Abstract
Cardiovascular disease (CVD) is the leading cause of death in the United States. Although the role of habitual lifestyle factors such as physical activity and dietary patterns in increasing CVD risk has long been appreciated, less is known about how acute daily activities may cumulatively contribute to long-term disease risk. Here, the term acute refers to metabolic responses occurring in a short period of time after eating, and the goal of this article is to review recently identified stressors that can occur after meals and during the sleep-wake cycle to affect macronutrient metabolism. It is hypothesized that these events, when repeated on a regular basis, contribute to the observed long-term behavioral risks identified in population studies. In this regard, developments in research methods have supported key advancements in 3 fields of macronutrient metabolism. The first of these research areas is the focus on the immediate postmeal metabolism, spanning from early intestinal adsorptive events to the impact of incretin hormones on these events. The second topic is a focus on the importance of meal components on postprandial vasculature function. Finally, some of the most exciting advances are being made in understanding dysregulation in metabolism early in the day, due to insufficient sleep, that may affect subsequent processing of nutrients throughout the day. Key future research questions are highlighted which will lead to a better understanding of the relations between nocturnal, basal (fasting), and early postmeal events, and aid in the development of optimal sleep and targeted dietary patterns to reduce cardiometabolic risk.
Keywords: meal metabolism, incretins, lipids, postprandial, vascular function, sleep restriction
Introduction
Cardiovascular disease (CVD)9 is the leading cause of deaths in the United States, accounting for ∼830,000 deaths annually (1). Obesity and insulin resistance are predisposing factors for CVD and type 2 diabetes, and much has been learned about the effects of lifestyle patterns that contribute to dysfunctional macronutrient metabolism. Epidemiologic findings provide clear support that patterns of behavior extending over years contribute to disease risk (2), and it is well recognized that the daily accumulation of metabolic events eventually disrupts metabolic homeostasis. In particular, accumulating evidence supports the critical role of acute postprandial responses to incrementally contribute to CVD risk (3), with postprandial hyperglycemia as an example; it better predicts CVD-related deaths compared with fasting glucose concentrations, regardless of the presence of diabetes (4). Because short-term metabolic events precede overt disease, examining metabolic stressors throughout the 24-h, sleep-wake-eating cycle may uncover disease risk that may be otherwise hidden while providing a basis for targeted strategies to mitigate this risk.
Recent advances in 3 distinct fields of macronutrient metabolism have resulted in the identification of key mediators that regulate the physiologic processing of meal nutrients and the manner in which short-term metabolic effects contribute to disease. Specifically, this review focuses first on discussing disease-associated alterations in the normal pathways of nutrient absorption and assimilation. Recent work has produced surprising discoveries of new pathways that control intestinal lipid processing and the role of incretins to regulate lipid absorption (5). A second area described here includes studies that link food processing to cardiometabolic disease risk by focusing on metabolic events that occur once food has been absorbed. Clinical and preclinical studies have uncovered mechanisms by which elevated postprandial concentrations of nutrients (e.g., glucose) promote vascular dysfunction through oxidative stress-related pathways (6). This research holds much promise to explain how meal metabolism, when repeated daily throughout a lifetime, contributes to the long-term development of cardiometabolic disease. A third focus of this review poses the question of whether metabolic events that occur in humans as they become fasted during the early hours of the morning (0200–0600) are influenced by sleep restriction. In addition discussed here is whether these events in the sleep-deprived state may also feed forward to impair subsequent nutrient handling throughout the day. This area has been one of active focus (7), as has the study of sleep restriction’s impact on diabetes and obesity risk (8, 9). The collective advances of these distinct, but complementary, research areas are supported by the use of stable isotopes and kinetic measurements such as insulin clamps and intravenous glucose tolerance tests (GTTs), meal-feeding paradigms to measure metabolic responses over time in nonsteady states, the development of pharmaceutical compounds to specifically target the action of gut hormones, the improvement of technical approaches to assess endothelial function in vivo (10), and the development of new technologies that include the expansion of clinical research units to test sleep disorders. As metabolic research advances, it is likely that discoveries yet to come will underscore the interplay between acute stressors and macronutrient metabolism. Accordingly, we also highlight key unanswered scientific questions that are expected to serve as the focus of future research.
Enterocyte Lipid Handling
The complexity of dietary fat absorption.
Measurements of the timing of carbohydrate absorption have shown that ∼75 g is completely absorbed within 3 h after the start of the meal (11). By contrast, to completely absorb a roughly equivalent caloric load of fat (40 g) may take up to 18 h of transit through the enterocyte. During this complex process, dietary FAs are resynthesized into TGs and packaged into apoB48-containing chylomicrons, before entering the blood through the thoracic duct of the heart (Figure 1) (12). Chylomicrons are secreted in both the fasting and fed states (13). In the fasting state, chylomicrons are smaller, ∼10,000 TGs/particle (14), whereas those secreted after meals can carry ∼50,000–100,000 TG molecules/particle (15). Thus, meal feeding increases the number of apoB48 particles secreted from the intestine to a smaller extent than it increases the number of TGs carried/particle (16).
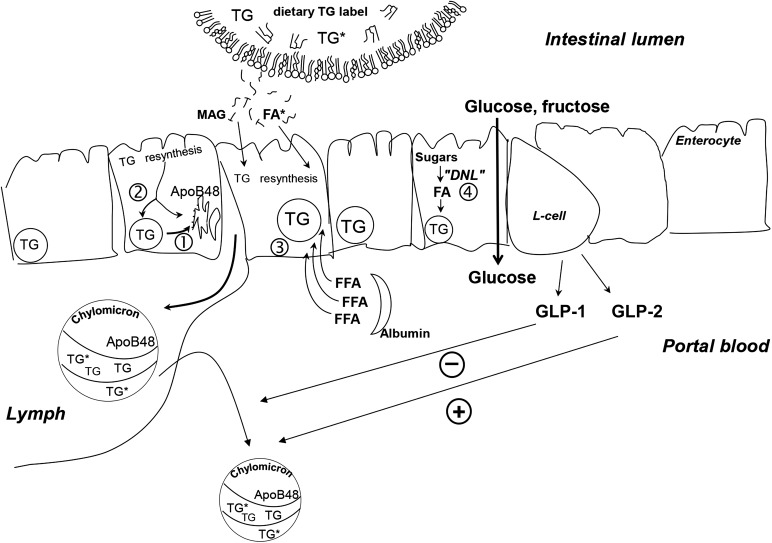
FIGURE 1.
Metabolic processes that support intestinal lipid absorption, chylomicron synthesis, and the role of GLP-1 and GLP-2 in glucose and lipid absorption. Numbered pathways depict 1) use of stored enterocyte TGs for chylomicron synthesis, 2) mixing of newly absorbed TGs into the stored lipid pool and direct use for chylomicron TG synthesis, 3) use of the plasma FFA pool for chylomicron TG synthesis, and 4) the potential for newly made FAs from dietary carbohydrates through the process of de novo lipogenesis. GLP-1 decreases chylomicron production (-). GLP-2 stimulates (+) the release of stored, preformed chylomicrons into the circulation. DNL, de novo lipogenesis; GLP, glucagon-like peptide; MAG, monoacylglycerol.
It is now recognized that at the onset of meal consumption, chylomicrons are immediately released that carry lipid previously stored within an enterocyte lipid pool (17, 18). Such cephalic phase lipid mobilization can occur 1) at the onset of eating a standard mixed meal (containing protein, carbohydrate, and fat); 2) after drinking a glucose solution (19); 3) while masticating, tasting, and expectorating (sham feeding) a whole food such as pizza (20); and 4) during sham feeding with a more simple stimulus such as cream cheese, which resulted in mobilization of a prestored, enterocyte lipid pool within 3 min (17). Further, in healthy individuals, chylomicrons are released, and a previous meal’s lipid can be detected in plasma if the cephalic phase stimulus occurs even 18 h after the last meal (17). The physiologic advantage or consequences of enterocyte lipid stores are unknown, but Parks and colleagues hypothesize that stores function to support constitutive chylomicron formation and secretion at a low rate in the fasting state and that this allows for a rapid ramping up of TG handling after large quantities of dietary fat are consumed in a high-fat meal. Furthermore, given that food consumption increases the release of intestinal hormones such as glucagon-like peptide 1 (GLP)-1 and GLP-2 and glucose-dependent insulinotropic peptide, early postprandial release of these incretins may provide a feed-forward signal to the periphery to increase fat storage (Figure 1). For instance, glucose-dependent insulinotropic peptide promotes FA esterification and adipocyte differentiation (21–23). In this way, early cephalic events may connect the size of the dietary lipid load with lipid secretion and body storage.
