Abstract
Anemia remains a widespread public health problem. Although iron deficiency is considered the leading cause of anemia globally, the cause of anemia varies considerably by country. To achieve global targets to reduce anemia, reliable estimates of the contribution of nutritional and non-nutritional causes of anemia are needed to guide interventions. Inflammation is known to affect many biomarkers used to assess micronutrient status and can thus lead to incorrect diagnosis of individuals and to overestimation or underestimation of the prevalence of deficiency in a population. Reliable assessment of iron status is particularly needed in settings with high infectious disease burden, given the call to screen for iron deficiency to mitigate potential adverse effects of iron supplementation. To address these information gaps, in 2012 the CDC, National Institute for Child Health and Human Development, and Global Alliance for Improved Nutrition formed a collaborative research group called Biomarkers Reflecting Inflammation and Nutrition Determinants of Anemia (BRINDA). Data from nationally and regionally representative nutrition surveys conducted in the past 10 y that included preschool children and/or women of childbearing age were pooled. Of 25 data sets considered for inclusion, 17 were included, representing ∼30,000 preschool children, 26,000 women of reproductive age, and 21,000 school-aged children from all 6 WHO geographic regions. This article provides an overview of the BRINDA project and describes key research questions and programmatic and research implications. Findings from this project will inform global guidelines on the assessment of anemia and micronutrient status and will guide the development of a research agenda for future longitudinal studies.
Keywords: Anemia, biomarkers, inflammation, iron, public health
Introduction
Anemia remains a widespread public health problem. Anemia is characterized by low hemoglobin concentration with subsequent impairment in meeting the oxygen delivery demands of tissues. On the basis of 2011 global estimates, 43% of preschool children (PSC)11 and 33% of nonpregnant women were anemic, with the highest burden in Africa and South Asia (1). Anemia accounts for nearly 9% of the world’s total disability and results in important health and economic consequences, including impaired immune response, maternal mortality, cognitive and developmental delays, and decreased productivity (2–4). Although iron deficiency is considered to be the most common cause of anemia, other important and common risk factors include other micronutrient deficiencies (e.g., vitamin A, folate, vitamin B-12), infections (e.g., intestinal parasites, schistosomiasis, malaria, HIV), and inherited red blood cell disorders (e.g., sickle cell, α-thalassemia). The cause of anemia is further complicated by other factors, including geography, age, and sex. Previous estimates on the risk factors for anemia are limited to regional data (5–8), data from indigenous populations (9), or data from single surveys (4). Aggregated estimates that used hemoglobin concentrations from national surveys are limited to modeling-estimation techniques, rather than to direct measurements of the many causes and risk factors thought to be associated with anemia (4). One of the six 2025 World Health Assembly Global Targets to improve maternal, infant, and young child nutrition is to reduce the prevalence of anemia by 50% (10). To achieve this ambitious goal, reliable estimates of the contribution of nutritional and non-nutritional risk factors for anemia are needed to guide interventions (2).
An important step in identifying the relative contribution of factors associated with anemia is being able to accurately measure these risk factors. In the case of micronutrient deficiencies, this is a unique challenge because several nutrient biomarkers are affected by inflammation (e.g., serum ferritin, retinol, and zinc) which can lead to incorrect diagnosis of individuals and to overestimation or underestimation of the prevalence of deficiency in a population (11–14). The assessment of iron status, in particular, has been a global priority since the 2006 WHO consultative group recommendations that routine iron supplementation of young children in malaria-endemic areas should not continue without appropriate screening for iron deficiency (15). Although accurate, field-friendly screening tests for iron deficiency are needed, so are approaches to better interpret existing measures of iron status (16, 17). The CDC and WHO recommend measuring inflammatory markers for assessment of population iron status with the use of serum ferritin and to either exclude individuals from analysis who are inflamed or to raise the cutoff of ferritin to define deficiency (18, 19); however, there are no universally accepted methods for accounting for inflammation in estimating micronutrient status. The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Biomarkers of Nutrition for Development (BOND) and Inflammation and Nutrition Science for Programs and Interpretation of Research Evidence (INSPIRE) projects have identified the role of inflammation on nutrient biomarkers as a critical research gap (12, 20).
To address these challenges, in 2012 the CDC, NICHD, and the Global Alliance for Improved Nutrition with support from the Bill & Melinda Gates Foundation formed a collaborative research group called Biomarkers Reflecting Inflammation and Nutrition Determinants of Anemia (BRINDA). This article summarizes preliminary findings presented at the 2015 Experimental Biology Symposium on the BRINDA project, including project rationale, overview, key questions, and programmatic and research implications. Further articles from the BRINDA project are forthcoming.
