Abstract
Mycoplasma pneumoniae is a major etiologic agent of acute lower respiratory infections. We evaluated the antimicrobial and immunologic effects of cethromycin (ABT-773), a ketolide antibiotic, for the treatment of M. pneumoniae pneumonia in a mouse model. Eight-week-old BALB/c mice were inoculated intranasally once with 106 CFU of M. pneumoniae on day 0. Treatment was started 24 h after inoculation. Groups of mice were treated subcutaneously with cethromycin at 25 mg/kg of body weight or with placebo daily until sacrifice. Five to ten mice per group were evaluated at days 1, 4, 7, and 10 after inoculation. Outcome variables included bronchoalveolar lavage (BAL) for M. pneumoniae quantitative culture and cytokine and chemokine concentration determinations by enzyme-linked immunosorbent assay (tumor necrosis factor alpha [TNF-α], gamma interferon [IFN-γ], interleukin-1β [IL-1β], IL-2, IL-4, IL-12, granulocyte-macrophage colony-stimulating factor, IL-8, monocyte chemoattractant protein 1 [MCP-1], and macrophage inflammatory protein 1α [MIP-1α]), histopathologic score of the lungs (HPS), and pulmonary function tests (PFT) using whole-body, unrestrained plethysmography at the baseline and post-methacholine exposure as indicators of airway obstruction (AO) and airway hyperresponsiveness (AHR), respectively. The cethromycin-treated mice had a greater reduction in M. pneumoniae culture titers than placebo-treated mice, reaching statistical significance on days 7 and 10 (P < 0.05). HPS was significantly reduced in cethromycin-treated mice compared with placebo-treated mice on days 4, 7, and 10 (P < 0.05). Cytokine concentrations in BAL samples were reduced in mice that received cethromycin, and the differences were statistically significant for 7 of the 10 cytokines measured (TNF-α, IFN-γ, IL-1β, IL-8, IL-12, MCP-1, and MIP-1α) on day 4 (P < 0.05). PFT values were improved in the cethromycin-treated mice, with AO and AHR significantly reduced on day 4 (P < 0.05). In this mouse model, treatment with cethromycin significantly reduced M. pneumoniae culture titers in BAL samples, cytokine and chemokine concentrations in BAL samples, histologic inflammation in the lungs, and disease severity as defined by AO and AHR.
Mycoplasma pneumoniae has been implicated in up to 41% of cases of community-acquired pneumonia in children and adults (6, 13, 22, 28, 38).
Macrolides and tetracyclines are considered the treatment of choice for M. pneumoniae respiratory tract infection (6, 12). However, it has been demonstrated that M. pneumoniae persists in the airway even after appropriate antibiotic therapy in studies with both humans and animals (8, 11, 20, 43, 44; S. Esposito, F. Blasi, R. Droghetti, S. Bosis, M. T. Panza, L. Allegra, and N. Pricipi, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. G-1542, 2003).
Despite the lack of eradication of the microorganism from the airway, appropriate antibiotic treatment significantly decreases the morbidity of pneumonia and shortens the duration of symptoms (8, 13, 20, 30, 40). Recent data also suggest that therapy with macrolide antibiotics can reduce the rate of recurrent wheezing and abnormal pulmonary function that result from acute M. pneumoniae infection (10, 29). The impact of chronic respiratory carriage of M. pneumoniae and its association with recurrent wheezing and asthma is being actively investigated (14, 15, 37; S. Biscardi, E. Marc, F. Moulin, E. Nicand, J. L. Iniquez, B. Boutnonnat-Faucher, J. Raymond, F. Brunet, and D. Gendrel, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr G-1550, 2001).
Cethromycin (ABT-773) belongs to the ketolide family, a new class of antibiotics derived from the macrolides, which represents a class of 14-membered ring macrolide agents characterized by a keto group at position 3 of the macrolactone ring, replacing the l-cladinose moiety of other members of the macrolide group (53). Cethromycin has activity against agents associated with community-acquired pneumonia, including the atypical bacteria (1, 3, 24, 31, 49). In vitro studies have demonstrated that cethromycin has excellent activity against M. pneumoniae, with MICs lower than those of macrolides (33, 48). However, there are no in vivo studies evaluating the activity and immunologic impact of therapy with ketolides against M. pneumoniae pneumonia.
The present study was designed to evaluate the in vivo activity of cethromycin against M. pneumoniae in a murine pneumonia model (19). The effect of therapy on the pulmonary immune response, as defined by cytokines and chemokines, was also evaluated to gain insight into the immunopathogenesis of M. pneumoniae disease and its treatment.
MATERIALS AND METHODS
Organism and growth conditions.
M. pneumoniae (ATCC 29342) was reconstituted in SP4 broth and subcultured after 24 to 48 h in a flask containing 20 ml of SP4 media at 37°C. When the broth turned an orange hue (approximately 72 h), the supernatant was decanted and 2 ml of fresh SP4 broth was added to the flask. A cell scraper was used to harvest the adherent mycoplasmas from the bottom of the flask. This achieved an M. pneumoniae concentration in the range of 106 to 107 CFU/ml. Aliquots were stored at −80°C. All SP4 media contained nystatin (50 U/ml) and ampicillin (1.0 mg/ml) to inhibit the growth of potential contaminants.
Animals and inoculation.
Mice were obtained from commercial vendors (Charles River), who confirmed their mycoplasma- and murine virus-free status. The Animal Resource Center at University of Texas Southwestern Medical Center performed quarterly health surveillance on sentinel mice housed in the mouse storage room. Sentinel mice were analyzed for antibodies against mouse hepatitis virus, Sendai virus, pneumonia virus of mice, reovirus type 3, mouse encephalitis virus (GD-7), mouse rotavirus (EDIM), minute virus of mice, and Mycoplasma pulmonis. Sentinel mice were also screened for pinworms and mites. Sentinel mice were free of these pathogens. Mice were housed in filter-top cages and allowed to acclimate to their new environment for 1 week. Methoxyflurane, an inhaled anesthetic, was used for inoculum sedation. Two-month-old female BALB/c mice were intranasally inoculated once (day 0) with 2 × 106 to 7 × 106 CFU of M. pneumoniae in 50 μl of SP4 broth. Directly comparable treatment and placebo groups were given M. pneumoniae inocula from the same vial. All mice were housed in the same animal room and received identical daily care. Animal guidelines were followed in accordance with the Institutional Care and Research Advisory Committee.
Cethromycin (ABT-773) administration.
Laboratory standard cethromycin powder (Abbott Laboratories, Chicago, Ill.) was formulated in 2% ethanol and 5% dextrose, with an adjusted pH of 6.0 to 6.5. Cethromycin (25 mg/kg of body weight; dosage, 0.5 mg in 0.25 ml per mouse) was started 24 h after M. pneumoniae inoculation and administered subcutaneously once daily for 10 days.