What constitutes a fatty intestine?
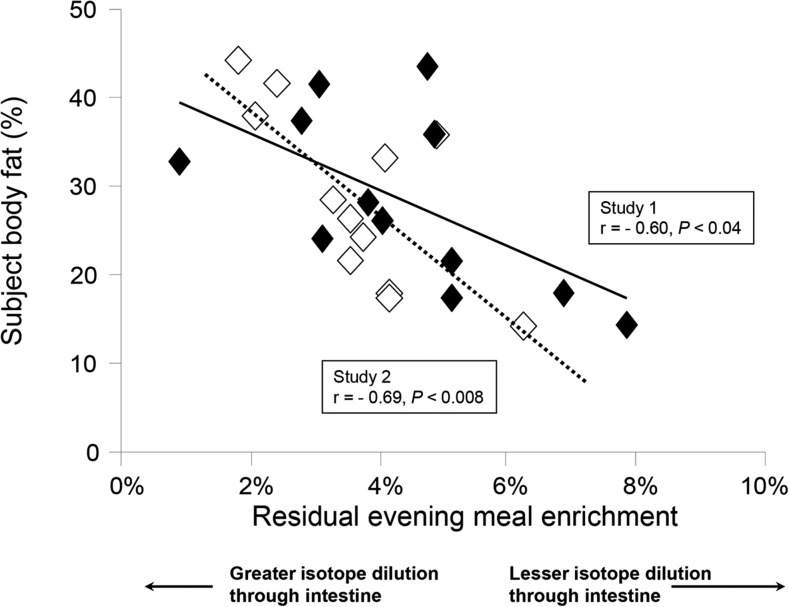
With the use of a stable isotope of TGs (deuterated tripalmitin) added to an evening meal, Chavez-Jauregui et al. (17) demonstrated that chylomicrons secreted the next morning in the fasted state carried the evening meal label. As shown in Figure 2, those subjects with the highest quantities of body fat exhibited the lowest enrichments of the label in the fasting state and also early in the postprandial process (within 1 h after food consumption). A greater dilution of the dietary label as it passed through the intestine suggested that subjects with greater body fat had greater quantities of stored lipid in the intestine. Interestingly, subjects performed this study of sham-feeding twice (either low-fat or high-fat cream cheese), and the observation was reproducible, suggesting that intestinal lipid stores are an individual characteristic of subjects. The metabolic syndrome shown to be associated with excess TGs stored in the liver and other ectopic sites (muscle, heart), and the data shown in Figure 2 suggest that being overweight may also be associated with excess lipid stored in the intestine.
FIGURE 2.
Relation between subject body and dilution of dietary fat isotope through the intestine. On 2 occasions, 12 healthy men and women consumed 2 g of 13C2 triolein with a standardized evening meal (34% fat, 57% carbohydrates). The next morning, chylomicrons were isolated in the fasting state, and TGs were analyzed for the presence of the label that originated from the evening meal. Body fat was measured by DXA. Open diamonds represent the data from the first study (sham feeding with low-fat cream cheese), and filled diamonds represent data from the second study (sham feeding with high-fat cream cheese). Adapted from reference 17 with permission.
Robertson et al. (19) have performed intestinal biopsies in subjects after consumption of a meal fat challenge, and electron micrographs of duodenal tissue provide direct evidence of large amounts of lipid stored intracellularly. Exploration of this process by taking intestinal biopsies is impractical in most human clinical studies. One potential method to estimate the size of intestinal lipid stores in humans in vivo is to use a combination of TG-labeled meals, analysis of plasma TGs by mass spectrometry, and calculation of the intracellular precursor FA pool enrichments with the use of a method called mass isotopomer distribution analysis (24). Such studies are currently under way to test the impact of subject body fat on enterocyte lipid handling.
Although stored intestinal lipid is used to support chylomicron-TG secretion early in the postprandial period, it may take up to 4 h after a meal for chylomicron lipid to begin to reflect the composition of the meal-TG FAs. One other source of FAs that can be used to support chylomicron-TG synthesis is the plasma FFA pool. Indirect data support the potential uptake and esterification of plasma FFAs through the basolateral membrane of the enterocyte (designated as pathway 3 in Figure 1). Lewis and colleagues (25) have infused intralipid and heparin to increase the plasma concentration of FFAs and found that apoB48 secretion was increased ∼66%. Physiologic states associated with adipose insulin resistance (e.g., sleep restriction, obesity), which also cause higher plasma FFA concentrations, may also be associated with higher fasting apoB48 concentrations (26). Direct evidence for enterocyte FFA uptake has yet to be generated by labeling plasma FFAs and detecting this label directly in TGs carried in newly secreted chylomicrons. One barrier to such studies is the lack of efficient methods to separate chylomicrons from VLDL, and this remains a key limitation for human studies in this field. Studies in rodents can circumvent this limitation by collecting lymphatic output in the fed state to assess apoB48 secretion (27). Thus, rodent models could be used to directly test the contribution of the plasma FFA pool to enterocyte chylomicron-TG synthesis.
A final source of FAs used for enterocyte TG synthesis is the process of de novo lipogenesis (Figure 1, pathway 4). Recently, Veilleux et al. (28) investigated de novo lipogenesis in intestinal tissue explants obtained from patients undergoing bariatric surgery. The tissue from subjects categorized as insulin resistant (HOMA-IR) had elevated rates of lipogenesis ex vivo as determined by the incorporation of 13C-acetate into lipids. Esterification of oleic acid into cellular lipids was also evident in secreted lipoproteins ex vivo. However, whether the intestinal lipogenic pathway can contribute to intestinal lipid storage and/or secretion during carbohydrate overfeeding in vivo in humans is unknown. Indirect evidence for this mechanism is supported by a recent study in healthy individuals (29) in which intraduodenal co-infusion of either glucose or fructose with a lipid emulsion resulted in increased production rates of apoB48 compared with co-infusion of the lipid emulsion and saline (6.0-fold and 1.6-fold, respectively). However, the investigators have proposed that this effect of sugars on chylomicron production may be a result of a shift of intracellular lipid from storage to the assembly pathway and that this is likely enhanced in response to hyperglycemia (30).
The Influence of Intestinal Hormones on apoB48 Synthesis
In addition to the sources of lipid used for chylomicron synthesis, some studies have uncovered the role of hormones in the control of apoB48 particle secretion. Lewis and colleagues (25) have demonstrated that insulin acutely inhibits apoB48 secretion in healthy humans (in part related to suppression of plasma FFAs), but this suppressive effect of insulin is blunted in individuals with type 2 diabetes (31). Thus, these studies provided the first evidence in humans that intestinal insulin resistance may underlie the overproduction of chylomicrons observed during these conditions. Most recently, studies have provided new insight into how gut hormones regulate chylomicron secretion. The intestinally derived peptides GLP-1 and GLP-2 are secreted by intestinal L cells in response to nutrient ingestion (Figure 1), particularly carbohydrates and fat (32, 33). Both peptides are degraded by the enzyme dipeptidyl peptidase-4 (DPP-4), although to a different extent with GLP-1 having a shorter half-life in the circulation (∼1.5 min) than GLP-2 (∼7 min), as reviewed by Holst et al. (34). Importantly, GLP-1 and GLP-2 were shown to exert opposing effects on regulating chylomicron secretion.
GLP-1 reduces chylomicron secretion.
The clinical use of incretin-based therapies (GLP-1 receptor agonists and DPP-4 inhibitors) in type 2 diabetes suggests a role for these agents in ameliorating fasting and postprandial hyperlipidemia. This emerging evidence was discussed comprehensively in a recent review that poses gut hormones as key regulators of intestinal-derived lipoprotein secretion (5); thus, this topic will not be further elaborated here. Of note, mechanistic studies in healthy humans have shown that exenatide (GLP-1 receptor agonist) and sitagliptin (DPP-4 inhibitor) can reduce intestinal, but not hepatic, lipoprotein production acutely, independent of glycemic status and weight loss (35, 36). These studies were conducted under a pancreatic clamp procedure, and nutrients were infused directly into the duodenum to isolate intestinal-specific effects, from other well-known pleiotropic effects of GLP-1 action such as changes in pancreatic hormones and gastric emptying. Current evidence therefore suggests that therapeutic regulation of GLP-1 action may provide benefits in reducing the secretion of atherogenic lipoproteins and CVD risk. Interestingly, in a recent large study, impairment of GLP-1 secretion was an event that may have occurred early in the progression of type 2 diabetes (37). Therefore, preventive strategies that increase GLP-1 secretion and action in overweight and obese, prediabetic individuals warrant further investigation.
