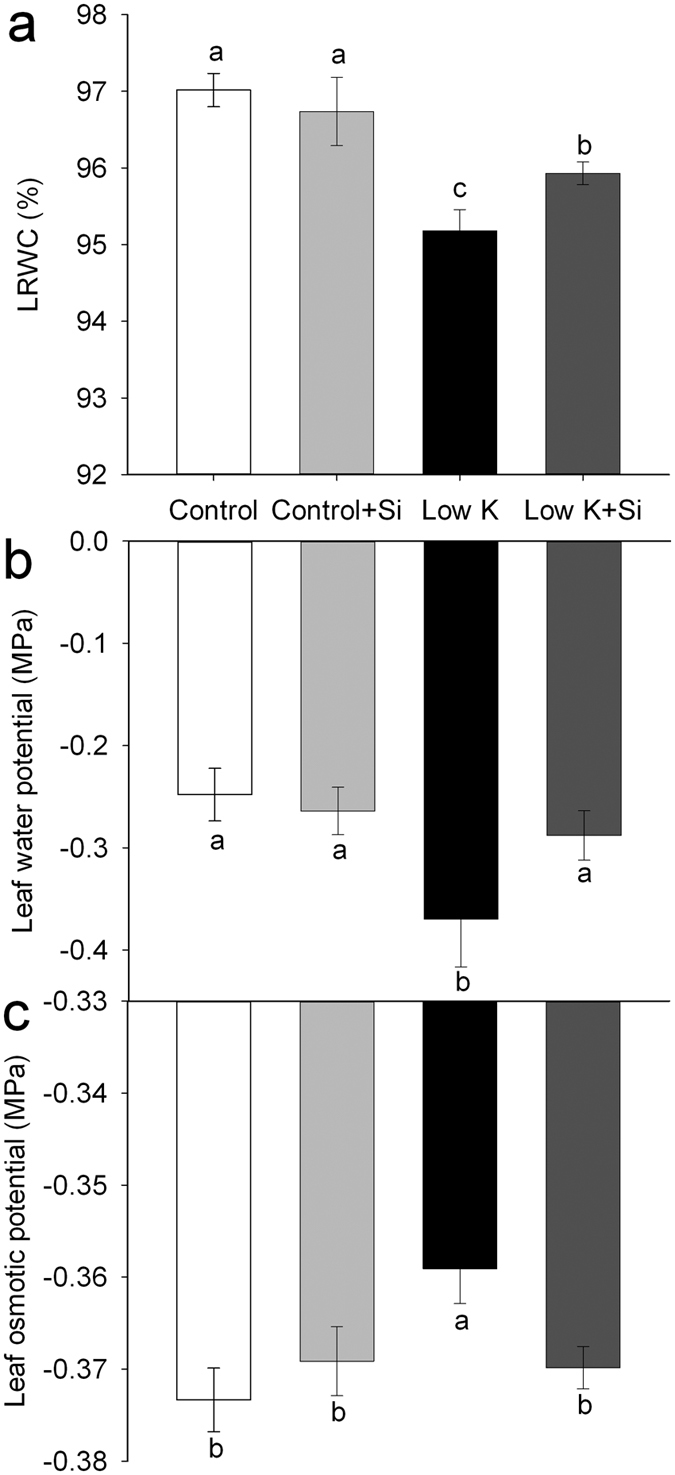
Effects of silicon (Si, 1 mM) on leaf relative water content (LRWC) (a), leaf water potential (b) and leaf osmotic potential (c) of sorghum plants grown under control (3 mM KCl)and low K (0.05 mM KCl) conditions. All parameters were measured after 15 days of treatment. Values are presented as the mean ± SE (n = 5). Different letters in one measure indicate statistically significant differences at P < 0.05.
Si application did not enhance the K concentration under the K-deficiency condition
Activated K+ uptake in plants by the induction of the expression of high-affinity transporters was considered to be a major mechanism of adaptation to K starvation29,30,31. As shown in Fig. 4, the K+ concentration in both shoots and roots sharply decreased after 15 days of K deficiency. Si application did not influence the K+ concentration in either shoots or roots under control or K-deficiency conditions. Total K+ uptake per plant was decreased by K deficiency, and was much lower in plants that did not receive Si treatment (Fig. 4). This result suggested that the increased total K+ absorption in Si-treated plants under K-deficiency conditions was the consequence of increase in biomass. The same K+ concentration between the Si-treated and -untreated plants indicates that Si alleviating the K deficiency was not due to a direct increase in K+ absorption.
Figure 4.
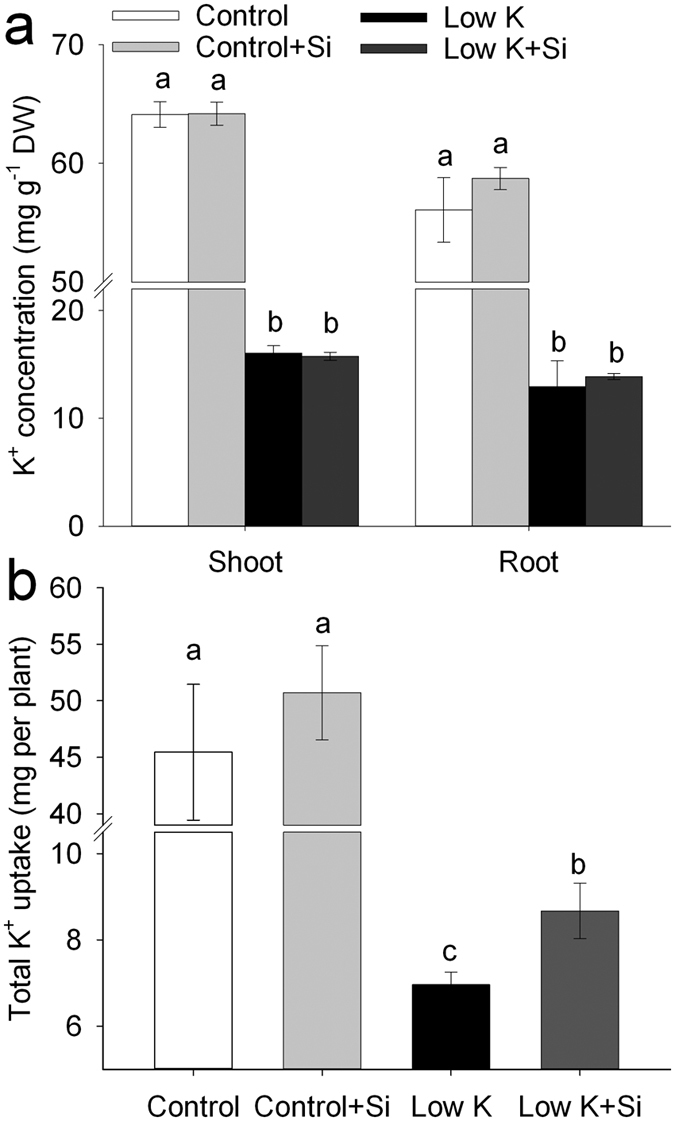
Effects of silicon (Si, 1 mM) on K+ concentration (a) and total K+ uptake (b) of sorghum plants grown under control (3 mM KCl)and low K (0.05 mM KCl) conditions. All parameters were measured after 15 days of treatment. Values are presented as the mean ± SE (n = 3). Different letters in one measure indicate statistically significant differences at P < 0.05.
