Abstract
Many studies have reported that olive oil–based lipid emulsion (LE) formulas of soybean oil, medium-chain triglycerides, olive oil, and fish oil (SMOF) may be a viable alternative for parenteral nutrition. However, some randomized controlled clinical trials (RCTs) have raised concerns regarding the nutritional benefits and safety of SMOFs. We searched principally the MEDLINE, Cumulative Index to Nursing and Allied Health Literature, Scopus, EMBASE, and Cochrane Central Register of Controlled Trials databases from inception to March 2014 for the relevant literature and conducted a meta-analysis of 15 selected RCTs that 1) compared either olive oil– or SMOF-based LEs with soybean oil–based LEs and 2) reported plasma concentrations of α-tocopherol, oleic acid, and ω-6 (n–6) and ω-3 (n–3) long-chain polyunsaturated fatty acids (PUFAs) and liver concentrations of total bilirubin and the enzymes alanine transaminase, aspartate transaminase, alkaline phosphatase, and γ-glutamyl transferase. The meta-analysis suggested that SMOF-based LEs were associated with higher plasma concentrations of plasma α-tocopherol, oleic acid, and the ω-3 PUFAs eicosapentaenoic and docosahexaenoic acid. Olive oil– and SMOF-based LEs correlated with lower plasma concentrations of long-chain ω-6 PUFAs and were similar to soybean oil–based LEs with regard to their effects on liver function indicators. In summary, olive oil– and SMOF-based LEs have nutritional advantages over soybean oil–based LEs and are similarly safe. However, their performance in clinical settings requires further investigation.
Keywords: lipid emulsion, parenteral nutrition, olive oil, SMOF, meta-analysis
Introduction
Parenteral nutrition regimes are commonly used to provide essential FAs, increase caloric intake in preterm infants (1, 2), restore immunologic competence (3), and reduce the frequency and severity of infectious complications in critically ill patients (4). However, compared with enteral nutrition, parenteral nutrition may increase the risk of infection and complications (5). The increase in associated complications may result from the development of hyperglycemia (6) or the high content of ω-6 long-chain (LC)10 PUFAs in conventional soybean oil–based lipid emulsions (LEs) (4, 7). It has been proposed that the safety of LEs could be improved by decreasing ω-6 LC PUFA content (8) and increasing the content of ω-3 LC PUFAs (9) and MUFAs (10). Thus, alternative LEs for parenteral nutrition have been developed (4, 11), including olive oil–based LEs or LEs containing a mixture of soybean oil, medium-chain TGs, olive oil, and fish oil (SMOF).
Limited data suggest that lowering the linoleic acid content of a parenteral nutrition formula by partly replacing soybean oil with olive oil may improve safety (12, 13). SMOF-based LEs have been reported to be safe and tolerable in surgical patients and premature neonates (14–18). However, it remains unclear how well olive oil– or SMOF-based LEs compare with conventional soybean oil–based LEs in terms of nutrition and safety for patients. Therefore, we conducted this systematic review and meta-analysis to determine the nutritional benefits and safety of olive oil– or SMOF-based LEs relative to conventional soybean oil–based LEs.
Methods
This meta-analysis was performed based on the Quality of Reporting of Meta-Analyses guidelines (19) and the recommendations of the Cochrane Collaboration (20).
Data sources and searches.
The electronic databases screened were MEDLINE (inception to March 2014); Cumulative Index to Nursing and Allied Health Literature (inception to March 2014); Scopus (inception to March 2014); EMBASE (inception to March 2014); and the Cochrane Library (March 2014, issue 3), including the Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, and Health Assessment Technology Database. Searches were limited to humans and performed for all languages, years, and types of publication.
The keywords olive; ClinOleic; soybean oil, medium-chain triglycerides, olive oil, fish oil; SMOF; SMOF lipid; and soybean, soy, or Intralipid in combination with parenteral nutrition, total parental nutrition, lipid emulsion, or intravenous feed. In addition, Y-JD, M-YL, and C-LD manually and independently screened the reference sections of relevant original articles, reviews, meta-analyses, and journals to guarantee the inclusion of appropriate randomized controlled trials (RCTs).
Study selection.
Trials were included if they met the following criteria: an RCT; at least 1 comparison with olive oil– or SMOF-based LEs with soybean oil–based LEs; and reported the nutritional benefits (plasma concentrations of antioxidant, ω-6 LC PUFAs, MUFAs, and ω-3 LC PUFAs) and liver enzyme concentrations (indicating safety). Corresponding authors of the RCTs with incomplete data (e.g., missing means, SDs) were contacted when necessary.
Data extraction.
Y-CS and C-LD independently screened the titles and abstracts of potentially eligible studies. The full-text articles were examined independently by WW and C-HZ to determine whether they met the inclusion criteria. Y-JD and M-YL independently extracted data (study characteristics and results) using data extraction forms. Point estimates for selected variables were extracted and checked by S-HX and FY. All discrepancies were rechecked, and consensus was reached through discussion with a third author (L-JS).
Outcome measures.
Based on the outcome measurements reported in the RCTs, we stratified the main outcomes as plasma concentrations of the antioxidant α-tocopherol, the ω-6 LC PUFAs α-linolenic and arachidonic acid, the ω-3 LC PUFAs EPA and DHA, the MUFA oleic acid, and liver enzyme concentrations of total bilirubin, alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), and γ-glutamyl transferase (GGT). The Jadad test (5 items) (21) was employed to assess methodological quality, which was classified as high (score 5), moderate (score 4), or low (scores 1–3).
Data synthesis and analysis.