GLP-2 stimulates the release of stored chylomicrons.
In contrast to the inhibitory effects of GLP-1 on chylomicron secretion, GLP-2 enhances the release of preformed chylomicrons from the intestine. In healthy individuals who are administered intraduodenal lipid infusions, GLP-2 increased apoB48 and TG-rich lipoprotein-TG concentrations within a half an hour of subcutaneous injection (38). GLP-2 injection also stimulated a rapid and transient release of prelabeled chylomicrons from a previous meal and in the absence of an additional meal. The increase was likely a result of the release of stored, presynthesized chylomicrons from the intestine into the circulation. However, it remains unknown whether endogenously secreted GLP-2 stimulates chylomicron secretion and whether this contributes to postprandial lipemia under a range of physiologic conditions.
Another question that remains to be answered is the net effect of gut peptides on intestinal lipoprotein secretion in the progression of type 2 diabetes. Under normal physiologic conditions, the half-life of GLP-2 is longer than GLP-1, which results in more sustained circulating concentrations of the former. Experimental studies in the hamster (39) and in healthy humans (36) suggest that prolonged co-infusion of GLP-1 and GLP-2 or pharmacologic inhibition of DPP-4 activity leads to sustained GLP-1 action and thus net reduction in postprandial lipemia. Yet, mechanisms by which GLP-1 and GLP-2 regulate chylomicron secretion remain unclear. Direct action of GLP-1 to reduce apoB48 secretion was shown in isolated enterocytes (40), but recent experimental evidence also suggests central nervous system regulation of chylomicron secretion by GLP-1 receptors as an alternative mechanism (41). Injection of GLP-2 in pigs and in humans increases intestinal blood flow which provides a potential explanation for how GLP-2 stimulates chylomicron secretion (42, 43). Understanding the factors that control fat absorption will provide novel therapeutic strategies to reduce postprandial excursions of atherogenic lipoproteins. Indeed, enterocyte regulation of dietary lipid entry was the focus of recent scientific efforts in drug development to treat hyperlipidemia and obesity (44, 45). Thus, future research in this area should address the following questions:
Do intestinal TG stores serve a regulatory function to control subsequent energy balance?
Do subjects with ectopic lipid stores in liver and muscle also have excess lipid stored in the intestine?
Does body fat or insulin resistance affect dietary FA absorption or secretion of intestinal hormones and do these events affect chylomicron production?
Does insulin resistance influence which signals control the release of stored intestinal lipid and is the vagus nerve involved?
Another mechanism by which postmeal macronutrient metabolites can affect CVD risk is via influences on the vasculature. The consumption of high-fat meals reduces brachial artery flow-mediated dilation (FMD) at the peak of postprandial lipemia, even in healthy subjects (46). Similarly, postprandial hyperglycemia was shown to transiently impair vascular function in normoglycemic and hyperglycemic adults (6). It is unclear whether reductions in postprandial FMD represent a normal (e.g., physiologic) response that may confer benefit somehow for nutrient disposal. However, studies that investigated prolonged exposure of high concentrations of nutrients to the vasculature support a net negative effect of nutrient toxicity. Through both fat- and glucose-related mechanisms, postprandial oxidative stress appears to play a central role in promoting vascular dysfunction. As described in the next section, postprandial hyperglycemia decreases NO bioavailability, which was the focus of recent advances in the field of postprandial metabolism.
Postprandial Hyperglycemia and Vascular Endothelial Dysfunction
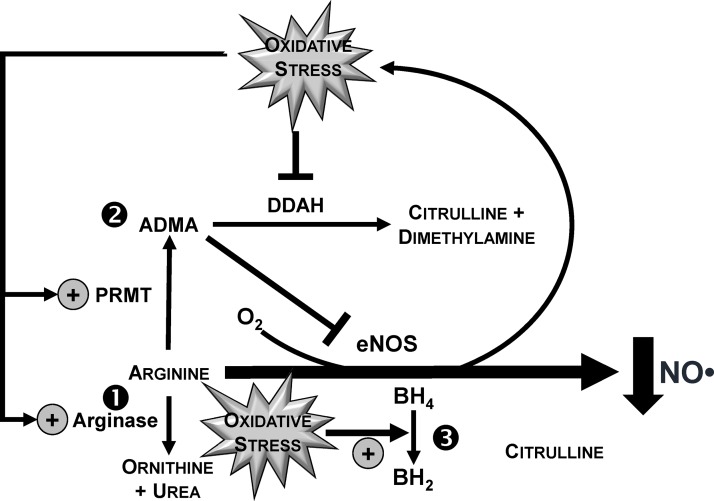
The mechanism by which postprandial hyperglycemia impairs vascular function is incompletely understood, but as reviewed (6) one major contributor is decreased NO bioavailability caused by oxidative stress (47–49). With the use of brachial artery FMD, postprandial hyperglycemia impairs vascular function, consistent with a mechanism of decreasing NO status in an oxidative stress-dependent manner (50). In a crossover design, healthy young men ingested 75 g of glucose or fructose before assessing vascular function at 30-min intervals for 3 h postprandially. Ingestion of glucose, but not fructose, transiently increased plasma glucose with a concomitant decrease in FMD responses. FMD responses were also inversely correlated with postprandial glycemia (r = −0.82, P < 0.05), supporting the concept that reductions in postmeal hyperglycemia would protect against vascular dysfunction. Malondialdehyde, a biomarker of lipid peroxidation that was measured by HPLC coupled to a fluorescence detector, also increased to a greater extent after glucose ingestion. Malondialdehyde was positively correlated to glycemic responses (r = 0.87, P < 0.05), whereas it was inversely correlated to FMD (r = −0.80, P < 0.05), supporting that the impairment in postprandial vascular function is an oxidative stress-dependent event. Oxidative stress is also likely to impair vascular function by increasing asymmetric dimethylarginine (ADMA) relative to Arg (50) (Figure 3). ADMA is an endogenously produced competitive inhibitor of endothelial NO synthase (eNOS) that outcompetes Arg for eNOS-mediated synthesis of NO (6). Although circulating ADMA is constitutively low at ∼0.5 μM, elevations of 0.1 μM raise CVD risk up to 5.3-fold (51), likely by lowering NO status (52). Consistent with this paradigm, hyperglycemia-induced vascular dysfunction after an oral glucose challenge was associated with increased plasma ADMA/Arg (50). Specifically, glucose ingestion decreased plasma Arg to a greater extent than fructose, whereas postprandial ADMA concentrations were unaffected. However, ingestion of glucose caused greater time-dependent increases in ADMA/Arg relative to that occurring in response to fructose ingestion. Thus, postprandial hyperglycemia is likely to reduce NO bioavailability by limiting Arg availability and increasing ADMA relative to Arg, which is expected to limit substrate availability for NO biosynthesis while competitively inhibiting eNOS. Although not yet investigated clinically, oxidative stress also decreases NO bioavailability by oxidizing tetrahydrobiopterin to dihydrobiopterin, which limits tetrahydrobiopterin binding to eNOS to prevent NO synthesis (53) (Figure 3). Studies in endothelial cells show that 48 h of high-glucose treatment decreases the ratio tetrahydrobiopterin:dihydrobiopterin (54). In healthy adults, intra-arterial infusion of 6R-tetrahydrobiopterin prevented decreases in endothelium-dependent vasodilation that were otherwise induced by an oral glucose challenge (55), supporting that postprandial hyperglycemia impairs vascular function by reducing tetrahydrobiopterin availability. However, the extent to which postprandial hyperglycemia directly diminishes vascular function by lowering NO bioavailability in a tetrahydrobiopterin-dependent manner requires investigation.
FIGURE 3.
Postprandial hyperglycemia induces oxidative stress responses that limit NO bioavailability. Studies in preclinical and clinical models support findings that oxidative stress downstream of acute hyperglycemia reduces NO bioavailability to the vascular endothelium by 1) increasing the catabolism of arginine in an arginase-dependent manner, 2) increasing the accumulation of ADMA by upregulating PRMT activity and downregulating DDAH activity, and 3) increasing the oxidation of BH4 to BH2. The net effect of greater ADMA relative to arginine is an increase in the competitive inhibition of eNOS binding toward arginine. ADMA, asymmetric dimethylarginine; BH2, dihydrobiopterin; BH4, tetrahydrobiopterin; DDAH, dimethylarginine dimethylaminohydrolase; eNOS, endothelial NO synthase; PRMT, protein arginine methyltransferase.
