Abstract
Adequate plasma, cellular, and tissue vitamin C concentrations are required for maintaining optimal health through suppression of oxidative stress and optimizing functions of certain enzymes that require vitamin C as a cofactor. Polymorphisms in the vitamin C transporter genes, compromising genes encoding sodium-dependent ascorbate transport proteins, and also genes encoding facilitative transporters of dehydroascorbic acid, are associated with plasma and tissue cellular ascorbate status and hence cellular redox balance. This review summarizes our current knowledge of the links between variations in vitamin C transporter genes and common chronic diseases. We conclude that emerging genetic knowledge has a good likelihood of defining future personalized dietary recommendations and interventions; however, further validations through biological studies as well as controlled dietary trials are required to identify predictive and actionable genetic biomarkers. We further advocate the need to consider genetic variation of vitamin C transporters in future clinical and epidemiologic studies on common complex diseases.
Keywords: antioxidants, genomics, inflammation, nutrigenomics, vitamin C
Introduction
Vitamin C is an essential nutrient and the most important plasma water-soluble antioxidant that plays critical roles in the biosynthesis of neurotransmitters and collagen, absorption of nonheme iron, detoxification of exogenous compounds and cytochrome P-450 activity, and regulation of hypoxia-inducible factor 1α (1, 2). In addition, it plays a major role as an antioxidant and free radical scavenger and protects against lipid peroxidation (3). Vitamin C has also been shown to function in sparing or reconstituting vitamin E for protection of lipid membranes (4, 5). Therefore, maintaining adequate plasma and tissue cellular vitamin C concentrations is crucial for normal metabolic function of the body and preventing many common complex diseases (6–14).
Epidemiologic studies show that individuals with reduced plasma vitamin C concentrations display an elevated risk of different chronic diseases (6). A review (15) of data from >90 epidemiologic studies that related the dietary intake of vitamin C to various types of cancer (breast, oral, gastric, esophageal, pancreatic, lung, cervical, and rectal) revealed a negative correlation in three-fourths of the studies. Besides, each 20-μM increase in plasma vitamin C concentration is associated with a 20% reduced risk of all-cause mortality (16) and a 9% relative decline in risk of heart failure (17).
A marginal vitamin C deficit (11 μM < plasma concentration < 24 μM) was estimated to affect up to 10% of adults in industrialized countries (13, 18, 19). Although vitamin C status is mainly determined by the dietary intake, it should be noted that a complex interplay of intrinsic metabolic factors, such as oxidative stress, inflammation, recycling, and transmembrane transport, contributes to the metabolic turnover and therefore vitamin C status (20). The metabolic turnover can be affected by genetic variations, and thus vitamin C status could be impaired even at dietary intake amounts that are currently regarded as adequate for the general population, if an individual carries a detrimental allele.
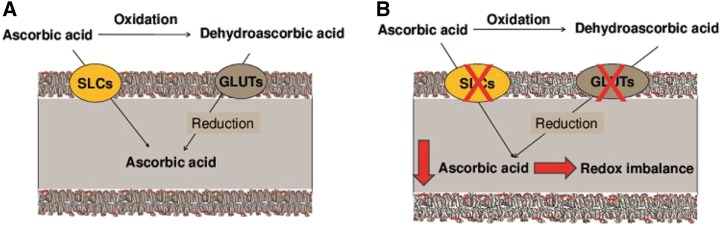
Transporters of the different forms of vitamin C directly regulate vitamin C intracellular bioavailability (Figure 1A). The elimination of selected ascorbic acid transporters in the mouse results in severely affected pharmacokinetics and reduced offspring viability (31) or even total offspring lethality (32). Therefore, variations in genes of the human vitamin C transporter pathways may affect disease development and outcomes (Figure 1B). This review summarizes existing knowledge on the variations in vitamin C transporter genes and disease associations. Two vitamin C transmembrane pathways are distinct by their substrates, where solute carriers (SLC) SLC23A1 and SLC23A2 mediate ascorbic acid transport, whereas dehydroascorbic acid is shuttled by the 4 members of the facilitative glucose transporter family GLUT1 (SLC2A1), GLUT2 (SLC2A2), GLUT3 (SLC2A3), and GLUT4 (SLC2A4), which will all be reviewed.
FIGURE 1.
(A) General schematic for cellular uptake of vitamin C. The concentration of ascorbic acid in the intracellular environment is tightly controlled through regulation of the transporters. Ascorbic acid (ascorbate) is the functional form of vitamin C, which is transported into the cell through SLCs. SLCs comprise SLC23A1 and SLC23A2, which have specific cell expression and precise subcellular localization. The SLC23A1 protein is responsible for active transport of ascorbate from the epical luminal surface of the intestinal tract and kidney (21, 22). The SLC23A2 protein, expressed in most human tissues, except lung and skeletal muscle (22, 23), is thought to regulate intracellular concentrations of ascorbate for subsequent protection of the cell from oxidative stress as well as promote the maturation of type I collagen. DHA, the oxidized form of ascorbate, is transported into the cell by some members of the facilitative GLUT family, including GLUT1 (SLC2A1), GLUT2 (SLC2A2), and GLUT3 (SLC2A3) (24–27), which have specific cell expression and transport activity. Within cells, DHA is immediately recycled back to ascorbate, and this process sustains the intracellular ascorbate and therefore redox balance (28–30). (B) A proposed mechanism for an association between variations in vitamin C transporters and common complex diseases, as well as genetic variation in each ascorbic acid transporter (SLCs) or dehydroascorbic acid transporter (GLUTs), could modulate transport of ascorbate or DHA, thereby resulting in reduced intracellular vitamin C, redox imbalance, and thus increased risk of common complex diseases. DHA, dehydroascorbic acid; GLUT, glucose transporter; SLC, solute carrier family.
Current Status of Knowledge
Sodium-dependent vitamin C transporters
The active transport of ascorbate across the cell membrane is generated by 2 sodium-dependent ascorbate transporters that were first cloned in 1999 (33). The 2 transporters, SLC23A1 and SLC23A2, mediate sodium and energy-dependent ascorbate transport against a concentration gradients into cells, resulting in intracellular concentrations that can be 50-fold higher than the extracellular fluids (33, 34). SLC23A1 and SLC23A2 cotransport Na+ and ascorbate with a 2:1 stoichiometry, using the electrochemical Na+ gradient (35, 36).
The SLC23A1 and SLC23A2 are responsible for the maintenance of vitamin C concentrations in nearly all cells (except erythrocytes), tissues, and extracellular fluids (20). The genetic patterns of both SLC23A1 and SLC23A2 share common intron/exon borders and have related coding sequence, but the genes differ 10-fold in size (16 kb compared with 160 kb, respectively) and in linkage disequilibrium (37). The encoded proteins of the 2 transporters are comparable in amino acid sequence and structure, but they have different tissue distributions (33, 37).
SLC23A1 expression is confined to epithelia, such as intestinal, renal, and hepatic tissues (33, 38), and it has the major role in whole-body ascorbate homeostasis, through its function as a sole apical ascorbic acid transporter in the proximal renal epithelial cell (31). SLC23A1 has low affinity [Michaelis constant (Km)4 of 65–252 μM] (21) and high capacity [the maximum rate achieved by the system, at saturating substrate concentrations (Vmax) of ~15 pmol · min−1 · cell−1] (39), establishing the ability of this transporter to maintain the whole-body homeostasis (21, 22).
The SLC23A1 locus on human chromosome 5q31.2 contains 16 exons (37, 40), spanning about 17.3 kb. A total of 1440 variations are listed in the Single Nucleotide Polymorphism Database of which 294 locate to the coding region (187 missense, 91 synonymous, 11 frameshift, 4 insertions). Many of the variations in SLC23A1 have not been verified in different populations, such as the HapMap cohorts (41), and most variations are neither reported in the literature nor functionally characterized. Genetic linkage throughout the locus is high, with some evidence of linkage blocks in the 5′ and 3′ of the gene (37). Variations in SLC23A1 seem to affect the vitamin C status, but current evidence remains inconclusive (42).
SLC23A2 is distributed in cells of most tissues (33) and contributes to delivering vitamin C into cells for some metal ion-dependent enzymatic reactions as well as protecting cells from oxidative stress (33, 43, 44). SLC23A2 has low capacity (~1 pmol · min−1 · cell−1) (21, 39) and high affinity (Km values of 8–69 μM) (21, 35, 45) for ascorbate transport, mediating uptake of ascorbate by cells of peripheral organs from the extracellular fluid (22, 23). A difference in membrane epithelial cells distribution of SLC23A1 and SLC23A2 suggests nonredundant functions for these 2 transporters (46, 47).