Intention-to-treat data were analyzed whenever available. Nonparametric tests (Mann-Whitney, Kruskal-Wallis) were used to compare continuous variables. A 2-sided P value ≤0.05 was considered significant. Because all outcomes were presented as continuous data (mean value or mean changes), the weighted mean differences and 95% CIs of each subgroup were calculated. I2 statistics were used to measure the heterogeneity of the RCTs. If the I2 value was <50%, a fixed-effects model was applied. If the I2 value was >50%, the random-effects model was then adopted (22). Means and SDs were used to calculate the weighted mean differences. When these values were illustrated in a graph without any description of the absolute value, we first tried to contact the authors and then used the measurements from the graph if we failed to get the original data from the authors. Data were analyzed using RevMan 5.0.25 in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (22), and the figures were processed by using GraphPad Prism 5.01 software.
Publication bias may lead to asymmetrical funnel plots (23). In this study, we visually assessed funnel plots calculated with RevMan to investigate potential publication bias (i.e., publication probability associated with the statistical significance of the study results).
Results
Study selection
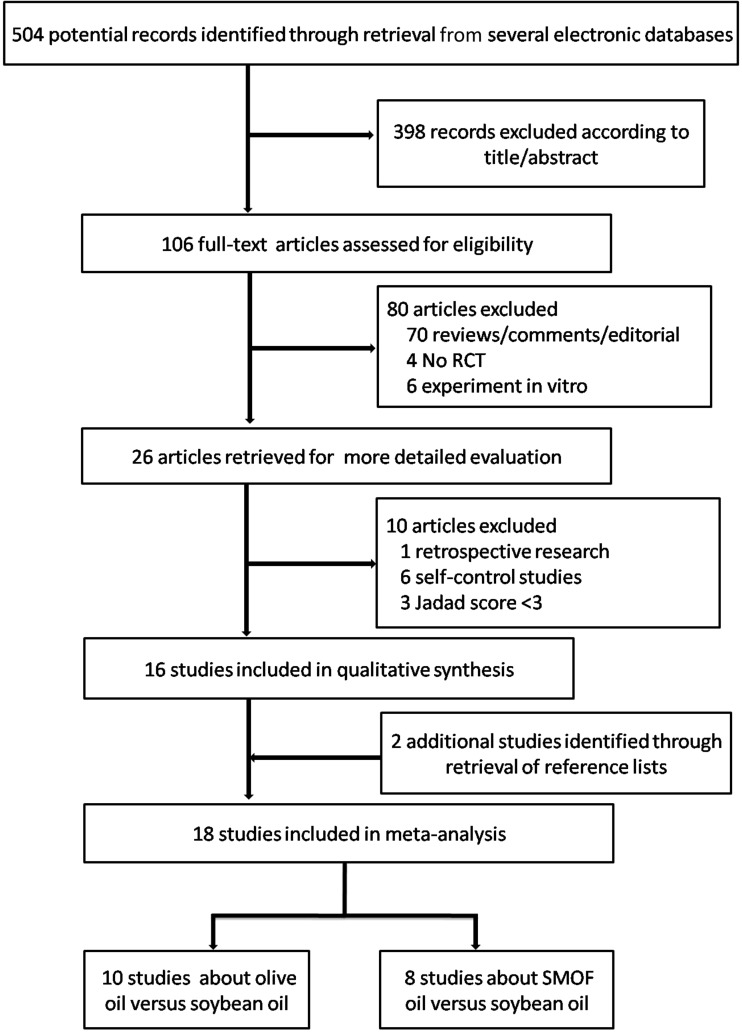
The literature search yielded 504 citations (Figure 1). On the basis of the title and abstract, 398 records were then excluded, leaving 106 publications for the second round of screening. An additional 90 papers were rejected after further examination because they were reviews, comments, editorials, retrospective research, or self-report studies or had a Jadad score <3. Three more papers were excluded because of insufficient data for this meta-analysis. Two additional eligible studies were identified by reviewing the reference lists. Finally, 15 trials—8 of which compared olive oil–based LEs with soybean oil–based LEs (12, 24–30) and 7 of which compared SMOF with soybean oil–based LEs (14, 16, 17, 31–34)—met our selection criteria and were included in the meta-analysis.
FIGURE 1.
A schematic of study selections. RCT, randomized controlled clinical trial.
Baseline characteristics and risk of bias
The compositions of soybean oil–, olive oil–, and SMOF-based LEs are listed in Table 1. The intervention, mean age, numbers of participants, and reasons and duration of LE administration for each study are shown in Table 2. Infants were investigated in 7 trials (12, 16, 24, 26, 29, 30, 34), children in 2 (28, 31), and adults in 6 (14, 17, 25, 27, 32, 33). Eleven studies were categorized as high quality [Jadad score = 5 (12, 14, 24, 25, 26, 27, 28, 30, 31, 32, 34)]; 1 was of middle quality [Jadad score = 4 (17)]; and 3 were of low quality [Jadad score = 3 (16, 29, 33)]. The Cochrane risk of bias assessments was consistent with the Jadad score evaluations (Supplemental Figures 1 and 2). The symmetric funnel plots suggested a low chance of publication bias (Supplemental Figures 3 and 4).
TABLE 1.