Dietary approaches to regulate postprandial vascular function.
Although postmeal hyperglycemia transiently impairs vascular function (6), few studies have investigated dietary approaches that may provide vasoprotective activities during the postprandial period. Such strategies could target vascular dysfunction directly by attenuating postprandial hyperglycemia or indirectly by mitigating pathogenic responses downstream of hyperglycemia that promote vascular dysfunction. Although ingestion of various carbohydrate types was examined for their effects on the postprandial glycemia index (56), few studies have linked their differential glycemic responses to postprandial vascular function. Actively under way are studies that examine starch ingestion (50 g) on vascular health to support that postprandial increases in blood glucose are attenuated (57) relative to the ingestion of glucose (75 g) (50). However, despite a lowering in the magnitude of hyperglycemia, postprandial brachial artery FMD after the ingestion of starch was lowered at the same time point that corresponded to peak blood glucose (60 min), suggesting that vascular function continues to be impaired despite reducing hyperglycemia. Continued studies are needed to examine whether attenuations in postprandial hyperglycemia also mitigate oxidative stress responses that would be expected to reduce NO bioavailability.
The involvement of oxidative stress in response to a glucose challenge supports dietary antioxidants in potentially mitigating impairments in vascular function independent of changes in hyperglycemia. Title et al. (58) demonstrated that co-administration of vitamin C (2 g) and α-tocopherol (800 IU) with an oral glucose challenge (75 g) maintained brachial artery FMD responses that were otherwise impaired by ingesting glucose alone. However, it remains unclear how these vasoprotective activities occurred. Neither plasma antioxidant was measured during the 4-h postprandial period, and the time to maximal plasma concentration of α-tocopherol occurs at ∼12 h after oral ingestion (59, 60). In contrast, vitamin C is water soluble and achieves peak plasma concentrations at ∼150 min after oral ingestion (61), supporting that the acute vasoprotective activities observed after co-antioxidant ingestion were largely mediated through vitamin C rather than their combined actions. To better evaluate vasoprotective effects of vitamin E, a crossover study was conducted in normoglycemic adults in which they completed an oral glucose challenge (75 g) without or with 5 d of prior supplementation of a γ-tocopherol-rich supplement (500 mg γ-tocopherol/d) (62). In response to short-term supplementation, plasma γ-tocopherol concentrations increased from 2.2 μM up to 6.6 μM, and γ-carboxyethyl-hydroxychromanol (γ-CEHC), the physiologic metabolite of γ-tocopherol, increased from 0.32 μM up to 3.1 μM; plasma α-tocopherol was unaffected. Although improvements in γ-tocopherol status did not affect postprandial hyperglycemic responses after an oral glucose challenge, supplementation protected against postprandial decreases in FMD responses that otherwise occurred after glucose ingestion (62). Maintenance of vascular function by vitamin E supplementation was also accompanied by a blunting in postprandial malondialdehyde concentrations and ration of ADMA to Arg. These findings suggest that γ-tocopherol and/or γ-CEHC protected against hyperglycemia-induced vascular impairments, likely in an oxidative stress-dependent manner that improves NO bioavailability but independent of any changes in glycemic responses. Additional research is needed to identify the independent and additive contributions of γ-tocopherol and γ-CEHC in regulating vascular dysfunction and the extent to which other dietary antioxidants similarly protect against postprandial hyperglycemia-mediated impairments in vascular health.
Inflammation during postprandial hyperglycemia-mediated vascular dysfunction.
Clear evidence implicates oxidative stress in contributing to hyperglycemia-mediated vascular dysfunction (6). Although studies in vitro consistently show that high-glucose treatments (typically ∼25 mM for 24 h) induce NFκB-dependent inflammatory responses, findings from clinical studies were equivocal with some showing induction of postprandial inflammation in response to acute hyperglycemia, whereas others show no changes in proinflammatory mediators (6). Studies in adults without diabetes support this relation with 2-h blood glucose concentrations after an oral GTT to predict the magnitude of circulating C-reactive protein (63). In healthy adults, plasma TNFα concentrations and NFκB binding activity increase at 1 h after an oral glucose challenge (64). In contrast, studies specifically aimed at defining postprandial vascular function show that a number of inflammatory proteins and adhesion molecules (i.e., C-reactive protein, TNFα, IL-6, IL-10, myeloperoxidase, intercellular adhesion molecule 1, vascular cell adhesion molecule 1, E-selectin) are unaffected in response to a 75-g glucose challenge (50) or supplementation of a γ-tocopherol-rich supplement before administration of a 75-g glucose challenge (62) in young, normoglycemic adults. Additional study is needed to address the role of inflammation on glycemia-mediated vascular dysfunction and whether these inconsistent study outcomes are due to differences in participant age or the presence of underlying morbidities that may exacerbate postprandial inflammation. Future research in this area should also focus on the following questions:
Are there additional NO-independent mechanisms during post-meal hyperglycemia-mediated vascular dysfunction?
How do mixed-meals affect hyperglycemia-induced impairments in postprandial vascular function?
What is the metabolic origin of reactive oxygen species that contribute to oxidative stress (e.g., mitochondrial, activated inflammatory cells)?
Although findings of meta-analyses support the use of brachial artery FMD in predicting future CVD events (65, 66), more research is needed to establish clear associations between postprandial FMD responses and the risk of CVD and the basic mechanisms to support these relations. Further, one of the most important research areas that has emerged over the past decade is the impact of circadian disruption on metabolism. In this regard, sleep restriction was also found to impair FMD responses, consistent with the growing epidemiologic evidence that associates short sleep duration with increased cardiovascular events (67). The final section of this review describes recent studies to investigate metabolic mechanisms by which lack of sleep increases type 2 diabetes and cardiometabolic risk.
Insufficient Sleep and Dysregulation of Nutrient Metabolism
Normal sleep is regulated by sleep-wake homeostasis and circadian rhythmicity, which are both known to affect peripheral energy metabolism via neuronal and hormonal pathways (68). Despite current recommendations that adults should sleep ≥7 h/night to promote optimal health (69), sleep curtailment has become an increasingly prevalent behavior in modern society. Estimates indicate that average sleep duration has decreased by 1.5–2.0 h in the past half century. According to the National Sleep Foundation (70), as many as one-third of Americans reported not getting enough sleep by comparing the hours of sleep they said they needed with the hours of sleep they were actually getting on workdays or weekdays. Overall, >50% of the respondents agreed that not getting enough sleep affected their job performance, relationships with family or friends, and ability to perform everyday activities.
Sleep restriction and insulin resistance.
In multiple epidemiologic studies, short sleep duration is associated with increased risk of obesity, type 2 diabetes, hypertension, and CVD, and these observational data were discussed comprehensively in 2 recent reviews in Advances in Nutrition (71, 72). Emerging data in this field have suggested that an increased consumption of energy dense and highly palatable foods in the sleep-deprived state is explained by alterations in hunger hormones, hedonic pathways, and the time of intake (71). Notably, the present discussion of this topic focuses on the nocturnal and subsequent nutrient handling throughout the day that occurs in humans after sleep restriction under experimental conditions. In well-controlled laboratory settings, insufficient sleep that results from either short sleep duration and/or circadian disruption is associated with marked reductions in insulin sensitivity and glucose intolerance (9, 73–79). Moreover, reduced sleep quality in healthy volunteers, induced experimentally by acoustic stimuli, resulted in decreased insulin sensitivity and glucose tolerance as assessed by an intravenous GTT (80). Potential mechanisms that play a role in the link between insufficient sleep and insulin resistance include alterations in brain glucose utilization, increased sympathetic activity, hyperactivity of the hypothalamic-pituitary axis, altered adipokine secretion, and elevated plasma FFAs. Acute elevations in circulating FFA concentrations are associated with insulin resistance (81–84). Under normal conditions, a marked diurnal variation in circulating FFA concentrations is observed in healthy subjects with peak concentrations occurring in the middle of the night while declining toward the morning (85).
