Abstract
The microbiota has recently been recognized as a driver of health that affects the immune, nervous, and metabolic systems. This influence is partially exerted through the metabolites produced, which may be relevant for optimal infant development and health. The gut microbiota begins developing early in life, and this initial colonization is remarkably important because it may influence long-term microbiota composition and activity. Considering that the microbiome may play a key role in health and disease, maintaining a protective microbiota could be critical in preventing dysbiosis-related diseases such as allergies, autoimmunity disorders, and metabolic syndrome. Breast milk and milk glycans in particular are thought to play a major role in shaping the early-life microbiota and promoting its development, thus affecting health. This review describes some of the effects the microbiota has on the host and discusses the role microbial metabolites play in shaping newborn health and development. We describe the gut microbiota structure and function during early life and the factors that determine its composition and hypothesize about the effects of human milk oligosaccharides and other prebiotic fibers on the neonatal microbiota.
Keywords: microbiota, human milk oligosaccharides, microbial metabolites, short-chain fatty acids, prebiotics
Introduction
The gut microbiota constitutes a complex ecosystem that harbors >1000 species with ∼7000 strains and contains >150 times more genes than the human genome (1). The interaction between the microbial ecosystem and the host represents a long evolutionary symbiosis that is essential for optimal health throughout life. The resident microbiota, with its broad genetic and metabolic diversity, exerts an effect on host metabolism, physiology, and immune system development (2). For this reason, the microbiota is now recognized as a “virtual organ” (3).
The microbiota encompasses 2 predominant bacterial phylotypes, bacteroidetes and firmicutes, with the phyla proteobacteria, actinobacteria, fusobacteria, and verrucomicrobia being present at a relatively lower abundance (1). Bacteroidetes are gram-negative, anaerobic, nonspore-forming bacteria that are enriched with enzymes to degrade carbohydrates; firmicutes are gram-positive, anaerobic, spore-forming bacteria that ferment simple sugars to produce a variety of SCFAs (4). The whole microbial distribution varies along the gastrointestinal tract, with microbial densities and diversities increasing both from the proximal to the distal gut and along the tissue-lumen axis (3). Although newborns were initially thought to be born sterile, it is now believed that the colonization of the gut starts during pregnancy and continues after birth until 2 years of age, when it reaches a relatively stable composition resembling that of an adult (5). Alterations in the normal microbial composition have been described in a whole range of health disorders from infancy to adulthood (2).
In this review, we consider different aspects of the microbiota’s influence on health. We summarize factors that contribute to the establishment of a protective microbiota early in life and describe the interactions between human milk oligosaccharides and the microbiota. We evaluate how alterations in early gut microbiota may promote the onset of health disorders. Finally, we provide insights into recent advances in the infant formula field.
Microbiota Axis
The influence of the virtual organ formed by the microbiota extends well beyond the gastrointestinal tract and expands throughout life. It can greatly affect many physiological aspects in the host, interacting with and regulating the activity of distal organs, including mainly the liver and brain. The microbiota exerts a strong influence over the liver and collaborates in maintaining gut-liver axis health. Alterations in the gut-liver axis contribute to the pathogenesis of obesity and nonalcoholic fatty liver disease, among others (6).
Microbiota-gut-brain axis.
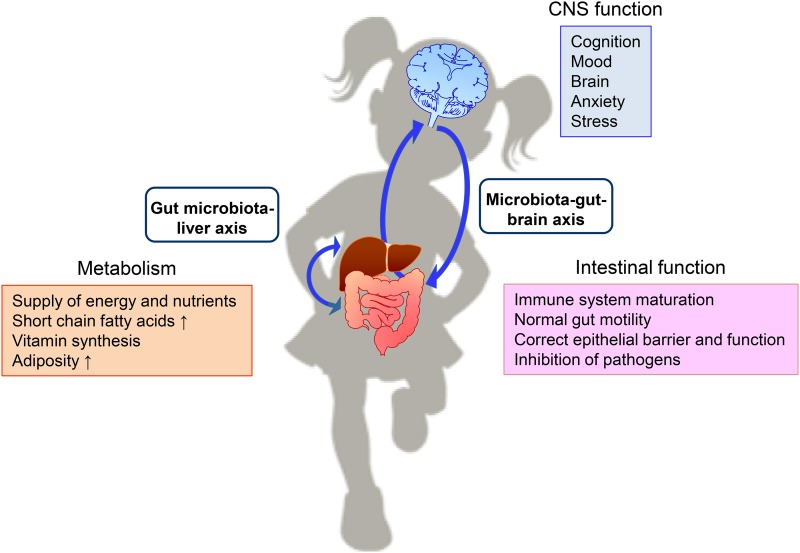
The gut and brain are in constant bidirectional communication through the gut-brain axis, which integrates neural pathways and immune and endocrine mechanisms in a complex relation essential for maintaining homeostasis. It is now evident that endogenous gut bacteria can act as signaling components within this system, and the term “microbiota-gut-brain axis” appeared to designate this communication, which begins early in life to modulate the immune system, central nervous system, and gastrointestinal functions (7, 8) (Figure 1).
FIGURE 1.
Interactions between the gut microbiota and host physiology. The gut microbiota exerts an effect on several aspects of host physiology through the 2 depicted axes. Thus, the microbiota is able to influence metabolism and brain functions and acts locally by modulating intestinal function (2, 3, 7). CNS, central nervous system.