Composition of LEs1
| Olive oil LE | SMOF LE | Soybean oil LE | |
| Soybean oil, g/L | 40 | 60 | 200 |
| Olive oil, g/L | 160 | 50 | 0 |
| MCTs, g/L | 0 | 60 | 0 |
| Fish oil, g/L | 0 | 30 | 0 |
| Egg phospholipids, g/L | 12 | 12 | 12 |
| Glycerol, g/L | 22.5 | 25 | 22.5 |
| pH | 7–8 | 7.5–8.8 | 7–8 |
| Osmolarity, mOsmol/L | 270 | 273 | 265 |
| α-Tocopherol, mg/L | 30 | 200 | 14 |
| Major FAs, % by wt | |||
| Oleic | 58.3 | 27.8 | 22.3 |
| Linoleic | 17.7 | 18.7 | 53 |
| α-Linoleic | 2 | 2.4 | 8 |
| Arachidonic | 0.3 | 0.5 | 0.3 |
| EPA | <0.02 | 2.4 | <0.02 |
| DHA | 0.23 | 2.2 | 0.34 |
LE, lipid emulsion; MCTs, medium-chain TGs; SMOF, soybean oil, medium-chain TGs, olive oil, and fish oil.
TABLE 2.
Main characteristics of the included studies1
| Mean age |
Participants, n |
LE administration |
||||||
| Reference | Location | Intervention2 | Treatment | Control | Enrolled | Completed | Reasons | Days |
| Antébi et al. (17) | France | SMOF LE | 70 y | 51 y | 20 | 20 | Major surgery | 5 |
| Cano et al. (27) | France | Olive oil LE | 73.2 y | 66.5 y | 41 | 35 | Malnourished hemodialysis | 35 |
| Deshpande et al. (12) | Australia | Olive oil LE | 26.14 wk | 25.92 wk | 50 | 45 | Premature birth | 5 |
| Gawecka et al. (26) | Poland | Olive oil LE | 27 wk | 27 wk | 44 | 38 | Premature birth | 14 |
| Gobel et al. (29) | Germany | Olive oil LE | 220 d | 224 d | 42 | 33 | Premature birth | 7 |
| Goulet et al. (28) | France | Olive oil LE | 4 y | 3 y | 18 | 18 | Short-bowel syndrome, intractable diarrhea, CIP | 60 |
| Goulet et al. (31) | Germany | SMOF LE | 30.3 mo | 38.8 mo | 28 | 28 | Short-bowel syndrome, CIP | 28 |
| Grimm et al. (33) | Germany | SMOF LE | 63.1 y | 61.1 y | 33 | 33 | Major abdominal surgery | 5 |
| Klek et al. (32) | Poland | SMOF LE | 53.2 y | 45.2 y | 75 | 62 | Short bowel syndrome, Crohn disease, malabsorption | 28 |
| Koksal et al. (24) | India | Olive oil LE | 30.2 wk | 30.4 wk | 64 | 64 | Premature birth | 7 |
| Mertes et al. (14) | Germany | SMOF LE | 60.5 y | 60.2 y | 249 | 199 | Elective abdominal or thoracic surgery | 5 |
| Rayyan et al. (34) | Germany | SMOF LE | 29.9 wk | 30.4 wk | 53 | 46 | Premature birth | 14 |
| Tomsits et al. (16) | Hungary | SMOF LE | 31.7 wk | 31.9 wk | 60 | 51 | Premature birth | 14 |
| Vahedi et al. (25) | France | Olive oil LE | 48 y | 53 y | 13 | 13 | Short-bowel syndrome, CIP | 90 |
| Webb et al. (30) | Australia | Olive oil LE | 37.0 wk | 36.7 wk | 80 | 78 | Critically ill | 5 |
CIP, chronic intestinal pseudo-obstruction; LE, lipid emulsion; SMOF, soybean oil, medium-chain TGs, olive oil, and fish oil.
Relative to soybean oil LE (control).
Nutritional benefits associated with olive oil– or SMOF-based LEs
The nutritional benefits of LE treatments were evaluated by measuring plasma concentrations of several final products described in the sections that follow.
Plasma concentrations of the antioxidant α-tocopherol.
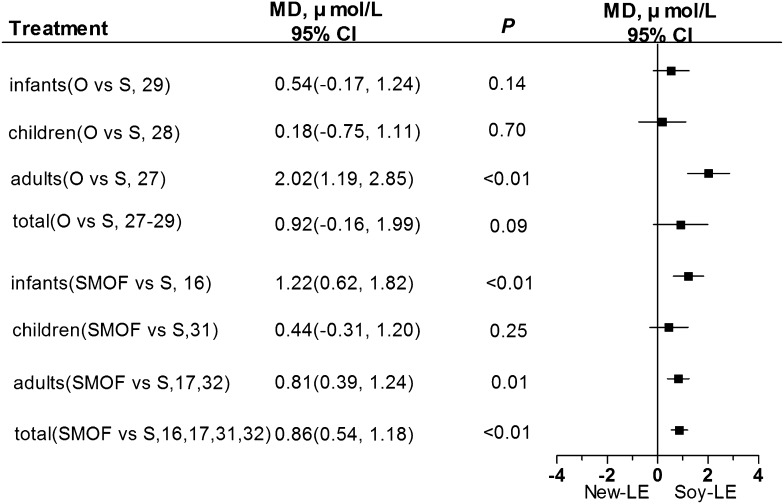
α-Tocopherol is an antioxidant in the blood (35) and has been associated with an anticancer effect via the nonantioxidant pathway (36). Plasma α-tocopherol concentrations of patients who received the olive oil–based LEs were higher than those of patients who were given the soybean oil–based LEs, but the difference was not statistically significant (P = 0.09; Figure 2).
FIGURE 2.
Concentrations of plasma α-tocopherol in infants, children, adults, or all individuals after olive oil– or SMOF-based LE administration compared with soybean oil–based LEs. LE, lipid emulsion; MD, mean difference; O, olive oil; S, soybean oil; SMOF, soybean oil, medium-chain TGs, olive oil, and fish oil.
However, the plasma α-tocopherol concentrations of patients who received the SMOF-based LEs were significantly higher than those of patients who were given the soybean oil–based LEs (Figure 2). Because the number of RCTs for each age group was not sufficient to stratify populations by age, it is not clear whether the increase in plasma α-tocopherol concentrations was age-dependent.