Since the manufacturer of cethromycin had not established a dosage regimen for humans at the time of our study initiation, we used a dosage that had been used previously in mouse studies (24, 51; I. C. Michelow, R. D. Hardy, K. Olsen, J. Iglehart, B. B. Rogers, H. Jafri, G. H. McCracken, and O. Ramilo, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. B-986, 2001; T. Fujikawa, S. Miyazaki, Y. Ishii, N. Furuya, A. Ohno, T. Matumoto, K. Takeda, and K. Yamaguchi, Abstr 41st Intersci. Conf. Antimcrob. Agents Chemother., abstr B-993, 2001). The pharmacokinetic profile of cethromycin was studied in rats and consisted of a mean peak concentration in plasma of 1.07 μg/ml and an area under the concentration-time curve (AUC) of 12.03 μg · h/ml following an oral dose of 25 mg/kg. It concentrated in the rat lung, with a lung tissue-to-plasma AUC ratio of 29, demonstrating properties similar to those of the macrolides. These pharmacokinetics were comparable between single and multiple doses (31). This dosage has been also evaluated with mice, obtaining a mean peak concentration in plasma of 1.77 μg/ml and a higher AUC of 40.7 μg · h/ml (24).
Azoulay-Dupuis et al. demonstrated with a mouse pneumonia model that cethromycin (ABT-773) had much higher maximum concentration in serum, AUC, and half-life in the lung than in the serum of Streptococcus pneumoniae-infected and noninfected Swiss mice after a single dose of 12.5 or 25 mg/kg by either gavage or the subcutaneous route, reaching lung-to-serum ratios of around 10. They also showed that the maximum concentration in serum/MIC and AUC/MIC ratios were higher in the lungs than in serum for the different strains of S. pneumoniae used (E. Azoulay-Dupuis, J. P. Bedos, P. Moine, J. Mohler, and C. Carbon, Abstr. Int. Congress Chemother. 2001, abstr. P19.023, 2001).
The placebo groups received the same treatment regimens with an identical solution not containing cethromycin.
The mycoplasma inoculum was sent to the Diagnostic Mycoplasma Laboratory (Birmingham, Ala.) to check for susceptibilities. The MIC of cethromycin was 0.000004 μg/ml, and those of azithromycin, clarithromycin, and erythromycin were 0.000125, 0.000125, and 0.02 μg/ml, respectively.
Experimental design and sample collection.
Groups of 5 to 10 mice per cethromycin treatment group at each time point and 5 to 15 mice per placebo group at each time point were sampled for Mycoplasma cultures, histopathology scores (HPS), cytokines, and chemokines. The pulmonary function tests had 4 to 8 mice per cethromycin treatment group at each time point and 4 to 12 mice per placebo group at each time point. Not all outcome variables were available for some mice due to culture contamination or insufficient quantity of bronchoalveolar lavage (BAL) fluid for cultures, cytokines, and chemokines.
Mice were anesthetized with an intraperitoneal injection of 75 mg of ketamine/kg and 5 mg of acepromazine/kg before cardiac puncture. BAL specimens were obtained by infusing 0.5 ml of SP4 broth through a 25-gauge needle into the lungs, via the trachea, followed by aspiration of this fluid into a syringe. Whole-lung specimens (including the trachea and both lungs) were collected and fixed with a 10% buffered formalin solution for histologic evaluation.
Culture.
Twenty-five microliters of undiluted sample and serial 10-fold dilutions in SP4 broth of BAL fluid (50 μl of undiluted sample was used for the initial dilution) were immediately cultured on SP4 agar plates at 37°C while the remainder of the undiluted BAL specimens were stored at −80°C. Quantification was performed by counting colonies on plated specimens and expressed as log10 CFU/milliliter.
Histopathology.
The HPS was determined by a single pathologist who was unaware of the treatment status of the animals from which specimens were taken. The HPS was based on the grading of peribronchiolar or bronchial infiltrate, bronchiolar or bronchial luminal exudate, perivascular infiltrate, and parenchymal pneumonia (neutrophilic alveolar infiltrate). This HPS system assigned values from 0 to 26 (the greater the score, the greater the inflammatory changes in the lung) and has been validated previously with the animal model (7, 19, 20, 21). The variation in HPS when the same slide was scored by the same pathologist on multiple occasions has been found to be 0 to 1.
BAL cytokines and chemokines.
BAL specimens were assessed for concentrations of cytokines and chemokines by enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, Minn.) or with the mouse cytokine multiplex antibody bead kit (Luminex; Biosource International). When ELISA was used, the limits of detection were as follows: tumor necrosis factor alpha (TNF-α), 5.1 pg/ml; mouse KC (functional interleukin-8 [IL-8]), 2.0 pg/ml; JE/monocyte chemoattractant protein 1 (MCP-1), 2.0 pg/ml; macrophage inflammatory protein 1α (MIP-1α), 1.5 pg/ml. For the mouse cytokine multiplex, the lower limits of detection were the following: IL-1β, 10 pg/ml; IL-2 (p40/p70), 15 pg/ml; IL-4, 5 pg/ml; IL-12, 15 pg/ml; granulocyte-macrophage colony-stimulating factor (GM-CSF), 10 pg/ml; gamma interferon (IFN-γ), 1 pg/ml. For statistical analysis, samples with optical density readings below the limit of the standard curve of the assay were assigned a value one-half that of the lowest detectable value. Correlations between results obtained with the mouse cytokine multiplex antibody bead kit (Luminex; Biosource International) and results obtained with the Biosource ELISA ranged from 0.86 to 0.95 for the different cytokines measured.
Plethysmography.
Whole-body, unrestrained plethysmography (Buxco, Troy, N.Y.) was utilized to monitor the respiratory dynamics of mice in a quantitative manner both before and after methacholine exposure (baseline plethysmography determined airway obstruction and methacholine plethysmography determined airway hyperreactivity). Prior to methacholine exposure, mice were allowed to acclimate to the chamber and then plethysmography readings were recorded to establish enhanced pause (Penh) baseline values. Next, the mice were exposed to aerosolized methacholine (50 mg/ml), and Penh values were recorded again. Penh is a dimensionless value that represents a function of the ratio of peak expiratory flow to peak inspiratory flow and a function of the timing of expiration. Penh correlates with pulmonary airflow resistance or obstruction. Penh, as measured by plethysmography, has been previously validated in animal models of airway hyperresponsiveness (15, 17, 19-21, 41, 18-20, 47).
Statistics.
For all statistical analysis, Sigma Stat 2000 software (SPSS Science) was used. The t test was used to compare values for cethromycin-treated animals versus placebo-treated animals at the same time point, if the data were normally distributed. In the instances where the data were not normally distributed, the Mann-Whitney rank sum test was used for comparisons. A comparison was considered statistically significant if the P value was ≤0.05.