This axis establishes a bidirectional communication between the brain and gut/microbiota. The latter produces metabolites or cytokines released into the blood stream and sends signals through the vagus nerve. In turn, the brain can influence the composition and function of the gut microbiota either indirectly via changes in gut motility, secretion, or intestinal permeability or directly via signaling molecules released into the gut lumen (8). Early-life events can modify the developing postnatal microbiota, leading to imbalances in the gut-brain communication and thus alterations in brain development and behavior (9). An adequately maturing microbiome during early life and a stable one in adulthood is necessary for appropriate signaling through the gut-brain axis and thereby for maintaining health.
Microbiota Metabolites
The microbiota provides the host with a range of metabolic capabilities that would not be accessible otherwise (Figure 1). It produces numerous metabolites that may act as autocrine or paracrine, thus modulating human health. Many of these microbial metabolites are essential for health and play a major role in regulating normal growth in infants (10). They are largely determined by diet composition and pattern of food intake. In fact, the structure of the microbiota itself is influenced by the diet because certain bacteria may be better adapted for utilizing specific substrates (5). Metabolites produced and/or modified by the microbiota include SCFAs, vitamins, bile acids, and choline, which are required for many aspects of host physiology. This section will cover those especially relevant for infant health.
SCFAs.
Nondigestible carbohydrates are fermented in the colon by a subset of anaerobic bacteria, yielding both energy for microbial growth and producing SCFAs, i.e., acetate, propionate, and butyrate. Bacteria that produce SCFAs include Bacteroides, Bifidobacterium, Lactobacillus, Faecalibacterium, and Ruminococcus, among others (11). SCFAs are essential for neonatal intestinal health because they provide energy and maintain gastrointestinal growth and development (12).
Within SCFAs, butyrate is a source of energy for the colonic epithelium, whereas acetate and propionate are carried into the bloodstream and become available to a variety of different organs (2). SCFAs are recognized by receptors expressed in gut endocrine cells that modulate the secretion of hormones involved in appetite control, thus linking microbial SCFAs and food intake (13). SCFAs may also play a role in the stimulation of leptin production by adipocytes by influencing feeding behavior (3). Recent reports have shown that propionate activates intestinal gluconeogenesis via the gut-brain neural circuit (14). Butyrate controls histone acetylation and hence influences host gene expression by remodeling chromatin structure (15). Locally, SCFAs acidify the colon lumen, limiting the growth of potential pathogens (13). Therefore, changes in the production rates of the major SCFAs by the colonic microbiota may be of particular importance if the fluctuations occur early in life, when epigenetic control is vital for function later in life (15).
Tryptophan.
Tryptophan is an essential amino acid that is crucial for differentiating and correcting central nervous system development and regulating normal newborn behavior. Its circulating concentrations are under the influence of the gut microbiota, probably through the modulation of the kynurenine pathway, the main physiological route for tryptophan metabolism (16).
Tryptophan constitutes a precursor of serotonin (5-hydroxytryptamine), a neurotransmitter that regulates gastrointestinal functions, mood, appetite, sleep, and anxiety (16). The gut microbiota can affect serotonin amounts indirectly by stimulating its release from intestinal enterochromaffin cells, thus influencing the gut-brain axis (16, 17).
Vitamins.
Certain bacteria of the human gut microbiota can synthesize vitamins essential for neonatal health, such as menaquinone (vitamin K-2), and many of the water-soluble B vitamins. In particular, many Bifidobacterium strains have shown the capability of producing vitamins in vitro (18).
Conjugated linoleic acid
CLA refers to a mixture of conjugated isomers of the essential FA linoleic acid, which has been associated with a variety of health benefits regarding obesity, diabetes, and immune function. CLA is produced by certain strains of different bacterial groups, such as Bifidobacteria, Lactobacillus, Propionibacterium, Enterococcus, and Lactococcus, albeit with different efficiencies, and is important for appropriate newborn growth and development (10).
Neurotransmitters.
Some bacteria can produce neuroactive metabolites ranging from serotonin and γ-aminobutyric acid to dopamine and norepinephrine, acetylcholine, and histamine and more newly described neurotransmitters such as agmatine. The modulation of these transmitters in the gut is another possible mechanism of action through which the microbiota could exert its effects on brain development and function (19).
Summary.
Metabolites produced by the gut microbiota can affect a wide variety of physiological and metabolic processes in organisms. Consequently, alterations in the host-microbiota relation may disrupt the subtle equilibrium of this symbiosis, resulting in disease—hence the importance of developing and maintaining a healthy microbiota throughout life.
Gut Microbiota Dynamics
Although it was generally considered that the intrauterine environment and newborn were sterile until delivery, there are indications of earlier microbial exposure because bacteria have been found in the umbilical cord, placenta, amniotic fluid, and meconium (20). At birth, however, once the neonate is exposed to several new microbes, the gut undergoes rapid colonization. The earliest colonizers are predominantly facultative anaerobes (Escherichia coli, Enterococcus), which, as oxygen is consumed, give way to strict anaerobes, including Bifidobacterium, Lactobacillus, Bacteroides, Clostridium, and sometimes Ruminococcus (21).