Plasma concentrations of ω-6 LC PUFAs.
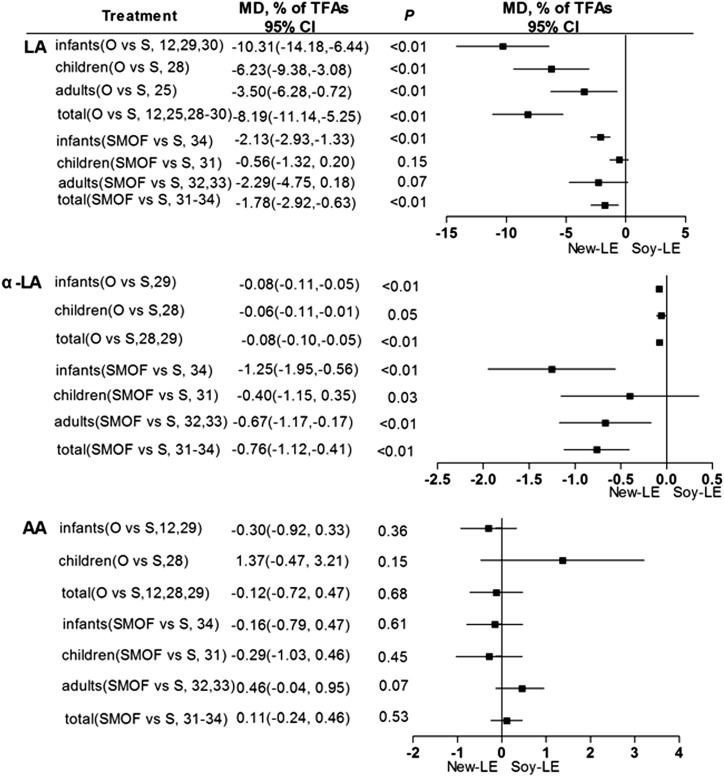
The ω-6 LC PUFAs investigated in this meta-analysis were linoleic, α-linoleic, and arachidonic acid. Although in humans linoleic and α-linoleic acid are essential, excessive intake of ω-6 LC PUFAs may increase lipid peroxidation and impair immune function (37).
For the entire sample population, patients given the olive oil–based LEs had significantly lower plasma linoleic and α-linoleic acid concentrations than did those who received soybean oil–based LEs (Figure 3). These alterations were also true for infants with regard to linoleic acid, but data for α-linoleic or arachidonic acid were too limited to support the conclusions. Similarly, the overall plasma concentrations of linoleic and α-linoleic acid were significantly lower in those given SMOF-based LEs than in those given soybean oil–based LEs (Figure 3), but potential differences between age groups could not be determined.
FIGURE 3.
Plasma concentrations of ω-6 long-chain PUFAs (linoleic, α-linoleic, and arachidonic acids) in infants, children, adults, and all individuals after olive oil– or SMOF-based LE administration compared with soybean oil–based LEs. AA, arachidonic acid; LA, linoleic acid; LE, lipid emulsion; MD, mean difference; O, olive oil; SMOF, soybean oil, medium-chain TGs, olive oil, and fish oil; S/Soy, soybean; TFAs, total FAs.
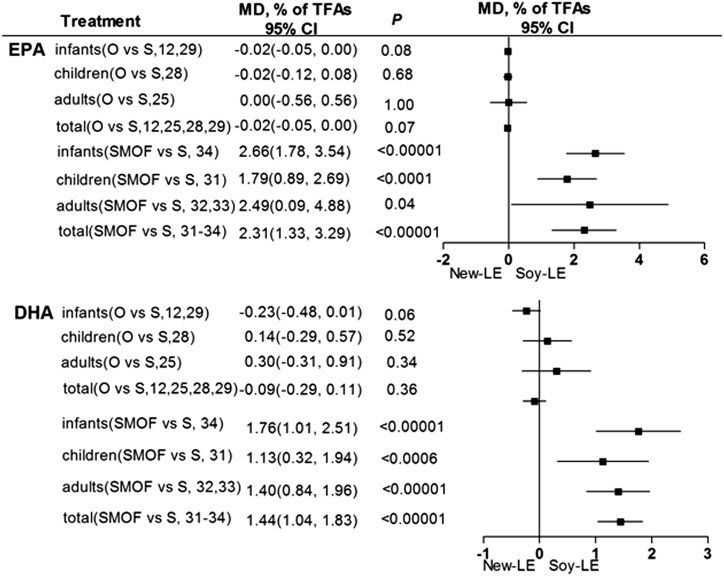
Plasma concentrations of ω-3 LC-PUFAs.
The importance of ω-3 LC-PUFAs, particularly the effect of EPA and DHA on visual and cognitive development, has been well acknowledged and established (38). Our analysis of the entire sample population indicated that olive oil–based LEs were associated with lower plasma EPA relative to that of soybean oil–based LEs, but the difference was not significant (P = 0.07; Figure 4). However, plasma EPA and DHA concentrations were significantly higher in those given SMOF-based LEs than in the group that received soybean oil–based LEs (Figure 4). Unfortunately, differences in EPA and DHA between age groups could not be confirmed.
FIGURE 4.
Plasma concentrations of ω-3 long-chain PUFAs (EPA/DHA) in infants, children, adults, and total individuals after olive oil– or SMOF-based LE administration compared with soybean-oil based LEs. LE, lipid emulsion; MD, mean difference; O, olive; SMOF, soybean oil, medium-chain TGs, olive oil, and fish oil; S/Soy, soybean; TFAs, total FAs.