The following variables had a normal distribution: Mycoplasma cultures, airway hyperreactivity, IL-4, IL-8, IL-12, and GM-CSF. Hence, the t test was used for these variables. HPS, airway obstruction, and the rest of the cytokines evaluated (TNF-α, IFN-γ, MCP-1, MIP-1α, IL-1, and IL-2) were not normally distributed; the Mann-Whitney rank sum test was used for these variables. The variables that had a normal distribution are presented as means with standard deviations, and the variables not normally distributed are presented as medians with 25th to 75th percentiles. Since some of the variables evaluated had normal distribution and others were not normally distributed, the correlations were measured by using Spearman rank order.
RESULTS
Visual.
No visual differences could be detected between the infected mice treated with cethromycin and the mice that received the placebo.
BAL culture.
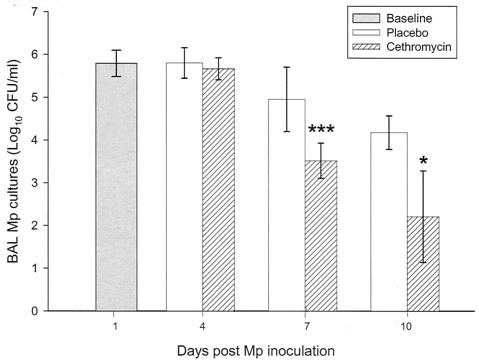
Quantitative M. pneumoniae BAL cultures of specimens from infected mice were lower at all times in the cethromycin treatment groups than in the placebo groups, reaching statistical significance on days 7 and 10 after infection. Despite these significant reductions, M. pneumoniae was not eradicated from the animals' airways in either group (Fig. 1).
FIG. 1.
Mean CFU of M. pneumoniae (Mp) in BAL cultures of infected mice treated with either cethromycin (25 mg/kg/day subcutaneously) or placebo for 10 days (therapy was started 1 day after infection). Error bars represent standard deviations. *, P < 0.05; ***, P < 0.001. n = 5 to 10 mice per cethromycin treatment group and 5 to 15 mice per placebo group.
Histopathology.
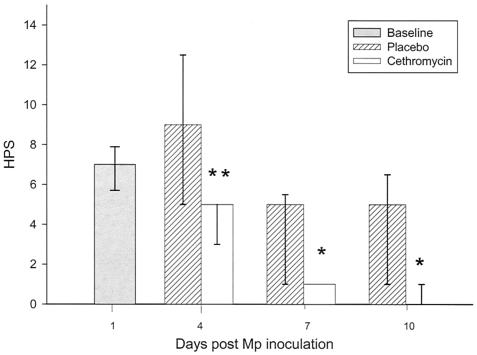
Mice treated with cethromycin had a significantly reduced HPS on days 4, 7, and 10 compared with mice in the placebo group (Fig. 2). In contrast to BAL cultures, lung inflammation was almost completely resolved by 10 days after inoculation in cethromycin-treated mice.
FIG. 2.
Median HPS of mice infected with M. pneumoniae (Mp) and treated with either cethromycin or placebo. Error bars represent 75th to 25th percentiles. *, P < 0.05; **, P < 0.01. n = 5 to 10 mice per cethromycin treatment group and 5 to 15 mice per placebo group.
BAL specimen cytokines and chemokines.
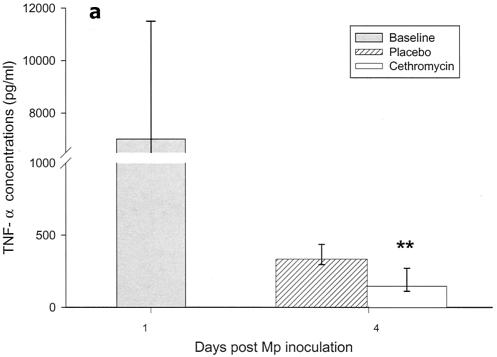
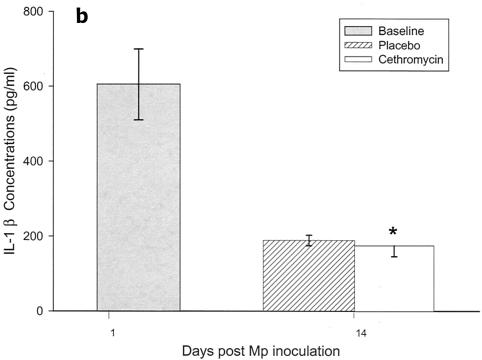
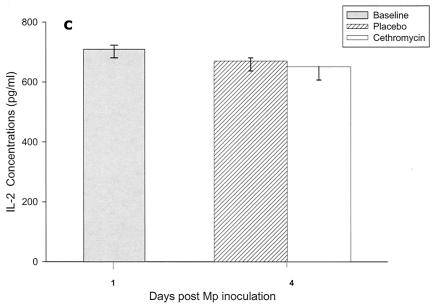
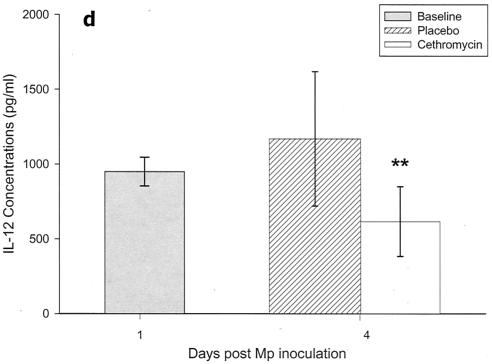
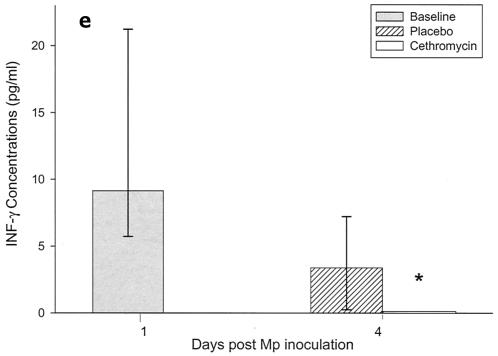
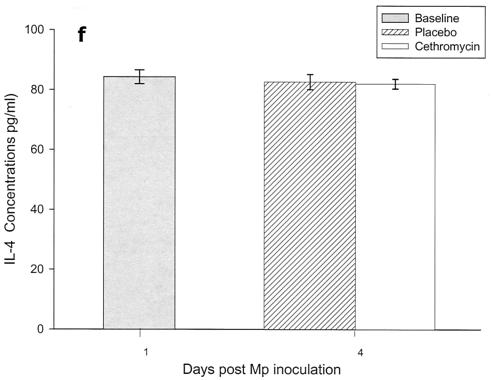
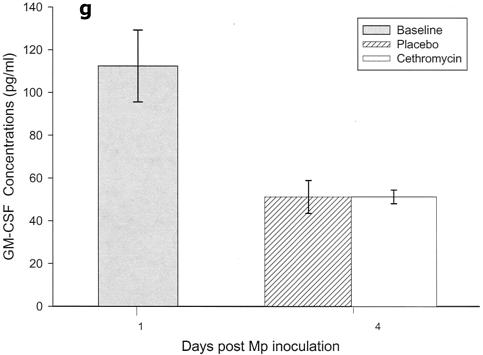
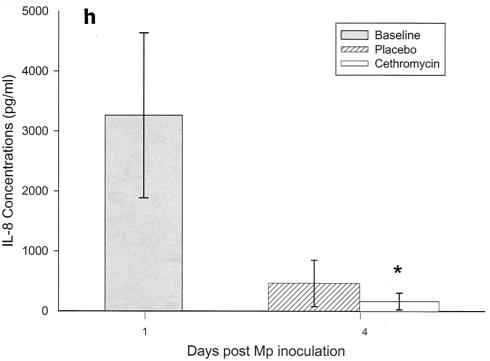
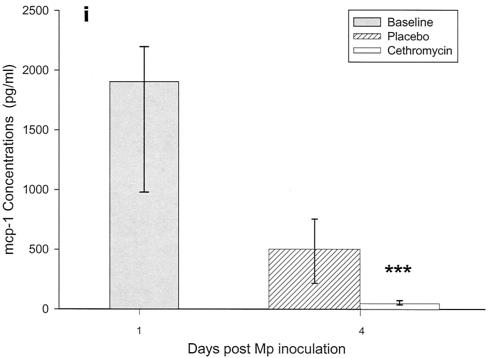
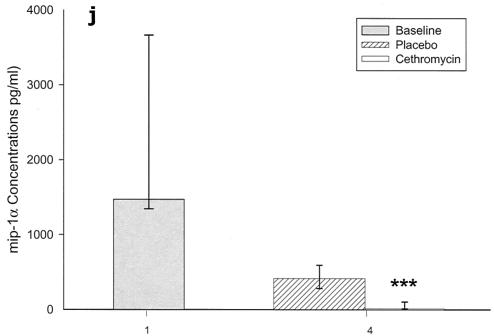