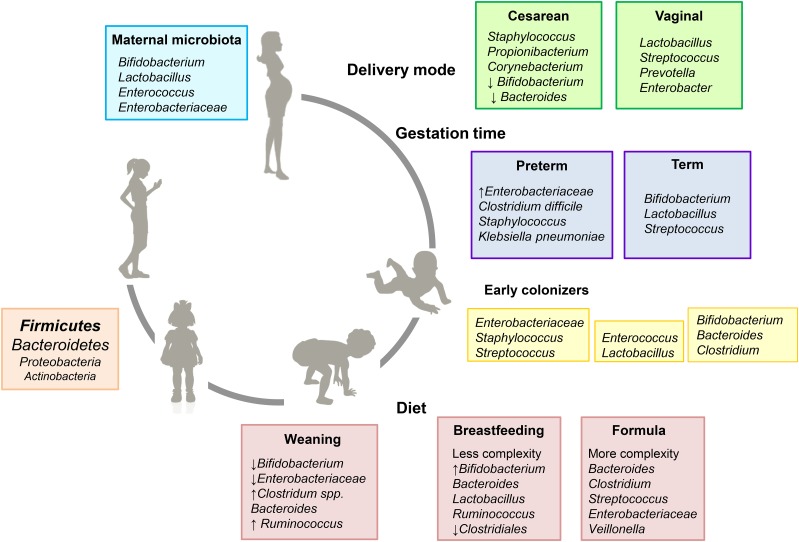
The gut microbiota composition of early infants has low diversity, is dynamic, and continues to develop until it becomes stable and adult-like at 2–3 y of age (21). The factors that influence the gut microbiota include mode of delivery, gestational age, feeding patterns, environment, antibiotic exposure, country of origin, and host genetics (22).
The first main factor that contributes to the colonization of the infant gut is delivery mode (Figure 2). Vaginally born infants are colonized with vaginal and fecal bacteria from the mother, whereas cesarean-born infants are mainly colonized by bacteria from the clinical environment (23). The latter, with a less diverse microbiota, harbor lower counts of Bifidobacterium spp. and Bacteroides fragilis but increased numbers of Clostridium difficile (21). These initial differences seem to have long-term effects on infant health, increasing the risk of developing allergy or obesity later in life (22).
FIGURE 2.
Evolution of the early-life gut microbiota and events influencing its composition. Factors such as the maternal microbiota, delivery mode, gestation time, and type of feeding strongly influence the microbiota. Colonization and expansion of the gut microbiota, shaped by diet, results in the establishment of an adult-like microbiota around 2–3 y of age, with firmicutes and bacteroidetes as the predominant phyla. Early life is a susceptible period when modifications in the gut microbiota composition can have long-term effects on health (5, 22).
Gestational time at birth greatly influences the establishment of the infant gut microbiota, as inferred from comparing fecal microbiota from term and preterm infants (Figure 2). Preterm infants showed higher amounts of facultative anaerobes belonging to Enterobacteriaceae, other potentially pathogenic bacteria such as C. difficile or Klebsiella pneumoniae, and low levels of Bifidobacterium and Bacteroides (24). In contrast, term infants had higher diversity in their fecal microbiota, with more common genera present, such as Bifidobacterium, Lactobacillus, and Streptococcus (25).
Feeding regimen has a crucial impact on gut microbiota composition (Figure 2) (5). Breast milk has been suggested to be a source of complex bacterial communities in infants who have been breastfed (26) and could contribute to early gut colonization (27). Bacterial transfer from the mother’s skin takes place during suckling, but several studies also support the enteromammary pathway hypothesis, in which bacteria from the maternal gut reach the mammary glands through maternal dendritic cells and macrophages. In fact, it has been reported that several gut bacterial species are shared between maternal feces, breast milk, and infant feces (28).
Decades ago, it was broadly accepted that breastfed and formula-fed infants had different microbiotas. The microbiota from infants fed traditional nonsupplemented formulas was reported to be more diverse, with higher proportions of Bacteroides, Clostridium, and Enterobacteriaceae compared with breastfed infants (29). The microbiota in the intestine of breastfed infants was described to contain higher proportions of Bifidobacterium and Lactobacillus than infants who were formula-fed (21, 29), although other studies found no significant differences (30, 31). Formulas have evolved over the past several years, and the addition of prebiotics has contributed to bringing the microbiota of formula-fed infants closer to that of breastfed infants (32, 33). During weaning, with the introduction of solid foods, infants are exposed to more complex carbohydrates and other nutrients that drive the development of an adult-like microbiota (34). Postweaning changes in the microbiota are more pronounced in breastfed infants, with a decrease in the proportions of Bifidobacteria, Enterobacteria, and Clostridium spp. Proportions of Bacteroides do not change and remain one of the most predominant groups in the infant gut microbiota (5).
Pediatric antibiotic use, in particular during prolonged periods, has been linked to disruptions in early microbiota colonization. Antibiotics can select for an altered community composition in the gut microbiota. Their use can alter the metabolism of the gut microbiota, and they have been described to increase adiposity both in mice and children (35).
Another factor that may influence the infant microbiota is the maternal gut microbiota itself. The mother’s microbiota is greatly remodeled during pregnancy (36). In addition, the metabolic health of the mother before or during pregnancy could have an effect on her infant’s gut microbiota. Alterations in the microbiota composition in mothers may thus be transferred to their infants and thus lead to an increased risk of metabolic disease in the latter (37). Although it is difficult to determine individual variables, it has been speculated that breastfeeding, natural birth, and lack of hospitalization relate to a more beneficial gut microbiota composition (37).