The SLC23A2 locus on human chromosome 20p13 contains 17 exons (37), spanning about 160 kb and is roughly 10 times bigger than SLC23A1. A total of 8165 variations are listed in dbSNP, of which 262 locate to the coding region (138 missense, 120 synonymous, 4 frameshift). Many of the variations in SLC23A2 have not been verified in different populations, such as the HapMap cohorts (41), and most of these variations are neither reported in the literature nor functionally characterized. Genetic linkage throughout the locus is moderate (37), but linkage blocks are not defined (37). Variations in SLC23A2 are yet to be reported to affect the vitamin C status.
When the patterns of single-nucleotide polymorphisms (SNPs) in SLC23A1 and SLC23A2 were compared, a substantial number of the SNPs in SLC23A1 were population specific in either Caucasians or African Americans, including 4 nonsynonymous SNPs; however, nearly all SNPs in SLC23A2 are shared between the 2 populations, African Americans and Caucasians (48). It was deduced that the SLC23A1 gene does tolerate variations better than SLC23A2, indicating a higher physiologic importance for the latter.
Polymorphisms in sodium-dependent vitamin C transporters and pathologic relevance
The risk association of several SNPs in SLC23A1 and SLC23A2 genes with a variety of common chronic diseases, including various cancers (49–55), inflammatory bowel disease (56), preterm delivery (57), coronary heart disease (58), and optic neuropathy (59, 60), has been evaluated (Table 1).
TABLE 1.
Phenotype-genotype associations of SNPs in the human sodium-dependent ascorbate transporter genes with chronic diseases1
| Gene | SNP | Allele, major/minor | Location | Disease | Population | Sample size, case/control, n | Findings | Study (reference) |
| SLC23A1 | rs11950646 | A/G | chr5: 139378785 | Follicular lymphoma | United States | 1292/1375 | ↑ Risk GG genotype | Skibola et al., 2008 (49) |
| Small lymphocytic lymphoma/chronic lymphocytic leukemia | Germany | 494/494 | ↑ Risk GG genotype | Skibola et al., 2008 (49) | ||||
| rs6596473 | G/C | chr5: 139374887 | Follicular lymphoma | United States | 1292/1375 | ↑ Risk CC genotype | Skibola et al., 2008 (49) | |
| Chronic lymphocytic leukemia, diffuse large B-cell lymphoma | Germany | 494/494 | ↑ Chronic lymphocytic leukemia risk; ↓ diffuse large B-cell lymphoma risk CC genotype | Skibola et al., 2008 (49) | ||||
| Lower concentration of ocular ascorbate | India | 60/— | ↑ Risk C-carrier | Senthilkumari et al., 2014 (60) | ||||
| rs10063949 | A/G | chr5: 139383837 | Inflammatory bowel disease | Canada | 311/142 | ↑ Crohn disease risk G-carrier | Amir Shaghaghi et al., 2014 (56) | |
| SLC23A2 | rs6133175 | A/G | chr20: 4911113 | Non-Hodgkin lymphoma, diffuse large B-cell lymphoma, small lymphocytic lymphoma | United States | 1292/1375 | ↑ Risk GG genotype | Skibola et al., 2008 (49) |
| rs1715364 | T/C | chr20: 4918250 | Small lymphocytic lymphoma, diffuse large B-cell lymphoma | United States | 1292/1375 | ↑ Risk CC genotype | Skibola et al., 2008 (49) | |
| Small lymphocytic lymphoma, diffuse large B-cell lymphoma | Germany | 494/494 | ↑ Risk CC genotype | Skibola et al., 2008 (49) | ||||
| rs1715385 | G/A | chr20: 4907024 | Non-Hodgkin lymphoma, diffuse large B-cell lymphoma | United States | 1292/1375 | ↑ Risk AA genotype | Skibola et al., 2008 (49) | |
| rs1776948 | G/A | chr20: 4950467 | Non-Hodgkin lymphoma, follicular lymphoma, small lymphocytic lymphoma | United States | 1292/1375 | ↑ Risk AA genotype | Skibola et al., 2008 (49) | |
| Non-Hodgkin lymphoma, follicular lymphoma | Germany | 494/494 | ↑ Risk AA genotype | Skibola et al., 2008 (49) | ||||
| rs6139587 | T/A | chr20: 4961828 | Non-Hodgkin lymphoma, small lymphocytic lymphoma | United States | 1292/1375 | ↑ Risk AA genotype | Skibola et al., 2008 (49) | |
| rs4987219 | G/C | chr20: 4884300 | Colorectal adenoma | United States | 656/665 | ↓ Risk C-carrier | Erichsen et al., 2008 (50) | |
| Human papillomavirus, head and neck squamous cell carcinomas association | United States | 319/495 | ↑ Risk G-carrier | Chen et al., 2009 (51) | ||||
| Esophageal squamous cell carcinoma | Japan | 49/— | ↑ Risk leukopenia C-carrier | Minegaki et al., 2014 (52) | ||||
| rs1110277 | T/C | chr20: 4874036 | Colorectal adenoma | United States | 656/665 | ↓ Risk C-carrier | Erichsen et al., 2008 (50) | |
| C/T | Esophageal squamous cell carcinoma | Japan | 49/— | ↑ Risk stomatitis T-carrier | Minegaki et al., 2014 (52) | |||
| rs12479919 | C/T | chr20: 5000094 | Gastric cancer | Poland | 279/414 | ↓ Risk TT genotype | Wright et al., 2009 (53) | |
| Bladder cancer | United States | 832/1191 | ↑ Risk CT genotype | Andrew et al., 2009 (55) | ||||
| Lower concentration of ocular ascorbate | India | 60/— | ↑ Risk TT genotype | Senthilkumari et al., 2014 (60) | ||||
| rs6116569 | C/T | chr20: 4884071 | Gastric cancer | Europe | 365/1284 | ↑ Risk T-carrier | Duell et al., 2013 (54) | |
| rs2681116 | G/A | chr20: 4970685 | Preterm delivery | United States | 271/572 | ↑ Risk GA genotypes | Erichsen et al., 2006 (57) | |
| rs6139591 | C/T | chr20: 4970713 | Preterm delivery | United States | 271/572 | ↑ Risk T-carrier | Erichsen et al., 2006 (57) | |
| Acute coronary syndrome | Denmark | 936/1580 | ↑ Risk TT genotype | Dalgard et al., 2013 (58) | ||||
| rs1776964 | C/T | chr20: 4880308 | Preterm delivery | United States | 271/572 | ↓ Risk TT genotype | Erichsen et al., 2006 (57) | |
| Acute coronary syndrome | Denmark | 936/1580 | ↑ Risk TT genotype | Dalgard et al., 2013 (58) | ||||
| rs1279383 | A/G | chr20: 5002446 | Primary open glaucoma | Mediterranean | 150/150 | ↑ Risk GG genotype | Zanon-Moreno et al., 2011 (59) |
chr, chromosome; SLC, solute carrier family; SNP, single-nucleotide polymorphism.
Cancer.
Vitamin C plasma and tissue concentrations have been postulated to affect relative cancer risk. The antioxidant effect of vitamin C may prevent cancers by inducing apoptosis and suppressing tumor cell growth (61–63) while counterbalancing DNA damage through scavenging of reactive oxygen species (64). Vitamin C also protects mucosal tissues from oxidative damage (65, 66) and plays an antitumorigenic role via sustaining proper collagen formation and matrix stabilization (67). As such, the risk association of vitamin C transporter genes with various intestinal cancers has been the key interest for several studies. In a study (50) with 656 patients with colorectal adenoma and 665 healthy controls, participants were genotyped for 4 SNPs in the SLC23A1 gene and 11 different SNPs in the SLC23A2 gene. No association between common SNPs in SLC23A1 and colorectal cancer was revealed. For SLC23A2, there was no association with SNPs, but the haplotype G-C (rs4987219 and rs1110277) was associated with a reduction in the risk of colorectal adenoma (50). In a study on gastric cancer (53), an association between 13 genetic variants of the SLC23A1 and SLC23A2 genes with the disease was examined. Among the 13 SNPs examined, gastric cancer was inversely associated with one SNP (rs12479919) in the SLC23A2 gene, whereas no association with variants in the SLC23A1 gene was determined. Compared with rs12479919-G/G genotypes, homozygotes for the minor allele A/A had a lower risk of gastric cancer (53). In the aforementioned study, a haplotype in the SLC23A2 gene, containing the common allele of the rs6139591, rs2681116, and rs14147458 SNPs, was inversely associated with gastric cancer (53). Likewise, in another study with 365 patients with gastric cancer and 1284 controls (54), the genotype rs6116569-C/T and the 2 haplotypes, CGTC (rs6052937, rs3787456, rs6116569, rs17339746) and ATC (rs6139587, rs6053005, rs2326576), in the SLC23A2 gene were associated with gastric cancer risk, whereas no association was found with variants in SLC23A1.