Cytokines and chemokines were measured in BAL samples on days 1 and 4 after inoculation. The M. pneumoniae-infected mice treated with cethromycin had significantly reduced concentrations of TNF-α, IL-1β, IL-12, and IFN-γ in BAL fluid at day 4 compared with mice in the placebo group (Fig. 3a, b, d, and e). IL-2, IL-4, and GM-CSF were not significantly modified by therapy with cethromycin (Fig. 3c, f, and g). Among the chemokines measured, KC (functional mouse IL-8), JE/MCP-1, and MIP-1α were significantly lower in treated mice than in placebo group mice at day 4 (Fig. 3h, i, and j).
FIG. 3.
(a to j) Mean and median cytokine and chemokine concentrations in BAL fluid of mice infected with M. pneumoniae (Mp) and treated with either cethromycin or placebo. Error bars represent standard deviations when variables have a normal distribution and 75th to 25th percentiles when variables do not have a normal distribution. *, P < 0.05; **, P < 0.01; ***, P < 0.001. n = 5 to 10 mice per cethromycin treatment group and 5 to 15 mice per placebo group.
Plethysmography.
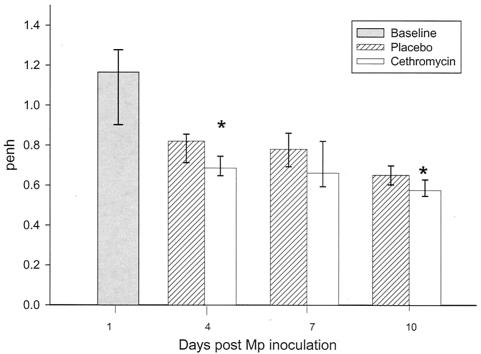
Airway obstruction, defined by baseline Penh values, in mice inoculated with M. pneumoniae was significantly reduced in cethromycin-treated animals compared with placebo group mice on days 4 and 10 (Fig. 4).
FIG. 4.
Median Penh of mice infected with M. pneumoniae (Mp) and treated with either cethromycin or placebo. Error bars represent 75th to 25th percentiles. *, P < 0.05. n = 5 to 10 mice per cethromycin treatment group and 5 to 15 mice per placebo group.
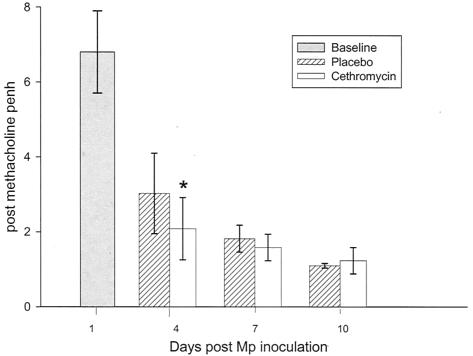
Airway hyperreactivity, defined by Penh values after exposure to methacholine, was reduced in mice treated with cethromycin compared with placebo group mice on days 4 and 7 and reached statistical significance on day 4 (Fig. 5).
FIG. 5.
Mean post-methacholine exposure Penh of mice infected with M. pneumoniae (Mp) and treated with either cethromycin or placebo. Error bars represent standard deviations. *, P < 0.05. n = 5 to 10 mice per cethromycin treatment group and 5 to 15 mice per placebo group.
Correlations (day 4).
The correlations between M. pneumoniae BAL cultures, HPS, pulmonary function, and cethromycin therapy are shown in Table 1. Correlations between the cytokines and chemokines with BAL M. pneumoniae cultures, HPS, pulmonary function, and cethromycin therapy are shown in Table 2. Correlations of interest among the cytokines and chemokines are shown in Table 3.
TABLE 1.
Correlations among outcome variables at day 4
| Variable | Correlation with:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HPS
|
AOa
|
AHRb
|
Cethromycin therapy
|
|||||||||
| r | P | n | r | P | n | r | P | n | r | P | n | |
| M. pneumoniae culture | 0.2 | 0.3 | 19 | 0.4 | 0.1 | 18 | 0.1 | 0.6 | 18 | −0.2 | 0.3 | 19 |
| HPS | 0.5 | 0.03 | 20 | 0.5 | 0.04 | 20 | −0.6 | <0.001 | 23 | |||
| AO | 0.7 | <0.001 | 20 | −0.6 | 0.007 | 20 | ||||||
| AHR | −0.4 | 0.08 | 20 | |||||||||
AO, airway obstruction.