Bioactive Molecules in Milk
As mentioned previously, breastfeeding is one of the factors that accounts for a beneficial gut microbiota. Human milk constitutes a unique source of nutrients and energy and has been shaped by mammalian evolution to provide not only optimal nutrition but also the bioactive components essential for immune maturation, metabolic and cognitive development, gut maturation, and optimal gut microbial colonization (38). The composition of human milk varies greatly among individuals and during lactation, with maternal lifestyle, nutritional and immunological status, dietary habits, and lactation time influencing its composition and quality (38).
The effects of breastfeeding on the infant intestinal microbiota cannot be attributed to a single compound because several milk factors are speculated to modulate it. There is a wide range of health-promoting constituents, including carbohydrates, human milk oligosaccharides (HMOs), nucleotides, fatty acids, immunoglobulins, cytokines, immune cells, lysozymes, lactoferrin, and other immunomodulatory factors. In particular, lactoferrin promotes the growth of bifidobacteria both in vitro and in animal models (39). Nucleotides, when added to infant formula, were also reported to improve the composition of the gut microbiota in formula-fed infants (40).
Potential Role of HMOs in Supporting the Growth of Beneficial Bacteria
HMOs are the third most abundant component in mature milk (5–15 g/L) (41). HMOs exhibit structural diversity (Figure 3), and their composition and concentration change significantly among different individuals and across lactation (42). They are resistant to digestion and reach the colon intact, where they can be expelled through the feces or fermented by the local microbiota (43). They can then function as prebiotics, selectively promoting the growth of beneficial bacteria in the gut while inhibiting the growth of pathogens (44).
FIGURE 3.
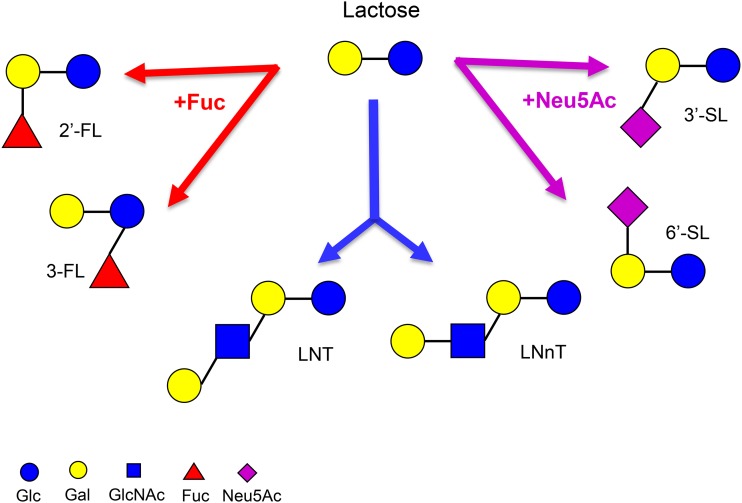
Selected HMO structures. HMOs are composed of 5 monosaccharides. Lactose can be fucosylated or sialylated to generate the following trisaccharides: the fucosyllactoses 2′-FL and 3-FL and the sialyllactoses 3′-SL and 6′-SL. It can be further elongated to generate tetrasaccharides, e.g., LNT and LNnT. Chains can be further elongated, fucosylated, and/or sialylated to generate more complex structures. FL, fucosyllactose; Fuc, fucose; Gal, galactose; Glc, glucose; GlcNAc, N-acetylglucosamine; HMO, human milk oligosaccharide; LNnT, lacto-N-neotetraose; LNT, lacto-N-tetraose; Neu5Ac, N-acetylneuraminic acid; SL, sialyllactose.
Beyond their prebiotic function, their recent detection in breastfed infants’ plasma compartments provides evidence of their systemic effects (45). In vitro studies suggest that HMOs can modulate immune cell responses or exhibit anti-inflammatory properties (46, 47). HMOs can act as decoys, preventing pathogenic bacteria from binding to intestinal cells (48). HMOs are also involved in gut motility through interactions with the enteric nervous system (49). Moreover, they may provide infants with sialic acid, which is important for brain development, and a role for fucosyllactose in learning and memory has recently been described (50).
As classic prebiotics, HMOs selectively promote the growth of specific bacteria (51). Data supporting this function derive from in vitro fermentation studies that used bacteria isolated from infant feces. Clinical data regarding the influence of feeding HMOs on the microbiota are anticipated (52).
The breastfed microbiota was traditionally characterized by a predominance of Bifidobacterium, which has long been associated with health (29), although some reports found no differences between breastfed and formula-fed infants (30). The predominance of bifidobacteria in breastfed infants has been partly attributed to the prebiotic effect of HMOs (53). The supplementation of formula with prebiotics, i.e., galactooligosacharides and fructooligosaccharides, has greatly contributed to reducing these differences and increasing Bifidobacterium and Lactobacillus counts (32, 33).
