Abstract
The intracellular pharmacokinetics and pharmacodynamics of oritavancin (LY333328) were studied in cultured cells. Oritavancin was avidly accumulated by J774 and THP-1 macrophages and rat fibroblasts and to a lesser extent by LLC-PK1 and Caco-2 cells. In J774 macrophages, the level of accumulation reached a plateau (at 370-fold the extracellular concentration) within 24 h and was partly defeated by a rise in serum protein levels. Efflux was incomplete (with a plateau at two-thirds of the original level at 6 h). In short-term kinetic studies, oritavancin uptake was linear for up to 4 h (as was the case for horseradish peroxidase and small latex beads, used as markers of the fluid phase and adsorptive endocytosis, respectively), which was in contrast to azithromycin and chloroquine uptake (which accumulate in cells by diffusion and segregation). The rates of clearance of oritavancin and latex beads were comparable (150 and 120 μl × mg of protein−1 × h−1, respectively) and were approximately 200 times higher than that of horseradish peroxidase. Oritavancin accumulation was partially reduced by monensin but was unaffected by acidic pH (these conditions abolished chloroquine accumulation). Cell-associated oritavancin was found in lysosomal fractions after homogenization of J774 macrophages and fractionation by isopycnic centrifugation. Oritavancin was bactericidal against intracellular Staphylococcus aureus (phagolysosomal infection) but was unable to control the intracellular growth of Listeria monocytogenes (cytosolic infection), even though its cellular concentration largely exceeded the MIC (0.02 mg/liter) and minimal bactericidal concentration (2 mg/liter). We conclude that oritavancin enters cells by adsorptive endocytosis (favored by its lipophilic side chain and/or the presence of three protonatable amines), which drives it to lysosomes, where it exerts antibiotic activity.
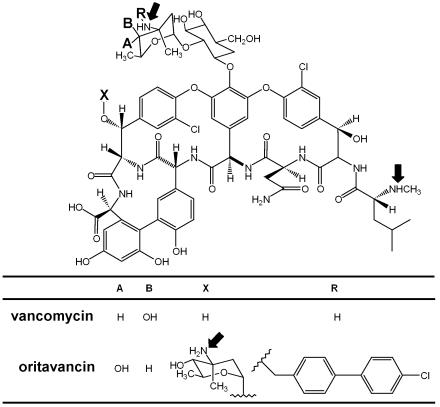
For more than a decade, vancomycin has remained one of the treatments of choice for infections caused by gram-positive organisms resistant to other common antibiotics, mostly methicillin-resistant staphylococci and enterococci. The emergence of resistance in enterococci in the late 1980s, however, triggered the development of new derivatives, among which oritavancin (LY333328) is one of the first to have entered large-scale clinical trials (43). Oritavancin (Fig. 1) is a semisynthetic derivative of vancomycin and differs from its parent compound by (i) the replacement of vancosamine by 4-epi-vancosamine, (ii) the presence of an additional 4-epi-vancosamine, and (iii) the addition of a p-chlorodiphenyl side chain to the additional 4-epi-vancosamine (9). Oritavancin therefore appears to be an amphipathic molecule with three protonatable amines. These modifications confer unusual pharmacodynamic and pharmacokinetic properties to oritavancin. It is indeed characterized by rapid, concentration-dependent bactericidal activity against most gram-positive organisms, whether they are susceptible or resistant to vancomycin (15), in association with a prolonged postantibiotic effect (2). This behavior is in sharp contrast to those of vancomycin and other conventional glycopeptides like teicoplanin, which have concentration-independent, slow bactericidal activities and short postantibiotic effects (15, 16). Oritavancin also shows a very prolonged terminal half-life (150 to 300 h, compared with a half-life of 6 h for vancomycin) and high levels of protein binding (>85%, compared with levels of protein binding of 10 to 55% for vancomycin). The persistence of oritavancin in the organism is believed to result from this high level of protein binding, analogous to the protein binding of teicoplanin (21), but it may also suggest the existence of deep storage compartments for the drug in the body. This triggered us to study the cellular pharmacokinetics and disposition of oritavancin in cultured cells by the approaches used previously to study the intracellular fates of other cationic antibiotics such as aminoglycosides (40), macrolides (4), and basic derivatives of β-lactam antibiotics (7, 27). The mechanism of accumulation was studied by using as comparators chloroquine and azithromycin, which accumulate in lysosomes by diffusion and segregation of their protonated forms (4, 12, 45), and with horseradish peroxidase (HRP) and small latex beads as markers of endocytosis (26, 41). Concentrating on macrophages and using previously established models (7, 31), we have also assessed the intracellular activities of oritavancin and vancomycin against phagolysosomal (Staphylococcus aureus) and cytosolic (Listeria monocytogenes) organisms using previously established models in an attempt to correlate accumulation, disposition, and expression of activity.
FIG. 1.
Chemical structure of oritavancin (LY333328) compared to that of vancomycin. Oritavancin differs from vancomycin by the replacement of the vancosamine sugar by a 4-epi-vancosamine (see the substituents at positions A and B), (ii) the presence of an additional 4-epi-vancosamine (substituent at position X), and (iii) a p-chlorodiphenyl side chain (substituent at position R). The arrows point to the protonatable aminated functions.
We found that oritavancin accumulates to exceptionally high levels in cells and is mainly found in lysosomes, where it exerts a bactericidal activity against S. aureus. In contrast, oritavancin is only poorly active against cytosolic bacteria such as L. monocytogenes.
MATERIALS AND METHODS
Cells and cell culture conditions.
All cell lines and primary cultures were obtained and maintained as described previously (27). J774 macrophages (a continuous reticulosarcoma cell line of murine origin which possesses many features of mouse macrophages [33]) were used for most experiments, whereas THP-1 cells (a human monomyelocytic cell type growing in suspension [38]), rat embryo fibroblasts (obtained by trypsinization of eviscerated 18-day-old Wistar rat fetuses ([39]), LLC-PK1 cells (a pig kidney proximal tubular cell line [ATCC CL-101] [25]), and Caco-2 cells (a human colorectal adenocarcinoma cell line [ATCC HTB-37] [37]) were used for comparison. All cultures were kept in an atmosphere of 95% air-5% CO2, and all media were supplemented with 10% fetal calf serum (FCS; referred to as complete culture medium) except in experiments exploring the influence of serum proteins on drug uptake. The viability of cells was checked under our experimental conditions by measuring the release of lactate dehydrogenase and taking as the criterion for viability the presence of lactate dehydrogenase at levels twice the maximum value measured in control cells (19) (values for controls, 3 to 7% of the total culture content).