AHR, airway hyperreactivity.
TABLE 2.
Correlations of cytokines and chemokines in BAL samples at day 4
| Cytokine or chemokine | Correlation with:
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
M. pneumoniae cultures
|
HPS
|
AOa
|
AHRb
|
Cethromycin therapy
|
|||||||||||
| r | P | n | r | P | n | r | P | n | r | P | n | r | P | n | |
| IL-1β | 0.2 | 0.4 | 15 | 0.5 | 0.05 | 19 | 0.3 | 0.2 | 16 | 0.6 | 0.02 | 16 | −0.5 | 0.03 | 19 |
| IL-2 | 0.3 | 0.3 | 15 | 0.5 | 0.01 | 19 | 0.4 | 0.2 | 16 | 0.4 | 0.1 | 16 | −0.4 | 0.09 | 19 |
| TNF-α | 0.2 | 0.3 | 19 | 0.7 | <0.001 | 23 | 0.4 | 0.09 | 20 | 0.3 | 0.2 | 20 | −0.6 | 0.005 | 23 |
| IL-12 | 0.5 | 0.06 | 15 | 0.5 | 0.02 | 19 | 0.6 | 0.01 | 16 | 0.4 | 0.2 | 16 | −0.7 | 0.002 | 19 |
| IFN-γ | 0.7 | 0.004 | 15 | 0.7 | <0.001 | 19 | 0.7 | <0.001 | 16 | 0.6 | 0.01 | 16 | −0.6 | 0.008 | 19 |
| IL-4 | 0.03 | 0.9 | 15 | 0.4 | 0.1 | 19 | 0.3 | 0.2 | 16 | 0.4 | 0.1 | 16 | −0.1 | 0.5 | 19 |
| GM-CSF | 0.02 | 0.9 | 15 | 0.3 | 0.1 | 19 | 0.08 | 0.7 | 16 | 0.3 | 0.2 | 16 | 0.05 | 0.8 | 19 |
| IL-8 | 0.2 | 0.4 | 19 | 0.3 | 0.1 | 23 | 0.4 | 0.1 | 20 | 0.4 | 0.04 | 20 | −0.4 | 0.003 | 23 |
| MCP-1 | 0.3 | 0.3 | 19 | 0.6 | 0.001 | 23 | 0.6 | 0.005 | 20 | 0.4 | 0.07 | 20 | −0.8 | <0.001 | 23 |
| MIP-1α | 0.2 | 0.3 | 19 | 0.6 | 0.007 | 22 | 0.5 | 0.05 | 19 | 0.2 | 0.3 | 19 | −0.8 | <0.001 | 22 |
AO, airway obstruction.
AHR, airway hyperreactivity.
TABLE 3.
Correlations of cytokines and chemokines in BAL samples at day 4
| Cytokine or chemokine | Correlation with:
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IL-1β
|
IL-2
|
TNF-α
|
IL-12
|
INF-γ
|
|||||||||||
| r | P | n | r | P | n | r | P | n | r | P | n | r | P | n | |
| IL-1β | 0.7 | 0.002 | 19 | 0.4 | 0.06 | 19 | 0.4 | 0.06 | 19 | 0.5 | 0.01 | 19 | |||
| IL-2 | 0.5 | 0.04 | 19 | 0.5 | 0.01 | 19 | 0.5 | 0.02 | 19 | ||||||
| TNF-α | 0.2 | 0.3 | 19 | 0.5 | 0.01 | 19 | |||||||||
| IL-12 | 0.7 | 0.001 | 19 | ||||||||||||
| MCP-1 | 0.07 | 0.7 | 19 | 0.3 | 0.1 | 19 | 0.5 | 0.01 | 19 | 0.8 | <0.001 | 19 | 0.8 | <0.001 | 19 |
| MIP-1α | 0.3 | 0.2 | 19 | 0.3 | 0.2 | 19 | 0.5 | 0.02 | 19 | 0.7 | <0.001 | 19 | 0.6 | 0.01 | 19 |
Cethromycin therapy inversely correlated with HPS and airway obstruction (Table 1). Regarding the BAL cytokines and chemokines measured, cethromycin correlated inversely with IL-1β, TNF-α, IL-12, IFN-γ, IL-8, MCP-1, and MIP-1α (Table 2).
DISCUSSION
Therapy with cethromycin resulted in statistically significant improvement of microbiological, histologic, respiratory, and immunologic markers of disease severity in the murine model of M. pneumoniae pneumonia.
Even though the BAL M. pneumoniae quantitative cultures were significantly reduced in the mice treated with cethromycin, M. pneumoniae was not eradicated from the airway, as has been previously documented, in response to other antibiotic regimens (3, 9, 20, 45). The significance of M. pneumoniae persistence in the airway is still not well defined, but it is hypothesized to play a role in chronic respiratory conditions, such as asthma (14, 15, 17, 18, 26, 50).
Cethromycin had a more pronounced effect on lung inflammation than on M. pneumoniae cultures at all of the time points evaluated in these experiments. On day 4, while M. pneumoniae cultures were not significantly reduced, HPS was significantly decreased. On day 10, lung histology had normalized while M. pneumoniae cultures were still positive. Similarly on day 4, cethromycin therapy had a significant effect on a broad range of pulmonary cytokines with significant reductions in concentrations of the proinflammatory cytokines, IL-1β, TNF-α, TH1, IFN-γ, and IL-12, and chemotactic cytokines, MIP-1α, MCP-1, and IL-8, without any significant effect on the concentrations of IL-2 and the TH2 cytokines evaluated, IL-4 and GM-CSF.
The cytokines that were reduced most significantly by therapy with cethromycin were among those with the strongest correlation with lung inflammation, suggesting that these cytokines play a significant role in orchestrating the acute pulmonary inflammatory response.
Among the proinflammatory cytokines, TNF-α was previously associated with disease severity in our laboratory's model (19) and an earlier study demonstrated that TNF-α released in response to M. pneumoniae could contribute to the enhancement of the cytotoxic activity of purified peritoneal macrophages and NK cells from BALB/c mice (2). Previous studies demonstrated that M. pneumoniae induced IL-1β gene expression following M. pneumoniae adherence to host target cells (52) or in the lungs of BALB/c mice acutely infected with M. pneumoniae (36). IL-1β and TNF-α share several activities, and in some situations, these two cytokines have been found to act synergistically (4, 39). In the present study, IL-1β and TNF-α concentrations were significantly correlated. IL-1β concentrations were strongly correlated with IL-2 concentrations, and both of these cytokines were in turn strongly correlated with histologic lung inflammation and abnormal pulmonary function tests. IL-1β is considered a multifunctional cytokine, and it activates T lymphocytes, enhancing the production of IL-2 and the expression of IL-2 receptors for lymphocyte stimulation (4). Lymphoid cell infiltration of the respiratory tract during mycoplasma infection suggests that lymphocyte activation is a key event in the progression of M. pneumoniae disease (10, 45).