Only certain bacterial species and strains have developed strategies for utilizing HMOs as a growth substrate, which suggests a coevolution of these microorganisms, the developing infant, and human milk (54). In vitro, only Bifidobacterium and Bacteroides strains were able to grow on HMOs as the sole carbon source (44). In contrast, other typical commensal bacteria, e.g., Clostridium spp., Enterococcus faecalis, Staphylococcus spp., Enterobacter spp., and E. coli, were unable to consume HMOs (55). To use HMOs effectively, bacteria harbor various catabolic strategies: some species preferentially import low-molecular-weight HMOs, whereas others export glycosidases for external hydrolysis (51). Some are also able to consume galactooligosacharides from infant formula (56). In addition, the preferential consumption of certain fucosylated or sialylated HMOs varies among different bacteria (57). The catabolism of the major HMOs by Bifidobacterium longum subps. infantis produces lactate and SCFAs, which reduces the pH significantly and diminishes the growth of putative pathogenic bacteria, therefore confirming the prebiotic effect of these glycans (58).
Health Benefits Derived from a Protective Microbiota
There are important interactions between human milk, the developing intestinal tract, and the gut microbiota. The intestinal epithelium of a newborn is immature, and changes leading to maturation are triggered by microbial colonization and linked to the diet and microbiota-derived metabolites (59). Breastfeeding is associated with a reduced risk of necrotizing enterocolitis (NEC), an inflammatory intestinal disorder affecting preterm infants (5). NEC has been associated with low gut microbial diversity, increased Proteobacteria, and decreased firmicutes numbers, although not all studies have corroborated such an association (60). HMOs have been suggested to reduce the incidence of NEC either by generating a protective microbiota or reducing the incidence of NEC on its own (61).
The balance between the microbiota, immune response, and tolerance mechanisms is essential for newborn gut health and for preventing gastrointestinal disease in adulthood. Alterations in normal bacterial colonization patterns early in life may change the immune development and cause predisposition to several diseases. Thus, lower microbial diversity in early infancy seems to lead the way for the development of allergic manifestations (62). In addition, changes in gut microbiota composition may influence the development of several autoimmune disorders in children, including type 1 diabetes and Crohn and celiac disease, as increasing evidence suggests (63). Early-life changes in gut microbiota composition can alter susceptibility to developing obesity later in life (64).
It remains to be demonstrated whether dysbiosis is really the cause or the consequence of a specific disease. Most studies agree on recognizing dysbiosis as a risk factor associated with many pediatric diseases. This highlights the importance of establishing a healthy microbiota early in life as well as maintaining a healthy microbial composition to prevent the onset of diseases during childhood and later in life.
Infant Formula
Considering that bovine milk constitutes the base for infant formula, unsupplemented formulas have a much lower content of bioactive molecules, e.g., oligosaccharides. HMOs are characterized by their great diversity, complexity, and abundance, whereas milk oligosaccharides in other mammals are present at smaller amounts and with less diverse structures (65). Bovine milk contains oligosaccharides, albeit at a lower concentration than human milk (0.05 g/L) and with less complexity and diversity (66). Contrary to HMOs, sialylation is predominant (70%) in bovine milk oligosaccharides, and fucosylated structures are present at much lower concentrations (67). Hence, formula-fed infants do not currently benefit from the advantages conferred by the HMO structures present in human milk.
Some years ago, the supplementation of infant formula with HMOs was unfeasible because of the lack of a large-scale synthesis at an affordable price. Recent advances in glycan synthesis have yielded industrial amounts of specific structures (the smallest but more abundant) at a reasonable cost. The more structurally complex HMOs still remain unavailable.
The understanding of human milk composition continues to evolve and helps to guide infant formula research. Since the late 1970s, researchers have observed that formula feeding influenced fecal microbial populations in a way that was different from breast milk (68). Efforts were thus directed to adding technologies, e.g., prebiotics, that would enhance the bifidogenic effect of formulas (69). At that time, the main prebiotics available were galactooligosacharides and fructooligosaccharides, which were able to improve bacterial communities in formula-fed infants (70). In fact, the addition of galactooligosacharides, fructooligosaccharides, or their combination had a stimulating effect on the growth of Bifidobacterium (32, 33, 71–73),
It is worth considering that studies that evaluated infant gut microbiota and the effects of prebiotic formula supplementation have sometimes rendered contradictory results. Differences may depend on laboratory methodology, formula composition, infant population, and individual intestinal microbiomes (72). With the appearance of high-throughput methods to study the microbiota, analyses became more sensitive and accurate than traditional culture-dependent methods (74). In summary, there are reasons to believe novel ingredients and techniques within the dairy industry may further contribute to minimize differences between formula and human milk.
Conclusions
The gut microbiota behaves like an organ, exerting a wide range of effects that extend beyond the gastrointestinal tract. Microbiota metabolites such as SCFAs, tryptophan, vitamins, and neurotransmitters may be important for newborns by supporting growth and development.
The gut microbiota is established early in life and matures during the first 2–3 y, whereupon it reaches an adult-like profile. Alterations in early microbiota are closely related to disease not only during childhood but also later in life. Because early life is a critical period when alterations may have a more pronounced long-lasting effect in health, this stage may provide a window of intervention for disease prevention.
Breast milk is one of the main factors driving the proliferation of a protective gut bacterial community enriched in bifidobacteria. Bioactive factors in human milk may promote the growth of beneficial bacteria and therefore ameliorate infant health. Although breast milk is considered the ideal nutrition for newborns, formulas represent an alternative for those who are unable or choose not to breastfeed.