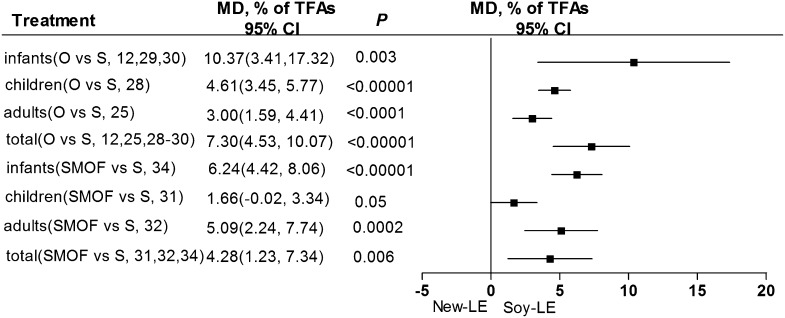
Plasma concentration of the MUFA oleic acid.
The MUFA investigated in our meta-analysis was oleic acid, which has been shown to effectively reduce plasma TG concentration and improve apolipoprotein metabolism (39). The plasma oleic acid concentration associated with either olive oil– or SMOF-based LE treatments was significantly higher than that of the soybean oil–based LE regimen (Figure 5). Furthermore, based on 3 RCTs that considered infants only, plasma oleic acid was significantly higher in those given olive oil–based LEs than in those given soybean oil–based LEs (Figure 5). Comparisons of groups stratified by age could not be performed between SMOF-based LEs and soybean oil–based LEs because of the lack of sufficient RCTs.
FIGURE 5.
Plasma concentrations of oleic acid (a MUFA) in infants, children, adults, and all individuals after olive oil– or SMOF-based LE administration compared with soybean oil–based LEs. LE, lipid emulsion; MD, mean difference; O, olive; SMOF, soybean oil, medium-chain TGs, olive oil, and fish oil; S/Soy, soybean; TFAs, total fat acids.
Safety of olive oil– or SMOF-based LEs as determined by liver function parameters
The safety of LEs remains a clinical concern of physicians. LEs, especially when used long term, may lead to moderate liver function impairments (40). We thus performed a meta-analysis of liver function parameters (total bilirubin, ALT, ALP, AST, and GGT) associated with the use of LE formulas (Supplemental Table 1).
ALP concentrations of patients given olive oil–based LEs were significantly higher than those of patients in the soybean oil–based LE group (Supplemental Table 1), whereas in the SMOF-based LE group both AST and ALP concentrations were significantly lower than the control. No other molecules were compared with soybean oil–based LEs. Interestingly, a meta-analysis of only adult patients showed that ALT and ALP were significantly lower in those who received SMOF-based LEs than in those given soybean oil–based LEs; GGT was also lower, but the difference was not significant (P = 0.08).
Discussion
This systematic review and meta-analysis was conducted to evaluate the nutritional benefits and safety of olive oil– or SMOF-based LEs relative to that of conventional soybean oil–based LEs for parenteral nutrition. After an extensive search of the relevant literature, 15 RCTs were identified, 8 of which compared olive oil–based LEs with soybean oil–based LEs (12, 24–30) and 7 of which compared SMOF with soybean oil–based LEs (14, 16, 17, 31–34). Nutritional benefits were judged on the basis of reported plasma concentrations of α-tocopherol, α-linolenic acid, arachidonic acid, EPA, DHA, and oleic acid. Conclusions regarding the safety of the formulations rested on the liver enzyme concentrations of total bilirubin, ALT, AST, ALP, and GGT. The meta-analysis showed that both olive oil– and SMOF-based LEs were associated with higher plasma concentrations of oleic acid (a MUFA) as well as lower plasma ω-6 LC PUFAs (α-linolenic and arachidonic acids) relative to conventional soybean oil–based LEs. This suggests that olive oil– and SMOF-based LEs may provide more nutritional benefit than conventional soybean oil–based LEs. In addition, SMOF-based LEs were shown to possess some advantages over olive oil–based LEs with regard to higher concentrations of plasma α-tocopherol, EPA, and DHA.
Nutritional benefits: antioxidant status, LC PUFA, and MUFA
Our analysis suggests that SMOF-based LEs lead to an increase in the antioxidant α-tocopherol. This may explain reduced neonatal morbidities in preterm infants who are at high risk of oxidative stress (41). Both olive oil– and SMOF-based LEs are associated with lower plasma ω-6 LC PUFAs (except for arachidonic acid), which may underlie the reduced risk of infectious side effects and immunosuppression.
Furthermore, SMOF-based LEs are associated with higher plasma concentrations of ω-3 LC PUFAs, including EPA and DHA. DHA is an important element that ensures and improves the normal growth of neurons (42); EPA serves as a precursor for the long-chain FA synthesized in the retina (43). It has also been demonstrated that feeding preterm infants with formulas containing EPA and DHA can effectively enhance cognitive and psychomotor development and visual acuity (44). Thus, SMOF-based LEs may offer better benefits to infants than either olive oil– or soybean oil–based LEs.
In this study, the olive oil– or SMOF-based LEs were associated with lower concentrations of linoleic acid. However, the concentrations for the ultimate metabolite linoleic acid (i.e., arachidonic acid in this study) are similar between these 3 LEs. One previous study suggested that the amounts of linoleic acid required for arachidonic acid synthesis in adulthood is low (<3.8% energy) (45). Another study suggested that dietary linoleic acid neither increases plasma arachidonic acid concentrations nor persistently contributes to prostaglandin synthesis (46). These findings may explain the inconsistent alterations in linoleic and arachidonic acid in this study. However, more efforts that focus on the infant population are needed.