Project Rationale and Overview
The BRINDA project is a multiagency and multicountry partnership designed to improve the interpretation of nutrient biomarkers in settings of inflammation and to generate context-specific estimates of risk factors for anemia (21). The rationale for BRINDA grew from an expressed need by the global nutrition community to improve assessment of micronutrient status. For example, observations from independent surveys suggested that the burden of iron deficiency anemia was lower than previously estimated and that non-nutritional causes may play an important role (6, 7). In addition, the Iron and Malaria Project, formed in 2007 to address issues that pertain to the safe and effective use of interventions to prevent iron deficiency in the context of infections, most prominently malaria, called for evidence on the use and interpretation of iron biomarkers (17). In 2010, the BOND program began as a collaboration between NICHD and the Bill & Melinda Gates Foundation, with the aim of developing consensus on accurate assessment methodologies for food and nutrition (20). BOND expert panels were charged with providing guidance on biomarkers of exposure, status, function, and effect for 6 priority micronutrients (folate, iodine, iron, vitamin A, vitamin B-12, and zinc). As a result of deliberations of the expert panels and input from the larger community on the need to address the cross-cutting issue of inflammation and nutrient assessment, the INPIRE project was initiated in 2012 (12). The goal of INSPIRE was to review the evidence for the relation between nutrition, immune function, and the inflammatory response. In addition, INSPIRE aimed to provide guidance on how to account for the impact of inflammation on selection, use, and interpretation of biomarker data. Although the working group summarized different approaches to account for the effects of inflammation on nutrient biomarkers, they concluded that more research is needed to achieve consensus on approaches (12). Finally, at the 2014 Micronutrient Forum, there was a call to the global nutrition community for more field-friendly and accurate biomarkers that are reliable in the presence of inflammation and other confounders (22). Given that nutrition is involved in all aspects of human biology, a deeper appreciation of nutrition from a systems biology perspective could be one important way to identify better biomarkers of nutrition (23).
The primary goal of BRINDA is to address knowledge gaps related to the assessment of nutritional status in diverse settings. Reliable data are essential for both policy making and practice to ensure that programs are appropriately designed and that nutritionally deficient populations are appropriately targeted. BRINDA has the following 2 broad primary objectives: 1) to examine the relation between inflammation and nutrient biomarkers (e.g., anemia, iron, and vitamin A biomarkers) and 2) to identify factors associated with anemia and their relative contribution to the prevalence of anemia. The project further established 2 tracks to examine key questions related to each objective (Table 1). The focus of the analyses is on high-risk population groups that include PSC, school-age children (SAC), and women of reproductive age (WRA). Separate analyses will be conducted for each nutritional biomarker and population subgroup for each country independently and will be pooled. To the best of our knowledge, the BRINDA project is the largest and most comprehensive project to date which uses individual participant data to systematically address these research questions.
TABLE 1.
BRINDA project key research questions1
| Tracks |
| Inflammation and biomarkers track |
| What factors (e.g., nutrition, infection, and demographic and environmental factors) are most strongly associated with inflammation across countries? |
| What is the relation between inflammation (as measured by C-reactive protein and α1-acid glycoprotein) and iron (e.g., ferritin, transferrin receptor, total body iron stores), vitamin A (e.g., retinol, retinol-binding protein), and anemia (hemoglobin) biomarkers? |
| How do approaches to adjust nutrient biomarkers for inflammation compare? |
| How do the effects of inflammation on nutrient biomarkers and the recommended approaches to account for this effect vary by population group and region? |
| Anemia track |
| What is the association of micronutrient deficiencies and undernutrition to mild, moderate, and severe anemia in different population groups? |
| What is the contribution of non-nutritional and genetic factors to mild, moderate, and severe anemia in different population groups? |
| What are the geographic differences in anemia causes, in particular between Asia and Africa? |
BRINDA, Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia.
Because BRINDA uses population studies of apparently healthy people, its focus is on nutritional assessment at the population level and not on clinical aspects of inflammation in individual patients. BRINDA defines biomarkers as objective measurements of normal biological processes, pathologic processes, or pharmacologic response to a therapeutic intervention (24). Although biomarkers may have biological utility, their measure must also be of use in clinical or programmatic settings (23).
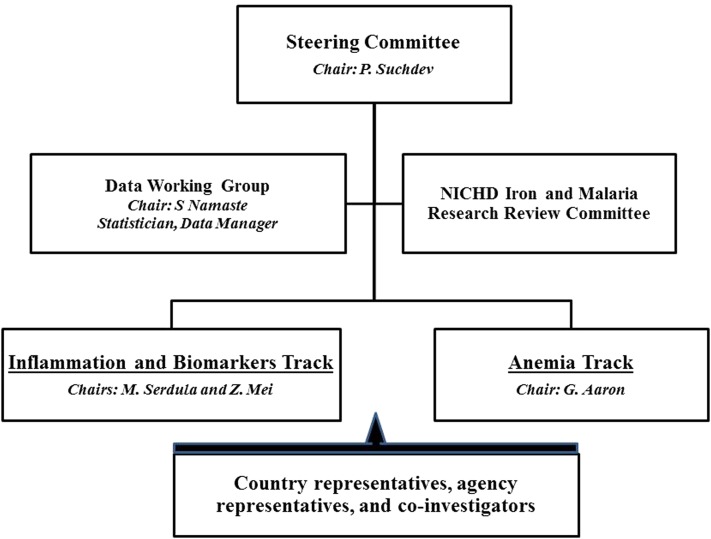
The organizational structure of BRINDA consists of a Steering Committee and Working Group (Figure 1). The Steering Committee, which consists of representatives from each of the 3 lead agencies, is responsible for project governance, ensuring quality, and liaising with representatives from partner organizations that have contributed data sets to the BRINDA project. The Working Group is composed of representatives from the countries or agencies and other collaborators with subject-matter expertise. A data management group, consisting of a data manager, statistician, and other coinvestigators, oversees data processing. The study was reviewed by the institutional review boards of NIH and was deemed nonhuman subjects research.