Si improved the plant-water status by maintaining the high level of whole-plant hydraulic conductance and root hydraulic conductance
The overall plant water uptake is represented by whole-plant hydraulic conductance (Kplant), which consists of leaf, stem, and root hydraulic conductance32. In this study, under normal growth conditions, Si application did not change Kplant. K deficiency largely decreased Kplant and Si application significantly reduced this decrease. Kplant in plants treated with Si was 31% (p < 0.05) higher than that of plants without Si under K deficient conditions (Fig. 5). These results showed that Si-treated plants had greater water uptake ability than untreated plants.
Figure 5. Effects of silicon (Si, 1 mM) on whole-plant hydraulic conductance (Kplant) of sorghum plants grown under control (3 mM KCl) and low K (0.05 mM KCl) conditions.
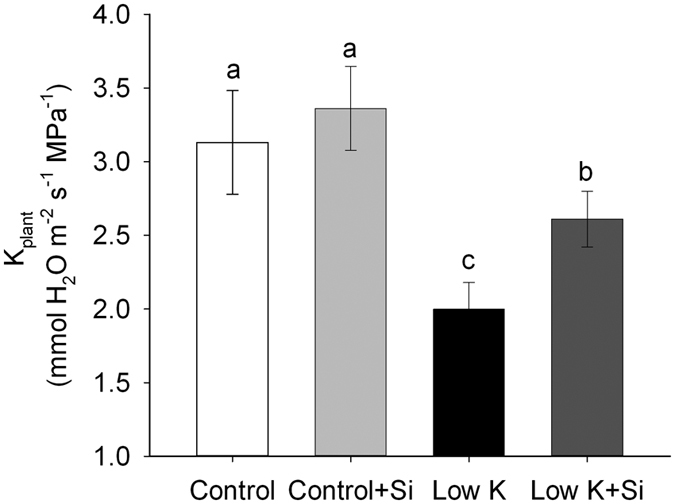
The Kplant was calculated by the transpiration rate determined gravimetrically divided by the difference between culture solution and leaf water potential. Values are presented as the mean ± SE (n = 6). Different letters in one measure indicate statistically significant differences at P < 0.05.
Root hydraulic conductance (Lpr) is usually the lowest within the liquid component of the soil–plant–air continuum33, and roots contribute the majority of the whole-plant hydraulic resistance34. In this study, under control conditions, Si application did not change the Lpr. However, K deficiency significantly decreased Lpr, and Si application significantly moderated this decrease. Lpr in Si-treated plants was 35% (p < 0.05) higher than that in untreated plants under the K deficiency conditions (Fig. 6). These results indicated that the higher Lpr in Si-treated plants could contribute to the Si-mediated increase in plant water uptake under K-deficiency condition.
Figure 6. Effects of silicon (Si, 1 mM) on root hydraulic conductance (Lpr) of sorghum plants grown under control (3 mM KCl) and low K (0.05 mM KCl) conditions.
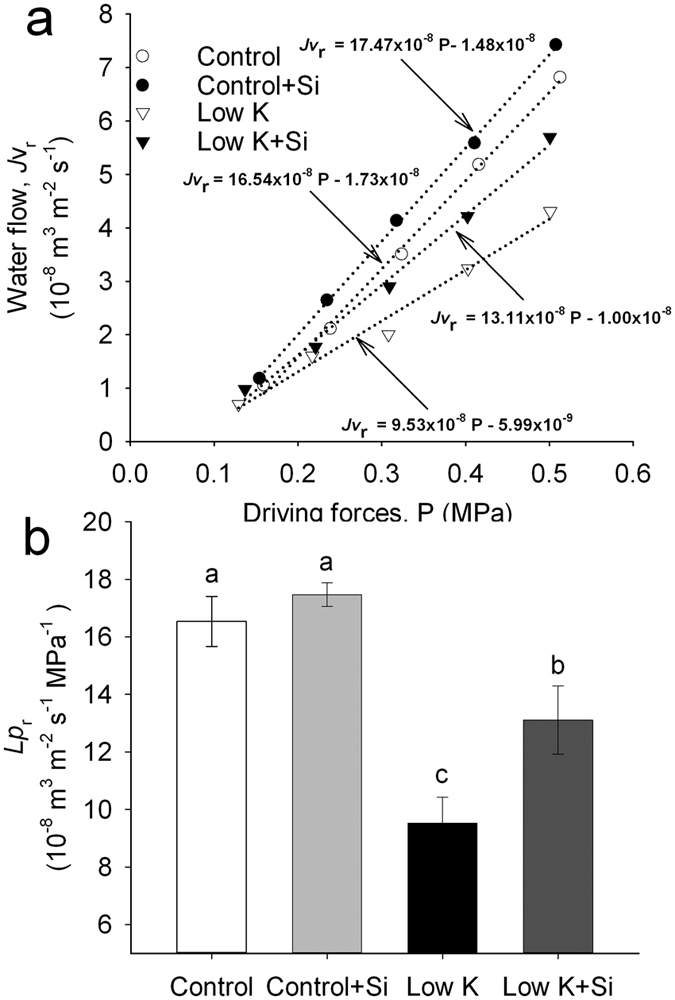
The Lpr was measured using a pressure chamber. Exuded sap was collected for 60 s for a given gas pressure and weighed, and the root surface area was then scanned and analyzed. The water flow, Jvr in m3 m−2 s−1, was obtained and Lpr (m3 m−2 s−1 MPa−1) is calculated from the slope of Jvr against the driving force P, consisting of Pgas and an osmotic gradient (a). The mean ± SE (n = 6) are shown in (b). Different letters in one measure indicate statistically significant differences at P < 0.05.
Si alleviated Lpr by enhancing aquaporin activity and xylem sap K+ concentration
In the process of water uptake by roots, water can move radially toward the xylem along three pathways: the apoplastic, symplastic, and transcellular pathways. The symplastic and transcellular pathways are collectively referred to as the “cell-to-cell pathway”35,36. The cell-to-cell pathway plays an important role in water transport in the roots under certain conditions and is driven by the osmotic gradient between the soil and root xylem sap34. In the present study, the K+ concentration in xylem sap was largely decreased after 15 days of K deficiency, but Si application significantly reduced the K-deficiency-induced decrease. The xylem sap K+ concentration in the Si-treated plant was 80% (p < 0.05) higher than that of untreated plants (Fig. 7a). The xylem sap osmotic potential was significantly enhanced after 15 days of K deficiency. However, under K deficiency conditions, the xylem sap osmotic potential of the plant was 32% (p < 0.05) lower in Si-treated plants than in untreated plants (Fig. 7b). The low osmotic potential in xylem sap indicates the higher osmotic gradient and driving force between the soil and the root xylem sap in Si-treated plants under the K-deficient condition. The contribution of K+ to the xylem sap osmotic potential was also investigated and the result showed that K+ accounts for 38% of the osmotic potential under the control condition with or without Si, 24% of the osmotic potential under K-deficiency conditions with Si treatment, and 17% under K-deficiency conditions without Si. The contribution of K+ to the osmotic potential in Si-treated plants was 41% (p < 0.05) higher than that of in Si-untreated plants under the K-deficiency condition (Fig. 7c).
Figure 7.