It is currently not possible to claim whether the alterations in the plasma measurements are beneficial or not. First, accumulating data from multiple lines of evidence suggest that oleic acid (a MUFA) is linked not only to health promotion but also to disease pathogenesis (47). Second, an increase in α-tocopherol cannot reliably reflect the antioxidant concentration as comprehensively as the measurement of total antioxidant potential (uric acid) (15). To conclude, more efforts are needed in future clinical trials to test this theory.
Safety
The safety of LEs is always on physicians’ minds, with hepatic function impairment being their primary concern. We found no significant difference in the various liver enzymes between the olive oil– and soybean oil–based LE treatments, except for significantly higher ALP that was still within the normal reference range (ALP <500 U/L). The cause of this laboratory finding has not yet been fully understood. Likewise, there was no significant difference in total bilirubin, ALT, or GGT between the SMOF- and soybean oil–based LE groups. Furthermore, there were significantly lower liver enzyme concentrations such as AST and ALP in SMOF-based LE groups than in soybean oil–based LE groups. Possible explanations for the improvement in liver function indicators may be the higher α-tocopherol content or more favorable amounts of ω-3 LC PUFAs associated with SMOF-based LEs than with the soybean oil–based LEs (17) or lower phytosterol load, which may be partly related to liver disease (48).
Strength and limitations
Because of the limited number of RCTs in this meta-analysis, we could not include clinical outcomes that resulted from beneficial biological alterations. On the basis of a search of the clinicaltrials.gov database, there are currently 8 ongoing trials to our knowledge evaluating the efficacy and safety of olive oil– or SMOF-based LEs relative to soybean oil–based LEs (Supplemental Table 2). Perhaps a definitive conclusion on these issues can be reached after the publication of these trials.
The main limitation of this meta-analysis is that the duration of LEs varied from 5 to 90 d. Future detailed RCTs focusing on the influence of treatment duration should be considered. Second, because of the limited number of RCTs that included data on age subgroups, we could not derive conclusions for some populations, such as infants.
Conclusions
Overall, the results of this meta-analysis indicate that both olive oil– and SMOF-based LEs provide better nutritional benefits than conventional soybean oil–based LEs, and SMOF-based LEs are associated with higher concentrations of the essential ω-3 LC PUFAs EPA and DHA. These findings also suggest that the safety profiles of these 3 LEs are similar. Future trials should focus on the clinical outcomes of each LE, which will help guide clinical recommendations.
Acknowledgments
All authors read and approved the final version of the manuscript.
Footnotes
Abbreviations used: ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; GGT, γ-glutamyl transferase; LC, long chain; LE, lipid emulsion; RCT, randomized controlled trial; SMOF, soybean oil, medium-chain TGs, olive oil, and fish oil.
References
- 1.Ibrahim HM, Jeroudi MA, Baier RJ, Dhanireddy R, Krouskop RW. Aggressive early total parental nutrition in low-birth-weight infants. J Perinatol 2004;24:482–6. [DOI] [PubMed] [Google Scholar]
- 2.Koletzko B, Goulet O, Hunt J, Krohn K, Shamir R. Guidelines on Paediatric Parenteral Nutrition of the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the European Society for Clinical Nutrition and Metabolism (ESPEN), supported by the European Society of Paediatric Research (ESPR). J Pediatr Gastroenterol Nutr 2005;41(Suppl 2):S1–87. [DOI] [PubMed] [Google Scholar]
- 3.Granato D, Blum S, Rossle C, Le Boucher J, Malnoe A, Dutot G. Effects of parenteral lipid emulsions with different fatty acid composition on immune cell functions in vitro. JPEN J Parenter Enteral Nutr 2000;24:113–8. [DOI] [PubMed] [Google Scholar]
- 4.Manzanares W, Dhaliwal R, Jurewitsch B, Stapleton RD, Jeejeebhoy KN, Heyland DK. Alternative lipid emulsions in the critically ill: a systematic review of the evidence. Intensive Care Med 2013;39:1683–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wei C, Hua J, Bin C, Klassen K. Impact of lipid emulsion containing fish oil on outcomes of surgical patients: systematic review of randomized controlled trials from Europe and Asia. Nutrition 2010;26:474–81. [DOI] [PubMed] [Google Scholar]
- 6.Yan CL, Huang YB, Chen CY, Huang GS, Yeh MK, Liaw WJ. Hyperglycemia is associated with poor outcomes in surgical critically ill patients receiving parenteral nutrition. Acta Anaesthesiol Taiwan 2013;51:67–72. [DOI] [PubMed] [Google Scholar]
