Abstract
Following a veterinary and behavioral survey of chimpanzees from a natural population in Uganda, leaf samples of Trichilia rubescens were collected because of the unusual method of ingestion observed. The methanolic crude extract of T. rubescens leaves exhibited significant antimalarial activity in vitro. Bioassay-directed fractionation provided two new limonoids, trichirubines A and B. A greater understanding of the role of secondary compounds in the primate diet may be helpful in recovering naturally occurring compounds of medicinal significance for human medicine.
Plant parts which have no apparent nutritive value and/or are rich in secondary compounds are ingested by chimpanzees (10, 14, 21, 22). Medicinal benefits of such ingestion have been suggested (8, 9, 20) by the observations of two unusual behaviors proposed to control intestinal parasite infection, namely swallowing whole bristly leaves and chewing the bitter pith of Vernonia amygdalina (7, 8, 18, 19). There is no chemical evidence to suggest a role of secondary compounds correlated with the leaf-swallowing behavior: more likely there is a mechanical effect of the surface of the whole rough hispid leaves leading to the expulsion of parasites. Instead of a physical effect, the benefit of bitter pith chewing is pharmacologically based on the activity of steroid glucosides (10, 14). In order to provide new information about self-medicative behavior of chimpanzees (Pan troglodytes schweinfurthii) and the phytochemistry of the plants ingested, field studies were conducted in Kanyawara, Kibale National Park, Uganda. The survey included behavioral data collection, as well as fecal and urine analysis of samples coming from identified chimpanzees. Particular attention was focused on sick individuals and unusual or occasional feeding behaviors. We report herein the bioassay-directed fractionation of the crude extracts of the plant leaves of Trichilia rubescens and elucidation of the structure of two limonoids, namely trichirubines A and B, which possess a significant anti-Plasmodium activity.
The study was conducted in the Kibale National Park (766 km2 between 0°13 to 0°41′N and 30°19′ to 30°22′E) in western Uganda. Data were collected between December 2000 and March 2001 (dry season) and in October 2001 (rainy season). The area contains mid-altitude moist forest, secondary forest, grassland, swamps, and plantations of Eucalyptus and pines, and it includes elements of lowland tropical rainforest, montane rainforest, and mixed deciduous rainforest. The elevation is between 800 and 1,500 m, and the rainfall averages 1,700 mm per year.
The Kanyawara community of wild chimpanzees (P. troglodytes schweinfurthii), including about 50 individuals, was observed. Feeding behaviors were recorded by 10-min focal-animal sessions and ad libitum observations (1). Health state was monitored daily by noninvasive methods consisting of clinical observations, coprological study of 252 stool samples, and urinalysis of 76 samples. We focused our behavioral observations on ill chimpanzees and unusual feeding behaviors, as plants eaten regularly and in large amounts probably did not contain highly active secondary compounds. Eighty-four crude extracts were made from the different parts of 24 plant species belonging to 13 botanical families. The crude extracts were evaluated for their antimalarial activities. After the first screening, plants exhibiting significant activities were submitted to chemical study in order to isolate the compounds responsible for bioactivities.
Air-dried powdered leaves (3 kg) of T. rubescens were macerated in heptane and then in methanol at 40°C. Filtration and vacuum concentration led to a dark-greenish extract (310 g).
Antimalarial activity was evaluated against intraerythrocytic asexual forms of Plasmodium falciparum. The chloroquine-resistant strain FcB1 of P. falciparum (50% inhibitory concentration [IC50] of chloroquine = 62 ng/ml) was maintained continuously in culture on human erythrocytes as described by Trager and Jensen (17). In vitro antiplasmodial activity was determined by a modification of the semiautomated microdilution technique of Desjardins et al. (6). Stock solutions of test extracts or compounds were prepared in dimethyl sulfoxide (DMSO) at a 10-mg/ml concentration. Drug solutions were serially diluted with culture medium and introduced to asynchronous parasite cultures (0.5% parasitemia and 1% final hematocrit) on 96-well plates for 24 h at 37°C, prior to the addition of 0.5 μCi of [3H]hypoxanthine (1 to 5 Ci/mmol; Amersham, Les Ulis, France) per well for 24 h. The growth inhibition for each drug concentration was determined by comparison of the radioactivity incorporated into the treated culture with that in the control culture (without drug) maintained on the same plate. The IC50 was obtained from the drug concentration-response curve and the results were expressed as the mean determined from three independent experiments. The DMSO concentration never exceeded 0.1% and did not inhibit the parasite growth.