FIGURE 1.
Organizational chart for the BRINDA project. BRINDA, Biomarkers Reflecting Inflammation and Nutrition Determinants of Anemia; NICHD, Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Selection of Data Sets and Key Indicators
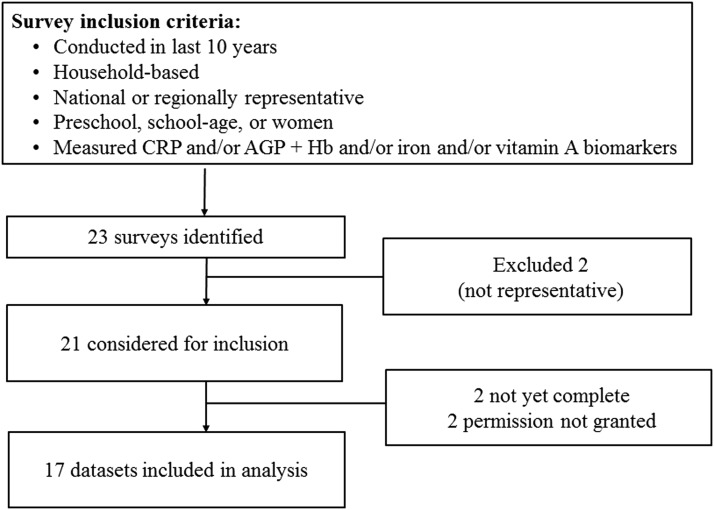
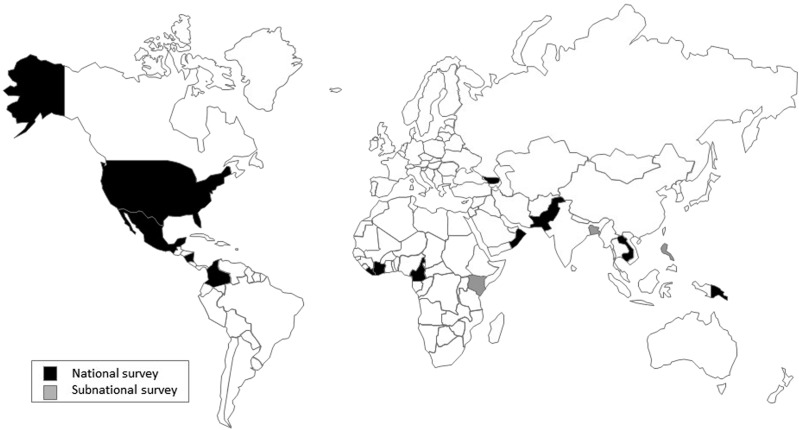
Only data sets from nationally and/or regionally representative household surveys were considered (Figure 2). Global Alliance for Improved Nutrition and CDC contributed data sets with appropriate permission from partners, and an informal call for additional data sets was made through partners to identify other surveys conducted by UNICEF, universities, and other nongovernmental organizations. All data sets had to contain ≥1 measure of inflammation [C-reactive protein (CRP) or α1-acid glycoprotein (AGP)] and ≥1 measure of anemia (hemoglobin), iron [ferritin or soluble transferrin receptor (sTfR)], or vitamin A [retinol or retinol-binding protein (RBP)] status among PSC (6–59 mo), SAC (6–14 y), and WRA (15–49 y). Additional inclusion criteria were 1) survey conducted in past 10 y; 2) household survey, excluding clinical studies; and 3) population-based, representative at the national, subnational, or regional level. Of 25 data sets considered for inclusion, 17 were included, representing ∼30,000 PSC, 26,000 WRA, and 21,000 SAC, from all 6 WHO regions (Figure 3).
FIGURE 2.
Selection of the BRINDA project data sets. AGP, α-1-acid glycoprotein; CRP, C-reactive protein; Hb, hemoglobin. BRINDA, Biomarkers Reflecting Inflammation and Nutrition Determinants of Anemia.
FIGURE 3.
BRINDA country data sets. Total sample size of ∼30,000 preschool children, 21,000 school-aged children, and 26,000 women of reproductive age from 17 data sets from 15 countries. BRINDA, Biomarkers Reflecting Inflammation and Nutrition Determinants of Anemia.