Formula composition has greatly improved within the past decade. The addition of prebiotics to infant formula has shown beneficial effects on the gut microbiota. Novel ingredients may further contribute to minimize differences between breastfed and formula-fed infants.
Acknowledgments
We gratefully acknowledge Rachael Buck, Maria Ramirez, Geralyn Duska-McEwen, and JoMay Chow for reviewing the manuscript and offering critical comments. All authors read and approved the final version of the manuscript.
References
- 1.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010;464:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature 2012;489:242–9. [DOI] [PubMed] [Google Scholar]
- 3.Evans JM, Morris LS, Marchesi JR. The gut microbiome: the role of a virtual organ in the endocrinology of the host. J Endocrinol 2013;218:R37–47. [DOI] [PubMed] [Google Scholar]
- 4.Fischbach MA, Sonnenburg JL. Eating for two: how metabolism establishes interspecies interactions in the gut. Cell Host Microbe 2011;10:336–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arrieta MC, Stiemsma LT, Amenyogbe N, Brown EM, Finlay B. The intestinal microbiome in early life: health and disease. Front Immunol 2014;5:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chassaing B, Etienne-Mesmin L, Gewirtz AT. Microbiota-liver axis in hepatic disease. Hepatology 2014;59:328–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol 2012;10:735–42. [DOI] [PubMed] [Google Scholar]
- 8.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 2012;13:701–12. [DOI] [PubMed] [Google Scholar]
- 9.Clarke G, O’Mahony S, Dinan T, Cryan J. Priming for health: gut microbiota acquired in early life regulates physiology, brain and behaviour. Acta Paediatr 2014;103:812–9. [DOI] [PubMed] [Google Scholar]
- 10.Patterson E, Cryan JF, Fitzgerald GF, Ross RP, Dinan TG, Stanton C. Gut microbiota, the pharmabiotics they produce and host health. Proc Nutr Soc 2014;73:477–89. [DOI] [PubMed] [Google Scholar]
- 11.Macfarlane GT, Macfarlane S. Bacteria, colonic fermentation, and gastrointestinal health. J AOAC Int 2012;95:50–60. [DOI] [PubMed] [Google Scholar]
- 12.Jacobi SK, Odle J. Nutritional factors influencing intestinal health of the neonate. Adv Nutr 2012;3:687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-gut microbiota metabolic interactions. Science 2012;336:1262–7. [DOI] [PubMed] [Google Scholar]
- 14.De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, Backhed F, Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014;156:84–96. [DOI] [PubMed] [Google Scholar]
- 15.Selkrig J, Wong P, Zhang X, Pettersson S. Metabolic tinkering by the gut microbiome: implications for brain development and function. Gut Microbes 2014;5:369–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res 2015;277:32–48. [DOI] [PubMed] [Google Scholar]
- 17.Clarke G, Stilling RM, Kennedy PJ, Stanton C, Cryan JF, Dinan TG. Minireview: gut microbiota: The neglected endocrine organ. Mol Endocrinol 2014;28:1221–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LeBlanc JG, Milani C, de Giori GS, Sesma F, van Sinderen D, Ventura M. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr Opin Biotechnol 2013;24:160–8. [DOI] [PubMed] [Google Scholar]
- 19.Lyte M. Microbial endocrinology: host-microbiota neuroendocrine interactions influencing brain and behavior. Gut Microbes 2014;5:381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rautava S, Luoto R, Salminen S, Isolauri E. Microbial contact during pregnancy, intestinal colonization and human disease. Nat Rev Gastroenterol Hepatol 2012;9:565–76. [DOI] [PubMed] [Google Scholar]
- 21.Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, van den Brandt PA, Stobberingh EE. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 2006;118:511–21. [DOI] [PubMed] [Google Scholar]
- 22.Scholtens PA, Oozeer R, Martin R, Amor KB, Knol J. The early settlers: intestinal microbiology in early life. Annu Rev Food Sci Technol 2012;3:425–47. [DOI] [PubMed] [Google Scholar]
- 23.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA 2010;107:11971–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arboleya S, Binetti A, Salazar N, Fernandez N, Solis G, Hernandez-Barranco A, Margolles A, de Los Reyes-Gavilan CG, Gueimonde M. Establishment and development of intestinal microbiota in preterm neonates. FEMS Microbiol Ecol 2012;79:763–72. [DOI] [PubMed] [Google Scholar]
- 25.Berrington JE, Stewart CJ, Embleton ND, Cummings SP. Gut microbiota in preterm infants: assessment and relevance to health and disease. Arch Dis Child Fetal Neonatal Ed 2013;98:F286–90. [DOI] [PubMed] [Google Scholar]
- 26.Hunt KM, Foster JA, Forney LJ, Schutte UM, Beck DL, Abdo Z, Fox LK, Williams JE, McGuire MK, McGuire MA. Characterization of the diversity and temporal stability of bacterial communities in human milk. PLoS One 2011;6:e21313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernández L, Langa S, Martin V, Maldonado A, Jimenez E, Martin R, Rodriguez JM. The human milk microbiota: origin and potential roles in health and disease. Pharmacol Res 2013;69:1–10. [DOI] [PubMed] [Google Scholar]