Of the cytokines and chemokines evaluated, MCP-1 and MIP-1α concentrations in BAL fluid had the strongest inverse correlation with cethromycin therapy. These chemokines significantly correlated with lung inflammation and abnormal pulmonary function tests, as has been previously demonstrated in this model (19). The production of β-chemokines has been described with other mycoplasma respiratory infections in mice and is likely responsible in part for the mononuclear cell recruitment in the lungs, which seems to be a key event in the pathogenesis of mycoplasma respiratory disease and in the later development of chronic inflammation (42).
The reduction of TH1 cytokine concentrations observed with cethromycin therapy suggests that these cytokines play an important role in the inflammatory response in the lung infected with mycoplasma (24, 32, 35). The importance of the TH1 cytokines in response to acute M. pneumoniae respiratory infection, particularly that of IFN-γ, was previously demonstrated (19). IFN-γ has also been postulated to be an important mediator of lung inflammation in mice infected with M. pulmonis (34). In addition to IFN-γ, the present study also evaluated the concentrations of IL-12. There was a significant correlation between IFN-γ and IL-12 concentrations, and both cytokines were associated with lung inflammation and abnormal pulmonary function tests, particularly airway obstruction.
To the contrary, we have found that the TH2 response is not as significant in the acute M. pneumoniae respiratory infection model (19). Regarding IL-4, we have not documented significant differences between infected and noninfected mice when measuring concentrations in BAL fluid within the first 7 days after inoculation. More recently, an experiment was conducted which compared M. pneumoniae-infected BALB/c and C57BL/6 mouse strains with their respective uninfected controls. No statistically significant differences were demonstrated in the concentrations of IL-2, IL-4, IL-5, and GM-CSF in the BAL specimens (M. Fonseca-Aten, A. M. Rios, A. Mejias, S. Chavez, K. Katz, A. M. Gomez, G. H. McCracken, and R. D. Hardy, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr B-1671, 2003).
IL-4 concentrations were not associated with markers of disease severity, and they were not affected by cethromycin therapy. The role of IL-4 in the innate immune system has not been well defined, and its contribution for first-time infections may be minimal (16). GM-CSF, which has been implicated in eliciting an allergic immune response with IL-5 and IL-3 (4), was not an important determinant of disease severity in this study, and its concentrations were not affected by cethromycin therapy. Human studies have revealed both TH1 and TH2 pulmonary host responses to be significant in M. pneumoniae pneumonia. The relative contributions of each are under investigation (9, 25, 27).
The discrepancy observed between the antimicrobial and anti-inflammatory effects of cethromycin therapy, also observed for clarithromycin therapy (20), demonstrates the importance of the host immune response, as opposed to the invading microbe, in the pathogenesis of M. pneumoniae infection (5, 23, 46). In this particular study, M. pneumoniae cultures were not associated with lung histopathology on day 4, whereas some of the cytokines and chemokines in the BAL fluid were strongly correlated with lung histopathology, supporting the immunopathological nature of M. pneumoniae infection.
Innate immunity provides the main mechanisms of defense after the initial encounter with M. pneumoniae in this model (5, 19, 21), and this response in turn will likely influence the type of adaptive immune response in the later stages of infection as well as in subsequent encounters with this microorganism (21). The impact of antibiotics, such as clarithromycin and cethromycin, appears to be beneficial to the host, despite their inability to achieve complete eradication of M. pneumoniae from the airway. Their effects on the acutely released cytokines and chemokines may not only hasten the resolution of the acute illness but may also contribute to improved long-term clinical outcomes, such as decreased recurrent reactive airway disease associated with chronic M. pneumoniae disease, even though M. pneumoniae is not completely eradicated from the airway.
Acknowledgments
This work was supported in part by a research grant from Abbott Laboratories.
REFERENCES
- 1.Andrews, J. M., T. M. Weller, J. P. Ashby, R. M. Walker, and R. Wise. 2000. The in vitro activity of ABT773, a new ketolide antimicrobial agent. J. Antimicrob. Chemother. 46:1017-1022. [DOI] [PubMed] [Google Scholar]
- 2.Arai, S., M. Furukawa, T. Munakata, K. Kuwano, H. Inoue, and T. Miyazaki. 1990. Enhancement of cytotoxicity of active macrophages by mycoplasma: role of mycoplasma-associated induction of tumor necrosis factor-alpha (TNF-alpha) in macrophages. Microbiol. Immunol. 34:231-243. [DOI] [PubMed] [Google Scholar]
- 3.Barry, A. L., P. C. Fuchs, and S. D. Brown. 2001. In vitro activity of the ketolide ABT-773. Antimicrob. Agents Chemother. 45:2922-2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borish, L. C., and J. W. Steinke. 2003. 2. Cytokines and chemokines. J. Allergy Clin. Immunol. 111:S460-S475. [DOI] [PubMed] [Google Scholar]
- 5.Cartner, S. C., J. R. Lindsey, J. Gibbs-Erwin, G. H. Cassell, and J. W. Simecka. 1998. Roles of innate and adaptive immunity in respiratory mycoplasmosis. Infect. Immun. 66:3485-3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cimolai, N. 1998. Mycoplasma pneumoniae respiratory infection. Pediatr. Rev. 19:327-332. [DOI] [PubMed] [Google Scholar]
- 7.Cimolai, N., G. P. Taylor, D. Mah, and B. J. Morrison. 1992. Definition and application of a histopathological scoring scheme for an animal model of acute Mycoplasma pneumoniae pulmonary infection. Microbiol. Immunol. 36:465-478. [DOI] [PubMed] [Google Scholar]