The 17 BRINDA data sets and available indicators in each data set are summarized in Table 2. Hemoglobin was measured in all data sets, as was a measure of iron status (ferritin in all countries except Papua New Guinea, and sTfR in all countries except Colombia, Nicaragua, Oman, and Pakistan). CRP and AGP were both measured in 9 data sets, CRP alone in 6 data sets, and AGP alone in 2 data sets. Vitamin A status, as defined by retinol or RBP, was measured in 14 data sets. Individual-level malaria data were collected in Cote d’Ivoire, Kenya (2007 and 2010), and Liberia. More detailed BRINDA methodology, summary of data, and analytical plan are beyond the scope of this article and will be described in subsequent publications.
TABLE 2.
Summary of data sets included in the BRINDA project1
| Sample size, n |
Summary of key indicators |
||||||||||
| Country | Year | Scope | PSC2 | SAC | WRA | Hb | CRP | AGP | Iron | Vitamin A | Malaria |
| Bangladesh | 2010 | Regional | 1493 | x | x | x | x | x | |||
| Cameroon | 2009 | National | 792 | 760 | x | x | x | x | x | ||
| Colombia | 2012 | National | 3866 | 8573 | 9083 | x | x | x | x | ||
| Cote d‘Ivoire | 2007 | National | 746 | 834 | x | x | x | x | x | x | |
| Georgia | 2009 | National | 2143 | 1688 | x | x | x | ||||
| Kenya | 2007 | Regional | 896 | x | x | x | x | x | x | ||
| Kenya | 2010 | Regional | 849 | x | x | x | x | x | x | ||
| Laos | 2006 | National | 482 | 816 | x | x | x | x | x | ||
| Liberia | 2011 | National | 1434 | 1942 | x | x | x | x | x | x | |
| Mexico | 2006 | National | 1592 | 4643 | 3032 | x | x | x | |||
| Mexico | 2012 | National | 2539 | 4332 | 3631 | x | x | x | x | ||
| Nicaragua | 2005 | National | 1424 | x | x | x | x | ||||
| Oman | 2004 | National | 183 | 349 | x | x | x | x | |||
| Pakistan | 2011 | National | 7557 | x | x | x | x | ||||
| PNG | 2005 | National | 872 | 749 | x | x | x | x | x | ||
| Philippines | 2011 | Regional | 1691 | x | x | x | x | x | |||
| United States | 2003–06 | National | 1315 | 3547 | 3196 | x | x | x | x | ||
AGP, α1-acid glycoprotein; BRINDA, Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia; CRP, C-reactive protein; Hb, hemoglobin; PNG, Papua New Guinea; PSC, preschool children aged 6–59 mo; SAC, school-age children aged 6–14 y; WRA, women of reproductive (nonpregnant) aged 15–49 y.
Ages of PSC were 6–11 mo in Bangladesh, 6–22 mo in Cameroon, 6–35 mo in Kenya, 6–35 mo in Liberia, and 12–59 mo in Mexico.
Approaches to Address the Influence of Inflammation on Iron Biomarkers
Several biomarkers are available to assess iron status, reflecting different components of iron metabolism (25). In 2007, the WHO and CDC held a joint consultation on markers to assess iron status in populations (18). The consultation resulted in the recommendation of ferritin as the primary measure of population iron status. sTfR was also identified as a promising measure that warranted continued evaluation.
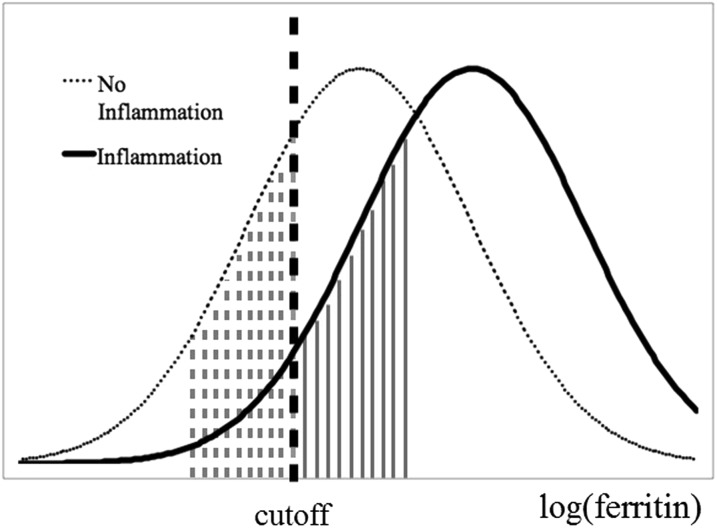
An important qualification to the current WHO recommendation is that, in settings with a high prevalence of infection or inflammation, the use of ferritin is fundamentally limited by the confounding role of inflammation. Ferritin not only reflects iron status but is also affected by the acute-phase response. The acute-phase response is a natural immunologic process that causes certain proteins in the body to fluctuate in response to infections and other causes of inflammation. Ferritin is a positive acute-phase protein (APP) and therefore increases in subclinically infected/inflamed individuals irrespective of iron status (11). We show a hypothetical example of the distribution of ferritin values in a population in 2 states: with and without inflammation (Figure 4). Without taking into account inflammation, previous studies have found that the prevalence of low iron stores can be underestimated by 14% (26).