Column chromatography on Merck silica gel 60 (reference no. 7736) was used for isolation and purification. High-performance liquid chromatography (HPLC) was carried out with a Waters HPLC instrument equipped with a UV photodiode array detector on C18 Thermohypersil Kromasil 250- by 21-mm 5-μm column at a flow rate of 16 ml/min. 1H nuclear magnetic resonance (NMR) (600 MHz), 13C NMR (150 MHz), and two-dimensional NMR were recorded on Bruker DRX 600 spectrometer. Mass spectra in the electron impact (EI) mode were recorded on a Kratos MS 50 instrument. Liquid chromatography-mass spectrometry (LC-MS) analyses in electrospray ionization (ESI) were conducted on a Thermoquest LCQ Deca ion trap coupled with an HPLC Thermofinnigan chain, using a Hypurity-C18 150- by 4.6-mm, 5-μm column at a flow rate of 1 ml/mn.
Column chromatography on silica gel of 51 g of methanolic extract eluted with a gradient of heptane-ethyl acetate (8:2 to 2:8) (column chromatography 1 [CC1]) led to 14 fractions. Fraction 10 (800 mg) was passed through a silica gel column eluted by heptane-acetone (8:2 to 1:9) (CC2), yielding 12 fractions. Fraction 10′ (81 mg) was a pure compound 1. The other active fraction, 7′ (IC50 = 0.2 μg/ml) was then purified by HPLC (45:55 H2O-acetonitrile).
Among unusual behaviors, we have observed the occasional ingestion of T. rubescens leaves by Kanyawara chimpanzees. We recorded five cases of consumption of T. rubescens Oliv. (Meliaceae) during the course of 700 h of observation. T. rubescens leaves were 1 of the 46 items consumed by the Kanyawara chimpanzees. The chimpanzee diet was dominated by fruit (81% of the feeding time was spent in fruit eating), and although the diet varied from month to month, only four plant species were consumed for more than 10% of the feeding time over the study period. Seventy-five percent of the feeding time was concentrated on six species: five for fruits and one for leaves. Of the 19 species on which chimpanzees spent 0.5% or more of feeding time, T. rubescens leaves were not present. Whereas the groups included two to six chimpanzees, in each bout of observation only one individual from the party ingested a few leaves of T. rubescens (mean of 5 leaves/min) for a short time (mean, 4 min 10 s; range, 2 to 7 min), leaving the shrub before having eaten all the leaves. The individual consuming T. rubescens leaves was different in each case. The other chimpanzees from the party did not even try to feed on the shrub after the consumer left it. Moreover this plant generally grows in a cluster so that leaves would be available for several chimpanzees. These observations suggest that chimpanzees eating T. rubescens leaves may be individuals with temporarily different criteria of food choice from other individuals in their party in spite of any symptom detected by health monitoring.
The plant part ingested was collected according to these observations. The crude MeOH extract exhibited an IC50 at 12 μg/ml. Fraction 10 obtained from CC1 yielded higher activity against P. falciparum (Table 1). Submitted to CC2, fraction 10 led to a pure compound 1 (81 mg), named trichirubine A, with a significant antimalarial activity (IC50 = 0.3 μg/ml) (Fig. 1).
TABLE 1.
Antimalarial activities from CC1 and CC2a
| Fraction | IC50 (μg/ml) |
|---|---|
| CC1 | |
| 1-5 | >30 |
| 6 | 14.2 |
| 7 | 29.9 |
| 8 | 20 |
| 9 | 12.6 |
| 10 | 0.9b |
| 11 | 2.6 |
| 12 | 3.7 |
| 13 | >30 |
| 14 | >30 |
| CC2 | |
| 1′ | 6.4 |
| 2′ | 5 |
| 3′ | 2.7 |
| 4′ | 0.4 |
| 5′ | 0.3 |
| 6′ | <0.2 |
| 7′ | 0.2b |
| 8′ | 0.2 |
| 9′ | 0.3 |
| 10′ | 0.3b |
| 11′ | 0.7 |
| 12′ | 1.2 |
See the text for details.