To address the role of FFAs in producing insulin resistance associated with sleep restriction, healthy young men were studied under controlled laboratory conditions with 4 consecutive nights of 8.5 h in bed (normal sleep) or 4.5 h in bed (sleep restriction) in a randomized order. Twenty-four-hour blood profiles of FFAs and hormones, including growth hormone, noradrenaline, and cortisol, were assessed simultaneously (86). Insulin sensitivity was measured with the use of a frequently sampled intravenous GTT. When sleep was restricted, FFA concentrations remained elevated throughout the night and early morning hours during the prolonged fasting state (86). However, subsequent daytime FFA concentrations were similar between sleep conditions, suggesting that sleep restriction may differentially affect FFA concentrations during fasting versus fed states. Sleep restriction was also associated with extended nocturnal growth hormone secretion at night, increased norepinephrine concentrations during the early nighttime and early morning hours, and increased evening cortisol concentrations. The elevation in FFAs was correlated with prolonged nocturnal growth hormone secretion (r = 0.81, P < 0.0001) and higher early morning noradrenaline concentrations (r = 0.55, P = 0.015). Insulin sensitivity was impaired by 23% after sleep restriction, and the reduction in insulin sensitivity correlated with the increase in nocturnal FFA concentrations (r = −0.52, P = 0.05). Elevations in norepinephrine concentrations support the hypothesis that augmented sympathetic drive may be a potential mediator of the metabolic consequences associated with sleep loss. In summary, this randomized, controlled laboratory study found that elevated FFAs contribute, at least in part, to insulin resistance and may lead to the elevated diabetes risk associated with long-term sleep restriction (86).
These more comprehensive findings extend earlier studies of sleep restriction when FFA concentrations were measured only in the morning hours over a limited number of time points (87, 88). These findings also suggest a mechanism of adipoctye cell resistance to the antilipolytic effects of insulin which is consistent with impaired intracellular insulin signaling in adipose tissue that occurs after sleep restriction (76, 89). Future mechanistic studies (e.g., utilization of pharmacologic agents to suppress FFAs) will be necessary to support a causal role for FFAs in insulin resistance induced by sleep loss. Circadian disruption has also been associated with adverse metabolic effects (79, 90), and evidence exists to support the importance of clock genes (CLOCK and BMAL1) in regulating lipolytic activity in adipose tissue (91).
Effects of sleep restriction on postprandial metabolism.
As discussed in the section above, most studies to date have focused on investigating the impact of insufficient sleep on metabolic events that occur early in the morning hours and during the fasted state. However, whether reduced insulin sensitivity in the sleep-deprived state may also feed forward to impair postprandial responses throughout the next day is not well understood. In the protocol of Broussard et al. (86) discussed above, healthy men underwent 4 nights of sleep restriction after which a 12-h period of metabolism was monitored, in the postprandial state. Sequential, identical, carbohydrate-rich meals were fed at breakfast (0900), lunch (1400), and dinner (1900). Postmeal glucose responses tended to be increased (P = 0.061), and insulin concentrations were significantly increased (P = 0.045) after breakfast only, suggesting that prolonged sleep restriction impaired glucose metabolism at breakfast, but that the sleep effect did not persist after the later meals. By contrast, another study in healthy men showed that metabolic impairments may accumulate throughout the day (92). In this study, Reynolds et al. (92) fed 3 identical meals (53% of energy from carbohydrate, 27% from fat, and 17% from protein) on the fifth night of sleep restriction (4 h in bed) and monitored 24-h interstitial glucose concentrations by a continuous glucose monitoring system. Relative to normal sleep (10 h in bed), sleep-restricted glucose concentrations were consistently elevated after each of the 3 meals, particularly after dinner during the nighttime hours. In other sleep restriction studies, subjects consumed additional energy after dinner, when awake (93, 94); thus, the sleep-restricted impairment in postprandial glucose metabolism may worsen health even more, if it also leads to increased energy intake of foods with high glycemic index at night after dinner. Interestingly, in a study that examined sex differences during sleep restriction, men exhibited a greater increase in energy intake during the late-night hours relative to women (94).
The effect of sleep restriction on postprandial lipid responses is also understudied. Wehrens et al. (88) investigated the effect of total sleep deprivation on lipid metabolism and found that postmeal TG responses were higher after a night of sleep recovery than after total sleep deprivation. These data suggest that metabolic abnormalities may appear after a stressor was removed and that inconsistent sleep patterns (switching between working nights and days) may be more detrimental than constant night-shift work. Another important area completely lacking in information, to our knowledge, is the impact of sleep restriction on amino acid metabolism. Given that sleep deprivation is associated with increased insulin resistance, more research is needed to identify whether it impairs whole-body protein synthesis and degradation. Future studies could be designed to address this issue and other questions, as follows:
What are the independent effects of dysregulated circadian timing on FFA metabolism?
Do men and woman respond differently to sleep restriction?
What are the effects of sleep restriction on sarcopenia in aging and disease processes?
Can correcting sleep restriction with adequate sleep improve cardiometabolic outcomes?
Conclusions and Future Directions
Dysfunctional metabolism of macronutrients characterizes the pathophysiology of obesity and insulin resistance, and the altered events downstream of metabolic flux clearly contribute to accelerated CVD progression. The influence of acute stressors on circadian energy metabolism has gained increased attention because these factors may be responsible for accumulated long-term disease risk (Figure 4). Understanding the regulation of enterocyte lipid handling throughout the day will provide novel therapeutic strategies to reduce CVD risk. Furthermore, clinical studies have provided clear evidence that prolonged postprandial hyperglycemia transiently impairs vascular function. In this regard, targeting postmeal hyperglycemia will be particularly important, given recent data to indicate that dietary energy intakes of Americans have increased by nearly 200 kcal/d, which was attributed partly to greater intakes of refined grains, sugars, and starches (96). Experimental laboratory studies have shown that insufficient sleep, which is increasingly common in modern society, is associated with adverse effects on energy metabolism that lead to poor nutrient handling early during the day and later during the nighttime hours. Further, it is important to appreciate that the variability among subjects in postprandial responses may be due to differences in enteral lipid handling, postabsorptive events in the vasculature, or lifestyle factors such as restricted sleep. As the field of postprandial metabolism advances, it is likely that future discoveries that underscore the interplay between acute stressors and macronutrient metabolism will become increasingly important. To support advancements in the clinical management of disease, future studies should focus on individual responses to meal components and on optimizing sleep and circadian factors to reduce cardiometabolic risk.
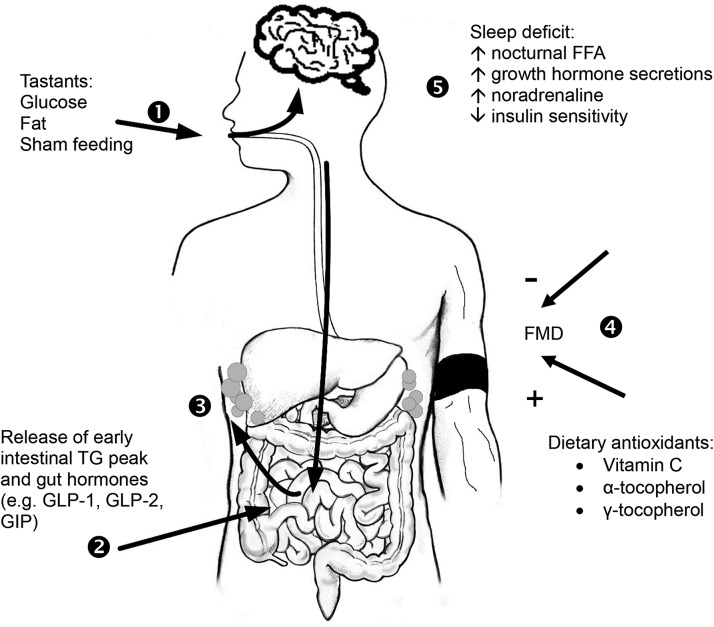
FIGURE 4.
The interplay between macronutrient metabolism and cardiometabolic risk may encompass the initial encounter of dietary fat and carbohydrate with taste buds through their eventual site of storage in the body and can be influenced by basal conditions present early in the day. 1) At the onset of a meal, putative taste receptors signal through the taste buds to the brain, and 2) the signal is hypothesized to be transported to the vagus nerve to cause the release of an early intestinal TG peak and gut hormones (e.g., GLP-1, GLP-2, GIP). 3) This taste/intestinal lipid axis may provide a potential feed-forward signal (arrow to adipose) of impeding dietary load by controlling lipid secretion and storage in adipose. 4) On feeding, postprandial excursions of lipids or glucose can impair FMD which is indicative of endothelial dysfunction, whereas dietary components that attenuate oxidative stress, for instance, exhibit vasoprotective activities during acute hyperglycemia. 5) Sleep deficit results in nocturnal FFAs that remain elevated throughout the next morning, along with prolonged nocturnal growth hormone secretion and higher early morning noradrenaline concentrations. These metabolic adaptations in the sleep-deprived state may accumulate to impair postmeal responses during the day. FMD, flow mediated dilation; GLP, glucagon-like peptide; GIP, gastric inhibitory peptide. Adapted from reference 95 with permission.