Uptake and efflux of drugs (oritavancin, vancomycin, chloroquine, and azithromycin), HRP, and Texas Red-labeled small latex beads.
For J774 macrophages, cells were grown to confluency and were then incubated with the drug, HRP, or latex beads for the appropriate times. Latex beads were provided as a suspension in water; a stock solution of the other compounds was prepared extemporaneously in water (or in 0.1 N HCl for azithromycin), and an aliquot of this solution was then added to the culture medium just before the start of the experiment. For the experiments involving azithromycin, we checked that the addition of HCl (typically 1 μl/ml) did not cause a significant change in the pH of the culture medium. For collection, the culture dishes were transferred onto crushed ice, the cell sheets were washed three times with ice-cold 0.9% NaCl, and the cells were detached by scraping them into distilled water. For the experiments with HRP, which adheres to the cell surface, the cells were washed with ice-cold 0.9% NaCl and then further washed at 4°C with HRP-free complete culture medium (two times) and again with 0.9% NaCl (five times) before collection (these conditions have been shown to eliminate at least 95% of the extracellular HRP [10, 41]). For the experiments with small latex beads, the cells were washed five times for 30 s each time with phosphate-buffered saline at 4°C prior to collection (41). For efflux studies, cells incubated with oritavancin were washed three times in ice-cold 0.9% NaCl, placed for appropriate times at 37°C in fresh complete culture medium, washed again, and collected as described above. For other adherent cells, uptake studies with oritavancin were performed as described above for J774 macrophages either at confluency (fibroblasts, Caco-2 cells) or at 80% confluency (LLC-PK1 cells). For cells growing in suspension (THP-1 cells), uptake studies were performed with a density of approximately 5 × 105 cells/ml, and the cells were collected by pelleting (325 × g; 8 min), followed by one wash in ice-cold phosphate-buffered saline and lysis of the final pellet in distilled water. All cell lysates were subjected to sonication (15 s at 100 W; Labsonic L; B. Braun Biotech International GmbH, Melsungen, Germany) to achieve complete homogenization. Cell drug contents were expressed as micrograms per milligram of cell protein, and these data were used to calculate an apparent cellular concentration and an apparent cellular concentration-to-extracellular concentration ratio, using a conversion factor of 5 μl of cell volume per mg of cell protein, as described previously (13, 24, 27, 40).
Cell fractionation studies (J774 cells).
The main subcellular organelles were separated by combined differential and isopycnic centrifugation, as previously described in detail (27), with minor modifications, as indicated below. In brief, the cells were incubated with oritavancin, chloroquine, or HRP for appropriate times and washed three times in ice-cold 0.9% NaCl and once with 0.25 M sucrose-1 mM EGTA-3 mM imidazole (pH 7.4). They were then homogenized in the same medium with an all-glass Dounce tissue grinder, and a cytoplasmic extract free of nuclei was obtained by three successive low-speed centrifugations (770, 625, and 500 × g; 10 min each), as described earlier (39). This extract was then further fractionated into a granular fraction containing the bulk of the cell organelles and membranes (MLP fraction) and a final supernatant fraction (S fraction) by centrifugation at 40,000 rpm (145,000 × g) for 30 min (Ti 50 rotor; Beckman Instruments, Inc., Fullerton, Calif.). This MLP fraction was resuspended in 0.25 M sucrose-1 mM EGTA-3 mM imidazole (pH 7.4) by use of 10 strokes of the loosely fitting pestle of the Dounce tissue grinder. One milliliter of this fraction was deposited on the top of a 10.9-ml linear sucrose gradient (density limits, 1.10 to 1.20 g/cm3) resting on a cushion of 600 μl of sucrose with a density of 1.34 g/cm3. Isopycnic equilibration was performed in a swinging-bucket SW 40 rotor spun at 20,000 rpm (50,000 × g) for 18 h at 4°C. After equilibration, 12 fractions of approximately 1 ml each were collected and weighed, and their densities were determined by refractometry. The amount of proteins (17) and oritavancin, chloroquine, or HRP in the fractions was determined in parallel with determination of the activities of enzymes that serve as markers of the main organelles, namely, inosine 5′-diphosphatase (E.C. 3.6.1.6), cytochrome c oxidase (E.C. 1.9.3.1), N-acetyl-β-glucosaminidase (E.C. 3.2.1.30), and cathepsin B (E.C. 3.4.33.1) (see references 5 and 27] for details and assay procedures and for the association of these enzymes with specific organelles). The results are expressed as the percentage of enzyme activity, protein, or drug recovered in each fraction as a function of the density. To compare the distribution profiles between gradients, histograms were standardized for densities of equal increments ranging from 1.065 to 1.230 g/cm3, as described earlier (27).
Assay of drugs, HRP, and small latex beads.
Oritavancin was assayed by liquid scintillation counting with 14C-labeled drug. In preliminary experiments, we checked that the levels of accumulation observed with the radiolabeled drug corresponded to those observed with a bioactive product. For this purpose, we used a microbiological assay with Micrococcus luteus ATCC 9341 (M. J. Rybak, G. P. Allen, R. L. Akins, E. A. Coyle, and J. R. Aeschlimann, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 337, 2000). In brief, samples were placed in wells cut in the seeded agar and allowed to diffuse at 4°C overnight before incubation. The plates were then heated at 37°C for 24 h, and the growth inhibition zones were read manually with a metering gauge. We then measured in parallel the oritavancin contents of cells incubated for 2 h at concentrations ranging from 10 to 50 mg/liter using both methods in parallel and found a correlation coefficient (R2) of 0.92 (n = 15). Azithromycin was assayed by a microbiological method with Bacillus subtilis, as described earlier (30). Vancomycin was assayed by a fluorescence polarization immunoassay (Abbott Diagnostic Laboratories, Chicago, Ill.) (28). Chloroquine was assayed by fluorimetry (λexcitation, 335 nm; λemission, 378 nm) in 40 mM Tris buffer at pH 10 (45). HRP was assayed by measurement of its enzymatic activity as described previously (35, 42). Texas Red-labeled small latex beads were assayed by fluorimetry (λexcitation, 575 nm; λemission, 610 nm).