FIGURE 4.
Hypothetical example of serum ferritin concentrations in populations with and without inflammation. A hypothetical population experiencing inflammation will exhibit increased ferritin concentrations shown by the rightward shift of the distribution of log(ferritin). In such a scenario the area shaded with solid lines represents the portion of the population misclassified as having sufficient iron stores while experiencing inflammation. These individuals would be correctly identified as having insufficient iron stores (and fall in the dashed line shaded area) if ferritin samples were taken when the population was not experiencing inflammation.
The WHO has provided some guidance on the use of ferritin to assess iron status in populations with widespread infection or inflammation (19). First, conduct the survey in seasons with low inflammation and, in the case of year-round inflammation, measure 2 additional APPs (CRP and AGP) concurrently with ferritin. Next, with the use of the APP data apply either a higher ferritin cutoff or exclude individuals with inflammation (Table 3). Little data support the use of a fixed, higher cutoff, and the sensitivity of this approach may be low. Furthermore, the exclusion approach may not be feasible in regions with high levels of inflammation because of diminishing sample sizes and introduction of selection bias.
TABLE 3.
Proposed methods to interpret ferritin values in the presence of inflammation1
| Proposed methods |
| Higher ferritin cutoff: Use a ferritin cutoff of <30 μg/L instead of <12 μg/L among populations with inflammation to define deficiency (18). |
| Exclude individuals: Stratify participants into those without and with inflammation (CRP > 5 mg/L and/or AGP > 1 g/L) and use ferritin values only among those without inflammation (19). |
| ICFs: Stratify individuals into those with and without inflammation. In the 4-group model, individuals are defined by no inflammation (CRP < 5 mg/L and AGP < 1 g/L), the incubation period (CRP > 5 mg/L), early convalescence (CRP > 5 mg/L and AGP > 1 g/L), and late convalescence (AGP > 1 g/L). A 2-group model can be used when only CRP or AGP was measured. ICFs are then generated by dividing the ferritin GM values of each inflammation group by the mean values in the non-inflammation group (26): ICFi = GMref/GMi. Subsequently, the ferritin values in individuals in the raised inflammation groups are multiplied by the ICFs matching their respective inflammation group. |
| Meta-analysis correction factors: Use the same approach as the ICF method but with the use of external correction factors generated from a meta-analysis (26). |
| Regression modeling: Run a linear regression analysis with ferritin as the dependent variable and CRP and/or AGP as the independent variables and use the slope of CRP and/or AGP to adjust for the effect of inflammation. A CRP and AGP reference value (e.g., maximum of lowest decile) may be required so that ferritin is not overadjusted at the lower values for CRP and AGP. Adjustment would be performed by using the following equation: Adjusted ferritin = unadjusted ferritin − β1(CRPobs − CRPref) − β2(AGPobs − AGPref). |
AGP, α1-acid glycoprotein; CRP, C-reactive protein; GM, geometric mean; GMi = geometric mean of those in the incubation, early, and late convalescence groups; GMref = geometric mean of those without inflammation; ICF, internal correction factor; obs, observed; ref, reference.
At present, there is no consensus method for adjusting ferritin values, but a number of investigators are exploring possible methods to address inflammation and, to a lesser extent, other potential confounders of ferritin (Table 3). Thurnham et al. (26) have proposed applying arithmetic correction factors that are based on study population data, with the use of a 2- or 4-level inflammation model. The same researchers further developed meta-analysis correction factors, based on 22 studies that can be applied to data. Most recently, Engle-Stone et al. (27) explored correction factors that were based on regression methods.
sTfR was proposed as a potential alternative biomarker to ferritin in settings where there is a high prevalence of low-grade inflammation because (unlike ferritin) it is not an APP (28). Although sTfR is potentially less influenced by inflammation, sTfR may be affected by inflammation through alternative mechanisms, such as iron-limited erythropoiesis and hypoxemia (29). Therefore, methods to adjust sTfR for inflammation should also be considered.
The BRINDA project researchers are exploring a series of questions to support recommendations on use of iron biomarkers in settings with a high prevalence of inflammation (Table 1). Preliminary results indicate that in settings with low inflammation adjustments have a minimal effect on ferritin and sTfR, whereas in settings with a high prevalence of inflammation there is a large impact on ferritin and to a lesser extent on sTfR values. It is difficult to predict the amount of inflammation in a given population; thus, inflammation biomarkers should be measured concurrently with nutrient biomarkers. On the question of which inflammation biomarkers to use (e.g., CRP, AGP, or both), the strength of the association between ferritin and sTfR with CRP or AGP appear similar, but the prevalence of elevated AGP is substantially higher than the prevalence of elevated CRP, so AGP appears to exert a greater impact on the nutritional biomarkers at the population level. Measuring both CRP and AGP at this early stage of exploration would be prudent. The regression approach has advantages over other methods because continuous data are used rather than arbitrary cutoffs, thus, taking into account the degree of inflammation in the adjustments.