- 8.Denny, F. W., W. A. Clyde, Jr., and W. P. Glezen. 1971. Mycoplasma pneumoniae disease: clinical spectrum, pathophysiology, epidemiology, and control. J Infect. Dis. 123:74-92. [DOI] [PubMed] [Google Scholar]
- 9.Esposito, S., R. Droghetti, S. Bosis, L. Claut, P. Marchisio, and N. Principi. 2002. Cytokine secretion in children with acute Mycoplasma pneumoniae infection and wheeze. Pediatr. Pulmonol. 34:122-127. [DOI] [PubMed] [Google Scholar]
- 10.Fernald, G. W. 1969. Immunologic aspects of experimental Mycoplasma pneumoniae infection. J. Infect. Dis. 119:255-266. [DOI] [PubMed] [Google Scholar]
- 11.Foy, H. M., J. T. Grayston, G. E. Kenny, E. R. Alexander, and R. McMahan. 1966. Epidemiology of Mycoplasma pneumoniae infection in families. JAMA 197:859-866. [PubMed] [Google Scholar]
- 12.Gendrel, D. 1997. Antibiotic treatment of Mycoplasma pneumoniae infections. Pediatr. Pulmonol. Suppl. 16:46-47. [PubMed] [Google Scholar]
- 13.Gendrel, D., J. Raymond, F. Moulin, J. L. Iniguez, S. Ravilly, F. Habib, P. Lebon, and G. Kalifa. 1997. Etiology and response to antibiotic therapy of community-acquired pneumonia in French children. Eur. J. Clin. Microbiol. Infect. Dis. 16:388-391. [DOI] [PubMed] [Google Scholar]
- 14.Gil, J. C., R. L. Cedillo, B. G. Mayagoitia, and M. D. Paz. 1993. Isolation of Mycoplasma pneumoniae from asthmatic patients. Ann. Allergy 70:23-25. [PubMed] [Google Scholar]
- 15.Gonzalo, J. A., C. M. Lloyd, D. Wen, J. P. Albar, T. N. Wells, A. Proudfoot, A. C. Martinez, M. Dorf, T. Bjerke, A. J. Coyle, and J. C. Gutierrez-Ramos. 1998. The coordinated action of CC chemokines in the lung orchestrates allergic inflammation and airway hyperresponsiveness. J. Exp. Med. 188:157-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haas, H., F. H. Falcone, M. J. Holland, G. Schramm, K. Haisch, B. F. Gibbs, A. Bufe, and M. Schlaak. 1999. Early interleukin-4: its role in the switch towards a Th2 response and IgE-mediated allergy. Int. Arch. Allergy Immunol. 119:86-94. [DOI] [PubMed] [Google Scholar]
- 17.Hamelmann, E., J. Schwarze, K. Takeda, A. Oshiba, G. L. Larsen, C. G. Irvin, and E. W. Gelfand. 1997. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am. J. Respir. Crit. Care Med. 156:766-775. [DOI] [PubMed] [Google Scholar]
- 18.Hardy, R. D., H. S. Jafri, K. Olsen, J. Hatfield, J. Iglehart, B. B. Rogers, P. Patel, G. Cassell, G. H. McCracken, and O. Ramilo. 2002. Mycoplasma pneumoniae induces chronic respiratory infection, airway hyperreactivity, and pulmonary inflammation: a murine model of infection-associated chronic reactive airway disease. Infect. Immun. 70:649-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardy, R. D., H. S. Jafri, K. Olsen, M. Wordemann, J. Hatfield, B. B. Rogers, P. Patel, L. Duffy, G. Cassell, G. H. McCracken, and O. Ramilo. 2001. Elevated cytokine and chemokine levels and prolonged pulmonary airflow resistance in a murine Mycoplasma pneumoniae pneumonia model: a microbiologic, histologic, immunologic, and respiratory plethysmographic profile. Infect. Immun. 69:3869-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hardy, R. D., A. M. Rios, S. Chavez-Bueno, H. S. Jafri, J. Hatfield, B. B. Rogers, G. H. McCracken, and O. Ramilo. 2003. Antimicrobial and immunologic activities of clarithromycin in a murine model of Mycoplasma pneumoniae-induced pneumonia. Antimicrob. Agents Chemother. 47:1614-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayakawa, M., H. Taguchi, S. Kamiya, Y. Fujioka, H. Watanabe, S. Kawai, and H. Kobayashi. 2002. Animal model of Mycoplasma pneumoniae infection using germfree mice. Clin. Diagn. Lab. Immunol. 9:669-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heiskanen-Kosma, T., M. Korppi, C. Jokinen, S. Kurki, L. Heiskanen, H. Juvonen, S. Kallinen, M. Sten, A. Tarkiainen, P. R. Ronnberg, M. Kleemola, P. H. Makela, and M. Leinonen. 1998. Etiology of childhood pneumonia: serologic results of a prospective, population-based study. Pediatr. Infect. Dis. J. 17:986-991. [DOI] [PubMed] [Google Scholar]
- 23.Jones, H. P., L. Tabor, X. Sun, M. D. Woolard, and J. W. Simecka. 2002. Depletion of CD8+ T cells exacerbates CD4+ Th cell-associated inflammatory lesions during murine mycoplasma respiratory disease. J. Immunol. 168:3493-3501. [DOI] [PubMed] [Google Scholar]
- 24.Kim, M. K., W. Zhou, P. R. Tessier, D. Xuan, M. Ye, C. H. Nightingale, and D. P. Nicolau. 2002. Bactericidal effect and pharmacodynamics of cethromycin (ABT-773) in a murine pneumococcal pneumonia model. Antimicrob. Agents Chemother. 46:3185-3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koh, Y. Y., Y. Park, H. J. Lee, and C. K. Kim. 2001. Levels of interleukin-2, interferon-gamma, and interleukin-4 in bronchoalveolar lavage fluid from patients with Mycoplasma pneumonia: implication of tendency toward increased immunoglobulin E production. Pediatrics 107:E39. [DOI] [PubMed] [Google Scholar]
- 26.Kraft, M., G. H. Cassell, J. E. Henson, H. Watson, J. Williamson, B. P. Marmion, C. A. Gaydos, and R. J. Martin. 1998. Detection of Mycoplasma pneumoniae in the airways of adults with chronic asthma. Am. J. Respir. Crit. Care Med. 158:998-1001. [DOI] [PubMed] [Google Scholar]
- 27.Lieberman, D., S. Livnat, F. Schlaeffer, A. Porath, S. Horowitz, and R. Levy. 1997. IL-1beta and IL-6 in community-acquired pneumonia: bacteremic pneumococcal pneumonia versus Mycoplasma pneumoniae pneumonia. Infection 25:90-94. [DOI] [PubMed] [Google Scholar]
- 28.Lieberman, D., F. Schlaeffer, I. Boldur, S. Horowitz, M. G. Friedman, M. Leiononen, O. Horovitz, E. Manor, and A. Porath. 1996. Multiple pathogens in adult patients admitted with community-acquired pneumonia: a one year prospective study of 346 consecutive patients. Thorax 51:179-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marc, E., M. Chaussain, F. Moulin, J. L. Iniguez, G. Kalifa, J. Raymond, and D. Gendrel. 2000. Reduced lung diffusion capacity after Mycoplasma pneumoniae pneumonia. Pediatr. Infect. Dis. J. 19:706-710. [DOI] [PubMed] [Google Scholar]