Cell infection studies (S. aureus and L. monocytogenes).
For the cell infection studies, we used the general procedures described earlier for J774 cells with S. aureus ATCC 25923 (24-h model [31]) or L. monocytogenes hemolysin-producing strain EGD serotype 1/2a (5-h model [7]). For S. aureus, gentamicin (0.5 mg/liter) was used to prevent extracellular growth in the control cultures. This was unnecessary for L. monocytogenes, since the model was limited to 5 h. The MICs and minimal bactericidal concentrations (MBCs) of the drugs for the S. aureus and L. monocytogenes strains used in the present study were determined in tryptic soy broth (L. monocytogenes) or Mueller-Hinton broth (S. aureus) by the procedures recommended by the NCCLS.
Data analyses.
Curve fitting and statistical analyses were performed with Prism software (version 2.01; GraphPad Software, San Diego, Calif.).
Reagents.
Oritavancin (LY333328 supplied as its fully hydrated diphosphate salt; potency, 80.6%) and [14C]oritavancin (3.5 μCi/mg; labeled on the chloro-biphenyl side chain) were obtained from Eli Lilly & Co., Indianapolis, Ind. The labeled drug was mixed with unlabeled oritavancin to obtain a specific activity of 0.6 μCi/mg. Gentamicin and vancomycin were procured as Geomycin and Vancocin, respectively (the commercial products registered for clinical use in Belgium and supplied by GlaxoSmithKline Belgium on behalf of Schering-Plough Belgium and Eli Lilly Benelux, respectively). Azithromycin (dihydrate-free base for microbiological standard; purity, 94%) was supplied by Pfizer s.a. (Brussels, Belgium) on behalf of Pfizer Inc. (Groton, Conn.). Chloroquine, HRP (grade II), carboxylate-modified polystyrene latex beads (average diameter, 0.1 μm) covalently bound to Texas Red, and monensin were purchased from Sigma-Aldrich-Fluka (St. Louis, Mo.). Cell culture media and FCS were purchased from Gibco Biocult (Paisley, Scotland). Human serum for opsonization was obtained from healthy volunteers as pooled samples stored as aliquots at −70°C until use. Unless stated otherwise, all other reagents were of analytic grade and were purchased from E. Merck AG (Darmstadt, Germany).
RESULTS
Accumulation and efflux of oritavancin.
The uptake and efflux of oritavancin were examined in J774 macrophages as a function of time at a fixed extracellular concentration (25 mg/liter). Figure 2 shows that accumulation at 37°C proceeded according to a one-phase exponential association process, reaching near saturation after approximately 20 h and a maximal cellular concentration of about 46 μg/mg of protein (corresponding to an apparent accumulation ratio of 370). Figure 2 also shows that oritavancin uptake was considerably slower at 4°C (less than 5% of the value at 37°C). Efflux of oritavancin was then examined in cells incubated for 24 h at 37°C with 25 mg of drug/liter and then transferred to oritavancin-free medium. Release proceeded according to a one-phase exponential decay (half-life, approximately 1.73 h) but was largely incomplete, with an apparent plateau value reached at an apparent cellular drug concentration of about two-thirds of the original value (more prolonged efflux experiments could not be performed because of a loss of cell viability). In sharp contrast to the results obtained with oritavancin, vancomycin accumulated only very slowly in J774 macrophages and reached an apparent cellular concentration-to-extracellular concentration ratio of only 8.0 ± 5.4 after 24 h of incubation (data not shown in Fig. 2). To establish whether the extensive accumulation of oritavancin was specific to J774 macrophages, these experiments were repeated with four other cell lines, three of which were not of the macrophage type. The levels of accumulation observed after 2 h of incubation are presented in Table 1. THP-1 macrophages and fibroblasts accumulated oritavancin to essentially the same extent as J774 macrophages. The levels of accumulation by the epithelial cell lines LLC-PK1 and Caco-2 were lower but nevertheless quite important.
FIG. 2.
Kinetics of accumulation and efflux of oritavancin in J774 macrophages incubated with an extracellular concentration of 25 mg/liter at 37°C in medium supplemented with 10% FCS (complete culture medium). Results are given as arithmetic means ± standard deviations (n = 3) of the cellular concentrations recorded (micrograms of oritavancin per milligram of cell protein; the apparent accumulation ratio for each value of the extracellular concentration can be calculated by multiplying the values on the ordinate by 200 [assuming a cell volume of 5 μl/mg of protein]). Left panel, influx (regression parameters [one-phase exponential association], R2 = 0.986, κ = 0.138 ± 0.014 h−1, and plateau = 46.2 ± 3.8); inset in left panel, influx kinetics over the first 6 h and comparison of cells incubated at 37 and 4°C; right panel, efflux from cells incubated with oritavancin for 24 h (regression parameters [one-phase exponential decay], R2 = 0.911, κ = 0.401 ± 0.252 h−1, and plateau = 30.7 ± 3.6). prot, protein.
TABLE 1.
Oritavancin accumulation by different cell typesa
| Cell type | Accumulation ratiob (no. of determinations) |
|---|---|
| J774 mouse macrophages | 66.4 ± 11.8 (12) |
| THP-1 human monocytes | 84.3 ± 7.0 (9) |
| Rat embryo fibroblasts | 72.4 ± 9.4 (6) |
| LLC-PK1 pig kidney proximal tubular cells | 37.8 ± 6.4 (3) |
| Caco-2 human colorectal cells | 13.8 ± 0.4 (3) |
The cells were incubated for 2 h at 37°C with 25 mg of the drug per liter in a medium containing 10% FCS.
Ratio of cellular concentration to extracellular concentration.