Variants in vitamin C transporter genes have also been associated with other type of cancers. In a population-based study with 832 patients with bladder cancer and 1191 healthy controls (55), variant rs12479919-C/T in SLC23A2 has been identified as a high-risk genotype for a gene-gene effect on bladder cancer. Indeed, the interaction of SLC23A2 (rs12479919) and SCARB1- rs4765621 (the gene scavenger receptor class B) showed the strongest effect on the higher risk of bladder cancer (55). In another study with 1292 patients and 1375 healthy controls (49), several SNPs in SLC23A1 and SLC23A2 have been associated with an increased risk of non-Hodgkin lymphoma. In this study, individuals with the SLC23A1 genotypes rs6596473-C/C and rs11950646-G/G showed an 80% elevated risk of lymphoma. Moreover, several SNPs in SLC23A2 (Table 1) as well as 2 haplotypes (AA: rs1776948, rs6139587 and AAC: rs1715385, rs6133175, rs1715364) in the gene were associated with increased risk of the disease (49). Authors conclude that both vitamin C uptake and storage are involved in the pathogenesis of lymphoma (49).
Variation in the SLC23A2 gene also affected the initiation or sustention of head and neck cancer in patients with human papillomavirus type 16 infection (51). In a study with 319 patients with head and neck cancer and 495 frequency-matched controls (51), the risk of the cancer associated with human papillomavirus type 16 was decreased among rs4987219-C/C homozygotes in the SLC23A2 gene compared with those with a wild-type allele. The authors suggest that the SNP modifies the risk of head and neck cancer associated with human papillomavirus type 16 infection through the role of ascorbate in the maintenance of the epidermal barrier, maturation of type I procollagen, intracellular antioxidant, or its immunostimulatory effect (51).
Vitamin C transporter genes not only have been associated with an increased risk of different types of cancer but also have been suggested as predictive biomarkers for therapies. In a study with 49 patients with esophageal squamous cell carcinoma (52), rs4987219 and rs1110277 in the SLC23A2 gene showed correlation with severe toxicities (acute stomatitis and leucopenia) (Table 1) after treatment with a definitive 5-fluorouracil/cisplatin-based chemoradiotherapy (52).
Inflammatory bowel disease.
In addition to associations with various intestinal cancers, a variation in ascorbate transporters is associated with inflammatory bowel disease, where oxidative damage plays a key role in the initiation and progression of the disease (68). In a study with 311 people with inflammatory bowel disease and 142 controls (56), the SNP rs10063949-G allele in the SLC23A1 gene was associated with an increased risk of Crohn disease. Specifically, rs10063949-A/G heterozygotes had a 2.5-fold elevated risk of Crohn disease, whereas rs10063949-G/G homozygotes had a 4.7-fold elevated risk compared with wild-type homozygotes (56).
Pregnancy complications.
Vitamin C deficiency (measured by dietary intake or ascorbic acid concentrations in serum, leukocytes, or cord blood) has been found in several epidemiologic investigations (14, 71–75) to be associated with premature rupture of membranes and preterm delivery (<37 wk of gestation), a leading cause of neonatal mortality and morbidity (57). In view of the necessity of vitamin C for preservation of collagen and potency of membrane tensile (57, 69), genetic variants in SLC23A1 and SLC23A2 have also been associated with the risk of preterm delivery. Associations have been found between haplotypes in the SLC23A1 gene and spontaneous preterm delivery (57). Moreover, a carrier of 1 or 2 minor alleles of variant rs6139591-T of the SLC23A2 gene showed a 1.7-fold and a 2.7-fold higher risk of spontaneous preterm birth, respectively (57). Likewise, heterozygous individuals for rs2681116-G/A in SLC23A2 showed a 1.9-fold increased risk of preterm birth, but analysis of the homozygous-carrying minor alleles (rs2681116-A/A) showed no effects. The authors speculate that the failure to detect a significant association between rs2681116-A/A homozygous individuals and the risk of preterm delivery was related to small numbers of the study population (57).
Coronary heart disease.
Variations in SLC23A2 have also been associated with acute coronary syndrome (58), where vitamin C is suggested to have cardioprotective influences due to its antioxidative effects and its beneficial effects on endothelial function and the collagen content of the atherosclerotic plaques (58, 74). A 5.4-fold elevated risk of acute coronary syndrome was observed (58) in women with the rs6139591-T/T genotype who had a low intake of dietary vitamin C. Moreover, women with the rs1776964-T/T genotype with a high intake of vitamin C had a 3.4-fold increased risk of acute coronary syndrome compared with C/C-homozygotes with low intake. Accordingly, the authors conclude that the effects of genotype may not be completely compensated by high dietary intake of vitamin C (58).
Optic neuropathy.
Lack of vitamin C antioxidant capacity is also associated with glaucomatous optic neuropathy, where oxidative stress is related to neuronal death (75, 76). Indeed, statistically significant lower concentrations of vitamin C have been observed in plasma (59), normal tension (77), and the secondary aqueous humor (78) of glaucomatous patients. In a study among 150 patients with open-angle glaucoma and 150 controls (59), genotype rs1279386-G/G in SLC23A2 was associated with a higher risk of the disease (1.7-fold) as well as lower plasma vitamin C concentration (mean ± SD values of 9.0 ± 1.4 μg/mL compared with 10.5 ± 1.6 μg/mL in patients and 10.9 ± 1.6 μg/mL compared with 12.1 ± 1.8 μg/mL in controls). In this study, no association was found between polymorphisms in the SLC23A1 gene with open-angle glaucoma (59). In another study (60), polymorphisms in the SLC23A1 and SLC23A2 genes were found to influence ascorbate concentration in the aqueous humor and lens nucleus of 60 patients undergoing small-incision cataract surgery. SNPs rs6596473 in the SLC23A1 gene and rs12479919 in the SLC23A2 gene showed an association with decreased ocular ascorbate concentration in carriers of the variant allele compared with the common homozygotes. For rs6596473, the per variant allele-C difference in aqueous humor ascorbate was −217 μmol/L, whereas for rs12479919, the per variant allele-T difference in lens nucleus ascorbate was 0.085 μmol/G (60) compared with homozygotes’ common allele (G/G and C/C, respectively).
All the studies mentioned above confirmed numerous minor frequency genotypes and haplotypes of the SLC23A1 and SLC23A2 genes, associated with various chronic diseases. Most findings were reported on an individual basis in cohorts of limited sizes. Therefore, it is warranted to validate these findings in larger cohorts to use it as actionable biomarker of the respective common complex diseases.
Facilitated diffusion vitamin C transporters
Dehydroascorbic acid (DHA) is one dietary source of vitamin C, beside ascorbate, that can be absorbed across the brush-border membrane. Upon entry into the enterocyte, DHA is reduced either enzymatically or chemically back to ascorbate and thus maintains a concentration gradient, favoring DHA uptake (23, 27). Local DHA absorption may be especially important during intestinal inflammatory conditions, where the immune cells’ oxidative burst increases extracellular oxidation of ascorbate to DHA (23, 79). The produced DHA is transported into enterocytes or other bystander cells, followed by immediate reduction to ascorbate, and thus boosts intracellular concentrations of the free radical scavenger (28–30). With regard to whole-body homeostasis, this might also prevent patients with chronic intestinal inflammation from becoming scorbutic (23, 27). Likewise, in any inflammatory condition throughout the body, where ascorbate gets oxidized to DHA in extracellular fluid, the produced DHA is taken up by specific facilitative diffusion transporters for various cells/tissues to elevate intracellular ascorbate (80–86).
SLC2A1 (GLUT1), SLC2A2 (GLUT 2), SLC2A3 (GLUT3), SLC2A4 (GLUT4), and SLC2A8 (GLUT8) are the 5 facilitated DHA transporters identified (24–27). They are members of the SLC2A solute carriers’ gene family, encoding for the glucose transporter (GLUT) proteins of facilitated sugar transporters. It is postulated that vitamin C accumulation in cells occurs in part through transport of DHA by the carriers of the SLC2A family. It should be noted that DHA diffusion to some specific cell types is competitively inhibited by excessive glucose in plasma (24, 26). However, this inhibition might not be relevant in tubular cells of the kidney and on the luminal surface of absorptive intestinal epithelia (26, 45, 87, 88). Moreover, DHA diffusion into cells might be impeded during high-glucose status through the lack of location of SLC2A transporters to the plasmalemma membrane.