Initial findings also showed that, depending on whether ferritin or sTfR were used, the prevalence of iron deficiency varied widely. The prevalence of iron deficiency was much higher with the use of sTfR than with ferritin in most countries, even after making adjustments. This is contrary to what would be expected on the basis of the different stages of iron deficiency that each of the biomarkers is supposed to reflect. Low levels of ferritin indicate insufficient iron reserves and indicate the beginning stages of iron deficiency. When iron stores are depleted, TfR production is augmented, and the quantity of the soluble form of transferrin receptors can be measured in the blood. The amount of sTfR is proportional to cellular iron demand and thus reflects early functional iron deficiency (30). Therefore, although sTfR appears to be less influenced by inflammation than ferritin, factors leading to erythropoiesis other than tissue iron deficiency may need to be taken into account and/or cutoffs revisited for sTfR (31, 32).
Risk Factors for Anemia in Different Settings
Although iron deficiency is considered to be the main cause of anemia globally, preliminary findings from the BRINDA project do indicate a high degree of heterogeneity by country and region in the proportion of anemia attributable to iron deficiency. Moreover, results show strong associations with anemia and inflammation in all contexts where inflammation is present. Further analyses of the BRINDA data sets will focus on country-level associations between anemia and population characteristics and will possibly develop adjustment factors for hemoglobin in the presence of inflammation.
The BRINDA anemia findings suggest the importance of determining context-specific risk factors related to anemia and the need to account for inflammation when present. From research to policy perspectives, such information is critical for informing the appropriate design of interventions and for evaluating progress. Strong coordination and collaboration between donors, policy makers, programs, and the academic community will be pivotal to ensure that 1) evidence generated is adequate to determine a wide range of risk factors associated with anemia and 2) on the basis of an understanding of this evidence, programs are designed and implemented to address risk factors that are relevant in the given context.
Research, Policy, and Programmatic Implications of BRINDA
The BRINDA project is a multisite, large-scale collaborative study that is expected to advance our understanding of the underlying causes of anemia in diverse populations and across geographical settings. In addition, BRINDA will identify methods to better interpret currently used iron and vitamin A biomarkers in populations with high rates of inflammation from recurrent infections and those with chronic low-grade inflammation.
Preliminary findings suggest that inflammation, as measured by elevated CRP and AGP, is common in many settings. As seen in prior studies, both CRP and AGP are positively correlated with ferritin and negatively correlated with hemoglobin and RBP across all countries. Ignoring inflammation would result in a substantial underestimation of iron deficiency prevalence with the use of serum ferritin and an overestimation of both anemia prevalence with the use of hemoglobin and vitamin A deficiency prevalence with the use of RBP. Because there appear to be no clear cutoffs for elevated CRP and AGP which predict their effect on nutrient biomarkers, the regression approach to account for the effects of inflammation is preferred because it better reflects the relation between CRP, AGP, and nutrient biomarkers (e.g., ferritin, TfR, RBP) and can be used to evaluate potential confounders of the effect of inflammation and biomarkers (e.g., malaria infection). A challenge of the regression approach is making it user friendly to optimize utilization, similar to what was recommended for altitude-adjusted hemoglobin concentrations (33).
Strengths of the BRINDA project include that it is a multiagency and multicountry partnership that uses previously collected data to achieve objectives that span across the research, policy, and program areas. In addition to the organizational partnership that leverages resources, the involvement of country representatives increases collective ownership. BRINDA uses comprehensive data from multiple countries with actively monitored nutrition programs, which increases the external validity of the research findings and their general applicability. Furthermore, the data set has a large sample size and contains high-quality laboratory analyses. A fundamental limitation of the project is that the data are cross-sectional, so causality cannot be determined on factors that influence nutrient biomarker levels or determinants of anemia. Data analysis and interpretation are challenged because not all data sets have all variables, and data are heterogeneous and from diverse geographic settings and population groups.
Despite these limitations, this project represents a step forward in developing more valid micronutrient deficiency prevalence estimates and in identifying the leading causes and risk factors for anemia. Next steps of the project include disseminating results to the nutrition community through scientific publications and programmatic guidance. For example, results of BRINDA will help inform the revision of the WHO and CDC guidelines on the use of serum ferritin to assess iron status and iron deficiency in populations (18, 34). We anticipate a second phase of the project to collect and compile additional data from partners and to answer additional research questions. It is our hope that the results of the BRINDA project will identify priority research gaps and will serve as a catalyst for future longitudinal and intervention studies. Furthermore, the insights gained from this project are anticipated to provide important results to the research, policy, and programmatic communities that will ultimately help guide global and country investments to prevent and control micronutrient deficiencies and anemia.