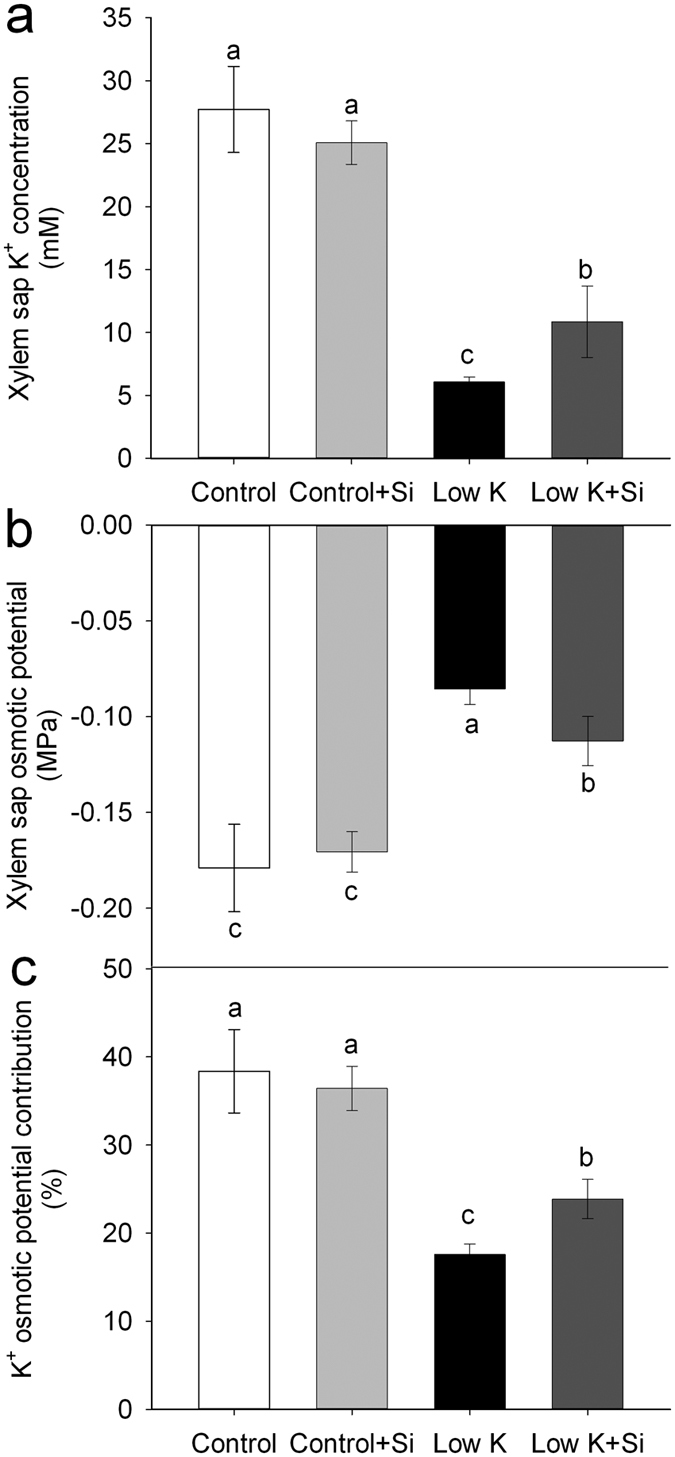
Effects of silicon (Si, 1 mM) on xylem sap K+ concentration (a), osmotic potential (b), and K+ osmotic potential contribution (c) of sorghum plants grown under control (3 mM KCl) and low K (0.05 mM KCl) conditions. All parameters were measured after 15 days of treatment. Values are presented as the mean ± SE (n = 3). Different letters in one measure indicate statistically significant differences at P < 0.05.
In addition to the osmotic gradient, it has been reported that the cell-to-cell pathway can be largely controlled by the activity of aquaporin33. In several studies, aquaporin activity has been assessed using inhibitors such as mercuric chloride (HgCl2), which blocks protein channels and results in a reduction of membrane permeability to water without any adverse effect on the tissue25. In this study, in the absence of HgCl2, the transpiration of Si-treated plants was higher than that of plants without Si treatment under K-deficient condition (Fig. 8). Under control conditions, the presence of HgCl2 decreased the transpiration rate slightly (approximately 6%) in plants that were treated both with and without Si. However, a significant inhibition by HgCl2 in plant transpiration (26% decrease, p < 0.05) was found under K-deficient condition. It was also found that the transpirations of plants both with and without Si were decreased by HgCl2 under the K-deficiency condition, but the differences between the Si-treated and -untreated plants were smaller compared with those before HgCl2 addition (Fig. 8). These results showed that aquaporin could be partly responsible for the enhanced Lpr under the K-deficiency condition.
Figure 8. Effect of aquaporin inhibitor (HgCl2) on the transpiration rate of sorghum plants with and without Si application grown under control (3 mM KCl) and low K (0.05 mM KCl) conditions.
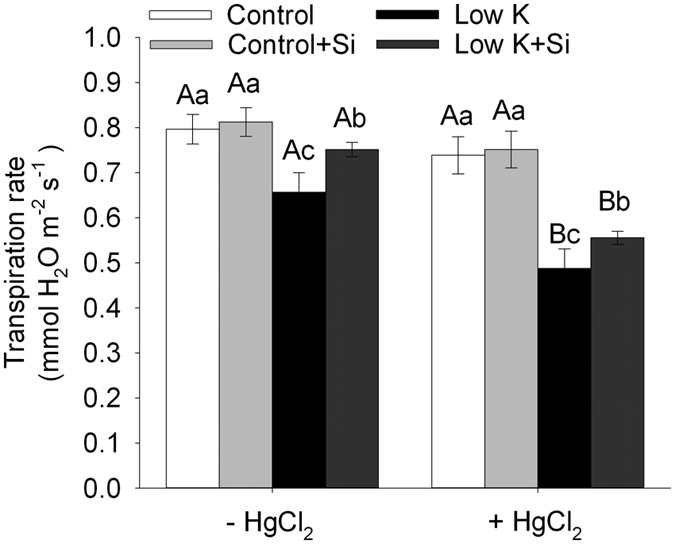
The seedlings were divided in two groups, as follows: one measured the normal transpiration rate, and the other was treated with 50 μM HgCl2 for 5 min and then rinsed with distilled water before measuring the transpiration rate in the culture solutions. Values are presented as the mean ± SE (n = 6). Different capital letters and lower cases indicate statistically significant differences at P < 0.05 between HgCl2 treatments or K and Si treatments.
Si application regulated the expression of aquaporins genes and K+-related genes
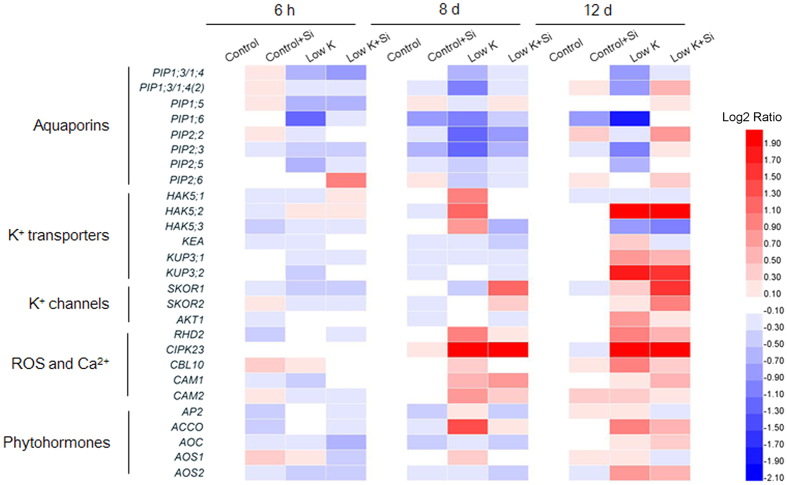
To investigate the molecular basis for Si alleviation of K-deficiency in plants, we examined the expression levels of aquaporins genes and putative genes homologous to Arabidopsis K+-related genes as reviewed by Wang and Wu37 including those encoding K+ transporters, K+ channels, regulatory factors, and signaling components in sorghum roots by qRT-PCR (Fig. 9 and Supplementary Table S3). Under control condition, the expression levels of tested genes showed no change by Si application. Under K-deficient condition, mRNA levels of plasma membrane intrinsic protein (PIP) PIP1;3/1;4(2), PIP1;6, PIP2;2, PIP2;3 decreased up to 2-fold, compared with levels under the control condition, whereas they remained unchanged under K-deficient plus Si treatment. Expression of putative high affinity K+ transporter (HAK) gene HAK5.2, which is regarded as the marker gene in Arabidopsis response to K+ deficiency37 and another K+-deficiency-induced K+ transporter K uptake permease (KUP) KUP3.2 increased by 7.0- and 3.3-fold, respectively, at the 12th day under K-deficient condition, whereas they increased less (4.4- and 2.9- fold) under K-deficient plus Si condition. For the putative sorghum stelar K+ outward rectifier (SKOR) gene SKOR1 and SKOR2, those are specifically expressed in the root stele (pericycle and stelar parenchyma) and encode the SKOR channel mediating K+ secretion from root cortex cells into the xylem38, their expression levels were not affected by K-deficient treatment, whereas they increased significantly by 2.8- and 2.1-fold under K-deficient plus Si condition, respectively.