Acknowledgments
We thank Elizabeth Miller for her art work. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: ADMA, asymmetric dimethylarginine; CEHC, carboxyethyl-hydroxychromanol; CVD, cardiovascular disease; DPP-4, dipeptidyl peptidase-4; eNOS, endothelial nitric oxide synthase; FMD, flow-mediated dilation; GLP, glucagon-like peptide; GTT, glucose tolerance test.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, et al. Executive summary: heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation 2010;121:948–54. [DOI] [PubMed] [Google Scholar]
- 2.Pearson TA, Blair SN, Daniels SR, Eckel RH, Fair JM, Fortmann SP, Franklin BA, Goldstein LB, Greenland P, Grundy SM, et al. AHA guidelines for primary prevention of cardiovascular disease and stroke: 2002 update: consensus panel guide to comprehensive risk reduction for adult patients without coronary or other atherosclerotic vascular diseases. American Heart Association science advisory and coordinating committee. Circulation 2002;106:388–91. [DOI] [PubMed] [Google Scholar]
- 3.Borén J, Matikainen N, Adiels M, Taskinen MR. Postprandial hypertriglyceridemia as a coronary risk factor. Clin Chim Acta 2014;431:131–42. [DOI] [PubMed] [Google Scholar]
- 4.DECODE Study Group; on behalf of the European Diabetes Epidemiology Group. Glucose tolerance and cardiovascular mortality: comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med 2001;161:397–405. [DOI] [PubMed] [Google Scholar]
- 5.Xiao C, Dash S, Morgantini C, Adeli K, Lewis GF. Gut peptides are novel regulators of intestinal lipoprotein secretion: experimental and pharmacological manipulation of lipoprotein metabolism. Diabetes 2015;64:2310–8. [DOI] [PubMed] [Google Scholar]
- 6.Mah E, Bruno RS. Postprandial hyperglycemia on vascular endothelial function: mechanisms and consequences. Nutr Res 2012;32:727–40. [DOI] [PubMed] [Google Scholar]
- 7.Miles JM, Wooldridge D, Grellner WJ, Windsor S, Isley WL, Klein S, Harris WS. Nocturnal and postprandial free fatty acid kinetics in normal and type 2 diabetic subjects: effects of insulin sensitization therapy. Diabetes 2003;52:675–81. [DOI] [PubMed] [Google Scholar]
- 8.Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol 2009;5:253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E.. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol (1985) 2005;99:2008–19. [DOI] [PubMed] [Google Scholar]
- 10.Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 2011;300:H2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer C, Dostou JM, Welle SL, Gerich JE. Role of human liver, kidney, and skeletal muscle in postprandial glucose homeostasis. Am J Physiol Endocrinol Metab 2002;282:E419–27. [DOI] [PubMed] [Google Scholar]
- 12.Mjos OD, Faergeman O, Hamilton RL, Havel RJ. Characterization of remnants produced during the metabolism of triglyceride-rich lipoproteins of blood plasma and intestinal lymph in the rat. J Clin Invest 1975;56:603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng C, Ikewaki K, Walsh BW, Sacks FM. Metabolism of apoB lipoproteins of intestinal and hepatic origin during constant feeding of small amounts of fat. J Lipid Res 2006;47:1771–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schneeman BO, Kotite L, Todd KM, Havel RJ. Relationships between the responses of triglyceride-rich lipoproteins in blood plasma containing apolipoproteins B-48 and B-100 to a fat-containing meal in normolipidemic humans. Proc Natl Acad Sci USA 1993;90:2069–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergeron N, Havel RJ. Influence of diets rich in saturated and omega-6 polyunsaturated fatty acids on the postprandial responses of apolipoproteins B-48, B-100, E, and lipids in triglyceride-rich lipoproteins. Arterioscler Thromb Vasc Biol 1995;15:2111–21. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi H, Fujimoto K, Cardelli JA, Nutting DF, Bergstedt S, Tso P. Fat feeding increases size, but not number, of chylomicrons produced by small intestine. Am J Physiol 1990;259:G709–19. [DOI] [PubMed] [Google Scholar]
- 17.Chavez-Jauregui RN, Mattes RD, Parks EJ. Dynamics of fat absorption and effect of sham feeding on postprandial lipema. Gastroenterology 2010;139:1538–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Timlin MT, Barrows B, Parks EJ. Increased dietary substrate delivery alters hepatic fatty acid recycling in healthy men. Diabetes 2005;54:2694–701. [DOI] [PubMed] [Google Scholar]
- 19.Robertson MD, Parkes M, Warren BF, Ferguson DJ, Jackson KG, Jewell DP, Frayn KN. Mobilisation of enterocyte fat stores by oral glucose in humans. Gut 2003;52:834–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heath RB, Jones R, Frayn KN, Robertson MD. Vagal stimulation exaggerates the inhibitory ghrelin response to oral fat in humans. J Endocrinol 2004;180:273–81. [DOI] [PubMed] [Google Scholar]
- 21.Getty-Kaushik L, Song DH, Boylan MO, Corkey BE, Wolfe MM. Glucose-dependent insulinotropic polypeptide modulates adipocyte lipolysis and reesterification. Obesity (Silver Spring) 2006;14:1124–31. [DOI] [PubMed] [Google Scholar]
- 22.Kim SJ, Nian C, McIntosh CH. GIP increases human adipocyte LPL expression through CREB and TORC2-mediated trans-activation of the LPL gene. J Lipid Res 2010;51:3145–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song DH, Getty-Kaushik L, Tseng E, Simon J, Corkey BE, Wolfe MM. Glucose-dependent insulinotropic polypeptide enhances adipocyte development and glucose uptake in part through Akt activation. Gastroenterology 2007;133:1796–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McLaren DG, Cardasis HL, Stout SJ, Wang SP, Mendoza V, Castro-Perez JM, Miller PL, Murphy BA, Cumiskey AM, Cleary MA, et al. Use of [13C18] oleic acid and mass isotopomer distribution analysis to study synthesis of plasma triglycerides in vivo: analytical and experimental considerations. Anal Chem 2013;85:6287–94. [DOI] [PubMed] [Google Scholar]
- 25.Pavlic M, Xiao C, Szeto L, Patterson BW, Lewis GF. Insulin acutely inhibits intestinal lipoprotein secretion in humans in part by suppressing plasma free fatty acids. Diabetes 2010;59:580–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duez H, Lamarche B, Uffelman KD, Valero R, Cohn JS, Lewis GF. Hyperinsulinemia is associated with increased production rate of intestinal apolipoprotein B-48-containing lipoproteins in humans. Arterioscler Thromb Vasc Biol 2006;26:1357–63. [DOI] [PubMed] [Google Scholar]
- 27.Borthwick F, Mangat R, Warnakula S, Jacome-Sosa M, Vine DF, Proctor SD. Simvastatin treatment upregulates intestinal lipid secretion pathways in a rodent model of the metabolic syndrome. Atherosclerosis 2014;232:141–8. [DOI] [PubMed] [Google Scholar]
- 28.Veilleux A, Grenier E, Marceau P, Carpentier AC, Richard D, Levy E. Intestinal lipid handling: evidence and implication of insulin signaling abnormalities in human obese subjects. Arterioscler Thromb Vasc Biol 2014;34:644–53. [DOI] [PubMed] [Google Scholar]
- 29.Xiao C, Dash S, Morgantini C, Lewis GF. Novel role of enteral monosaccharides in intestinal lipoprotein production in healthy humans. Arterioscler Thromb Vasc Biol 2013;33:1056–62. [DOI] [PubMed] [Google Scholar]
- 30.Morgantini C, Xiao C, Dash S, Lewis GF. Dietary carbohydrates and intestinal lipoprotein production. Curr Opin Clin Nutr Metab Care 2014;17:355–9. [DOI] [PubMed] [Google Scholar]
- 31.Nogueira JP, Maraninchi M, Béliard S, Padilla N, Duvillard L, Mancini J, Nicolay A, Xiao C, Vialettes B, Lewis GF, et al. Absence of acute inhibitory effect of insulin on chylomicron production in type 2 diabetes. Arterioscler Thromb Vasc Biol 2012;32:1039–44. [DOI] [PubMed] [Google Scholar]