- 28.Jost T, Lacroix C, Braegger CP, Rochat F, Chassard C. Vertical mother-neonate transfer of maternal gut bacteria via breastfeeding. Environ Microbiol 2014;16:2891–904. [DOI] [PubMed] [Google Scholar]
- 29.Harmsen HJ, Wildeboer-Veloo AC, Raangs GC, Wagendorp AA, Klijn N, Bindels JG, Welling GW. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J Pediatr Gastroenterol Nutr 2000;30:61–7. [DOI] [PubMed] [Google Scholar]
- 30.Adlerberth I, Wold AE. Establishment of the gut microbiota in western infants. Acta Paediatr 2009;98:229–38. [DOI] [PubMed] [Google Scholar]
- 31.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol 2007;5:e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veereman-Wauters G, Staelens S, Van de Broek H, Plaskie K, Wesling F, Roger LC, McCartney AL, Assam P. Physiological and bifidogenic effects of prebiotic supplements in infant formulae. J Pediatr Gastroenterol Nutr 2011;52:763–71. [DOI] [PubMed] [Google Scholar]
- 33.Sierra C, Bernal MJ, Blasco J, Martinez R, Dalmau J, Ortuno I, Espin B, Vasallo MI, Gil D, Vidal ML, et al. Prebiotic effect during the first year of life in healthy infants fed formula containing gos as the only prebiotic: a multicentre, randomised, double-blind and placebo-controlled trial. Eur J Nutr 2015;54:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, Angenent LT, Ley RE. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci USA 2011;108 Suppl 1:4578–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schulfer A, Blaser MJ. Risks of antibiotic exposures early in life on the developing microbiome. PLoS Pathog 2015;11:e1004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koren O, Goodrich JK, Cullender TC, Spor A, Laitinen K, Backhed HK, Gonzalez A, Werner JJ, Angenent LT, Knight R, et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 2012;150:470–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kerr CA, Grice DM, Tran CD, Bauer DC, Li D, Hendry P, Hannan GN. Early life events influence whole-of-life metabolic health via gut microflora and gut permeability. Crit Rev Microbiol 2015;41:326–40. [DOI] [PubMed] [Google Scholar]
- 38.Le Huërou-Luron I, Blat S, Boudry G. Breast- v. formula-feeding: impacts on the digestive tract and immediate and long-term health effects. Nutr Res Rev 2010;23:23–36. [DOI] [PubMed] [Google Scholar]
- 39.Maga EA, Weimer BC, Murray JD. Dissecting the role of milk components on gut microbiota composition. Gut Microbes 2013;4:136–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singhal A, Macfarlane G, Macfarlane S, Lanigan J, Kennedy K, Elias-Jones A, Stephenson T, Dudek P, Lucas A. Dietary nucleotides and fecal microbiota in formula-fed infants: a randomized controlled trial. Am J Clin Nutr 2008;87:1785–92. [DOI] [PubMed] [Google Scholar]
- 41.Kunz C, Rudloff S, Baier W, Klein N, Strobel S. Oligosaccharides in human milk: structural, functional, and metabolic aspects. Annu Rev Nutr 2000;20:699–722. [DOI] [PubMed] [Google Scholar]
- 42.Erney RM, Malone WT, Skelding MB, Marcon AA, Kleman-Leyer KM, O’Ryan ML, Ruiz-Palacios G, Hilty MD, Pickering LK, Prieto PA. Variability of human milk neutral oligosaccharides in a diverse population. J Pediatr Gastroenterol Nutr 2000;30:181–92. [DOI] [PubMed] [Google Scholar]
- 43.Rudloff S, Kunz C. Milk oligosaccharides and metabolism in infants. Adv Nutr 2012;3:398S–405S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marcobal A, Sonnenburg JL. Human milk oligosaccharide consumption by intestinal microbiota. Clin Microbiol Infect 2012;18 Suppl 4:12–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goehring KC, Kennedy AD, Prieto PA, Buck RH. Direct evidence for the presence of human milk oligosaccharides in the circulation of breastfed infants. PLoS One 2014;9:e101692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bode L. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology 2012;22:1147–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duska-McEwen G, Senft AP, Ruetschilling TL, Barrett EG, Buck RH. Human milk oligosaccharides enhance innate immunity to respiratory syncytial virus and influenza in vitro. Food Nutr Sci 2014;5:1387–98. [Google Scholar]
- 48.Wang B. Molecular mechanism underlying sialic acid as an essential nutrient for brain development and cognition. Adv Nutr 2012;3:465S–72S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bienenstock J, Buck RH, Linke H, Forsythe P, Stanisz AM, Kunze WA. Fucosylated but not sialylated milk oligosaccharides diminish colon motor contractions. PLoS One 2013;8:e76236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vázquez E, Barranco A, Ramirez M, Gruart A, Delgado-Garcia JM, Martinez-Lara E, Blanco S, Martin MJ, Castanys E, Buck R, et al. Effects of a human milk oligosaccharide, 2′-fucosyllactose, on hippocampal long-term potentiation and learning capabilities in rodents. J Nutr Biochem 2015;26:455–65. [DOI] [PubMed] [Google Scholar]