Figure 9. Effects of silicon (Si, 1 mM) on expression of aquaporin genes and putative genes homologous to Arabidopsis K+-related genes in root of sorghum plants with and without Si application grown under control (3 mM KCl) and low K (0.05 mM KCl) conditions.
Each treatment includes three replications and each replication includes three technical replications. Colors indicate change of transcript level in the treated samples with respect to the control samples (red for up-regulation, blue for down-regulation. See color bar at the right of figure).
ROS, Ca2+, ethylene, and jasmonic acid have been considered the major signals in plant responses to K+-deficiency stress37. In this study, the putative NADPH oxidase gene root hair defective 2 (RHD2), which is involved in ROS generation39, is significantly up-regulated at the 8th and 12th day under K-deficient condition, whereas the degree of up-regulation was less under K-deficient plus Si condition. Similar changes in mRNA levels were also found in putative sorghum calcineurin B-like protein 10 (CBL10), CBL-interacting serine/threonine-protein kinase 23 (CIPK23), and calmodulin 2 (CaM2), those involved in the perception and transduction of K+-deficiency-induced Ca2+ signals, 1-aminocyclopropane-1-carboxylate (ACC) oxidase (ACCO), encoding the ethylene synthesis enzyme ACC oxidase, and allene oxide synthase (AOS), encoding the jasmonic biosynthetic enzyme allene oxide synthase37.
The up-regulation of the aquaporins genes by Si application under K-deficient condition could be partly responsible for the enhanced aquaporin activity. The up-regulation of the SKOR genes and the down-regulation of the HAK5 gene could contribute to xylem sap K+ concentration under K-deficient condition. Also, Si negatively regulated the Arabidopsis K transporter 1 (AKT1), an inward-rectifying channel, by down regulation of the CIPK23 gene, and resulted in inhibition of the K+ translocation out of xylem. Moreover, the down-regulation of the K+-deficiency-responsive genes by Si under K deficient condition suggested that the K deficiency was moderated by Si application, and this moderation was not due to the increase in K uptake by the up-regulation of K+ transport proteins at the transcriptional level.
Discussion
Severe K deficiency first inhibits plant growth, and leaf necrosis appears in old leaves that had a prolonged exposure time26. In the present study, Si application significantly inhibited the decrease in total dry weight under K-deficiency condition, and leaf necrosis in old leaves was also alleviated in Si-treated plants compared with Si-untreated plants (Fig.1a, Supplementary Fig. S1), indicating that Si application alleviated the K deficiency. The effect of Si on alleviating the K deficiency was also investigated under serial K concentrations (from 6 mM to 0 mM, see Supplementary Fig. S2). The result showed that Si could only alleviate K deficiency when the K concentration was lower than 1.5 mM, and the biggest effect occurred when the K concentration was set between 0.5 mM and 0.01 mM. The results also showed that Si only plays a role when the plant growth was obviously inhibited by K deficiency. Therefore, Si enhanced plant growth specifically under the condition of K deficiency. It is of note that although Si showed a significant effect on alleviating K deficiency, it cannot entirely eliminate the adverse effect of K deficiency under a severe K-deficiency condition.
The major mechanism of adaptation to K starvation in plants is the activation of K+ uptake through the induction of expression of high affinity transporters29,30,31. A previous study showed that Si application enhanced plant K+ concentration4. However, in this study, the K+ concentration in both the root and shoot was not affected by Si application. And consistently, the putative HAK5 and AKT1, which mediate almost all K+ absorption in Arabidopsis roots37, were not up-regulated by Si compared with that without Si application under K deficient condition (Fig. 9). Severe K deficiency was also reported to decrease the photosynthetic rate, and maintenance of a high photosynthetic rate is a base of accumulation of more biomass under K deficiency21,27,28. In this study, although the K concentration in plants that were treated with and without Si was low and at the same level under K deficiency, the photosynthetic rate and dry weight in Si-treated plants were much higher than in those without Si, suggesting that the Si-alleviated K-deficiency-induced growth inhibition was not directly due to the increase in K uptake.
Severe K deficiency generally induces stomatal conductance and lowers transpiration rate, which results in a lower photosynthetic rate21,27,28. In this study, plants treated with Si maintained a higher stomatal conductance and transpiration rate, which is coincident with the higher photosynthetic rate (Fig. 2). These results suggest that the Si-improved plant-water status could be the major reason for the increased photosynthetic rate under the K-deficient conditon. Water status, as reflected by LRWC, is the basis of metabolic activity in tissues15,16. It is widely accepted that K deficiency increases a plant’s susceptibility to the water stress and that plants with an adequate K supply have better hydrated tissues than those with K deficiency37. In this study, K deficiency decreased the LRWC and water potential, which was moderated by Si application. The results of this study suggest that Si alleviates K deficiency by improving plant-water status.
Plant water-status involves the balance between the differences in water uptake through the roots and the transpiration in leaves15,16. Severe K deficiency has been reported to favor stomatal closure and decrease the transpiration rate22,40. However, the stomatal conductance and transpiration rates were maintained at higher levels in Si-treated plants compared with untreated plants under K-deficiency treatment. This suggests that Si’s improvement of the plant-water status is a result of an increase in water uptake, but not because of a reduction in water loss.
The overall plant water uptake is represented by whole-plant hydraulic conductance (Kplant), which consists of leaf, stem and root hydraulic conductance32. In this study, K deficiency caused a decrease in Kplant that was moderated by Si application (Fig. 5). The seedlings used in the experiment did not form stems. Therefore, Kplant was only affected by the root and leaf hydraulic conductance. Kleaf was not measured in this study due to technical limitations, but it tends to be similar to leaf water potential41. In the present study, the leaf water potential of sorghum with Si was higher than that without Si under K-deficiency condition, suggesting that plants with Si application maintained a higher Kleaf than without Si. Root hydraulic conductance (Lpr) is usually the lowest within the liquid component of the soil–plant–air continuum33 and roots contribute the majority of the whole plant hydraulic resistance34. In addition, the shoot/root ratio was not affected by Si under the K-deficiency conditon (Fig. 1c). Therefore, the similar extent of Si-enhanced Kplant (31%) and Lpr (35%) (Figs 5 and 7) indicated that Si enhanced the plant water uptake was mainly ascribed to the enhancement of the Lpr.