- 32.Elliott RM, Morgan LM, Tredger JA, Deacon S, Wright J, Marks V. Glucagon-like peptide-1 (7–36)amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: acute post-prandial and 24-h secretion patterns. J Endocrinol 1993;138:159–66. [DOI] [PubMed] [Google Scholar]
- 33.Xiao Q, Boushey RP, Drucker DJ, Brubaker PL. Secretion of the intestinotropic hormone glucagon-like peptide 2 is differentially regulated by nutrients in humans. Gastroenterology 1999;117:99–105. [DOI] [PubMed] [Google Scholar]
- 34.Holst JJ. Gut hormones as pharmaceuticals. From enteroglucagon to GLP-1 and GLP-2. Regul Pept 2000;93:45–51. [DOI] [PubMed] [Google Scholar]
- 35.Xiao C, Bandsma RH, Dash S, Szeto L, Lewis GF. Exenatide, a glucagon-like peptide-1 receptor agonist, acutely inhibits intestinal lipoprotein production in healthy humans. Arterioscler Thromb Vasc Biol 2012;32:1513–9. [DOI] [PubMed] [Google Scholar]
- 36.Xiao C, Dash S, Morgantini C, Patterson BW, Lewis GF. Sitagliptin, a DPP-4 inhibitor, acutely inhibits intestinal lipoprotein particle secretion in healthy humans. Diabetes 2014;63:2394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Færch K, Torekov SS, Vistisen D, Johansen NB, Witte DR, Jonsson A, Pedersen O, Hansen T, Lauritzen T, Sandbaek A, et al. GLP-1 response to oral glucose is reduced in prediabetes, screen-detected type 2 diabetes, and obesity and influenced by sex: the ADDITION-PRO study. Diabetes 2015;64:2513–25. [DOI] [PubMed] [Google Scholar]
- 38.Dash S, Xiao C, Morgantini C, Connelly PW, Patterson BW, Lewis GF. Glucagon-like peptide-2 regulates release of chylomicrons from the intestine. Gastroenterology 2014;147:1275–1284.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hein GJ, Baker C, Hsieh J, Farr S, Adeli K. GLP-1 and GLP-2 as yin and yang of intestinal lipoprotein production: evidence for predominance of GLP-2-stimulated postprandial lipemia in normal and insulin-resistant states. Diabetes 2013;62:373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsieh J, Longuet C, Baker CL, Qin B, Federico LM, Drucker DJ, Adeli K. The glucagon-like peptide 1 receptor is essential for postprandial lipoprotein synthesis and secretion in hamsters and mice. Diabetologia 2010;53:552–61. [DOI] [PubMed] [Google Scholar]
- 41.Farr S, Baker C, Naples M, Taher J, Iqbal J, Hussain M, Adeli K. Central nervous system regulation of intestinal lipoprotein metabolism by glucagon-like peptide-1 via a brain-gut axis. Arterioscler Thromb Vasc Biol 2015;35:1092–100. [DOI] [PubMed] [Google Scholar]
- 42.Guan X, Karpen HE, Stephens J, Bukowski JT, Niu S, Zhang G, Stoll B, Finegold MJ, Holst JJ, Hadsell D, et al. GLP-2 receptor localizes to enteric neurons and endocrine cells expressing vasoactive peptides and mediates increased blood flow. Gastroenterology 2006;130:150–64. [DOI] [PubMed] [Google Scholar]
- 43.Bremholm L, Hornum M, Andersen UB, Holst JJ. The effect of glucagon-like peptide-2 on arterial blood flow and cardiac parameters. Regul Pept 2010;159:67–71. [DOI] [PubMed] [Google Scholar]
- 44.Denison H, Nilsson C, Kujacic M, Lofgren L, Karlsson C, Knutsson M, Eriksson JW. Proof of mechanism for the DGAT1 inhibitor AZD7687: results from a first-time-in-human single-dose study. Diabetes Obes Metab 2013;15:136–43. [DOI] [PubMed] [Google Scholar]
- 45.Meyers CD, Tremblay K, Amer A, Chen J, Jiang L, Gaudet D. Effect of the DGAT1 inhibitor pradigastat on triglyceride and apoB48 levels in patients with familial chylomicronemia syndrome. Lipids Health Dis 2015;14:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson BD, Padilla J, Harris RA, Wallace JP. Vascular consequences of a high-fat meal in physically active and inactive adults. Appl Physiol Nutr Metab 2011;36:368–75. [DOI] [PubMed] [Google Scholar]
- 47.Ceriello A, Bortolotti N, Crescentini A, Motz E, Lizzio S, Russo A, Ezsol Z, Tonutti L, Taboga C. Antioxidant defences are reduced during the oral glucose tolerance test in normal and non-insulin-dependent diabetic subjects. Eur J Clin Invest 1998;28:329–33. [DOI] [PubMed] [Google Scholar]
- 48.Ceriello A, Taboga C, Tonutti L, Quagliaro L, Piconi L, Bais B, Da Ros R, Motz E. Evidence for an independent and cumulative effect of postprandial hypertriglyceridemia and hyperglycemia on endothelial dysfunction and oxidative stress generation: effects of short- and long-term simvastatin treatment. Circulation 2002;106:1211–8. [DOI] [PubMed] [Google Scholar]
- 49.Kawano H, Motoyama T, Hirashima O, Hirai N, Miyao Y, Sakamoto T, Kugiyama K, Ogawa H, Yasue H. Hyperglycemia rapidly suppresses flow-mediated endothelium-dependent vasodilation of brachial artery. J Am Coll Cardiol 1999;34:146–54. [DOI] [PubMed] [Google Scholar]
- 50.Mah E, Noh SK, Ballard KD, Matos ME, Volek JS, Bruno RS. Postprandial hyperglycemia impairs vascular endothelial function in healthy men by inducing lipid peroxidation and increasing asymmetric dimethylarginine:arginine. J Nutr 2011;141:1961–8. [DOI] [PubMed] [Google Scholar]
- 51.De Gennaro Colonna V, Bianchi M, Pascale V, Ferrario P, Morelli F, Pascale W, Tomasoni L, Turiel M. Asymmetric dimethylarginine (ADMA): an endogenous inhibitor of nitric oxide synthase and a novel cardiovascular risk molecule. Med Sci Monit 2009;15:RA91–101. [PubMed] [Google Scholar]
- 52.Böger RH. The emerging role of asymmetric dimethylarginine as a novel cardiovascular risk factor. Cardiovasc Res 2003;59:824–33. [DOI] [PubMed] [Google Scholar]
- 53.Daff S. NO synthase: structures and mechanisms. Nitric Oxide 2010;23:1–11. [DOI] [PubMed] [Google Scholar]
- 54.Crabtree MJ, Smith CL, Lam G, Goligorsky MS, Gross SS. Ratio of 5,6,7,8-tetrahydrobiopterin to 7,8-dihydrobiopterin in endothelial cells determines glucose-elicited changes in NO vs. superoxide production by eNOS. Am J Physiol Heart Circ Physiol 2008;294:H1530–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ihlemann N, Rask-Madsen C, Perner A, Dominguez H, Hermann T, Kober L, Torp-Pedersen C. Tetrahydrobiopterin restores endothelial dysfunction induced by an oral glucose challenge in healthy subjects. Am J Physiol Heart Circ Physiol 2003;285:H875–82. [DOI] [PubMed] [Google Scholar]
- 56.Brand-Miller JC, Stockmann K, Atkinson F, Petocz P, Denyer G. Glycemic index, postprandial glycemia, and the shape of the curve in healthy subjects: analysis of a database of more than 1,000 foods. Am J Clin Nutr 2009;89:97–105. [DOI] [PubMed] [Google Scholar]
- 57.Sapper T, Mah E, Ahn-Jarvis J, Vodovotz Y, Bruno R. Postprandial hyperglycemia-induced vascular endothelial dysfunction is unaffected by a green tea containing starch confection. FASEB J 2015;29(1 Suppl):608.3. [Google Scholar]
- 58.Title LM, Cummings PM, Giddens K, Nassar BA. Oral glucose loading acutely attenuates endothelium-dependent vasodilation in healthy adults without diabetes: an effect prevented by vitamins C and E. J Am Coll Cardiol 2000;36:2185–91. [DOI] [PubMed] [Google Scholar]
- 59.Leonard SW, Paterson E, Atkinson JK, Ramakrishnan R, Cross CE, Traber MG. Studies in humans using deuterium-labeled alpha- and gamma-tocopherols demonstrate faster plasma gamma-tocopherol disappearance and greater gamma-metabolite production. Free Radic Biol Med 2005;38:857–66. [DOI] [PubMed] [Google Scholar]
- 60.Bruno RS, Leonard SW, Atkinson J, Montine TJ, Ramakrishnan R, Bray TM, Traber MG. Faster plasma vitamin E disappearance in smokers is normalized by vitamin C supplementation. Free Radic Biol Med 2006;40:689–97. [DOI] [PubMed] [Google Scholar]
- 61.Padayatty SJ, Sun H, Wang Y, Riordan HD, Hewitt SM, Katz A, Wesley RA, Levine M. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med 2004;140:533–7. [DOI] [PubMed] [Google Scholar]