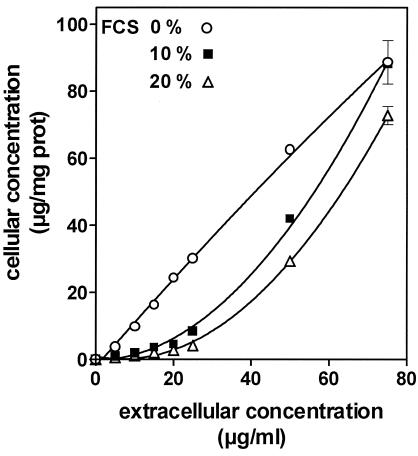
Because oritavancin is known to bind to serum proteins, we examined in detail its accumulation (2 h of incubation with increasing extracellular concentrations) in the absence and in the presence of calf serum (10 and 20%). Figure 3 shows that the uptake by cells incubated with the complete culture medium (10% FCS) for 2 h did not proceed linearly with the extracellular concentration but, rather, proceeded in an apparent cooperative fashion. This was not observed with cells incubated in serum-free medium, for which uptake was quasilinearly related to the extracellular concentration and was therefore considerably higher in the range of 10 to 50 mg/liter. Conversely, increasing the FCS concentration to 20% made the uptake process less effective over the range of concentrations investigated.
FIG. 3.
Accumulation of oritavancin in J774 macrophages incubated for 2 h at 37°C with increasing extracellular concentrations of oritavancin in serum-free medium or in medium supplemented with 10 or 20% FCS. Results are given as arithmetic means ± standard deviations (n = 3) of the cellular concentrations recorded (micrograms of oritavancin per milligram of cell protein; the apparent accumulation ratio for each value of the extracellular concentration can be calculated by multiplying the values on the ordinate by 200 [based on a cell volume of 5 μl/mg of protein]). prot, protein.
Subcellular distribution of oritavancin.
In preliminary experiments, we observed that about 80% of the cell-associated oritavancin was recovered in the granular (MLP) fraction, with only 5% recovered in the supernatant (S) fraction and the remaining recovered in the fraction with nuclei and unbroken cells. The MLP fraction was therefore analyzed by isopycnic centrifugation to determine the distribution of oritavancin among the organelles present in that fraction (mainly mitochondria, lysosomes, endoplasmic reticula, and plasma membranes). Figure 4 (controls) shows the distribution of marker enzymes of lysosomes (N-acetyl-β-glucosaminidase), mitochondria (cytochrome c oxidase), and plasma and endoplasmic reticulum membranes (inosine 5′-diphosphatase) in control cells. These profiles are well distinct from one another (an assay for cathepsin B was also performed in these experiments, and the results showed a profile similar to that for N-acetyl-β-glucosaminidase). Figure 4 (treated cells) shows the results of three experiments in which cells were incubated for 2 h with either oritavancin (20 mg/liter), chloroquine (20 mg/liter), or HRP (2 g/liter). For cells incubated with oritavancin first, it appeared that the drug and N-acetyl-β-glucosaminidase showed essentially common behaviors in all fractions, with densities less than 1.15. Chloroquine, a known lysosomotropic agent, also showed a large overlap of distribution with that of N-acetyl-β-glucosaminidase, but the latter equilibrated at a somewhat lower density than it did in control cells. HRP was also codistributed with N-acetyl-β-glucosaminidase, with a slight but detectable shift of density of the latter enzyme toward higher densities, however.
FIG. 4.
Density distribution of marker enzymes (N-acetyl-β-glucosaminidase [NABGase; lysosomes], cytochrome c oxidase [mitochondria], and inosine 5′-diphosphatase [plama and endoplasmic reticulum membranes]), drugs, and HRP after isopycnic centrifugation of granule (MLP) fractions prepared from homogenates of J774 cells in linear sucrose gradients. The ordinate shows the percentage of each constituent recovered in each fraction. Left panels, distribution of marker enzymes in control macrophages; right panels, distributions of N-acetyl-β-glucosaminidase (thin lines) and oritavancin, chloroquine, or HRP (thick lines) in cells incubated for 2 h with oritavancin (20 mg/liter), chloroquine (20 mg/liter), or HRP (2 g/liter). The vertical arrowheads point to the median density of the N-acetyl-β-glucosaminidase distribution in both control cells (top left panel) and treated cells (all three right panels).
Mechanism of lysosomal accumulation of oritavancin.
Having established that the oritavancin accumulated by J774 macrophages was primarily associated with lysosomes, we investigated the mechanisms of its uptake by short-term kinetic and pharmacological approaches. Figure 5 compares the kinetics of oritavancin accumulation with those of HRP and small latex beads (taken as tracers of fluid phase and adsorptive pinocytosis, respectively [26, 35, 41]) on the one hand and of chloroquine and azithromycin (taken as examples of molecules diffusing through membranes and accumulating in lysosomes by proton trapping [4, 12, 45]) on the other. Oritavancin, HRP, and small latex beads accumulated in a linear fashion over time. The rates of clearance of oritavancin and small latex beads were quite similar (∼150 and ∼120 μl × mg of protein−1 × h−1) and were considerably higher than that of HRP (∼0.7 μl × mg of protein−1 × h−1). In contrast, the uptake kinetics of chloroquine and azithromycin over the first 4 h were not linear but followed a one-phase exponential association process with similar time constants (approximately 1 h−1) for both drugs.
FIG. 5.
Kinetics of accumulation over a 4-h period of oritavancin (extracellular concentration, 25 mg/liter), 0.1-μm-diameter latex beads (extracellular concentration, 230 × 1012 beads/liter), and HRP (extracellular concentration, 2 g/liter) (upper panel) and of chloroquine (extracellular concentration, 12 mg/liter) and azithromycin (extracellular concentration, 25 mg/liter) (lower panel) in J774 macrophages incubated at 37°C in medium supplemented with 10% FCS. Results are given as arithmetic means ± standard deviations (n = 3) of the apparent cell accumulation levels (ratio of the apparent cellular concentration to the extracellular concentration). The data were fitted to a linear equation (oritavancin, latex beads, HRP) or a one-phase exponential association (chroroquine, azithromycin). Regression parameters are as follows: for oritavancin, R2 = 0.995, slope = 31.42 ± 1.43 h−1; for latex beads, R2 = 0.929, slope = 25.73 ± 1.65 h−1; for HRP, R2 = 0.997, slope = 0.15 ± 0.01 h−1; for azithromycin, R2 = 0.941, κ = 0.89 ± 0.27 h−1, plateau = 116.6 ± 12.8; and for chloroquine, R2 = 0.966, κ = 1.24 ± 0.23 h−1, plateau = 546.8 ± 31.7.