Gas exchange rates and photosynthesis are limited by the hydraulic conductance of the liquid water pathway from the soil to the leaves42,43,44,45. In this study, K deficiency caused a significant decrease in the LRWC (Fig. 3). The whole-plant transpiration rate and leaf transpiration was decreased by 18% and 57%, respectively, suggesting that water is a serious limitation for photosynthesis under the K-deficient condition. However, the plant hydraulic conductance was 31% higher in Si-treated plants than Si-untreated plants under the K-deficient condition. Similarly, the whole-plant transpiration rate and leaf transpiration was increased by 14% and 94%, respectively (Figs 2 and 8). Meanwhile, the photosynthesis rate of the leaf was enhanced by 139% in Si-treated plants compared to untreated plants. Although there is not a clear quantitative relationship between the plant water conductance and the growth rate or photosynthetic rate under K deficiency, the highly consistent tendency for plant water conductance, transpiration rate, photosynthesis, and dry weight suggests that the alleviation of the plant-water status through the enhancement of the water hydraulic conductance played an important role in Si-alleviated K deficiency.
Lpr represents the root water uptake capacity and it is determined by root surface, root anatomy and root water permeability46. In the root water uptake, the cell to-cell pathway can be largely controlled by the activity of aquaporins, which respond relatively rapidly and reversibly, causing changes in Lpr33. In this study, plant transpiration with and without Si is decreased by HgCl2, but the difference between Si-treated and -untreated plants was reduced after HgCl2 treatment, and aquaporin contributed to 29% of the Si-induced increase in transpiration. Liu et al.24,25 reported that aquaporin regulation accounts for the mechanism of Si-induced alleviation of the plant-water status under drought and salt stress conditions. However, in the present study, 71% of the Si-induced increase in transpiration remained in addition to the aquaporin-regulated water uptake. Thus, other important approaches that mediate the Si-enhanced water uptake under K-deficiency condition must be considered.
The cell-to-cell pathway plays an important role in water transport in the roots under certain conditions, and is driven by the osmotic gradient between the soil and the root xylem sap34. In this study, the osmotic potential of root xylem sap was 20% lower in Si-treated plants than in Si-untreated plants (Fig. 7). This resulted in an increase in the osmotic gradient between the soil and the root xylem. The osmotic gradient between the cultivated solution and the xylem in plants treated with Si was −0.092 MPa; it was −0.073 MPa in Si-untreated plants under the K-deficiency condition. Thus, the osmotic gradient was 26% higher in Si-treated plants than Si-untreated plants.
In addition to serving as an important component of the xylem osmotic potential, xylem K+ plays a critical role in modifying the xylem hydraulic conductance and water relations in plants in response to ion-mediated volume changes of pectin in pit membranes47. Nardini et al.48 reported that a 30 to 60% increase in stem hydraulic conductance in Laurus nobilis is associated with an increase in the xylem sap K+, from 3 to 12 mM. In another experiment on laurel plants, short-term K fertilization led to an increase in xylem sap K+ and caused a rapid (within 24 h) 45% increase in the transpiration rate and a 30% increase in plant hydraulic conductance19. Cation-mediated enhancement of xylem hydraulic conductivity has been reported for both herbaceous and woody species49. In this study, the K+ concentration in the root xylem sap was 80% higher in Si-treated plants than in untreated plants under K-deficiency condition. Although we cannot estimate the contribution of the enhanced K+ concentration to xylem hydraulic conductance and Lpr, based on the previous study, the huge discrepancy in the xylem sap K concentration between plants treated with and without Si is expected to lead to much higher plant hydraulic conductance and transpiration rates in Si-treated plants.
To date, there is no information available on how Si is involved in regulating important metabolic pathways in plants because of the absence of evidence of organic Si in plant tissue. However, there is some evidence that supports the idea that Si can be involved in regulating the plant stress response. For example, the expression of nearly 4,000 genes was affected in Si-treated Arabidopsis that was infected with a fungal pathogen50. Liu et al.24 also suggested that reactive oxygen species (ROS) could participate in the Si-regulated aquaporin activity under salt stress. In this study, the aquaporins genes were up-regulated by Si application under K-deficient condition, which could be partly responsible for the enhanced aquaporin activity (Fig. 9 and Supplementary Table S3). The SKOR genes mediate K+ secretion from root cortex cells into the xylem. Pilot et al. reported that the level of SKOR transcripts decreased after K+ shortage in Arabidopsis51. However, other studies showed that SKOR did not transcriptionally respond to K+ deprivation52, and also short-term K+ resupply after long-term K+ starvation in Arabidopsis53. In addition, OsSKOR gene was up-regulated under low-K+ stress31. These inconsistent results suggest that transcriptional response of SKOR to K+ deficiency may differ among different species and K+ deficiency treatments. In this study, the expression level of SKOR showed no change under low K condition, but the it was up-regulated by Si application under K-deficient condition. In addition, the HAK5, CIPK23 and RHD2 genes were down-regulated by Si application under K-deficient condition. Those changes contribute to xylem sap K+ concentration under K-deficient condition (Fig. 9 and Supplementary Table S3). Therefore, it appears that Si can regulate the K-deficiency responses at the transcriptional level directly or indirectly. Recently, the K-deficiency response signals have been clarified in model plants, such as Arabidopsis. In particular, it has been shown that ROS, Ca2+, ethylene, and jasmonic acid are involved in plant signaling responses to K+ deficiency37,54,55,56,57. Although no previous studies have reported how Si is involved in plant signaling regulation under various stresses, the application of Si that influences the ROS generation and elimination has been widely reported24,58. In this study, genes related to ROS metabolism, Ca2+ signal perception, and ethylene and jasmonic acid synthesis are down-regulated by Si application under K-deficient condition (Fig. 9 and Supplementary Table S3). Thus, it appears that Si can also influence the plant signaling regulation under K-deficient stress. Therefore, although we do not yet have a clear picture of how Si regulates the K concentration in xylem under K deficiency, based on the present and previous studies, we propose the following model: under the K-deficient condition, Si directly or indirectly influence the plant signaling regulation, which are involved in regulating the K channels and transporter gene expression and post-transcriptional regulation. The regulation of K channels and transporters, especially the outward-rectifying channel SKOR, which mediate K concentration in the xylem under the K-deficient condition, may lead to enhanced K concentration in the xylem.
Conclusion
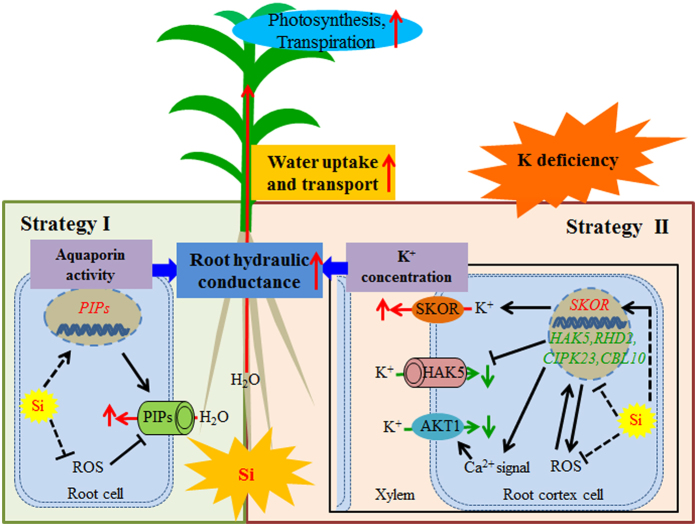
K is widely involved in regulating the plant-water status, and severe K deficiency induces plant dehydration. In this study, Si application alleviated the K deficiency while maintaining higher relative water content, water potential, transpiration and plant hydraulic conductance. Combining the present findings with those of previous studies, a model describing how Si is involved in alleviating K deficiency by moderating plant-water status was proposed (Fig. 10). (1) Under the K-deficient condition, Si activated the expression of SKOR, down-regulated the expression of HAK5 and negatively regulated the AKT1 channel, which resulted in the concentrating of K in xylem. (2) The higher K concentration in the xylem sap contributed to the increase in the osmotic gradient and the xylem hydraulic conductance, leading to enhanced root hydraulic conductance. (3) Si could also participate in regulating the aquaporin activity, resulting in increased root hydraulic conductance. (4) The higher root hydraulic conductance resulted in higher whole-plant hydraulic conductance, which contributed to an increase in water uptake and transport, resulting in an alleviation of K-deficiency-induced plant dehydration, and alleviating the K deficiency. However, the K+-related genes, whose product are almost all hypothetical proteins, used for expression analysis were gotten from homology searching based on information in Arabidopsis, rice and maize. Furthermore, besides transcriptional regulation, posttranscriptional regulation also plays a critical role in plant response to K deficiency, especially for the K+ channels. Thus, the mechanisms underlying the Si-mediated K+ concentration in the xylem require further study.