The DHA-GLUT transporters show tissue- and cell-specific expression as well as various affinities and efficiencies in DHA transport (24, 26, 89, 90). SLC2A1 is expressed in an extensive variety of cells throughout the body, with a particularly high expression in endothelial and epithelial-like barriers of the brain, peripheral nerve, eye, placenta, and lactating mammary gland (24, 91, 92), and exhibits a DHA transport activity defined by a Km of 1.1 mM and a Vmax of 108 pmol · min−1 · oocyte−1 (24). SLC2A2 is mainly expressed in the brain, spleen, kidney, pancreas, liver, and basolateral membranes of intestinal epithelial cells (90, 91, 93) and transports DHA with a Km of 2.33 mM and a Vmax of 25.9 pmol · min−1 · oocyte−1 (27). SLC2A3 is expressed particularly in the brain, neurons, and intestinal epithelial cells (24, 91) and has DHA transport activity defined by a Km of 1.7 mM and a Vmax of 241 pmol · min−1 · oocyte−1 (24). SLC2A4 is mainly found in adipose tissues as well as skeletal and cardiac muscle cells (26, 91) with a DHA transport activity showing a Km of 0.98 mM and a Vmax of 66 pmol · min−1 · oocyte−1 (26). GLUT8 is expressed in the testis, blastocyst, brain, muscle, and adipose tissues with a DHA transport activity defined by a Km of 3.23 mM and a Vmax of 10.1 pmol · min−1 · oocyte−1 (27).
Polymorphisms in facilitative diffusion vitamin C transporters and pathologic relevance
Genetic variation in the DHA-GLUT transporter genes is associated with various common complex diseases, which could be attributed to not only disturbed monosaccharide transport but also disturbed transport of alternative substrates, such as DHA. The link between diabetes-related traits and impaired glucose metabolism is not the main focus of this section of the review. Our focus is to review the association studies with respect to DHA-GLUT variation and common complex disease, other than directly to diabetes-related traits (e.g., fasting blood glucose). The number of these studies is relatively limited (Table 2).
TABLE 2.
Phenotype-genotype association of SNPs in the human dehydroascorbic acid transporter genes with chronic diseases1
| Gene | SNP | Allele, major/minor | Location | Disease | Population | Sample size, case/control, n | Findings | Study (reference) |
| SLC2A1 | rs841847 | C/T | chr1: 42937037 | Diabetic albuminuria and macroalbuminuria | n1 = African American, n2 = European American | n1 = 2156/—, n2 = 8122/ 9453 | ↑ Risk TT genotype (n2) | Hsu et al., 2010 (94) |
| rs841846 | A/G | chr1: 42938000 | Severe diabetic retinopathy | African American | 473 | ↑ Risk (not specified) | Roy et al., 2009 (95) | |
| rs3754218 | G/T | chr1: 42933897 | Renal cell carcinoma | England | 92/99 | ↑ Risk GT genotype | Page et al., 2005 (96) | |
| rs3820589 | A/T | chr1: 42960373 | Renal cell carcinoma | England | 92/99 | ↑ Risk T-carrier | Page et al., 2005 (96) | |
| rs4658 | C/G | chr1: 42926579 | Nonalcoholic fatty liver disease | Spain | 520/521 | ↑ Risk GG genotype | Vazquez-Chantada et al., 2013 (97) | |
| rs841856 | G/T | chr1: 42934442 | Nonalcoholic fatty liver disease | Spain | 520/521 | ↑ Risk TT genotype | Vazquez-Chantada et al., 2013 (97) | |
| rs2229682 | G/A | chr1: 42929964 | Spina bifida meningomyelocele | Hispanic and Caucasian, American | 507/184 | ↑ Risk A-carrier | Davidson et al., 2008 (98) | |
| SLC2A2 | rs5393 | C/A | chr3: 171027131 | Impaired glucose tolerance | Finland | 259/248 | ↑ Risk AA genotype | Laukkanen et al., 2005 (99) |
| rs5394 | C/T | chr3: 171027104 | Impaired glucose tolerance | Finland | 259/248 | ↑ Risk of type 2 diabetes T-carrier | Laukkanen et al., 2005 (99) | |
| rs5404 | G/A | chr3, 171007166 | Impaired glucose tolerance | Finland | 259/248 | ↑ Risk of type 2 diabetes A-carrier | Laukkanen et al., 2005 (99) | |
| rs5400 | A/G | chr3: 171014511 | Impaired glucose tolerance | Finland | 259/248 | ↑ Risk A-carrier | Laukkanen et al., 2005 (99) | |
| Type 2 diabetes | Finland | 1170/983 | ↑ risk GG genotype | Willer et al., 2007 (100) | ||||
| Prostate cancer | United States | 6642 | ↓ Risk G-carrier | Meyer et al., 2010 (101) | ||||
| rs11920090 | T/A | chr3 170999732 | Healthy individuals | Europe | 76,558/— | ↑ Risk higher fasting glucode concentration and type 2 diabetes A-carrier | Dupuis et al., 2010 (102) | |
| History of CVD | Denmark | 6049/— (interstudy) | ↑ Risk A-carrier | Borglykke et al., 2012 (103) | ||||
| History of CVD | Denmark | 9572/— (pooled analyses) | ↑ Risk A-carrier | Borglykke et al., 2012 (103) | ||||
| History of CVD | Denmark | 3523/— (Monica study) | ↑ Risk A-carrier | Borglykke et al., 2012 (103) | ||||
| rs5398 | T/C | chr3: 17099804 | Negative mood delusions | n1 = German, n2 = European American | n1 = 927/2168, n2 = 1247/1434 | ↑ Risk C-carrier | Meier et al., 2012 (104) | |
| rs1499821 | A/G | chr3: 172207423 | Negative mood delusions | n1 = German, n2 = European American | n1 = 927/2168, n2 = 1247/1434 | ↑ Risk G-carrier | Meier et al., 2012 (104) | |
| rs11924032 | A/G | chr3: 172217793 | Negative mood delusions | n1 = German,, n2 = European American | n1 = 927/2168, n2 = 1247/1434 | ↑ Risk G-carrier | Meier et al., 2012 (104) | |
| rs9875793 | A/G | chr3: 170686573 | Negative mood delusions, bipolar disorder | n1 = German n2 = European American | n1 = 927/2168, n2 = 1247/1434 | ↑ Risk G-carrier | Meier et al., 2012 (104) | |
| rs8192675 | G/A | chr3: 171007094 | Negative mood delusions | n1 = German, n2 = European American | n1 = 927/2168, n2 = 1247/1434 | ↑ Risk A-carrier | Meier et al., 2012 (104) | |
| Hypertension | n1 = African American, n2 = European American | n1 = 167, n2 = 237 | ↓ High-density lipoprotein A-carrier (n2) | Le et al., 2013 (105) | ||||
| SLC2A4 | rs5417 | C/A | chr17, 7281743 | Obstructive sleep apnea syndrome | China | 412/156 | ↑ Risk A-carrier | Yin et al., 2014 (106) |
| SLC2A5 | rs5438 | G/A | chr1: 9069561 | Hypertension | n1 = African American, n2 = European American | n1 = 167, n2 = 237 | ↑ Serum uric acid GA genotype (n2) | Le et al., 2013 (105) |
chr, chromosome; CVD, cardiovascular disease; SLC, solute carrier family; SNP, single-nucleotide polymorphism.
Diabetes complications.
A variety of studies have found associations between variations in DHA-GLUT genes and diabetes-related traits (99, 100, 102), as well as diabetes complications such as albuminuria (94), retinopathy (95, 107), and nephropathy (108), in which etiology might involve modulations to DHA transport. With regard to vitamin C metabolism, excess glucose during conditions of uncontrolled diabetes may competitively block uptake of DHA through facilitative GLUTs and thus impair the transport of DHA by cells and affect the intracellular redox imbalance (23). As such, considering diabetes as a well-established risk factor for cardiovascular disease (CVD) in a study with 2383 incidence cases of CVD (fatal and nonfatal) (103), the contribution of 46 type 2 diabetes-related SNPs to CVD incidence was examined. Of the 46 genetic variants examined, the variant rs11920090 in SLC2A2 was associated with incident CVD, independent of baseline diabetes status (103).
Cancer.
Variants in DHA-GLUT genes have been proposed to have diverse effects on the relative risk of renal and prostate cancers; however, the overall studies are limited (96, 101), with no observed association in one study (109). In a study with 92 patients with renal cell carcinoma and 99 healthy controls (96), carriers of the minor allele rs3820589-T as well as heterozygotes for rs3754218-G/T in SLC2A1 showed higher incidences of renal cancer. On the other hand, in a study with 6642 patients with prostate cancer (101) (participants in the Atherosclerosis Risk in Communities Study), SNP rs5400-G in SLC2A2 was associated with a 24% lower cancer risk in Caucasians but not in African Americans. The authors suggest that, despite uncertainty about the mechanism involved in the observed association, SLC2A2 may be involved in prostate cancer progression, with several reports linking several large-scale duplications on chromosome 3q, the region containing SLC2A2, with prostate cancer.
Psychological disorders.