Acknowledgments
We thank the BRINDA Working Group (Yaw Addo, Deena Alasfour, Fayrouz Ashour, Christine Clewes, Reina Engle-Stone, Roland Kupka, Leila Larson, Nino Lortkipanidze, Barbara MacDonald, Purnima Menon, Rebecca Merrill, Zuguo Mei, Pura Rayco-Solon, Rahul Rawat, Fabian Rohner, Ofelia Saniel, Olga Lucia Sarmiento, Mary Serdula, Saleh Al Shammakhi, Victor Temple, Andres Tschanne, Ravi Varadhan, Anne Williams, James Wirth, Miriam Yiannakis) and Steering Committee (Grant Aaron, Rafael Flores-Ayala, Sorrel Namaste, Daniel Raiten, Parminder Suchdev) for their contributions. We thank participants of the BRINDA project for the contribution of data, Juergen Erhardt for advice on laboratory methodology, and the Iron and Malaria Research Review Committee (Bernard Brabin, Gary Brittenham, James Cook, Kathryn Dewey, Victor Gordeuk, Richard Hurrell, Sean Lynch, Theonest Mutabingwa, Andrew Prentice, Neeru Singh) for providing scientific insight. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AGP, α1-acid glycoprotein; APP, acute-phase protein; BOND, Biomarkers of Nutrition for Development; BRINDA, Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia; CRP, C-reactive protein; INSPIRE, Inflammation and Nutrition Science for Programs and Interpretation of Research Evidence; NICHD, Eunice Kennedy Shriver National Institute of Child Health and Human Development; PSC, preschool children; RBP, retinol-binding protein; SAC, school-age children; sTfR, soluble transferrin receptor; WRA, women of reproductive age.
References
- 1.Stevens G, Finucane M, De-Regil L, Paciorek C, Flaxman S, Branca F, Pablo Peña-Rosas J, Bhutta Z, Ezzati M; Nutrition Impact Model Study Group (Anaemia). Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: a systematic analysis of population-representative data. Lancet Glob Health 2013;1:e16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balarajan Y, Ramakrishnan U, Ozaltin E, Shankar AH, Subramanian SV. Anaemia in low-income and middle-income countries. Lancet 2011;378:2123–35. [DOI] [PubMed] [Google Scholar]
- 3.Walker SP, Wachs TD, Gardner JM, Lozoff B, Wasserman GA, Pollitt E, Carter JA; International Child Development Steering Group. Child development: risk factors for adverse outcomes in developing countries. Lancet 2007;369:145–57. [DOI] [PubMed] [Google Scholar]
- 4.Kassebaum NJ, Jasrasaria R, Naghavi M, Wulf SK, Johns N, Lozano R, Regan M, Weatherall D, Chou DP, Eisele TP, et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014;123:615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kounnavong S, Sunahara T, Hashizume M, Okumura J, Moji K, Boupha B, Yamamoto T. Anemia and related factors in preschool children in the southern rural Lao People’s Democratic Republic. Trop Med Health 2011;39:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foote EM, Sullivan KM, Ruth LJ, Oremo J, Sadumah I, Williams TN, Suchdev PS. Determinants of anemia among preschool children in rural, western Kenya. Am J Trop Med Hyg 2013;88:757–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.George J, Yiannakis M, Main B, Devenish R, Anderson C, An US, Williams SM, Gibson RS. Genetic hemoglobin disorders, infection, and deficiencies of iron and vitamin A determine anemia in young Cambodian children. J Nutr 2012;142:781–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasricha SR, Black J, Muthayya S, Shet A, Bhat V, Nagaraj S, Prashanth NS, Sudarshan H, Biggs BA, Shet AS. Determinants of anemia among young children in rural India. Pediatrics 2010;126:e140–9. [DOI] [PubMed] [Google Scholar]
- 9.Khambalia AZ, Aimone AM, Zlotkin SH. Burden of anemia among indigenous populations. Nutr Rev 2011;69:693–719. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Comprehensive Implementation Plan on Maternal, Infant and Young Child Nutrition. Geneva: WHO, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomkins A. Assessing micronutrient status in the presence of inflammation. J Nutr 2003;133(5 Suppl 2)1649S–55S. [DOI] [PubMed] [Google Scholar]
- 12.Raiten DJ, Sakr Ashour FA, Ross AC, Meydani SN, Dawson HD, Stephensen CB, Brabin BJ, Suchdev PS, van Ommen B, Group IC. Inflammation and Nutritional Science for Programs/Policies and Interpretation of Research Evidence (INSPIRE). J Nutr 2015;145:1039S–108S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haynes BM, Pfeiffer CM, Sternberg MR, Schleicher RL. Selected physiologic variables are weakly to moderately associated with 29 biomarkers of diet and nutrition, NHANES 2003–2006. J Nutr 2013;143:1001S–10S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Northrop-Clewes CA. Interpreting indicators of iron status during an acute phase response–lessons from malaria and human immunodeficiency virus. Ann Clin Biochem 2008;45:18–32. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. Conclusions and recommendations of the WHO Consultation on prevention and control of iron deficiency in infants and young children in malaria-endemic areas. Food Nutr Bull 2007;28(4 Suppl)S621–7. [DOI] [PubMed] [Google Scholar]
- 16.Suchdev PS, Leeds IL, McFarland DA, Flores R. Is it time to change guidelines for iron supplementation in malarial areas? J Nutr 2010;140:875–6. [DOI] [PubMed] [Google Scholar]
- 17.Raiten DJ, Namaste S, Brabin B. Considerations for the safe and effective use of iron interventions in areas of malaria burden - executive summary. Int J Vitam Nutr Res 2011;81:57–71. [DOI] [PubMed] [Google Scholar]
- 18.WHO/CDC. Assessing the iron status of populations: report of a joint World Health Organization/Centers for Disease Control and Prevention technical consultation on the assessment of iron status at the population level. Geneva, Switzerland, 2004.