- 51.Pacheco AR, Barile D, Underwood MA, Mills DA. The impact of the milk glycobiome on the neonate gut microbiota. Annu Rev Anim Biosci 2015;3:419–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marriage BJ, Buck RH, Goehring KC, Oliver JS, Williams JA. Infants fed a lower calorie formula with 2′-fucosyllactose (2′-fl) show growth and 2′-fl uptake like breast-fed infants. J Pediatr Gastroenterol Nutr 2015. July 6 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garrido D, Barile D, Mills DA. A molecular basis for bifidobacterial enrichment in the infant gastrointestinal tract. Adv Nutr 2012;3:415S–21S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sela DA, Mills DA. Nursing our microbiota: molecular linkages between bifidobacteria and milk oligosaccharides. Trends Microbiol 2010;18:298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoeflinger JL, Davis SR, Chow J, Miller M. In vitro impact of human milk oligosaccharides on <i>enterobacteriaceae growth. J Agric Food Chem 2015;63:3295–302. [DOI] [PubMed] [Google Scholar]
- 56.Garrido D, Ruiz-Moyano S, Jimenez-Espinoza R, Eom H-J, Block DE, Mills DA. Utilization of galactooligosaccharides by bifidobacterium longum subsp. Infantis isolates. Food Microbiol 2013;33:262–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu Z-T, Chen C, Newburg DS. Utilization of major fucosylated and sialylated human milk oligosaccharides by isolated human gut microbes. Glycobiology 2013;23:1281–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu ZT, Chen C, Kling DE, Liu B, McCoy JM, Merighi M, Heidtman M, Newburg DS. The principal fucosylated oligosaccharides of human milk exhibit prebiotic properties on cultured infant microbiota. Glycobiology 2013;23:169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walker WA, Iyengar RS. Breast milk, microbiota, and intestinal immune homeostasis. Pediatr Res 2015;77:220–8. [DOI] [PubMed] [Google Scholar]
- 60.Torrazza RM, Neu J. The altered gut microbiome and necrotizing enterocolitis. Clin Perinatol 2013;40:93–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jantscher-Krenn E, Zherebtsov M, Nissan C, Goth K, Guner YS, Naidu N, Choudhury B, Grishin AV, Ford HR, Bode L. The human milk oligosaccharide disialyllacto-n-tetraose prevents necrotising enterocolitis in neonatal rats. Gut 2012;61:1417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wopereis H, Oozeer R, Knipping K, Belzer C, Knol J. The first thousand days—intestinal microbiology of early life: establishing a symbiosis. Pediatr Allergy Immunol 2014;25:428–38. [DOI] [PubMed] [Google Scholar]
- 63.McLean MH, Dieguez D Jr, Miller LM, Young HA. Does the microbiota play a role in the pathogenesis of autoimmune diseases? Gut 2015;64:332–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cox LM, Blaser MJ. Pathways in microbe-induced obesity. Cell Metab 2013;17:883–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Urashima T, Asakuma S, Leo F, Fukuda K, Messer M, Oftedal OT. The predominance of type i oligosaccharides is a feature specific to human breast milk. Adv Nutr 2012;3:473S–82S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Urashima T, Taufik E, Fukuda K, Asakuma S. Recent advances in studies on milk oligosaccharides of cows and other domestic farm animals. Biosci Biotechnol Biochem 2013;77:455–66. [DOI] [PubMed] [Google Scholar]
- 67.Mehra R, Barile D, Marotta M, Lebrilla CB, Chu C, German JB. Novel high-molecular weight fucosylated milk oligosaccharides identified in dairy streams. PLoS One 2014;9:e96040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bullen CL, Tearle PV, Stewart MG. The effect of “humanised” milks and supplemented breast feeding on the faecal flora of infants. J Med Microbiol 1977;10:403–13. [DOI] [PubMed] [Google Scholar]
- 69.Howard MD, Gordon DT, Pace LW, Garleb KA, Kerley MS. Effects of dietary supplementation with fructooligosaccharides on colonic microbiota populations and epithelial cell proliferation in neonatal pigs. J Pediatr Gastroenterol Nutr 1995;21:297–303. [DOI] [PubMed] [Google Scholar]
- 70.Boehm G, Moro G. Structural and functional aspects of prebiotics used in infant nutrition. J Nutr 2008;138:1818S–28S. [DOI] [PubMed] [Google Scholar]
- 71.Holscher HD, Faust KL, Czerkies LA, Litov R, Ziegler EE, Lessin H, Hatch T, Sun S, Tappenden KA. Effects of prebiotic-containing infant formula on gastrointestinal tolerance and fecal microbiota in a randomized controlled trial. JPEN J Parenter Enteral Nutr 2012;36:95S–105S. [DOI] [PubMed] [Google Scholar]
- 72.Stiverson J, Williams T, Chen J, Adams S, Hustead D, Price P, Guerrieri J, Deacon J, Yu Z. Prebiotic oligosaccharides: comparative evaluation using in vitro cultures of infants’ fecal microbiomes. Appl Environ Microbiol 2014;80:7388–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Paineau D, Respondek F, Menet V, Sauvage R, Bornet F, Wagner A. Effects of short-chain fructooligosaccharides on faecal bifidobacteria and specific immune response in formula-fed term infants: a randomized, double-blind, placebo-controlled trial. J Nutr Sci Vitaminol (Tokyo) 2014;60:167–75. [DOI] [PubMed] [Google Scholar]
- 74.Kuczynski J, Lauber CL, Walters WA, Parfrey LW, Clemente JC, Gevers D, Knight R. Experimental and analytical tools for studying the human microbiome. Nat Rev Genet 2012;13:47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]