Variants in the SLC2A2 gene were associated with bipolar disorder, which is a severe psychiatric condition with fundamental and distinctive alteration in emotion regulation and perception (104). In a study with 2174 patients with bipolar disorder and 3601 healthy controls (104), the minor alleles for several variants in SLC2A2 (rs5398-C, rs1499821-G, rs8192675-A, rs11924032-G, rs9875793-G) were associated with higher susceptibility to the disease or its complications. The functions of ascorbate in the central nervous system and the brain have been extensively reviewed (110). Neurons have high amounts of oxidative metabolism, 10-fold higher rates than supporting glia, which make them particularly vulnerable to ascorbate deficiency (111, 112). The neuronal sensitivity to a low supply of ascorbate is most apparent in neurodegenerative disease conditions in which there is excess oxidant stress and a high oxidation rate of ascorbate to DHA (110). Radiotracer experiments have confirmed that DHA enters the brain and is converted to ascorbate (113). Therefore, in neurodegenerative diseases such as bipolar disorder, DHA-GLUT transporters, including SLC2A2, which is highly expressed in the brain, may play a key role to uptake of DHA, thus increasing cerebral ascorbate concentrations to counter the oxidative stress resulting from the disease.
Liver disease.
Genetic variants in SLC2A1 are observed to actively contribute to nonalcoholic fatty liver disease (NAFLD), independent of diabetes or obesity (97). In a study of 520 patients with NAFLD and 521 healthy controls as well as 4414 individuals with type 2 diabetes and 4567 matched controls (97), genotypes rs4658-G/G and rs841856-T/T of SLC2A1 showed an association with an increased risk of NAFLD but not of diabetes. In this study, gene expression analysis demonstrated a considerable downregulation of SLC2A1 in the livers of patients with NAFLD. Moreover, in vitro silencing of SLC2A1 resulted in increased oxidative stress and a higher lipid accumulation (97). SLC2A1 is involved in the DHA transport into mitochondria, resulting in mitochondrial vitamin C recycling and elevating protection against reactive oxygen species (97, 114). The mitochondrion has a key role in progression of NAFLD through impairing fatty liver homeostasis as well as inducing overproduction of reactive oxygen species and thus lipid peroxidation (95, 115). Variation in SLC2A1 results in mitochondrial redox imbalance and hence could increase reactive oxygen species and regulate the proinflammatory environment at early stages of the disease (97).
Future Directions
Previously, observational studies have demonstrated that low vitamin C status increases the risk of many common chronic diseases. Today, genetic association studies on transporters of both vitamin C transport pathways support and expand on these observational findings. This review stresses the importance of considering and investigating genetic variations affecting overall status but also local tissue and cell concentrations of vitamin C to sustain health and prevent common complex diseases. As research progresses, it will be determined if human genetic variation on vitamin C transporters affects local or systemic pharmacokinetics. If pharmacokinetics is affected, recommendations will need to be adjusted for individuals or population subgroups of certain genotypes. This is apparent through the differential distributions of functional SNPs between African American and Caucasian individuals. Studies on variation in the genes coding different forms of vitamin C transporters are progressing, and the evidence could be incorporated into future dietary guidelines. However, the emerging evidence, as previously proposed by others (6, 42, 116), needs further replications, biological proof, and dietary intervention studies in targeted individuals carrying the specific variants to stand as valid diagnostic biomarkers. Moreover, the emerging fields of epigenetics and microbial analyses will contribute to the understanding of systematic interactions, and future studies will have to find a way to integrate genetics, epigenetics, and metagenomics data.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: CVD, cardiovascular disease; DHA, dehydroascorbic acid; GLUT, glucose transporter; Km, Michaelis constant; NAFLD, nonalcoholic fatty liver disease; SLC, solute carrier family; SNP, single-nucleotide polymorphism; Vmax, the maximum rate achieved by the system, at saturating substrate concentrations.
References
- 1.Levine M. New concepts in the biology and biochemistry of ascorbic acid. N Engl J Med 1986;314:892–902. [DOI] [PubMed] [Google Scholar]
- 2.Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci USA 1989;86:6377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frei B, Stocker R, Ames BN. Antioxidant defenses and lipid peroxidation in human blood plasma. Proc Natl Acad Sci USA 1988;85:9748–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niki E. Interaction of ascorbate and alpha-tocopherol. Ann N Y Acad Sci 1987;498:186–99. [DOI] [PubMed] [Google Scholar]
- 5.Packer JE, Slater TF, Willson RL. Direct observation of a free radical interaction between vitamin E and vitamin C. Nature 1979;278:737–8. [DOI] [PubMed] [Google Scholar]
- 6.Frei B, Birlouez-Aragon I, Lykkesfeldt J. Authors’ perspective: what is the optimum intake of vitamin C in humans? Crit Rev Food Sci Nutr 2012;52:815–29. [DOI] [PubMed] [Google Scholar]
- 7.Agarwal M, Mehta PK, Dwyer JH, Dwyer KM, Shircore AM, Nordstrom CK, Sun P, Paul-Labrador M, Yang Y, Merz CN. Differing relations to early atherosclerosis between vitamin C from supplements vs. food in the Los Angeles Atherosclerosis Study: a prospective cohort study. Open Cardiovasc Med J 2012;6:113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Langlois M, Duprez D, Delanghe J, De Buyzere M, Clement DL. Serum vitamin C concentration is low in peripheral arterial disease and is associated with inflammation and severity of atherosclerosis. Circulation 2001;103:1863–8. [DOI] [PubMed] [Google Scholar]
- 9.Myint PK, Luben RN, Welch AA, Bingham SA, Wareham NJ, Khaw KT. Plasma vitamin C concentrations predict risk of incident stroke over 10 y in 20 649 participants of the European Prospective Investigation into Cancer Norfolk prospective population study. Am J Clin Nutr 2008;87:64–9. [DOI] [PubMed] [Google Scholar]
- 10.Simon JA, Hudes ES. Serum ascorbic acid and other correlates of gallbladder disease among US adults. Am J Public Health 1998;88:1208–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simon JA, Hudes ES, Browner WS. Serum ascorbic acid and cardiovascular disease prevalence in U.S. adults. Epidemiology 1998;9:316–21. [PubMed] [Google Scholar]
- 12.Tveden-Nyborg P, Lykkesfeldt J. Does vitamin C deficiency increase lifestyle-associated vascular disease progression? Evidence based on experimental and clinical studies. Antioxid Redox Signal 2013;19:2084–104. [DOI] [PubMed] [Google Scholar]
- 13.Schleicher RL, Carroll MD, Ford ES, Lacher DA. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr 2009;90:1252–63. [DOI] [PubMed] [Google Scholar]
- 14.Siega-Riz AM, Promislow JH, Savitz DA, Thorp JM Jr, McDonald T. Vitamin C intake and the risk of preterm delivery. Am J Obstet Gynecol 2003;189:519–25. [DOI] [PubMed] [Google Scholar]
- 15.Block G. Vitamin C and cancer prevention: the epidemiologic evidence. Am J Clin Nutr 1991;53:270S–82S. [DOI] [PubMed] [Google Scholar]
- 16.Khaw KT, Bingham S, Welch A, Luben R, Wareham N, Oakes S, Day N. Relation between plasma ascorbic acid and mortality in men and women in EPIC-Norfolk prospective study: a prospective population study. European Prospective Investigation into Cancer and Nutrition. Lancet 2001;357:657–63. [DOI] [PubMed] [Google Scholar]