We next examined the influences of the extracellular pH and monensin on the accumulation of oritavancin in comparison with that of chloroquine. Acid pH is known to depress the cellular accumulation of weak organic bases that accumulate in lysosomes by diffusion and segregation of their protonated forms due to the corresponding change in the lysosome-extracellular pH gradient (12). In this context, the levels of accumulation of oritavancin (25 mg/liter) and chloroquine (20 mg/liter) were examined after 2 h of incubation at pH 6 and 8 and compared with those at pH 7.4, yielding values of 128% ± 7.5% and 86.5% ± 5.3%, respectively, for oritavancin and 18.1% ± 1.5% and 140.4% ± 10.7%, respectively, for chloroquine. Monensin is a proton ionophore that (i) collapses transmembrane gradients (36), suppressing almost completely the accumulation of weak organic bases (41), and (ii) partly defeats pinocytosis by stimulating the regurgitation of the contents of early endosomes (10). Cells were preincubated with 20 μM monensin for 1 h and then exposed to oritavancin (25 mg/liter), HRP (2 g/liter), or chloroquine (20 mg/liter) for 2 h in the continuing presence of monensin. Oritavancin and HRP accumulated to 28.5% ± 2.0% and 29.9% ± 2.7% of the control value (no monensin treatment), respectively; this decrease in the level of HRP uptake is similar to that observed previously in J774 macrophages (41), whereas the accumulation of chloroquine was reduced to 4.8% ± 0.6% of the control value.
Intracellular activity of oritavancin against phagolysosomal (S. aureus) and cytosolic (L. monocytogenes) bacteria.
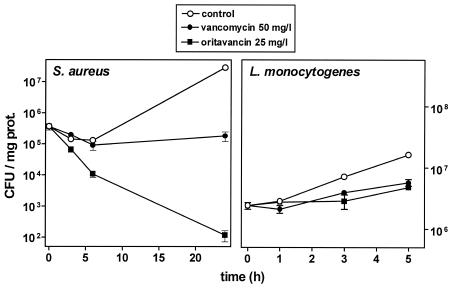
The MICs and MBCs of oritavancin in comparison with those of vancomycin for the S. aureus and L. monocytogenes strains used in this study are shown in Table 2. They illustrate (i) the bactericidal character of glycopeptides against S. aureus (MBC/MIC ratio = 4) and (ii) the high degree of sensitivity of L. monocytogenes to oritavancin. In contrast, vancomycin was not bactericidal against L. monocytogenes. Oritavancin and vancomycin were used at extracellular concentrations of 25 mg/liter, as in our pharmacokinetic studies, and 50 mg/liter, respectively, with the latter concentration mimicking the maximum level observed in the sera of patients receiving the conventional dose of vancomycin (15 mg/kg). Figure 6 shows that oritavancin, as already reported (31), exerted a marked bactericidal effect against intracellular S. aureus. This effect developed rapidly, yielding an almost 4-log-unit decrease in the level of postphagocytosis within 24 h. In contrast, the effect of vancomycin was essentially static. After 5 h of incubation, neither oritavancin nor vancomycin was capable of preventing the intracellular growth of L. monocytogenes, which proceeded at approximately 60% of the rate observed in the controls.
TABLE 2.
MICs and MBCs of oritavancin and vancomycin for the bacterial strains used in this study
| Antibiotic |
S. aureus
|
L. monocytogenes
|
||
|---|---|---|---|---|
| MIC (μg/ml) | MBC (μg/ml) | MIC (μg/ml) | MBC (μg/ml) | |
| Oritavancin | 0.25 | 1 | 0.02 | 2 |
| Vancomycin | 1 | 4 | 1 | >64 |
FIG. 6.
Variation of the number of viable (CFU) S. aureus (left panel) or L. monocytogenes (right panel) organisms phagocytosed by J774 macrophages upon subsequent incubation of the cells in the presence of 50 mg of vancomycin per liter or 25 mg of oritavancin per liter or under control conditions (gentamicin at 0.5 mg/liter [left panel] or no antibiotic [right panel]). Values are given as arithmetic means of CFU per milligram of protein ± standard deviations (n = 3).
DISCUSSION
The levels of accumulation and subcellular dispositions of aminoglycosides, macrolides, and fluoroquinolones have been extensively studied in eucaryotic cells, yielding useful pharmacodynamic and toxicodynamic information (4, 14, 30, 40). In contrast, little is known so far about the levels of accumulation and subcellular dispositions of glycopeptide antibiotics and those of vancomycin in particular (3, 44). The present study has focused on oritavancin, a semisynthetic derivative of vancomycin, and shows that it accumulates to exceptionally high levels in both macrophages and fibroblasts, in sharp contrast to its parent compound, vancomycin. Most of the cell-associated oritavancin must be truly intracellular, since (i) the level of accumulation is drastically reduced at 4°C (a condition which should not prevent the drug binding at the membrane, as shown for gentamicin [11] and pivaloyl- or phthalimidomethyl esters of ampicillin [8]) and (ii) the drug distributes in the same fractions as lysosomes upon isopycnic centrifugation of the granular cell fraction, with no drug or only a minor proportion of the drug found in the soluble fraction.
Accumulation of exogenous compounds in lysosomes has been shown to result from two main mechanisms, namely, endocytosis and diffusion-segregation (12, 34). The first mechanism is demonstrated for many nonpermeant molecules and can be distinguished between the fluid phase and adsorptive endocytosis, depending on the capacity of the substance to bind or not to bind to the pericellular membrane (20, 32). Fluid-phase pinocytosis is exemplified here by HRP, which has been widely used and validated as a typical marker of this cellular activity in J774 macrophages (41). Receptor-mediated or adsorptive endocytosis is typically observed in J774 macrophages for substances such as transferrin or immunoglobulin G (which have specific receptors) or small latex beads (which bind nonspecifically at the cell surface [41]). The second mechanism is observed with amphiphilic, cationic molecules that are able to diffuse through membranes in their unprotonated form but that get sequestered in lysosomes as protonated molecules because of the acid pH created therein through the activity of an ATP-dependent proton pump (22). This mechanism is exemplified here by azithromycin and chloroquine, which have been extensively studied in this context (4, 41, 45).