- 19.WHO. Serum ferritin concentrations for the assessment of iron status and iron deficiency in populations. Vitamin and Mineral Nutrition Information System. Geneva: World Health Organization, 2011.
- 20.Raiten DJ, Namaste S, Brabin B, Combs G Jr, L’Abbe MR, Wasantwisut E, Darnton-Hill I. Executive summary–biomarkers of nutrition for development: building a consensus. Am J Clin Nutr 2011;94:633S–50S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Namaste S, Suchdev PS, Aaron G. Biomarkers Reflecting Inflammation and Nutrition Determinants of Anemia (BRINDA): project overview. FASEB J 2013;27 lb270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forum M. Bridging Discovery and Delivery: Micronutrient Forum Global Conference 2014. Basel, 2014.
- 23.Raiten DJ, Combs GF. Biomarkers and bio-indicators: providing clarity in the face of complexity. Sight Life Mag 2015;29(1):39–44. [Google Scholar]
- 24.Strimbu K, Tavel JA. What are biomarkers? Curr Opin HIV AIDS 2010;5:463–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muñoz M, Villar I, Garcia-Erce JA. An update on iron physiology. World J Gastroenterol 2009;15:4617–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thurnham DI, McCabe LD, Haldar S, Wieringa FT, Northrop-Clewes CA, McCabe GP. Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: a meta-analysis. Am J Clin Nutr 2010;92:546–55. [DOI] [PubMed] [Google Scholar]
- 27.Engle-Stone R, Nankap M, Ndjebayi AO, Erhardt JG, Brown KH. Plasma ferritin and soluble transferrin receptor concentrations and body iron stores identify similar risk factors for iron deficiency but result in different estimates of the national prevalence of iron deficiency and iron-deficiency anemia among women and children in Cameroon. J Nutr 2013;143:369–77. [DOI] [PubMed] [Google Scholar]
- 28.Abraham K, Muller C, Gruters A, Wahn U, Schweigert FJ. Minimal inflammation, acute phase response and avoidance of misclassification of vitamin A and iron status in infants–importance of a high-sensitivity C-reactive protein (CRP) assay. Int J Vitam Nutr Res 2003;73:423–30. [DOI] [PubMed] [Google Scholar]
- 29.Kasvosve I, Gomo ZA, Nathoo KJ, Matibe P, Mudenge B, Loyevsky M, Nekhai S, Gordeuk VR. Association of serum transferrin receptor concentration with markers of inflammation in Zimbabwean children. Clin Chim Acta 2006;371:130–6. [DOI] [PubMed] [Google Scholar]
- 30.Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood 2003;101:3359–64. [DOI] [PubMed] [Google Scholar]
- 31.Verhoef H, West CE, Ndeto P, Burema J, Beguin Y, Kok FJ. Serum transferrin receptor concentration indicates increased erythropoiesis in Kenyan children with asymptomatic malaria. Am J Clin Nutr 2001;74:767–75. [DOI] [PubMed] [Google Scholar]
- 32.Mei Z, Pfeiffer CM, Looker AC, Flores-Ayala RC, Lacher DA, Mirel LB, Grummer-Strawn LM. Serum soluble transferrin receptor concentrations in US preschool children and non-pregnant women of childbearing age from the National Health and Nutrition Examination Survey 2003–2010. Clin Chim Acta 2012;413:1479–84. [DOI] [PubMed] [Google Scholar]
- 33.Nestel P, INACG Steering Committee. Adjusting hemoglobin values in program surveys. Washington (DC): INACG, 2002. [Google Scholar]
- 34.Garcia-Casal MN, Peña-Rosas JP, Pasricha S-R. Rethinking ferritin cutoffs for iron deficiency and overload. Lancet Haematol 2014;1(3):e92–4 10.1016/S2352–3026(14)00025–8. [DOI] [PubMed] [Google Scholar]