- 17.Pfister R, Sharp SJ, Luben R, Wareham NJ, Khaw KT. Plasma vitamin C predicts incident heart failure in men and women in European Prospective Investigation into Cancer and Nutrition–Norfolk prospective study. Am Heart J 2011;162:246–53. [DOI] [PubMed] [Google Scholar]
- 18.Jenab M, Riboli E, Ferrari P, Sabate J, Slimani N, Norat T, Friesen M, Tjonneland A, Olsen A, Overvad K, et al. Plasma and dietary vitamin C levels and risk of gastric cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST). Carcinogenesis 2006;27:2250–7. [DOI] [PubMed] [Google Scholar]
- 19.Simon JA, Hudes ES. Serum ascorbic acid and cardiovascular disease prevalence in U.S. adults: the Third National Health and Nutrition Examination Survey (NHANES III). Ann Epidemiol 1999;9:358–65. [DOI] [PubMed] [Google Scholar]
- 20.Lindblad M, Tveden-Nyborg P, Lykkesfeldt J. Regulation of vitamin C homeostasis during deficiency. Nutrients 2013;5:2860–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daruwala R, Song J, Koh WS, Rumsey SC, Levine M. Cloning and functional characterization of the human sodium-dependent vitamin C transporters hSVCT1 and hSVCT2. FEBS Lett 1999;460:480–4. [DOI] [PubMed] [Google Scholar]
- 22.Savini I, Rossi A, Pierro C, Avigliano L, Catani MV. SVCT1 and SVCT2: key proteins for vitamin C uptake. Amino Acids 2008;34:347–55. [DOI] [PubMed] [Google Scholar]
- 23.Wilson JX. Regulation of vitamin C transport. Annu Rev Nutr 2005;25:105–25. [DOI] [PubMed] [Google Scholar]
- 24.Rumsey SC, Kwon O, Xu GW, Burant CF, Simpson I, Levine M. Glucose transporter isoforms GLUT1 and GLUT3 transport dehydroascorbic acid. J Biol Chem 1997;272:18982–9. [DOI] [PubMed] [Google Scholar]
- 25.Montel-Hagen A, Kinet S, Manel N, Mongellaz C, Prohaska R, Battini JL, Delaunay J, Sitbon M, Taylor N. Erythrocyte Glut1 triggers dehydroascorbic acid uptake in mammals unable to synthesize vitamin C. Cell 2008;132:1039–48. [DOI] [PubMed] [Google Scholar]
- 26.Rumsey SC, Daruwala R, Al-Hasani H, Zarnowski MJ, Simpson IA, Levine M. Dehydroascorbic acid transport by GLUT4 in Xenopus oocytes and isolated rat adipocytes. J Biol Chem 2000;275:28246–53. [DOI] [PubMed] [Google Scholar]
- 27.Corpe CP, Eck P, Wang J, Al-Hasani H, Levine M. Intestinal dehydroascorbic acid (DHA) transport mediated by the facilitative sugar transporters, GLUT2 and GLUT8. J Biol Chem 2013;288:9092–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lykkesfeldt J. Increased oxidative damage in vitamin C deficiency is accompanied by induction of ascorbic acid recycling capacity in young but not mature guinea pigs. Free Radic Res 2002;36:567–74. [DOI] [PubMed] [Google Scholar]
- 29.Buettner GR. The pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbate. Arch Biochem Biophys 1993;300:535–43. [DOI] [PubMed] [Google Scholar]
- 30.Linster CL, Van Schaftingen E. Glucuronate, the precursor of vitamin C, is directly formed from UDP-glucuronate in liver. FEBS J 2006;273:1516–27. [DOI] [PubMed] [Google Scholar]
- 31.Corpe CP, Tu H, Eck P, Wang J, Faulhaber-Walter R, Schnermann J, Margolis S, Padayatty S, Sun H, Wang Y, et al. Vitamin C transporter Slc23a1 links renal reabsorption, vitamin C tissue accumulation, and perinatal survival in mice. J Clin Invest 2010;120:1069–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sotiriou S, Gispert S, Cheng J, Wang Y, Chen A, Hoogstraten-Miller S, Miller GF, Kwon O, Levine M, Guttentag SH, et al. Ascorbic-acid transporter Slc23a1 is essential for vitamin C transport into the brain and for perinatal survival. Nat Med 2002;8:514–7. [DOI] [PubMed] [Google Scholar]
- 33.Tsukaguchi H, Tokui T, Mackenzie B, Berger UV, Chen XZ, Wang Y, Brubaker RF, Hediger MA. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature 1999;399:70–5. [DOI] [PubMed] [Google Scholar]
- 34.Welch RW, Bergsten P, Butler JD, Levine M. Ascorbic acid accumulation and transport in human fibroblasts. Biochem J 1993;294:505–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Godoy A, Ormazabal V, Moraga-Cid G, Zuniga FA, Sotomayor P, Barra V, Vasquez O, Montecinos V, Mardones L, Guzman C, et al. Mechanistic insights and functional determinants of the transport cycle of the ascorbic acid transporter SVCT2: activation by sodium and absolute dependence on bivalent cations. J Biol Chem 2007;282:615–24. [DOI] [PubMed] [Google Scholar]
- 36.Mackenzie B, Illing AC, Hediger MA. Transport model of the human Na+-coupled L-ascorbic acid (vitamin C) transporter SVCT1. Am J Physiol Cell Physiol 2008;294:C451–9. [DOI] [PubMed] [Google Scholar]
- 37.Eck P, Erichsen HC, Taylor JG, Yeager M, Hughes AL, Levine M, Chanock S. Comparison of the genomic structure and variation in the two human sodium-dependent vitamin C transporters, SLC23A1 and SLC23A2. Hum Genet 2004;115:285–94. [DOI] [PubMed] [Google Scholar]
- 38.Wang H, Dutta B, Huang W, Devoe LD, Leibach FH, Ganapathy V, Prasad PD. Human Na(+)-dependent vitamin C transporter 1 (hSVCT1): primary structure, functional characteristics and evidence for a non-functional splice variant. Biochim Biophys Acta 1999;1461:1–9. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Mackenzie B, Tsukaguchi H, Weremowicz S, Morton CC, Hediger MA. Human vitamin C (L-ascorbic acid) transporter SVCT1. Biochem Biophys Res Commun 2000;267:488–94. [DOI] [PubMed] [Google Scholar]
- 40.Shaghaghi MA, Yurkova N, Tu H, Levine M, Eck P. Identification and functional characterization of an alternative 5′ exon of the sodium dependent ascorbic acid transporter SLC23A1. J Hum Nutr Food Sci 2013;1:1001. [Google Scholar]
- 41.International HapMap Consortium. The International HapMap Project. Nature 2003;426:789–96. [DOI] [PubMed] [Google Scholar]
- 42.Michels AJ, Hagen TM, Frei B. Human genetic variation influences vitamin C homeostasis by altering vitamin C transport and antioxidant enzyme function. Annu Rev Nutr 2013;33:45–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clark AG, Rohrbaugh AL, Otterness I, Kraus VB. The effects of ascorbic acid on cartilage metabolism in guinea pig articular cartilage explants. Matrix Biol 2002;21:175–84. [DOI] [PubMed] [Google Scholar]
- 44.Rajan DP, Huang W, Dutta B, Devoe LD, Leibach FH, Ganapathy V, Prasad PD. Human placental sodium-dependent vitamin C transporter (SVCT2): molecular cloning and transport function. Biochem Biophys Res Commun 1999;262:762–8. [DOI] [PubMed] [Google Scholar]
- 45.Liang WJ, Johnson D, Jarvis SM. Vitamin C transport systems of mammalian cells. Mol Membr Biol 2001;18:87–95. [DOI] [PubMed] [Google Scholar]
- 46.Boyer JC, Campbell CE, Sigurdson WJ, Kuo SM. Polarized localization of vitamin C transporters, SVCT1 and SVCT2, in epithelial cells. Biochem Biophys Res Commun 2005;334:150–6. [DOI] [PubMed] [Google Scholar]
- 47.Varma S, Sobey K, Campbell CE, Kuo SM. Hierarchal contribution of N- and C-terminal sequences to the differential localization of homologous sodium-dependent vitamin C transporters, SVCT1 and SVCT2, in epithelial cells. Biochemistry 2009;48:2969–80. [DOI] [PubMed] [Google Scholar]