- 7.Siqueira J, Smiley D, Newton C, Le NA, Gosmanov AR, Spiegelman R, Peng L, Osteen SJ, Jones DP, Quyyumi AA, et al. . Substitution of standard soybean oil with olive oil-based lipid emulsion in parenteral nutrition: comparison of vascular, metabolic, and inflammatory effects. J Clin Endocrinol Metab 2011;96:3207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Ascenzo R, D'Egidio S, Angelini L, Bellagamba MP, Manna M, Pompilio A, Cogo PE, Carnielli VP. Parenteral nutrition of preterm infants with a lipid emulsion containing 10% fish oil: effect on plasma lipids and long-chain polyunsaturated fatty acids. J Pediatr 2011;159:33–8 e1. [DOI] [PubMed] [Google Scholar]
- 9.Tillman EM, Helms RA. Omega-3 long chain polyunsaturated Fatty acids for treatment of parenteral nutrition-associated liver disease: a review of the literature. J Pediatr Pharmacol Ther 2011;16:31–8. [PMC free article] [PubMed] [Google Scholar]
- 10.Yaqoob P. Monounsaturated fatty acids in parenteral nutrition; evaluation of risks and benefits. Br J Nutr 2005;94:867–8. [DOI] [PubMed] [Google Scholar]
- 11.Ren T, Cong L, Wang Y, Tang Y, Tian B, Lin X, Zhang Y, Tang X. Lipid emulsions in parenteral nutrition: current applications and future developments. Expert Opin Drug Deliv 2013;10:1533–49. [DOI] [PubMed] [Google Scholar]
- 12.Deshpande GC, Simmer K, Mori T, Croft K. Parenteral lipid emulsions based on olive oil compared with soybean oil in preterm (<28 weeks’ gestation) neonates: a randomised controlled trial. J Pediatr Gastroenterol Nutr 2009;49:619–25. [DOI] [PubMed] [Google Scholar]
- 13.Sala-Vila A, Barbosa VM, Calder PC. Olive oil in parenteral nutrition. Curr Opin Clin Nutr Metab Care 2007;10:165–74. [DOI] [PubMed] [Google Scholar]
- 14.Mertes N, Grimm H, Furst P, Stehle P. Safety and efficacy of a new parenteral lipid emulsion (SMOFlipid) in surgical patients: a randomized, double-blind, multicenter study. Ann Nutr Metab 2006;50:253–9. [DOI] [PubMed] [Google Scholar]
- 15.Skouroliakou M, Konstantinou D, Koutri K, Kakavelaki C, Stathopoulou M, Antoniadi M, Xemelidis N, Kona V, Markantonis S. A double-blind, randomized clinical trial of the effect of omega-3 fatty acids on the oxidative stress of preterm neonates fed through parenteral nutrition. Eur J Clin Nutr 2010;64:940–7. [DOI] [PubMed] [Google Scholar]
- 16.Tomsits E, Pataki M, Tolgyesi A, Fekete G, Rischak K, Szollar L. Safety and efficacy of a lipid emulsion containing a mixture of soybean oil, medium-chain triglycerides, olive oil, and fish oil: a randomised, double-blind clinical trial in premature infants requiring parenteral nutrition. J Pediatr Gastroenterol Nutr 2010;51:514–21. [DOI] [PubMed] [Google Scholar]
- 17.Antébi H, Mansoor O, Ferrier C, Tetegan M, Morvan C, Rangaraj J, Alcindor LG. Liver function and plasma antioxidant status in intensive care unit patients requiring total parenteral nutrition: comparison of 2 fat emulsions. JPEN J Parenter Enteral Nutr 2004;28:142–8. [DOI] [PubMed] [Google Scholar]
- 18.Piper SN, Schade I, Beschmann RB, Maleck WH, Boldt J, Rohm KD. Hepatocellular integrity after parenteral nutrition: comparison of a fish-oil-containing lipid emulsion with an olive-soybean oil-based lipid emulsion. Eur J Anaesthesiol 2009;26:1076–82. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet 1999;354:1896–900. [DOI] [PubMed] [Google Scholar]
- 20.Bero L, Rennie D. The Cochrane Collaboration Preparing, maintaining, and disseminating systematic reviews of the effects of health care. JAMA 1995;274:1935–8. [DOI] [PubMed] [Google Scholar]
- 21.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–12. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JPTGS, Green S. Cochrane handbook for systematic reviews of interventions version 5.0.2. London: The Cochrane Collaboration; 2009. [Google Scholar]
- 23.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Köksal N, Kavurt AV, Cetinkaya M, Ozarda Y, Ozkan H. Comparison of lipid emulsions on antioxidant capacity in preterm infants receiving parenteral nutrition. Pediatr Int 2011;53:562–6. [DOI] [PubMed] [Google Scholar]
- 25.Vahedi K, Atlan P, Joly F, Le Brun A, Evard D, Perennec V, Roux-Haguenau D, Bereziat G, Messing B. A 3-month double-blind randomised study comparing an olive oil- with a soyabean oil-based intravenous lipid emulsion in home parenteral nutrition patients. Br J Nutr 2005;94:909–16. [DOI] [PubMed] [Google Scholar]
- 26.Gawecka A, Michalkiewicz J, Kornacka MK, Luckiewicz B, Kubiszewska I. Immunologic properties differ in preterm infants fed olive oil vs soy-based lipid emulsions during parenteral nutrition. JPEN J Parenter Enteral Nutr 2008;32:448–53. [DOI] [PubMed] [Google Scholar]
- 27.Cano NJ, Saingra Y, Dupuy AM, Lorec-Penet AM, Portugal H, Lairon D, Cristol JP, Come A, Le Brun A, Atlan P, et al. . Intradialytic parenteral nutrition: comparison of olive oil versus soybean oil-based lipid emulsions. Br J Nutr 2006;95:152–9. [DOI] [PubMed] [Google Scholar]
- 28.Goulet O, de Potter S, Antebi H, Driss F, Colomb V, Bereziat G, Alcindor LG, Corriol O, Le Brun A, Dutot G, et al. . Long-term efficacy and safety of a new olive oil-based intravenous fat emulsion in pediatric patients: a double-blind randomized study. Am J Clin Nutr 1999;70:338–45. [DOI] [PubMed] [Google Scholar]
- 29.Göbel Y, Koletzko B, Bohles HJ, Engelsberger I, Forget D, Le Brun A, Peters J, Zimmermann A. Parenteral fat emulsions based on olive and soybean oils: a randomized clinical trial in preterm infants. J Pediatr Gastroenterol Nutr 2003;37:161–7. [DOI] [PubMed] [Google Scholar]