The data that we have presented here strongly suggest that oritavancin accumulates in J774 macrophages by the endocytic route, since (i) its kinetics of uptake over the first 4 h follow the same model as those of HRP or small latex beads and is clearly distinct from the kinetics of uptake of azithromycin and chloroquine, and (ii) the accumulation of oritavancin is only partially impaired by monensin and is impaired to the same extent as HRP, while monensin almost entirely suppresses the accumulation of chloroquine (this study) and that of azithromycin (41). The fact that the rate of uptake of oritavancin is similar to that of latex beads and much higher than that of HRP suggests that the occurrence of binding sites at the cell surface allows oritavancin to enter by receptor-mediated or adsorptive endocytosis. No binding sites, however, were evidenced here in the fractionation studies. This may result from elution of the membrane-bound oritavancin during the washing of the cells and the homogenization and fractionation procedures. In this context, the defeating effect on accumulation exerted by serum in the presence of low drug concentrations may indicate a competition between serum proteins and cell membrane binding sites. Conversely, the increased levels of accumulation observed at acid pH could reflect a higher level of binding of the cationic compound oritavancin to the cell membrane, which is globally negatively charged.
Whatever the mechanism of accumulation in lysosomes, oritavancin seems largely, if not exclusively, confined to these organelles. This can be inferred from the results of our studies concerning its intracellular activity. We indeed demonstrate that oritavancin has a marked bactericidal effect against a phagolysosomal microorganism (S. aureus) (31) but no or only negligible activity against a cytosolic bacterium (L. monocytogenes) (23), even though the latter is highly susceptible to oritavancin in vitro. This is in sharp contrast to fluoroquinolones, which show activities against both types of bacteria in the same cellular model (29).
Most of our studies were performed with culture medium containing 10% FCS, because this is required for optimal growth and long-term maintenance of most eucaryotic cells, including J774 macrophages. Oritavancin, however, is known to tightly bind to serum proteins, but such binding is difficult to study because of a tendency of the molecule to stick to various materials, such as dialysis membranes and filters, that must be used to perform these experiments (T. Nicas, personal communication). Studies by the dextran-charcoal method, however, suggest that the level of protein binding of oritavancin in human serum spiked with total drug concentrations of up to 91 mg/liter is about 85 to 90% (A. Rowe and T. J. Brown, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-2193, 2001). Since the mean peak concentrations in serum measured in ongoing clinical trials is 31 mg/liter (45; data on file from Intermune, Inc.), we may estimate that the free concentration in human serum is about 3 to 5 mg/liter. The data from Fig. 3 show that cells exposed to 5 mg of oritavancin per liter in the absence of serum display a drug concentration of about 4 μg/mg of protein. A similar level is obtained for cells exposed to 15 to 25 mg of oritavancin per liter in the presence of 10% FCS. Thus, our standard conditions may actually reproduce the levels of free drug exposure that could be observed in humans.
In a larger context, the unusual cellular pharmacokinetic and pharmacodynamic properties of oritavancin open interesting perspectives in terms of both structure-activity relationships and the potential uses of this molecule in clinics. It is indeed possible that the lipophilic character of oritavancin is what causes not only its fast uptake and high level of retention (as suggested by the present study) but also its rapid bactericidal effect (1, 46). In this context, it is interesting that the few available reports on teicoplanin, another glycopeptide with lipophilic properties and high levels of protein binding, also point to a marked accumulation of this antibiotic (6, 18). Teicoplanin, however, is not more bactericidal than vancomycin. The performance of accumulation and efflux studies and microbiological evaluations with glycopeptide derivatives with systematic variations in their lipophilic-hydrophilic balances would be interesting in this context. With respect to clinical applications, our data appear to be of interest, since they demonstrate that oritavancin has useful activity against intracellular bacteria under incubation conditions (25 mg/liter with 10% FCS) that are probably pertinent to those observed in humans when the influence of protein binding on drug accumulation is taken into account (see above). Targeted animal studies and clinical trials would therefore be most welcome in this context. We must, however, note that the intraphagocytic activity of oritavancin is considerably lower than that which can be anticipated on the basis of its accumulation, as already reported (31). Further study of the mechanisms of this defeating effect may be worthwhile. Finally, the high level of cellular accumulation may suggest the existence of storage compartments (in relation to the prolonged terminal half-life of the molecule), which could constitute potential targets for toxicity, even though major side effects have not been reported so far.
Acknowledgments
We are grateful to N. Aguilera, M. C. Cambier, and F. Renoird for skillful technical assistance. We thank F. N′Kuli (Unité de Biologie Cellulaire, Université Catholique de Louvain) for technical advice in the fractionation studies, P. Wallemacq (Cliniques Universitaires St. Luc, Brussels, Belgium) for determination of vancomycin concentrations, and M. J. Rybak (Wayne State University, Detroit, Mich.) for providing us with experimental details about the microbiological assay for oritavancin. We also thank Eli Lilly & Co. for the kind gift of oritavancin.
F.V.B. and D.T. are chercheur qualifié and collaborateur scientifique, respectively; C.S. and H.C. were a postdoctoral fellow and an aspirant of the Belgian Fonds National de la Recherche Scientifique, respectively; and S.C. was a boursier of the Belgian Fonds pour la Formation à la Recherche dans l'Industrie et l'Agriculture.
This work was supported by the Fonds Spéciaux de Recherches of the Université Catholique de Louvain and by a grant-in-aid from Eli Lilly & Co.