- 48.Eck P, Erichsen HC, Taylor JG, Corpe C, Chanock SJ, Levine M. Genomic and functional analysis of the sodium-dependent vitamin C transporter SLC23A1-SVCT1. Genes Nutr 2007;2:143–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Skibola CF, Bracci PM, Halperin E, Nieters A, Hubbard A, Paynter RA, Skibola DR, Agana L, Becker N, Tressler P, et al. Polymorphisms in the estrogen receptor 1 and vitamin C and matrix metalloproteinase gene families are associated with susceptibility to lymphoma. PLoS One 2008;3:e2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Erichsen HC, Peters U, Eck P, Welch R, Schoen RE, Yeager M, Levine M, Hayes RB, Chanock S. Genetic variation in sodium-dependent vitamin C transporters SLC23A1 and SLC23A2 and risk of advanced colorectal adenoma. Nutr Cancer 2008;60:652–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen AA, Marsit CJ, Christensen BC, Houseman EA, McClean MD, Smith JF, Bryan JT, Posner MR, Nelson HH, Kelsey KT. Genetic variation in the vitamin C transporter, SLC23A2, modifies the risk of HPV16-associated head and neck cancer. Carcinogenesis 2009;30:977–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Minegaki T, Kuwahara A, Yamamori M, Nakamura T, Okuno T, Miki I, Omatsu H, Tamura T, Hirai M, Azuma T, et al. Genetic polymorphisms in SLC23A2 as predictive biomarkers of severe acute toxicities after treatment with a definitive 5-fluorouracil/cisplatin-based chemoradiotherapy in Japanese patients with esophageal squamous cell carcinoma. Int J Med Sci 2014;11:321–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wright ME, Andreotti G, Lissowska J, Yeager M, Zatonski W, Chanock SJ, Chow WH, Hou L. Genetic variation in sodium-dependent ascorbic acid transporters and risk of gastric cancer in Poland. Eur J Cancer 2009;45:1824–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duell EJ, Lujan-Barroso L, Llivina C, Munoz X, Jenab M, Boutron-Ruault MC, Clavel-Chapelon F, Racine A, Boeing H, Buijsse B, et al. Vitamin C transporter gene (SLC23A1 and SLC23A2) polymorphisms, plasma vitamin C levels, and gastric cancer risk in the EPIC cohort. Genes Nutr 2013;8:549–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Andrew AS, Gui J, Sanderson AC, Mason RA, Morlock EV, Schned AR, Kelsey KT, Marsit CJ, Moore JH, Karagas MR. Bladder cancer SNP panel predicts susceptibility and survival. Hum Genet 2009;125:527–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Amir Shaghaghi M, Bernstein CN, Serrano León A, El-Gabalawy H, Eck P. Polymorphisms in the sodium-dependent ascorbate transporter gene SLC23A1 are associated with susceptibility to Crohn disease. Am J Clin Nutr 2014;99:378–83. [DOI] [PubMed] [Google Scholar]
- 57.Erichsen HC, Engel SA, Eck PK, Welch R, Yeager M, Levine M, Siega-Riz AM, Olshan AF, Chanock SJ. Genetic variation in the sodium-dependent vitamin C transporters, SLC23A1, and SLC23A2 and risk for preterm delivery. Am J Epidemiol 2006;163:245–54. [DOI] [PubMed] [Google Scholar]
- 58.Dalgård C, Christiansen L, Vogel U, Dethlefsen C, Tjonneland A, Overvad K. Variation in the sodium-dependent vitamin C transporter 2 gene is associated with risk of acute coronary syndrome among women. PLoS One 2013;8:e70421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zanon-Moreno V, Ciancotti-Olivares L, Asencio J, Sanz P, Ortega-Azorin C, Pinazo-Duran MD, Corella D. Association between a SLC23A2 gene variation, plasma vitamin C levels, and risk of glaucoma in a Mediterranean population. Mol Vis 2011;17:2997–3004. [PMC free article] [PubMed] [Google Scholar]
- 60.Senthilkumari S, Talwar B, Dharmalingam K, Ravindran RD, Jayanthi R, Sundaresan P, Saravanan C, Young IS, Dangour AD, Fletcher AE. Polymorphisms in sodium-dependent vitamin C transporter genes and plasma, aqueous humor and lens nucleus ascorbate concentrations in an ascorbate depleted setting. Exp Eye Res 2014;124:24–30. [DOI] [PubMed] [Google Scholar]
- 61.Lee SK, Kang JS, Jung da J, Hur DY, Kim JE, Hahm E, Bae S, Kim HW, Kim D, Cho BJ, et al. Vitamin C suppresses proliferation of the human melanoma cell SK-MEL-2 through the inhibition of cyclooxygenase-2 (COX-2) expression and the modulation of insulin-like growth factor II (IGF-II) production. J Cell Physiol 2008;216:180–8. [DOI] [PubMed] [Google Scholar]
- 62.Cui Y, Lu Z, Bai L, Shi Z, Zhao WE, Zhao B. beta-Carotene induces apoptosis and up-regulates peroxisome proliferator-activated receptor gamma expression and reactive oxygen species production in MCF-7 cancer cells. Eur J Cancer 2007;43:2590–601. [DOI] [PubMed] [Google Scholar]
- 63.Yu W, Sanders BG, Kline K. RRR-alpha-tocopheryl succinate-induced apoptosis of human breast cancer cells involves Bax translocation to mitochondria. Cancer Res 2003;63:2483–91. [PubMed] [Google Scholar]
- 64.Seifried HE, Anderson DE, Sorkin BC, Costello RB. Free radicals: the pros and cons of antioxidants. Executive summary report. J Nutr 2004;134:3143S–63S. [DOI] [PubMed] [Google Scholar]
- 65.Drake IM, Davies MJ, Mapstone NP, Dixon MF, Schorah CJ, White KL, Chalmers DM, Axon AT. Ascorbic acid may protect against human gastric cancer by scavenging mucosal oxygen radicals. Carcinogenesis 1996;17:559–62. [DOI] [PubMed] [Google Scholar]
- 66.Sasazuki S, Hayashi T, Nakachi K, Sasaki S, Tsubono Y, Okubo S, Hayashi M, Tsugane S. Protective effect of vitamin C on oxidative stress: a randomized controlled trial. Int J Vitam Nutr Res 2008;78:121–8. [DOI] [PubMed] [Google Scholar]
- 67.Catani MV, Savini I, Rossi A, Melino G, Avigliano L. Biological role of vitamin C in keratinocytes. Nutr Rev 2005;63:81–90. [DOI] [PubMed] [Google Scholar]
- 68.Kruidenier L, Verspaget HW. Review article: oxidative stress as a pathogenic factor in inflammatory bowel disease–radicals or ridiculous? Aliment Pharmacol Ther 2002;16:1997–2015. [DOI] [PubMed] [Google Scholar]
- 69.Wideman GL, Baird GH, Bolding OT. Ascorbic acid deficiency and premature rupture of fetal membranes. Am J Obstet Gynecol 1964;88:592–5. [DOI] [PubMed] [Google Scholar]
- 70.Guajardo L, Beharry KD, Modanlou HD, Aranda JV. Ascorbic acid concentrations in umbilical cord veins and arteries of preterm and term newborns. Biol Neonate 1995;68:1–9. [DOI] [PubMed] [Google Scholar]
- 71.Steyn PS, Odendaal HJ, Schoeman J, Stander C, Fanie N, Grove D. A randomised, double-blind placebo-controlled trial of ascorbic acid supplementation for the prevention of preterm labour. J Obstet Gynaecol 2003;23:150–5. [DOI] [PubMed] [Google Scholar]
- 72.Barrett BM, Sowell A, Gunter E, Wang M. Potential role of ascorbic acid and beta-carotene in the prevention of preterm rupture of fetal membranes. Int J Vitam Nutr Res 1994;64:192–7. [PubMed] [Google Scholar]
- 73.Casanueva E, Magana L, Pfeffer F, Baez A. Incidence of premature rupture of membranes in pregnant women with low leukocyte levels of vitamin C. Eur J Clin Nutr 1991;45:401–5. [PubMed] [Google Scholar]
- 74.Nakata Y, Maeda N. Vulnerable atherosclerotic plaque morphology in apolipoprotein E-deficient mice unable to make ascorbic acid. Circulation 2002;105:1485–90. [DOI] [PubMed] [Google Scholar]
- 75.Izzotti A, Bagnis A, Sacca SC. The role of oxidative stress in glaucoma. Mutat Res 2006;612:105–14. [DOI] [PubMed] [Google Scholar]
- 76.Kang JH, Pasquale LR, Willett W, Rosner B, Egan KM, Faberowski N, Hankinson SE. Antioxidant intake and primary open-angle glaucoma: a prospective study. Am J Epidemiol 2003;158:337–46. [DOI] [PubMed] [Google Scholar]
- 77.Yuki K, Murat D, Kimura I, Ohtake Y, Tsubota K. Reduced-serum vitamin C and increased uric acid levels in normal-tension glaucoma. Graefes Arch Clin Exp Ophthalmol 2010;248:243–8. [DOI] [PubMed] [Google Scholar]
- 78.Leite MT, Prata TS, Kera CZ, Miranda DV, de Moraes Barros SB, Melo LA Jr. Ascorbic acid concentration is reduced in the secondary aqueous humour of glaucomatous patients. Clin Experiment Ophthalmol 2009;37:402–6. [DOI] [PubMed] [Google Scholar]
- 79.Schorah CJ. The transport of vitamin C and effects of disease. Proc Nutr Soc 1992;51:189–98. [DOI] [PubMed] [Google Scholar]
- 80.Daskalopoulos R, Korcok J, Tao L, Wilson JX. Accumulation of intracellular ascorbate from dehydroascorbic acid by astrocytes is decreased after oxidative stress and restored by propofol. Glia 2002;39:124–32. [DOI] [PubMed] [Google Scholar]
- 81.Himmelreich U, Drew KN, Serianni AS, Kuchel PW. 13C NMR studies of vitamin C transport and its redox cycling in human erythrocytes. Biochemistry 1998;37:7578–88. [DOI] [PubMed] [Google Scholar]
- 82.Holmes ME, Mwanjewe J, Samson SE, Haist JV, Wilson JX, Dixon SJ, Karmazyn M, Grover AK. Dehydroascorbic acid uptake by coronary artery smooth muscle: effect of intracellular acidification. Biochem J 2002;362:507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kodaman PH, Behrman HR. Hormone-regulated and glucose-sensitive transport of dehydroascorbic acid in immature rat granulosa cells. Endocrinology 1999;140:3659–65. [DOI] [PubMed] [Google Scholar]