Figure 10. A schematic model for silicon (Si) moderated the K deficiency in sorghum by improving the water uptake and transport.
Two strategies are involved in Si enhance the root hydraulic conductance under K-deficient condition. For strategy I, Si could enhance the aquaporin activity by both up-regulating the expression of aquaporin genes (PIPs) and alleviating the decrease of aquaporin activity caused by ROS (Reactive Oxygen Species). For strategy II, Si could activate the K+ translocation to xylem by the activation the expression of SKOR gene, which mediate K+ secretion from root cortex cells into the xylem; Si could also down-regulate the expression of HAK5 gene and may interact with Ca2+ and ROS signals to negatively regulate the AKT1 channel to inhibit the K+ translocation out of xylem (red for up-regulation, green for down-regulation). The enhanced aquaporin activity and K+ concentration in xylem resulted in increased root hydraulic conductance and higher whole-plant hydraulic conductance, which contributed to an increase in water uptake and transport. The improved water uptake and transport alleviates the K-deficiency-induced plant dehydration and moderates the K deficiency.
Materials and Methods
Seedling cultivation and Si and K deficiency treatment
Sorghum cultivar (Sorghum bicolor [L.] Moench. cv. Gadambalia) was used in this study. This cultivar can uptake a large amount of Si, and Si application can improve its water status under drought and salt stress conditions24,25. The seedlings were cultivated in a growth chamber that was set to a 14/10 h day/night cycle at a day/night temperature of 28/25 °C with 40 to 50% relative humidity. The amount of photosynthetically active radiation (PAR) to the upper plant was 600 μmol m−2 s−1.
Sterilized seeds were germinated in an incubator at 24 °C under dark conditions. After germination for 4 days, uniform seedlings (6 ± 0.5 cm)were selected and transplanted into a plastic container (40 × 28 × 14 cm) with 8 liters of one-quarter strength Hoagland culture solution with no KNO3, NO3− was provided by Ca(NO3)2 and the K was supplied by KCl. The experiment included four treatments: control, control + Si, low K and low K + Si. The K concentration was 0.05 mM in the low K (K deficiency) treatments and 3 mM in the control (K sufficiency) treatment, supplied by KCl. Under 3 mM K concentration, the growth of seedlings showed no difference compared to that under 6 mM K (normal K concentration in Hoagland solution). The growth of seedlings showed obvious inhibition under the 0.05 mM K condition (Supplemental Fig. S1). The pH of the culture solution was adjusted to 6.053. Si was set to 0 mM in the without Si treatment and 1mM H2SiO3 in the Si treatments. H2SiO3 was produced by passing sodium silicate solution through a column filled with cation-exchange resins58. The culture solution was renewed every three days and continuously aerated. Fifteen days after transplantation, samples were collected and the parameters listed below were measured.
Dry weight and root/shoot ratio
Fifteen days after transplantation, the plant was harvested. The shoots and roots were separated and dried at 75 °C for 72 h. The total weight and root/shoot ratio were calculated. Each treatment included 10 plants.
Photosynthetic rate, stomatal conductance and transpiration rate
Fifteen days after transplantation, the upper fully expanded leaves were selected to measure the photosynthetic rate, stomatal conductance and transpiration rate using a portable photosynthesis system (Li-6400; LI-COR Inc., Lincoln, NE, USA) between 10:00 AM and 12:00 AM. The leaf was placed in a 6 cm2 chamber at a photo flux density of 1000 μmol m−2s−1. Each treatment included six plants.
Leaf relative water content, leaf water potential and osmotic potential
Fifteen days after transplantation, the upper fully expanded leaves were sampled and LRWC was measured and calculated according to Liu et al.25. Upper fully expanded leaves were covered with aluminum foil before excision from the plant and the water potential was measured using a pressure chamber (Model 3500, Soil Moisture Corp., Santa Barbara, CA, USA). The leaf osmotic potential of the upper fully expanded leaves was measured using a dew point microvolt meter (Model 5600, Wescor, Logan, UT, USA). Each treatment included five replications.
K+ concentration and total K+ uptake in the tissue
Dried shoot and root samples were ground to powder, weighed and then digested using nitric acid at 320 °C for 5 h. K+ concentrations were measured using an atomic adsorption spectrometer with a flame photometer (ZL5100, PerkinElmer, Inc., USA)59. The K+ absorption was calculated based on the dry weight and tissue K+ concentration. Each treatment included three replications.
Whole-plant hydraulic conductance
Kplant was calculated according to the following equation32:
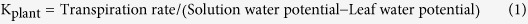 |
The transpiration rate was determined gravimetrically. The leaf water potential was measured as described above. In hydroponic culture, the culture solution water potential was −0.032 MPa in the control, −0.034 MPa in control + Si, −0.018 MPa in low K, and −0.019 MPa in low K + Si treatment groups. Each treatment included six replications.
Transpiration rate in response to aquaporin inhibitor
Aquaporin-mediated water transport was investigated by measuring changes in the transpiration rate in response to an aquaporin inhibitor (HgCl2), according to Knipfer et al.60. The seedlings were divided into two groups: one group (6 plants) was used for measuring the transpiration rate, and the other group (6 plants) was treated with 50 μM HgCl2 for 5 min and then rinsed with distilled water (devoid of HgCl2) before measuring the transpiration rate in the culture solutions. The transpiration rate was determined gravimetrically.
Root hydraulic conductance
Lpr based on the root surface area was measured using a pressure chamber (Model 3500, Soil Moisture Corp., Santa Barbara, CA, USA), according to the method of Miyamoto et al.61. The roots were enclosed in a steel chamber and pneumatic pressure was applied to the root medium. The pressure in the chamber was increased in steps of 0.1 MPa up to a pressure of 0.5 MPa. Exuded sap was collected for 60 s at a given gas pressure and weighed. The root surface area was then scanned and analyzed using WinRHIZO PRO 2009 software (Regent Inc., Canada). The water flow, Jvr in m3 m−2 s−1, was obtained. The osmotic potential of the collected exuded sap was measured as described above and Lpr (m3 m−2 s−1 MPa−1) was calculated from the slope of Jvr against driving force P, consisting of Pgas and the osmotic gradient, according to the following equation:
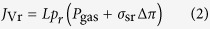 |
In this equation, σsr is the root reflection coefficient for nutrient salts in the xylem, which was estimated to be σsr = 0.4, according to Miyamoto et al.56. Each treatment included six plants.