Processed fraction.
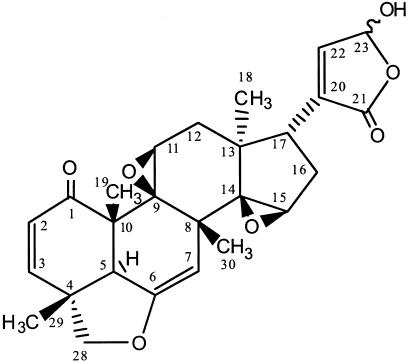
FIG. 1.
Trichirubine A 1.
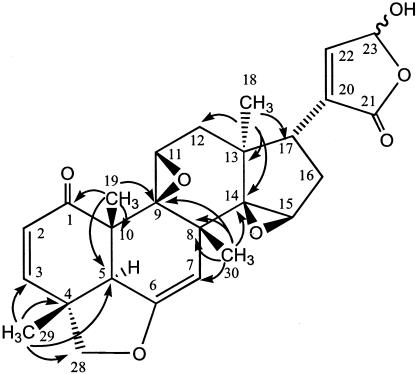
Trichirubine A 1 was obtained as a yellow amorphous solid, [α]D25 +44 (c 0.1, CHCl3). EI mass spectrometry (EIMS) and ESI-MS gave a molecular ion peak at m/z 452.2 corresponding to the molecular formula C26H28O7. NMR spectra were obtained in CD2Cl2, but epimerization induced a doubling of the signals (Table 2). The structure of compound 1 was first determined on the basis of heteronuclear multiple-bond correlations (HMBCs). The correlations of the methyls with α and β carbons led to the skeleton drawn in Fig. 2. The HMBCs of the carbonyl C-1 with H-2 and H-3 implied the presence of a ring C-1, C-2, C-3, C-4, C-5, and C-10. The correlations obtained from both correlated spectroscopy (H,H COSY) and HMBC spectra prove the cycles B, C, and D. The lactone group was determined as located at C-17 on the HMBC correlations (H-17 to C-20, C-21 and C-22). Two epoxide groups were supported by very large coupling constants (JCH = 177 Hz and 184 Hz) observed for C-11 and C-15, respectively. The stereochemistry was determined by nuclear Overhauser effect (NOE) correlations.
TABLE 2.
1H (CD2Cl2, 600 MHz) and 13C (CD2Cl2, 150 MHz) NNMR data of trichirubine A 1
| Atoma | Chemical shift (ppm)b
|
J (Hz) | No. of protons; multiplicity for each signal | |
|---|---|---|---|---|
| δ13C | δ1H | |||
| 1* | 198.82; 198.84 | |||
| 2* | 132.21; 133.33 | 5.84 | 10.2 | 1; d |
| 3* | 150.82; 150.86 | 7.07 | 10.2 | 1; d |
| 4 | 44.35 | |||
| 5 | 53.87 | 3.28 | 2.6 | 1; d |
| 6 | 150.55 | |||
| 7* | 100.27 | 4.68; 4.69 | 2.6 | 1; d |
| 8 | 41.65 | |||
| 9 | 66.35 | |||
| 10 | 47.62 | |||
| 11 | 59.26 | 3.52 | 8.0; 2.0 | 1; dd |
| 12α* | 35.13; 35.30 | 2.13 | 15.0 | 1; m |
| 12β* | 35.13; 35.30 | 1.99 | 15.0; 8.0 | 1; dd |
| 13 | 41.65 | |||
| 14 | 71.87 | |||
| 15 | 54.82 | 3.36 | 1; s | |
| 16α* | 30.52; 30.60 | 1.89 | 11.5; 11.5 | 1; dd |
| 1.91 | 11.5; 11.5 | 1; dd | ||
| 16β* | 30.52; 30.60 | 2.10 | 1; m | |
| 17* | 41.20 | 2.42; 2.44 | 11.5; 6.6; 1.5 | 1; ddd |
| 18* | 19.43 | 0.73; 0.76 | 3; s | |
| 19 | 19.10 | 1.41 | 3; s | |
| 20* | 137.59; 137.75 | |||
| 21* | 171.25; 171.47 | |||
| 22* | 146.70; 146.84 | 6.79 | 1.5 | 1; d |
| 23* | 96.90 | 6.06; 6.11 | 1; bs | |
| 97.17 | 1; bs | |||
| 28α* | 81.56; 81.59 | 3.90 | 7.8 | 1; d |
| 28β* | 81.56; 81.59 | 4.10 | 7.8 | 1; d |
| 29* | 21.48; 21.51 | 1.34 | 3; s | |
| 30* | 24.05 | 1.09; 1.10 | 3; s | |
*, doubled signal.