- 84.Mendiratta S, Qu ZC, May JM. Erythrocyte ascorbate recycling: antioxidant effects in blood. Free Radic Biol Med 1998;24:789–97. [DOI] [PubMed] [Google Scholar]
- 85.Qutob S, Dixon SJ, Wilson JX. Insulin stimulates vitamin C recycling and ascorbate accumulation in osteoblastic cells. Endocrinology 1998;139:51–6. [DOI] [PubMed] [Google Scholar]
- 86.Upston JM, Karjalainen A, Bygrave FL, Stocker R. Efflux of hepatic ascorbate: a potential contributor to the maintenance of plasma vitamin C. Biochem J 1999;342:49–56. [PMC free article] [PubMed] [Google Scholar]
- 87.Malo C, Wilson JX. Glucose modulates vitamin C transport in adult human small intestinal brush border membrane vesicles. J Nutr 2000;130:63–9. [DOI] [PubMed] [Google Scholar]
- 88.Vera JC, Rivas CI, Velasquez FV, Zhang RH, Concha II, Golde DW. Resolution of the facilitated transport of dehydroascorbic acid from its intracellular accumulation as ascorbic acid. J Biol Chem 1995;270:23706–12. [DOI] [PubMed] [Google Scholar]
- 89.Vera JC, Rivas CI, Fischbarg J, Golde DW. Mammalian facilitative hexose transporters mediate the transport of dehydroascorbic acid. Nature 1993;364:79–82. [DOI] [PubMed] [Google Scholar]
- 90.Mardones L, Ormazabal V, Romo X, Jana C, Binder P, Pena E, Vergara M, Zuniga FA. The glucose transporter-2 (GLUT2) is a low affinity dehydroascorbic acid transporter. Biochem Biophys Res Commun 2011;410:7–12. [DOI] [PubMed] [Google Scholar]
- 91.Zhao FQ, Keating AF. Functional properties and genomics of glucose transporters. Curr Genomics 2007;8:113–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhao FQ, Glimm DR, Kennelly JJ. Distribution of mammalian facilitative glucose transporter messenger RNA in bovine tissues. Int J Biochem 1993;25:1897–903. [DOI] [PubMed] [Google Scholar]
- 93.Kellett GL, Brot-Laroche E. Apical GLUT2: a major pathway of intestinal sugar absorption. Diabetes 2005;54:3056–62. [DOI] [PubMed] [Google Scholar]
- 94.Hsu CC, Kao WL, Steffes MW, Gambir T, Brancati FL, Heilig CW, Shuldiner AR, Boerwinkle EA, Coresh J. Genetic variation of glucose transporter-1 (GLUT1) and albuminuria in 10,278 European Americans and African Americans: a case-control study in the Atherosclerosis Risk in Communities (ARIC) study. BMC Med Genet 2011;12:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Roy MS, Hallman DM, Fu YP, Machado M, Hanis CL. Assessment of 193 candidate genes for retinopathy in African Americans with type 1 diabetes. Arch Ophthalmol 2009;127:605–12. [DOI] [PubMed] [Google Scholar]
- 96.Page T, Hodgkinson AD, Ollerenshaw M, Hammonds JC, Demaine AG. Glucose transporter polymorphisms are associated with clear-cell renal carcinoma. Cancer Genet Cytogenet 2005;163:151–5. [DOI] [PubMed] [Google Scholar]
- 97.Vazquez-Chantada M, Gonzalez-Lahera A, Martinez-Arranz I, Garcia-Monzon C, Regueiro MM, Garcia-Rodriguez JL, Schlangen KA, Mendibil I, Rodriguez-Ezpeleta N, Lozano JJ, et al. Solute carrier family 2 member 1 is involved in the development of nonalcoholic fatty liver disease. Hepatology 2013;57:505–14. [DOI] [PubMed] [Google Scholar]
- 98.Davidson CM, Northrup H, King TM, Fletcher JM, Townsend I, Tyerman GH, Au KS. Genes in glucose metabolism and association with spina bifida. Reprod Sci 2008;15:51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Laukkanen O, Lindstrom J, Eriksson J, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Tuomilehto J, Uusitupa M, Laakso M, et al. Polymorphisms in the SLC2A2 (GLUT2) gene are associated with the conversion from impaired glucose tolerance to type 2 diabetes: the Finnish Diabetes Prevention Study. Diabetes 2005;54:2256–60. [DOI] [PubMed] [Google Scholar]
- 100.Willer CJ, Bonnycastle LL, Conneely KN, Duren WL, Jackson AU, Scott LJ, Narisu N, Chines PS, Skol A, Stringham HM, et al. Screening of 134 single nucleotide polymorphisms (SNPs) previously associated with type 2 diabetes replicates association with 12 SNPs in nine genes. Diabetes 2007;56:256–64. [DOI] [PubMed] [Google Scholar]
- 101.Meyer TE, Boerwinkle E, Morrison AC, Volcik KA, Sanderson M, Coker AL, Pankow JS, Folsom AR. Diabetes genes and prostate cancer in the Atherosclerosis Risk in Communities study. Cancer Epidemiol Biomarkers Prev 2010;19:558–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, Wheeler E, Glazer NL, Bouatia-Naji N, Gloyn AL, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet 2010;42:105–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Borglykke A, Grarup N, Sparso T, Linneberg A, Fenger M, Jeppesen J, Hansen T, Pedersen O, Jorgensen T. Genetic variant SLC2A2 [corrected] is associated with risk of cardiovascular disease—assessing the individual and cumulative effect of 46 type 2 diabetes related genetic variants. PLoS One 2012;7:e50418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Meier S, Mattheisen M, Vassos E, Strohmaier J, Treutlein J, Josef F, Breuer R, Degenhardt F, Muhleisen TW, Muller-Myhsok B, et al. Genome-wide significant association between a ‘negative mood delusions’ dimension in bipolar disorder and genetic variation on chromosome 3q26.1. Transl Psychiatry 2012;2:e165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Le MT, Lobmeyer MT, Campbell M, Cheng J, Wang Z, Turner ST, Chapman AB, Boerwinkle E, Gums JG, Gong Y, et al. Impact of genetic polymorphisms of SLC2A2, SLC2A5, and KHK on metabolic phenotypes in hypertensive individuals. PLoS One 2013;8:e52062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yin T, Li NF, Heizhati M, Zhang J, Zhang J, Zhou L, Chang G. Association of glucose transporter 4 genetic polymorphisms with obstructive sleep apnea syndrome in Han Chinese general population: a cross-section study. Lipids Health Dis 2014;13:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Abhary S, Hewitt AW, Burdon KP, Craig JE. A systematic meta-analysis of genetic association studies for diabetic retinopathy. Diabetes 2009;58:2137–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hodgkinson AD, Millward BA, Demaine AG. Polymorphisms of the glucose transporter (GLUT1) gene are associated with diabetic nephropathy. Kidney Int 2001;59:985–9. [DOI] [PubMed] [Google Scholar]
- 109.Campa D, Husing A, Chang-Claude J, Dostal L, Boeing H, Kroger J, Tjonneland A, Roswall N, Overvad K, Dahm CC, et al. Genetic variability of the fatty acid synthase pathway is not associated with prostate cancer risk in the European Prospective Investigation on Cancer (EPIC). Eur J Cancer 2011;47:420–7. [DOI] [PubMed] [Google Scholar]
- 110.Harrison FE, May JM. Vitamin C function in the brain: vital role of the ascorbate transporter SVCT2. Free Radic Biol Med 2009;46:719–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wilson JX. Antioxidant defense of the brain: a role for astrocytes. Can J Physiol Pharmacol 1997;75:1149–63. [PubMed] [Google Scholar]
- 112.Hediger MA. New view at C. Nat Med 2002;8:445–6. [DOI] [PubMed] [Google Scholar]
- 113.Agus DB, Gambhir SS, Pardridge WM, Spielholz C, Baselga J, Vera JC, Golde DW. Vitamin C crosses the blood-brain barrier in the oxidized form through the glucose transporters. J Clin Invest 1997;100:2842–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kc S, Carcamo JM, Golde DW. Vitamin C enters mitochondria via facilitative glucose transporter 1 (Glut1) and confers mitochondrial protection against oxidative injury. FASEB J 2005;19:1657–67. [DOI] [PubMed] [Google Scholar]
- 115.Serviddio G, Bellanti F, Vendemiale G, Altomare E. Mitochondrial dysfunction in nonalcoholic steatohepatitis. Expert Rev Gastroenterol Hepatol 2011;5:233–44. [DOI] [PubMed] [Google Scholar]
- 116.Lykkesfeldt J, Poulsen HE. Is vitamin C supplementation beneficial? Lessons learned from randomised controlled trials. Br J Nutr 2010;103:1251–9. [DOI] [PubMed] [Google Scholar]