Xylem sap K+ concentration and osmotic potential
Xylem sap was extracted between 10:00 AM and 12:00 AM according to Liu et al.25. Plants were rapidly cut using a razor blade at the base of the root system. The roots were enclosed in a steel chamber and the surface of the cut was washed with distilled water and blotted dry to remove any disrupted and residual cell. The pneumatic pressure applied to the root medium in the chamber was increased to 0.2 MPa until xylem sap flowed out from the excised tip. The first droplet was discarded to avoid contamination, and then 50 μl of sap was collected from each plant to determine the xylem sap K+ concentration and osmotic potential. The xylem sap osmotic potential was measured using a dew point microvolt meter and the ion concentration was measured by an atomic adsorption spectrometer with a flame photometer as described above. Each treatment included three replications.
Gene expression analysis
Roots were sampled at the 8th day (visible K+ starvation symptoms appeared) and the 12th day (significant differences between the plants with or without Si application under K deficiency appeared) after transplantation. In addition, 12 days after transplantation, one group of seedlings (6 plants) under control and control + Si conditions were transplanted to low K and low K+ Si conditions, respectively; roots were sampled also after K deficiency treatment for 6 hours to investigate the short-term starvation responses. The expression of 8 sorghum plasma membrane intrinsic protein (PIP) aquaporin genes according to Liu et al.25 and 19 putative genes homologous to Arabidopsis K+-related genes based on information in Arabidopsis, rice (Oryza sativa) and maize (Zea mays), were analyzed using quantitative RT-PCR. RNA was isolated and gene expression was analyzed using Actin1 as constitutive controls25. Each treatment included three replications and each replication included three technical replications. The genes and the sequences of their specific primers are listed in Supplemental Table S1. The protein sequence similarities between putative K+-related genes and those in Arabidopsis, rice and maize are listed in Supplemental Table S2.
Statistical analysis
Data were analyzed using an analysis of variance (ANOVA) using the Statistical Analysis System (SAS version 8.0) software. The differences between the means were compared using the Tukey-Kramer test (p < 0.05). All experiments were repeated at least three times. All plots were created using SigmaPlot12.0 and the different letters indicating statistically significant differences at P < 0.05 are indicated in the figures.
Additional Information
How to cite this article: Chen, D. et al. Silicon moderated the K deficiency by improving the plant-water status in sorghum. Sci. Rep. 6, 22882; doi: 10.1038/srep22882 (2016).
Supplementary Material
Acknowledgments
This study was supported by Youth Innovation Promotion Association of the Chinese Academy of Sciences (2013307), National Key Technology Support Program of China (2015BAD22B01) and National Basic Research Program of China (2015CB150402).
Footnotes
Author Contributions S.W. conceived and designed research. D.C. and B.C. conducted the experiment, collected and analyzed the data. S.W. and D.C. prepared the draft. P.L. helped in data collection and draft preparation. X.D., L.Y. and S.Z helped in drafting the manuscript and interpretation of results and revised the manuscript. D.C. and B.C. contributed equally to this work. All authors read and approved the manuscript.
References
- Maathuis F. J. M. Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol. 12, 250–258 (2009). [DOI] [PubMed] [Google Scholar]
- Hafsi C., Debez A. & Abdelly C. Potassium deficiency in plants: effects and signaling cascades. Acta Physiol. Plant. 36, 1055–1070 (2014). [Google Scholar]
- Sharma T., Dreyer I. & Riedelsberger J. The role of K+ channels in uptake and redistribution of potassium in the model plant Arabidopsis thaliana. Front. Plant Sci. 4, 224 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao B., Han X. & Zhang W. The ameliorative effect of silicon on soybean seedlings grown in potassium-deficient medium. Ann. Bot. 105, 967–973 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anschutz U., Becker D. & Shabala S. Going beyond nutrition: Regulation of potassium homoeostasis as a common denominator of plant adaptive responses to environment. J. Plant Physiol. 171, 670–687 (2014). [DOI] [PubMed] [Google Scholar]
- De Boer A. H. Potassium translocation into the root xylem. Plant Biology 1, 36–45 (1999). [Google Scholar]
- Wang Y. & Wu W. Genetic approaches for improvement of the crop potassium acquisition and utilization efficiency. Curr. Opin. Plant Biol. 25, 46–52 (2015). [DOI] [PubMed] [Google Scholar]
- Epstein E. The anomaly of silicon in plant biology. Proc. Natl. Acad. Sci. USA 91, 11–17 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E. Silicon. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 641–664 (1999). [DOI] [PubMed] [Google Scholar]
- Epstein E. Silicon: its manifold roles in plants. Ann. Appl. Biol. 155, 155 (2009). [Google Scholar]
- Guntzer F. Benefits of plant silicon for crops: a review. Agron. Sustain. Dev. 32, 201–213 (2012). [Google Scholar]
- Pavlovic J. et al. Silicon alleviates iron deficiency in cucumber by promoting mobilization of iron in the root apoplast. New Phytol. 198, 1096–1107 (2013). [DOI] [PubMed] [Google Scholar]
- Hernandez-Apaolaza L. Can silicon partially alleviate micronutrient deficiency in plants? a review. Planta 240, 447–458 (2014). [DOI] [PubMed] [Google Scholar]
- Eneji A. E. et al. Growth and nutrient use in four grasses under drought stress as mediated by silicon fertilizers. J. Plant Nutr. 31, 355–365 (2008). [Google Scholar]
- Mali M. & Aery N. C. Influence of silicon on growth, relative water contents and uptake of silicon, calcium and potassium in wheat grown in nutrient solution. J. Plant Nutr. 31, 1867–1876 (2008). [Google Scholar]
- Flower D. J. & Ludlow M. M. Contribution of osmotic adjustment to the dehydration tolerance of water-stressed pigeon pea (Cajanus cajan (L.) millsp.) leaves. Plant Cell Environ. 9, 33–40 (1986). [Google Scholar]
- Hsiao T. C. & Läuchli A. Role of potassium in plant–water relations in Advances in Plant Nutrition (ed. Tinker B., Läuchli A.) Vol. 2, 281–311 (Praeger Scientific, New York, 1986). [Google Scholar]
- Shabala S. Regulation of potassium transport in leaves: from molecular to tissue level. Ann. Bot. 92, 627–634 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läuchli A. Mechanism of nutrient fluxes at membranes of the rootsurface and their regulation in the whole plant in Roots, Nutrient and Water Influx, and Plant Growth (ed. Barber S. A., Bouldin D. R.) 1–25 (ASA Special Publication, No. 49, Madison, 1984). [Google Scholar]
- Oddo E. et al. Short-term effects of potassium fertilization on the hydraulic conductance of Laurus nobilis L. Tree Physiol. 31, 131–138 (2011). [DOI] [PubMed] [Google Scholar]
- Kanai S. et al. Potassium deficiency affects water status and photosynthetic rate of the vegetative sink in green house tomato prior to its effects on source activity. Plant Sci. 180, 368–374 (2011). [DOI] [PubMed] [Google Scholar]
- Fournier J. M., Roldan A. M., Sanchez C., Alexandre G. & Benlloch M. K+ starvation increases water uptake in whole sunflower plants. Plant Sci. 168, 823–829 (2005). [Google Scholar]
- Romero-Aranda M. R., Jurado O. & Cuartero J. Silicon alleviates the deleterious salt effect on tomato plant growth by improving plant water status. J. Plant Physiol. 163, 847–855 (2006). [DOI] [PubMed] [Google Scholar]