The chemical shifts are expressed in ppm relative to tetramethylsilane.
FIG. 2.
HMBC correlations of trichirubine A 1.
Compound 2 (2 mg), named trichirubine B (Fig. 3), was isolated from the fraction 7′ (IC50 = 0.2 μg/ml). The small quantity isolated enabled us to determine the compound's structure, but the activity of the pure product after experiments including heating and acid addition could not be determined with certainty.
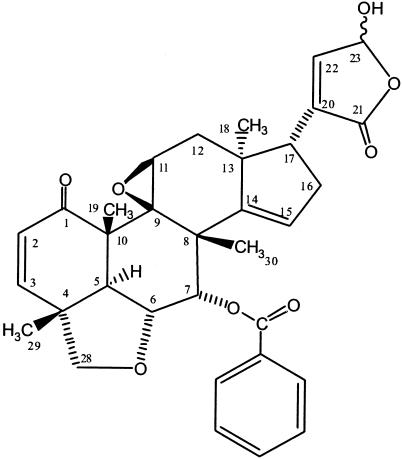
FIG. 3.
Trichirubine B 2.
Trichirubine B 2 was obtained as a white amorphous solid. EIMS and ESI-MS gave a molecular ion peak at m/z 558.04 corresponding to the molecular formula C33H34O8. Because of the small quantity isolated, the 13C NMR spectrum could not be recorded. The 13C chemical shifts were determined by using the HMBC spectrum in which the doubling of signals was not observed due to the weak resolution in the carbon dimension. NMR spectra were also obtained in pyridine to confirm the determination of terpenic structure, while the spectrum in CD2Cl2 with trifluoroacetic acid (TFA) allowed lactone determination. Even with TFA, 1H signals were still broad and epimers were undistinguishable (Table 3). Compound 2 was related to compound 1, but Me-30, Me-18, and H-17 showed cross-correlation with a carbon at δ 151.37, suggesting that C-14 is an sp2 carbon. An additional phenyl group appeared in 1H NMR spectrum as well as an additional carbonyl (δ 167.50) linked to H-7 according to HMBC correlation. The structure of compound 2 has thus been assigned on the basis of this spectral data.
TABLE 3.
1H(CD2Cl2, 600 MHz) and 13C (CD2Cl2, 150 MHz) NMR data of trichirubine B 2
| Atom(s) | Chemical shift (ppm)a
|
J (Hz) | No. of protons; multiplicity for each signal | |
|---|---|---|---|---|
| δ 13C | δ 1H | |||
| 1 | 202.5 | |||
| 2 | 131.0 | 6.11 | 9.7 | 1; d |
| 3 | 154.0 | 7.23 | 9.7 | 1; d |
| 4 | 43.0 | |||
| 5 | 49.9 | 3.11 | 12.7 | 1; d |
| 6 | 72.6 | 4.73 | 12.7; 3.6 | 1; dd |
| 7 | 79.2 | 5.85 | 3.6 | 1; d |
| 8 | 45.7 | |||
| 9 | 65.4 | |||
| 10 | 47.9 | |||
| 11 | 60.5 | 3.93 | 1; m | |
| 12 | 39.6 | 2.03 | 9.0 | 1; m |
| 2.19 | 9.0 | 1; m | ||
| 13 | 45.8 | |||
| 14 | 151.3 | |||
| 15 | 126.9 | 5.84 | 1; bs | |
| 16 | 34.4 | 2.35 | 1; m | |
| 17 | 49.4 | 2.79 | 1; m | |
| 18 | 24.3 | 0.62 | 3; s | |
| 19 | 18.4 | 1.64 | 3; s | |
| 20 | 138.3 | |||
| 21 | 173.5 | |||
| 22b | 6.82 | 1; bs | ||
| 23 | 97.8 | 6.10 | 1; bs | |
| 28 | 80.2 | 3.56 | 7.5 | 1; d |
| 3.88 | 7.5 | 1; d | ||
| 29 | 21.1 | 1.37 | 3; s | |
| 30 | 26.6 | 1.64 | 3; s | |
| 1′ | 167.5 | |||
| 2′ | 128.7 | |||
| 3′ 7′ | 130.1 | 7.91 | bd | |
| 4′ 6′ | 129.3 | 7.47 | bt | |
| 5′ | 134.3 | 7.62 | bt | |
The chemical shifts are expressed in ppm relative to tetramethylsilane.