REFERENCES
- 1.Allen, N. E., J. N. Hobbs, Jr., and T. I. Nicas. 1996. Inhibition of peptidoglycan biosynthesis in vancomycin-susceptible and -resistant bacteria by a semisynthetic glycopeptide antibiotic. Antimicrob. Agents Chemother. 40:2356-2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baltch, A. L., R. P. Smith, W. J. Ritz, and L. H. Bopp. 1998. Comparison of inhibitory and bactericidal activities and postantibiotic effects of LY333328 and ampicillin used singly and in combination against vancomycin-resistant Enterococcus faecium. Antimicrob. Agents Chemother. 42:2564-2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beauchamp, D., P. Gourde, M. Simard, and M. G. Bergeron. 1992. Subcellular localization of tobramycin and vancomycin given alone and in combination in proximal tubular cells, determined by immunogold labeling. Antimicrob. Agents Chemother. 36:2204-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlier, M. B., I. Garcia-Luque, J. P. Montenez, P. M. Tulkens, and J. Piret. 1994. Accumulation, release and subcellular localization of azithromycin in phagocytic and non-phagocytic cells in culture. Int. J. Tissue React. 16:211-220. [PubMed] [Google Scholar]
- 5.Carlier, M. B., A. Zenebergh, and P. M. Tulkens. 1987. Cellular uptake and subcellular distribution of roxithromycin and erythromycin in phagocytic cells. J. Antimicrob. Chemother. 20(Suppl. B):47-56. [DOI] [PubMed] [Google Scholar]
- 6.Carlone, N. A., A. M. Cuffini, M. Ferrero, V. Tullio, and G. Avetta. 1989. Cellular uptake, and intracellular bactericidal activity of teicoplanin in human macrophages. J. Antimicrob. Chemother. 23:849-859. [DOI] [PubMed] [Google Scholar]
- 7.Chanteux, H., M. P. Mingeot-Leclercq, E. Sonveaux, F. Van Bambeke, and P. M. Tulkens. 2003. Intracellular accumulation and activity of ampicillin used as free drug and as its phthalimidomethyl or pivaloyloxymethyl ester (pivampicillin) against Listeria monocytogenes in J774 macrophages. J. Antimicrob. Chemother. 52:610-615. [DOI] [PubMed] [Google Scholar]
- 8.Chanteux, H., I. Paternotte, M. P. Mingeot-Leclercq, R. Brasseur, E. Sonveaux, and P. M. Tulkens. 2003. Cell handling, membrane-binding properties, and membrane-penetration modeling approaches of pivampicillin and phthalimidomethylampicillin, two basic esters of ampicillin, in comparison with chloroquine and azithromycin. Pharm. Res. 20:624-631. [DOI] [PubMed] [Google Scholar]
- 9.Cooper, R. D., N. J. Snyder, M. J. Zweifel, M. A. Staszak, S. C. Wilkie, T. I. Nicas, D. L. Mullen, T. F. Butler, M. J. Rodriguez, B. E. Huff, and R. C. Thompson. 1996. Reductive alkylation of glycopeptide antibiotics: synthesis and antibacterial activity. J. Antibiot. (Tokyo) 49:575-581. [DOI] [PubMed] [Google Scholar]
- 10.Cupers, P., A. Veithen, A. Kiss, P. Baudhuin, and P. J. Courtoy. 1994. Clathrin polymerization is not required for bulk-phase endocytosis in rat fetal fibroblasts. J. Cell Biol. 127:725-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Decorti, G., N. Malusa, G. Furlan, L. Candussio, and F. B. Klugmann. 1999. Endocytosis of gentamicin in a proximal tubular renal cell line. Life Sci. 65:1115-1124. [DOI] [PubMed] [Google Scholar]
- 12.de Duve, C., T. de Barsy, B. Poole, A. Trouet, P. Tulkens, and F. Van Hoof. 1974. Lysosomotropic agents. Biochem. Pharmacol. 23:2495-2531. [DOI] [PubMed] [Google Scholar]
- 13.El Mouedden, M., G. Laurent, M. P. Mingeot-Leclercq, and P. M. Tulkens. 2000. Gentamicin-induced apoptosis in renal cell lines and embryonic rat fibroblasts. Toxicol. Sci. 56:229-239. [DOI] [PubMed] [Google Scholar]
- 14.Garcia, I., A. Pascual, S. Ballesta, and E. J. Perea. 2000. Uptake and intracellular activity of ofloxacin isomers in human phagocytic and non-phagocytic cells. Int. J. Antimicrob. Agents 15:201-205. [DOI] [PubMed] [Google Scholar]
- 15.Harland, S., S. E. Tebbs, and T. S. Elliott. 1998. Evaluation of the in-vitro activity of the glycopeptide antibiotic LY333328 in comparison with vancomycin and teicoplanin. J. Antimicrob. Chemother. 41:273-276. [DOI] [PubMed] [Google Scholar]
- 16.Lowdin, E., I. Odenholt, and O. Cars. 1998. In vitro studies of pharmacodynamic properties of vancomycin against Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob. Agents Chemother. 42:2739-2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 18.Maderazo, E. G., S. P. Breaux, C. L. Woronick, R. Quintiliani, and C. H. Nightingale. 1988. High teicoplanin uptake by human neutrophils. Chemotherapy 34:248-255. [DOI] [PubMed] [Google Scholar]
- 19.Montenez, J. P., F. Van Bambeke, J. Piret, R. Brasseur, P. M. Tulkens, and M. P. Mingeot-Leclercq. 1999. Interactions of macrolide antibiotics (erythromycin A, roxithromycin, erythromycylamine [dirithromycin], and azithromycin) with phospholipids: computer-aided conformational analysis and studies on acellular and cell culture models. Toxicol. Appl. Pharmacol. 156:129-140. [DOI] [PubMed] [Google Scholar]
- 20.Mukherjee, S., R. N. Ghosh, and F. R. Maxfield. 1997. Endocytosis. Physiol. Rev. 77:759-803. [DOI] [PubMed] [Google Scholar]
- 21.Nicas, T. I., M. L. Zeckel, and D. K. Braun. 1997. Beyond vancomycin: new therapies to meet the challenge of glycopeptide resistance. Trends Microbiol. 5:240-249. [DOI] [PubMed] [Google Scholar]