- 30.McCracken, G. H., Jr. 1986. Current status of antibiotic treatment for Mycoplasma pneumoniae infections. Pediatr. Infect. Dis. 5:167-171. [DOI] [PubMed] [Google Scholar]
- 31.Mitten, M. J., J. Meulbroek, M. Nukkala, L. Paige, K. Jarvis, A. Oleksijew, A. Tovcimak, L. Hernandez, J. D. Alder, P. Ewing, Y. S. Or, Z. Ma, A. M. Nilius, K. Mollison, and R. K. Flamm. 2001. Efficacies of ABT-773, a new ketolide, against experimental bacterial infections. Antimicrob. Agents Chemother. 45:2585-2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Narita, M., H. Tanaka, S. Abe, S. Yamada, M. Kubota, and T. Togashi. 2000. Close association between pulmonary disease manifestation in Mycoplasma pneumoniae infection and enhanced local production of interleukin-18 in the lung, independent of gamma interferon. Clin. Diagn. Lab. Immunol. 7:909-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nilius, A. M., M. H. Bui, L. Almer, D. Hensey-Rudloff, J. Beyer, Z. Ma, Y. S. Or, and R. K. Flamm. 2001. Comparative in vitro activity of ABT-773, a novel antibacterial ketolide. Antimicrob. Agents Chemother. 45:2163-2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishimoto, M., A. Akashi, K. Kuwano, C. C. Tseng, K. Ohizumi, and S. Arai. 1994. Gene expression of tumor necrosis factor alpha and interferon gamma in the lungs of Mycoplasma pulmonis-infected mice. Microbiol. Immunol. 38:345-352. [DOI] [PubMed] [Google Scholar]
- 35.Opitz, O., K. Pietsch, S. Ehlers, and E. Jacobs. 1996. Cytokine gene expression in immune mice reinfected with Mycoplasma pneumoniae: the role of T cell subsets in aggravating the inflammatory response. Immunobiology 196:575-587. [DOI] [PubMed] [Google Scholar]
- 36.Pietsch, K., S. Ehlers, and E. Jacobs. 1994. Cytokine gene expression in the lungs of BALB/c mice during primary and secondary intranasal infection with Mycoplasma pneumoniae. Microbiology 140:2043-2048. [DOI] [PubMed] [Google Scholar]
- 37.Principi, N., and S. Esposito. 2001. Emerging role of Mycoplasma pneumoniae and Chlamydia pneumoniae in paediatric respiratory-tract infections. Lancet Infect. Dis. 1:334-344. [DOI] [PubMed] [Google Scholar]
- 38.Principi, N., S. Esposito, F. Blasi, and L. Allegra. 2001. Role of Mycoplasma pneumoniae and Chlamydia pneumoniae in children with community-acquired lower respiratory tract infections. Clin. Infect. Dis. 32:1281-1289. [DOI] [PubMed] [Google Scholar]
- 39.Ramilo, O., X. Saez-Llorens, J. Mertsola, H. Jafari, K. D. Olsen, E. J. Hansen, M. Yoshinaga, S. Ohkawara, H. Nariuchi, and G. H. McCracken, Jr. 1990. Tumor necrosis factor alpha/cachectin and interleukin 1 beta initiate meningeal inflammation. J. Exp. Med. 172:497-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sabato, A. R., A. J. Martin, B. P. Marmion, T. W. Kok, and D. M. Cooper. 1984. Mycoplasma pneumoniae: acute illness, antibiotics, and subsequent pulmonary function. Arch. Dis. Child. 59:1034-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwarze, J., E. Hamelmann, K. L. Bradley, K. Takeda, and E. W. Gelfand. 1997. Respiratory syncytial virus infection results in airway hyperresponsiveness and enhanced airway sensitization to allergen. J. Clin. Investig. 100:226-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simecka, J. W. 1999. Beta-chemokines are produced in lungs of mice with mycoplasma respiratory disease. Curr. Microbiol. 39:163-167. [DOI] [PubMed] [Google Scholar]
- 43.Slotkin, R. I., W. A. Clyde, Jr., and F. W. Denny. 1967. The effect of antibiotics on Mycoplasma pneumoniae in vitro and in vivo. Am. J. Epidemiol. 86:225-237. [DOI] [PubMed] [Google Scholar]
- 44.Smith, C. B., W. T. Friedewald, and R. M. Chanock. 1967. Shedding of Mycoplasma pneumoniae after tetracycline and erythromycin therapy. N. Engl. J. Med. 276:1172-1175. [DOI] [PubMed] [Google Scholar]
- 45.Stanbridge, E. J. 1982. Mycoplasma-lymphocyte interactions and their possible role in immunopathologic manifestations of mycoplasmal disease. Rev Infect. Dis. 4(Suppl.):S219-S226. [DOI] [PubMed] [Google Scholar]
- 46.Taylor, G., D. Taylor-Robinson, and G. W. Fernald. 1974. Reduction in the severity of Mycoplasma pneumoniae-induced pneumonia in hamsters by immunosuppressive treatment with antithymocyte sera. J. Med. Microbiol. 7:343-348. [DOI] [PubMed] [Google Scholar]
- 47.van Schaik, S. M., G. Enhorning, I. Vargas, and R. C. Welliver. 1998. Respiratory syncytial virus affects pulmonary function in BALB/c mice. J. Infect. Dis. 177:269-276. [DOI] [PubMed] [Google Scholar]
- 48.Waites, K. B., D. M. Crabb, and L. B. Duffy. 2003. In vitro activities of ABT-773 and other antimicrobials against human mycoplasmas. Antimicrob. Agents Chemother. 47:39-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wiland, E., A. Wojda, M. Kamieniczna, A. Latos-Bielenska, P. Jdrzejczak, and M. Kurpisz. 2001. Idiopathic infertility in married couples in the light of cytogenetic analysis and sperm penetration assay. Folia Histochem. Cytobiol 39:35-41. [PubMed] [Google Scholar]
- 50.Wongtim, S., and S. Mogmued. 1995. Methacholine inhalation challenge in patients with post-Mycoplasma pneumoniae pneumonia. Asian Pac. J. Allergy Immunol. 13:5-10. [PubMed] [Google Scholar]
- 51.Xuan, D., M. Ye, M. Kim, C. H. Nightingale, and D. P. Nicolau. 2002. Pharmacokinetics of ABT-773, a new semi-synthetic ketolide in neutropenic lung-infected mice: a population approach. J. Pharm. Pharmacol. 54:71-75. [DOI] [PubMed] [Google Scholar]
- 52.Yang, J., W. C. Hooper, D. J. Phillips, and D. F. Talkington. 2002. Regulation of proinflammatory cytokines in human lung epithelial cells infected with Mycoplasma pneumoniae. Infect. Immun. 70:3649-3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhanel, G. G., M. Walters, A. Noreddin, L. M. Vercaigne, A. Wierzbowski, J. M. Embil, A. S. Gin, S. Douthwaite, and D. J. Hoban. 2002. The ketolides: a critical review. Drugs 62:1771-1804. [DOI] [PubMed] [Google Scholar]