- 62.Mah E, Noh SK, Ballard KD, Park HJ, Volek JS, Bruno RS. Supplementation of a gamma-tocopherol-rich mixture of tocopherols in healthy men protects against vascular endothelial dysfunction induced by postprandial hyperglycemia. J Nutr Biochem 2013;24:196–203. [DOI] [PubMed] [Google Scholar]
- 63.Festa A, D’Agostino R Jr, Tracy RP, Haffner SM. C-reactive protein is more strongly related to post-glucose load glucose than to fasting glucose in non-diabetic subjects; the Insulin Resistance Atherosclerosis Study. Diabet Med 2002;19:939–43. [DOI] [PubMed] [Google Scholar]
- 64.Aljada A, Friedman J, Ghanim H, Mohanty P, Hofmeyer D, Chaudhuri A, Dandona P. Glucose ingestion induces an increase in intranuclear nuclear factor kappaB, a fall in cellular inhibitor kappaB, and an increase in tumor necrosis factor alpha messenger RNA by mononuclear cells in healthy human subjects. Metabolism 2006;55:1177–85. [DOI] [PubMed] [Google Scholar]
- 65.Ras RT, Streppel MT, Draijer R, Zock PL. Flow-mediated dilation and cardiovascular risk prediction: a systematic review with meta-analysis. Int J Cardiol 2013;168:344–51. [DOI] [PubMed] [Google Scholar]
- 66.Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging 2010;26:631–40. [DOI] [PubMed] [Google Scholar]
- 67.Calvin AD, Covassin N, Kremers WK, Adachi T, Macedo P, Albuquerque FN, Bukartyk J, Davison DE, Levine JA, Singh P, et al. Experimental sleep restriction causes endothelial dysfunction in healthy humans. J Am Heart Assoc 2014;3:e001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van Cauter E, Tasali E. Endocrine physiology in relation to sleep and sleep disturbances. In: Kryger M, Roth T, Dement W, editors. Principles and practice of sleep disorders. Philadelphia: Elsevier and Saunders; 2011. p. 291–311.
- 69.Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, Buysse D, Dinges DF, Gangwisch J, Grandner MA, Kushida C, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep 2015;38:843–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Centers for Disease Control and Prevention. Unhealthy sleep-related behaviors - 12 states, 2009. MMWR Morb Mortal Wkly Rep 2011;6(8):233–8. [PubMed] [Google Scholar]
- 71.Dashti HS, Scheer FA, Jacques PF, Lamon-Fava S, Ordovás JM. Short sleep duration and dietary intake: epidemiologic evidence, mechanisms, and health implications. Adv Nutr 2015;6:648–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Golem DL, Martin-Biggers JT, Koenings MM, Davis KF, Byrd-Bredbenner C. An integrative review of sleep for nutrition professionals. Adv Nutr 2014;5:742–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet 1999;354:1435–9. [DOI] [PubMed] [Google Scholar]
- 74.Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes 2010;59:2126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nedeltcheva AV, Kessler L, Imperial J, Penev PD. Exposure to recurrent sleep restriction in the setting of high caloric intake and physical inactivity results in increased insulin resistance and reduced glucose tolerance. J Clin Endocrinol Metab 2009;94:3242–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Broussard JL, Ehrmann DA, Van Cauter E, Tasali E, Brady MJ. Impaired insulin signaling in human adipocytes after experimental sleep restriction: a randomized, crossover study. Ann Intern Med 2012;157:549–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Donga E, van Dijk M, van Dijk JG, Biermasz NR, Lammers GJ, van Kralingen KW, Corssmit EP, Romijn JA. A single night of partial sleep deprivation induces insulin resistance in multiple metabolic pathways in healthy subjects. J Clin Endocrinol Metab 2010;95:2963–8. [DOI] [PubMed] [Google Scholar]
- 78.Buxton OM, Cain SW, O’Connor SP, Porter JH, Duffy JF, Wang W, Czeisler CA, Shea SA. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med 2012;4:129ra43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA 2009;106:4453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci USA 2008;105:1044–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boden G, Lebed B, Schatz M, Homko C, Lemieux S. Effects of acute changes of plasma free fatty acids on intramyocellular fat content and insulin resistance in healthy subjects. Diabetes 2001;50:1612–7. [DOI] [PubMed] [Google Scholar]
- 82.Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, Shulman GI. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest 1996;97:2859–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chavez AO, Kamath S, Jani R, Sharma LK, Monroy A, Abdul-Ghani MA, Centonze VE, Sathyanarayana P, Coletta DK, Jenkinson CP, et al. Effect of short-term free Fatty acids elevation on mitochondrial function in skeletal muscle of healthy individuals. J Clin Endocrinol Metab 2010;95:422–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yoshino J, Almeda-Valdes P, Patterson BW, Okunade AL, Imai S, Mittendorfer B, Klein S. Diurnal variation in insulin sensitivity of glucose metabolism is associated with diurnal variations in whole-body and cellular fatty acid metabolism in metabolically normal women. J Clin Endocrinol Metab 2014;99:E1666–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schlierf G, Dorow E. Diurnal patterns of triglycerides, free fatty acids, blood sugar, and insulin during carbohydrate-induction in man and their modification by nocturnal suppression of lipolysis. J Clin Invest 1973;52:732–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Broussard JL, Chapotot F, Abraham V, Day A, Delebecque F, Whitmore HR, Tasali E. Sleep restriction increases free fatty acids in healthy men. Diabetologia 2015;58:791–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nedeltcheva AV, Imperial JG, Penev PD. Effects of sleep restriction on glucose control and insulin secretion during diet-induced weight loss. Obesity (Silver Spring) 2012;20:1379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wehrens SM, Hampton SM, Finn RE, Skene DJ. Effect of total sleep deprivation on postprandial metabolic and insulin responses in shift workers and non-shift workers. J Endocrinol 2010;206:205–15. [DOI] [PubMed] [Google Scholar]
- 89.Cedernaes J, Osler ME, Voisin S, Broman JE, Vogel H, Dickson SL, Zierath JR, Schiöth HB, Benedict C. Acute sleep loss induces tissue-specific epigenetic and transcriptional alterations to circadian clock genes in men. J Clin Endocrinol Metab 2015;100:E1255–61. [DOI] [PubMed] [Google Scholar]
- 90.Maury E, Hong HK, Bass J. Circadian disruption in the pathogenesis of metabolic syndrome. Diabetes Metab 2014;40:338–46. [DOI] [PubMed] [Google Scholar]
- 91.Shostak A, Meyer-Kovac J, Oster H. Circadian regulation of lipid mobilization in white adipose tissues. Diabetes 2013;62:2195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Reynolds AC, Dorrian J, Liu PY, Van Dongen HP, Wittert GA, Harmer LJ, Banks S. Impact of five nights of sleep restriction on glucose metabolism, leptin and testosterone in young adult men. PLoS One 2012;7:e41218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Markwald RR, Melanson EL, Smith MR, Higgins J, Perreault L, Eckel RH, Wright KP Jr. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci USA 2013;110:5695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Spaeth AM, Dinges DF, Goel N. Sex and race differences in caloric intake during sleep restriction in healthy adults. Am J Clin Nutr 2014;100:559–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ioannides-Demos LL, Piccenna L, McNeil JJ. Pharmacotherapies for obesity: past, current, and future therapies. J Obes 2011;2011:179674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, et al. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation 2014;129:e28–292. [DOI] [PMC free article] [PubMed] [Google Scholar]