- 22.Ohkuma, S., and B. Poole. 1978. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc. Natl. Acad. Sci. USA 75:3327-3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ouadrhiri, Y., B. Scorneaux, Y. Sibille, and P. M. Tulkens. 1999. Mechanism of the intracellular killing and modulation of antibiotic susceptibility of Listeria monocytogenes in THP-1 macrophages activated by gamma interferon. Antimicrob. Agents Chemother. 43:1242-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ouadrhiri, Y., Y. Sibille, and P. M. Tulkens. 1999. Modulation of intracellular growth of Listeria monocytogenes in human enterocyte Caco-2 cells by interferon-gamma and interleukin-6: role of nitric oxide and cooperation with antibiotics. J. Infect. Dis. 180:1195-1204. [DOI] [PubMed] [Google Scholar]
- 25.Perantoni, A., and J. J. Berman. 1979. Properties of Wilms' tumor line (TuWi) and pig kidney line (LLC-PK1) typical of normal kidney tubular epithelium. In Vitro 15:446-454. [DOI] [PubMed] [Google Scholar]
- 26.Rejman, J., V. Oberle, I. S. Zuhorn, and D. Hoekstra. 2004. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem. J. 377(Pt 1):159-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Renard, C., H. J. Vanderhaeghe, P. J. Claes, A. Zenebergh, and P. M. Tulkens. 1987. Influence of conversion of penicillin G into a basic derivative on its accumulation and subcellular localization in cultured macrophages. Antimicrob. Agents Chemother. 31:410-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwenzer, K. S., C. H. Wang, and J. P. Anhalt. 1983. Automated fluorescence polarization immunoassay for monitoring vancomycin. Ther. Drug Monit. 5:341-345. [DOI] [PubMed] [Google Scholar]
- 29.Seral, C., S. Carryn, P. M. Tulkens, and F. Van Bambeke. 2003. Influence of P-glycoprotein and MRP efflux pump inhibitors on the intracellular activity of azithromycin and ciprofloxacin in macrophages infected by Listeria monocytogenes or Staphylococcus aureus. J. Antimicrob. Chemother. 51:1167-1173. [DOI] [PubMed] [Google Scholar]
- 30.Seral, C., J. M. Michot, H. Chanteux, M. P. Mingeot-Leclercq, P. M. Tulkens, and F. Van Bambeke. 2003. Influence of P-glycoprotein inhibitors on the accumulation of macrolides in J774 murine macrophages. Antimicrob. Agents Chemother. 47:1047-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seral, C., F. Van Bambeke, and P. M. Tulkens. 2003. Quantitative analysis of the activity of antibiotics (gentamicin, azithromycin, telithromycin, ciprofloxacin, moxifloxacin, oritavancin [LY333328]) against intracellular Staphylococcus aureus in mouse J774 macrophages. Antimicrob. Agents Chemother. 47:2283-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silverstein, S. C., R. M. Steinman, and Z. A. Cohn. 1977. Endocytosis. Annu. Rev. Biochem. 46:669-722. [DOI] [PubMed] [Google Scholar]
- 33.Snyderman, R., M. C. Pike, D. G. Fischer, and H. S. Koren. 1977. Biologic and biochemical activities of continuous macrophage cell lines P388D1 and J774.1. J. Immunol. 119:2060-2066. [PubMed] [Google Scholar]
- 34.Steinberg, T. H. 1994. Cellular transport of drugs. Clin. Infect. Dis. 19:916-921. [DOI] [PubMed] [Google Scholar]
- 35.Steinman, R. M., J. M. Silver, and Z. A. Cohn. 1974. Pinocytosis in fibroblasts. Quantitative studies in vitro. J. Cell Biol. 63:949-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tartakoff, A. M. 1983. Perturbation of vesicular traffic with the carboxylic ionophore monensin. Cell 32:1026-1028. [DOI] [PubMed] [Google Scholar]
- 37.Trainer, D. L., T. Kline, F. L. McCabe, L. F. Faucette, J. Feild, M. Chaikin, M. Anzano, D. Rieman, S. Hoffstein, and D. J. Li. 1988. Biological characterization and oncogene expression in human colorectal carcinoma cell lines. Int. J. Cancer 41:287-296. [DOI] [PubMed] [Google Scholar]
- 38.Tsuchiya, S., M. Yamabe, Y. Yamaguchi, Y. Kobayashi, T. Konno, and K. Tada. 1980. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 26:171-176. [DOI] [PubMed] [Google Scholar]
- 39.Tulkens, P., H. Beaufay, and A. Trouet. 1974. Analytical fractionation of homogenates from cultured rat embryo fibroblasts. J. Cell Biol. 63:383-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tulkens, P., and A. Trouet. 1978. The uptake and intracellular accumulation of aminoglycoside antibiotics in lysosomes of cultured rat fibroblasts. Biochem. Pharmacol. 27:415-424. [DOI] [PubMed] [Google Scholar]
- 41.Tyteca, D., P. Van Der Smissen, M. Mettlen, F. Van Bambeke, P. M. Tulkens, M. P. Mingeot-Leclercq, and P. J. Courtoy. 2002. Azithromycin, a lysosomotropic antibiotic, has distinct effects on fluid-phase and receptor-mediated endocytosis, but does not impair phagocytosis in J774 macrophages. Exp. Cell Res. 281:86-100. [DOI] [PubMed] [Google Scholar]
- 42.Tyteca, D., P. Van Der Smissen, F. Van Bambeke, K. Leys, P. M. Tulkens, P. J. Courtoy, and M. P. Mingeot-Leclercq. 2001. Azithromycin, a lysosomotropic antibiotic, impairs fluid-phase pinocytosis in cultured fibroblasts. Eur. J. Cell Biol. 80:466-478. [DOI] [PubMed] [Google Scholar]
- 43.Van Bambeke, F., Y. Van Laethem, P. Courvalin, and P. M. Tulkens. 2004. Glycopeptide antibiotics: from conventional molecules to new derivatives. Pharmacological properties and clinical use. Drugs 64:913-936. [DOI] [PubMed] [Google Scholar]
- 44.Van der Auwera, P., T. Matsumoto, and M. Husson. 1988. Intraphagocytic penetration of antibiotics. J. Antimicrob. Chemother. 22:185-192. [DOI] [PubMed] [Google Scholar]
- 45.Wibo, M., and B. Poole. 1974. Protein degradation in cultured cells. II. The uptake of chloroquine by rat fibroblasts and the inhibition of cellular protein degradation and cathepsin B1. J. Cell Biol. 63:430-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams, D. H. 1996. The glycopeptide story—how to kill the deadly ‘superbugs.’ Nat. Prod. Rep. 13:469-477. [DOI] [PubMed] [Google Scholar]