- Liu P. et al. Enhanced root hydraulic conductance by aquaporin regulation accounts for silicon alleviated salt-induced osmotic stress in Sorghum bicolor L. Environ. Exp. Bot. 111, 42–51 (2015). [Google Scholar]
- Liu P. et al. Aquaporin-mediated increase in root hydraulic conductance is involved in silicon-induced improved root water uptake under osmotic stress in Sorghum bicolor L. J. Exp. Bot. 65, 4747–4756 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengel K. & Kirkby E. A. Principles of Plant Nutrition (International Potash Institute, Bern, 1987). [Google Scholar]
- Bednarz C. W., Oosterhuis D. M. & Evans R. D. Leaf photosynthesis and carbon isotope discrimination of cotton in response to potassium deficiency. Environ. Exp. Bot. 39, 131–139 (1998). [Google Scholar]
- Tomemori H., Hamamura K. & Tabane K. Interactive effects of sodium and potassium on the growth and photosynthesis of spinach and komatsuna. Plant Prod. Sci. 5, 281–285 (2002). [Google Scholar]
- Ashley M. K., Grant M. & Grabov A. Plant responses to potassium deficiencies: a role for potassium transport proteins. J. Exp. Bot. 57, 425–436 (2006). [DOI] [PubMed] [Google Scholar]
- Siddiqi M. Y. & Glass A. D. M. Regulation of K+ influx in barley: evidence for a direct control of influx by K+ concentration of root cells. J. Exp. Bot. 38, 935–947 (1987). [Google Scholar]
- Ma T., Wu W. & Wang Y. Transcriptome analysis of rice root responses to potassium deficiency. BMC Plant Biol. 12, 161 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martre P. et al. Plasma membrane aquaporins play a significant role during recovery from water deficit. Plant Physiol. 130, 2101–2110 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeleur R. K. et al. The role of plasma membrane intrinsic protein aquaporins in water transport through roots: diurnal and drought stress responses reveal different strategies between isohydric and anisohydric cultivars of grapevine. Plant Physiol. 149, 445–460 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javot H. & Maurel C. The role of aquaporins in root water uptake. Ann. Bot. 90, 301–313 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steudle E. Water uptake by roots: effects of water deficit. J. Exp. Bot. 51, 1531–1542 (2000). [DOI] [PubMed] [Google Scholar]
- Steudle E. Water uptake by plant roots: an integration of views. Plant and Soil 226, 45–56 (2000). [Google Scholar]
- Wang Y. & Wu W. H. Potassium transport and signaling in higher plants. Annu. Rev. Plant Biol. 64, 451–476 (2013). [DOI] [PubMed] [Google Scholar]
- Gaymard F. et al. Identification and disruption of a plant shaker-like outward channel involved in K+ release into the xylem sap. Cell 94, 647–655 (1998). [DOI] [PubMed] [Google Scholar]
- Shin R. & Schachtman D. P. Hydrogen peroxide mediates plant root cell response to nutrient deprivation. Proc. Natl. Acad. Sci. USA 101, 8827–8832 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengel K. & Kirkby E. A. Principles of Plant Nutrition (Kluwer Academic Publishers, Dordrecht, 2001). [Google Scholar]
- Brodribb T. J. & Holbrook N. M. Stomatal closure during leaf dehydration, correlation with other leaf physiological traits. Plant Physiol. 132, 2166–2173 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb T. J., Holbrook N. M., Zwieniecki M. A. & Palma B. Leaf hydraulic capacity in ferns, conifers and angiosperms: impacts on photosynthetic maxima. New Phytol. 165, 839–846 (2005). [DOI] [PubMed] [Google Scholar]
- Hubbard R. M., Ryan M. G., Stiller V. & Sperry J. S. Stomatal conductance and photosynthesis vary linearly with plant hydraulic conductance in ponderosa pine. Plant Cell Environ. 24, 113–121 (2001). [Google Scholar]
- Nardini A. & Salleo S. Limitation of stomatal conductance by hydraulic traits: sensing or preventing xylem cavitation? Trees 15, 14–24 (2000). [Google Scholar]
- Sperry J. S. Hydraulic constraints on plant gas exchange. Agr. Forest Meteorol. 104, 13–23 (2000). [Google Scholar]
- Sutka M. et al. Natural variation of root hydraulics in Arabidopsis grown in normal and salt-stressed conditions. Plant Physiol. 155, 1264–1276 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwieniecki M. A., Melcher P. J. & Michele Holbrook N. M. Hydrogel control of xylem hydraulic resistance in plants. Science 291, 1059–1062 (2001). [DOI] [PubMed] [Google Scholar]
- Nardini A., Grego F., Trifilo P. & Salleo S. Changes of xylem sap ionic content and stem hydraulics in response to irradiance in Laurus nobilis. Tree Physiol. 30, 628–635 (2010). [DOI] [PubMed] [Google Scholar]
- Jansen S. et al. Do quantitative vessel and pit characters account for ion-mediated changes in the hydraulic conductance of angiosperm xylem? New Phytol. 189, 218–228 (2011). [DOI] [PubMed] [Google Scholar]
- Fauteux F., Chain F., Belzile F., Menzies J. G. & Belanger R. R. The protective role of silicon in the Arabidopsis-powdery mildew pathosystem. Proc. Natl. Acad. Sci. USA 103, 17554–17559 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilot G., Gaymard F., Mouline K., Chérel I. & Sentenac H. Regulated expression of arabidopsis shaker K+ channel genes involved in K+ uptake and distribution in the plant. Plant Mol. Biol. 51, 773–787 (2003). [DOI] [PubMed] [Google Scholar]
- Gierth M. & Schroeder S. I. The potassium transporter AtHAK5 functions in K+ deprivation-induced high-affinity K+ uptake and AKT1 K+ channel contribution to K+ uptake kinetics in arabidopsis roots. Plant Physiol. 137, 1105–1114 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick A., Rainer B. & Anna A. The potassium-dependent transcriptome of arabidopsis reveals a prominent role of jasmonic acid in nutrient signaling. Plant Physiol. 136, 2556–2576 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J. et al. A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell 125, 1347–1360 (2006). [DOI] [PubMed] [Google Scholar]
- Wang Y., He L., Li H., Xu J. & Wu W. Potassium channel alpha-subunit AtKC1 negatively regulates AKT1-mediated K+ uptake in Arabidopsis roots under low-K+ stress. Cell Res. 20, 826–837 (2010). [DOI] [PubMed] [Google Scholar]
- Cherel I., Lefoulon C., Boeglin M. & Sentenac H. Molecular mechanisms involved in plant adaptation to low K+ availability. J. Exp. Bot. 65, 833–848 (2014). [DOI] [PubMed] [Google Scholar]
- Liu L., Ren H., Chen L., Wang Y. & Wu W. A protein kinase, Calcineurin B-Like Protein-Interacting Protein Kinase9, interacts with calcium sensor Calcineurin B-Like Protein3 and regulates potassium homeostasis under low-potassium stress in Arabidopsis. Plant Physiol. 161, 266–277 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L. N., Wang S. W., Li J. Y., Tanaka K. & Oka M. Application of silicon improves salt tolerance through ameliorating osmotic and ionic stresses in the seedling of Sorghum bicolor. Acta Physiol. Plant. 35, 3099–3107 (2013). [Google Scholar]
- Chen D. Q., Yin L. N., Deng X. P. & Wang S. W. Silicon increases salt tolerance by influencing the two-phase growth response to salinity in wheat (Triticum aestivum L.). Acta Physiol. Plant. 36, 2531–2535 (2014). [Google Scholar]
- Knipfer T., Besse M., Verdeil J. L. & Fricke W. Aquaporin-facilitated water uptake in barley (Hordeum vulgare L.) roots. J. Exp. Bot. 62, 4115–4126 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto N., Steudle E., Hirasawa T. & Lafitte R. Hydraulic conductivity of rice roots. J. Exp. Bot. 52, 1835–1846 (2001). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.