- 30.Webb AN, Hardy P, Peterkin M, Lee O, Shalley H, Croft KD, Mori TA, Heine RG, Bines JE. Tolerability and safety of olive oil-based lipid emulsion in critically ill neonates: a blinded randomized trial. Nutrition 2008;24:1057–64. [DOI] [PubMed] [Google Scholar]
- 31.Goulet O, Antebi H, Wolf C, Talbotec C, Alcindor LG, Corriol O, Lamor M, Colomb-Jung V. A new intravenous fat emulsion containing soybean oil, medium-chain triglycerides, olive oil, and fish oil: a single-center, double-blind randomized study on efficacy and safety in pediatric patients receiving home parenteral nutrition. JPEN J Parenter Enteral Nutr 2010;34:485–95. [DOI] [PubMed] [Google Scholar]
- 32.Klek S, Chambrier C, Singer P, Rubin M, Bowling T, Staun M, Joly F, Rasmussen H, Strauss BJ, Wanten G, et al. . Four-week parenteral nutrition using a third generation lipid emulsion (SMOFlipid)—a double-blind, randomised, multicentre study in adults. Clin Nutr 2013;32:224–31. [DOI] [PubMed] [Google Scholar]
- 33.Grimm H, Mertes N, Goeters C, Schlotzer E, Mayer K, Grimminger F, Furst P. Improved fatty acid and leukotriene pattern with a novel lipid emulsion in surgical patients. Eur J Nutr 2006;45:55–60. [DOI] [PubMed] [Google Scholar]
- 34.Rayyan M, Devlieger H, Jochum F, Allegaert K. Short-term use of parenteral nutrition with a lipid emulsion containing a mixture of soybean oil, olive oil, medium-chain triglycerides, and fish oil: a randomized double-blind study in preterm infants. JPEN J Parenter Enteral Nutr 2012;36:81S–94S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waitzberg DL, Lotierzo PH, Logullo AF, Torrinhas RS, Pereira CC, Meier R. Parenteral lipid emulsions and phagocytic systems. Br J Nutr 2002;87(Suppl 1):S49–57. [DOI] [PubMed] [Google Scholar]
- 36.Palmblad J, Brostrom O, Lahnborg G, Uden AM, Venizelos N. Neutrophil functions during total parenteral nutrition and intralipid infusion. Am J Clin Nutr 1982;35:1430–6. [DOI] [PubMed] [Google Scholar]
- 37.Martínez M, Ballabriga A. Effects of parenteral nutrition with high doses of linoleate on the developing human liver and brain. Lipids 1987;22:133–8. [DOI] [PubMed] [Google Scholar]
- 38.Antébi H, Pages N, Zimmermann L, Bourcier C, Flechet B, Alcindor LG. Resistance to oxidation of native lipoproteins and erythrocyte membrane lipids in rats with iron overload. Ann Nutr Metab 1995;39:63–8. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y, Wang J, Zhang R, Zhang Y, Xu Q, Zhang J, Zheng Z, Yu X, Jing H, Nosaka N, et al. . A good response to oil with medium- and long-chain fatty acids in body fat and blood lipid profiles of male hypertriglyceridemic subjects. Asia Pac J Clin Nutr 2009;18:351–8. [PubMed] [Google Scholar]
- 40.Gura KM, Lee S, Valim C, Zhou J, Kim S, Modi BP, Arsenault DA, Strijbosch RA, Lopes S, Duggan C, et al. . Safety and efficacy of a fish-oil-based fat emulsion in the treatment of parenteral nutrition-associated liver disease. Pediatrics 2008;121:e678–86. [DOI] [PubMed] [Google Scholar]
- 41.Krohn K, Koletzko B. Parenteral lipid emulsions in paediatrics. Curr Opin Clin Nutr Metab Care 2006;9:319–23. [DOI] [PubMed] [Google Scholar]
- 42.Eilander A, Hundscheid DC, Osendarp SJ, Transler C, Zock PL. Effects of n-3 long chain polyunsaturated fatty acid supplementation on visual and cognitive development throughout childhood: a review of human studies. Prostaglandins Leukot Essent Fatty Acids 2007;76:189–203. [DOI] [PubMed] [Google Scholar]
- 43.Barbosa VM, Miles EA, Calhau C, Lafuente E, Calder PC. Effects of a fish oil containing lipid emulsion on plasma phospholipid fatty acids, inflammatory markers, and clinical outcomes in septic patients: a randomized, controlled clinical trial. Crit Care 2010;14:R5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Birch EE, Hoffman DR, Uauy R, Birch DG, Prestidge C. Visual acuity and the essentiality of docosahexaenoic acid and arachidonic acid in the diet of term infants. Pediatr Res 1998;44:201–9. [DOI] [PubMed] [Google Scholar]
- 45.Angela Liou Y, Innis SM. Dietary linoleic acid has no effect on arachidonic acid, but increases n-6 eicosadienoic acid, and lowers dihomo-gamma-linolenic and eicosapentaenoic acid in plasma of adult men. Prostaglandins Leukot Essent Fatty Acids 2009;80:201–6. [DOI] [PubMed] [Google Scholar]
- 46.Adam O, Wolfram G, Zollner N. Influence of dietary linoleic acid intake with different fat intakes on arachidonic acid concentrations in plasma and platelet lipids and eicosanoid biosynthesis in female volunteers. Ann Nutr Metab 2003;47:31–6. [DOI] [PubMed] [Google Scholar]
- 47.Safinya CR. Structures of lipid-DNA complexes: supramolecular assembly and gene delivery. Curr Opin Struct Biol 2001;11:440–8. [DOI] [PubMed] [Google Scholar]
- 48.Carter BA, Shulman RJ. Mechanisms of disease: update on the molecular etiology and fundamentals of parenteral nutrition associated cholestasis. Nat Clin Pract Gastroenterol Hepatol 2007;4:277–87. [DOI] [PubMed] [Google Scholar]