The chemical shift of C-22 cannot be obtained.
The genus Trichilia is known for its content in various limonoids, many of which are active against insects (4). In vitro antimalarial activities have recently been reported for limonoids from other Meliaceae: bark and seeds of Khaya grandifolia (2), bark of Khaya senegalensis (13), leaves of Azadirachta indica (11), and Cedrela odorata wood (3). The most active limonoid, gedunin, isolated from Khaya grandifolia, Cedrela odorata, and Azadirachta indica (3, 16), had a better in vitro activity than chloroquine against clones sensitive to chloroquine (15). Its in vitro activity evaluated against a chloroquine-resistant strain of P. falciparum (IC50 = 0.72 μg/ml) was roughly equivalent to that of trichirubine A (12), but its in vivo activity, initially poor (3), might be improved: for example, when combined with chloroquine (2), by synergism with dillapiol, a cytochrome P-450 3A4 inhibitor, or preparation of a more stable compound (16). Antimalarial activities may be related to the presence of reactive groups on ring A (4). As in the case of gedunin, the carbonyl group in C-3 and unsaturation in C-1/C-2 are currently observed in ring A of limonoids. The present structures of compounds 1 and 2 are original for two reasons: (i) the locations of these reactive groups, respectively, in C-1 and C-2/C3, as observed for limonoids isolated recently by deCarvalho et al. (5); and (ii) the presence of a lactone ring compared to the usual furan ring, as observed in limonoids and particularly in limonoids isolated by deCarvalho et al. (5). Those compounds also extracted from Trichilia rubescens leaves were shown to have potential activity in cystic fibrosis (5).
New evidence has been provided to support the previous hypothesis that chimpanzee diet contains secondary metabolites that may be useful to health maintenance. The study of self-medication in apes based in veterinary and behavioral survey might provide a novel approach to the discovery of new bioactive natural products useful in human therapy.
Acknowledgments
We acknowledge Richard Wrangham, codirector of the Kibale Chimpanzee Project, for valuable support in providing data and sharing experience and Claude-Marcel Hladik for important collaboration with the primatological survey. We are grateful to the Uganda Wildlife Authority and to the Uganda National Council for Science and Technology for permission to work in the Kibale National Park. For his kind and helpful collaboration in antimalarial screening, we thank Mehdi Labaied. For their valuable assistance with the fieldwork, S.K. expresses her sincere gratitude to the teams of K.C.P. and M.U.B.F.S. for their cooperation in the field.
Acknowledgments for funding are due to Centre National de la Recherche Scientifique and Museum National d'Histoire Naturelle, France.
We are indebted to the anonymous reviewers who provided interesting comments to improve the manuscript.
REFERENCES
- 1.Altmann, J. 1974. Observational study of behavior: sampling methods. Behaviour 48:1-41. [DOI] [PubMed] [Google Scholar]
- 2.Bickii, J., N. Njifutie, J. Ayafor Foyere, L. K. Basco, and P. Ringwald. 2000. In vitro antimalarial activity of limonoids from Khaya grandifolia C.D.C. (Meliaceae). J. Ethnopharmacol. 69:27-33. [DOI] [PubMed] [Google Scholar]
- 3.Bray, D. H., D. C. Warhurst, J. D. Connolly, M. J. O'Neill, and J. D. Phillipson. 1990. Plants as source of antimalarial drugs. Part 7. Activity of some species of Meliaceae plants and their constituent limonoids. Phytother. Res. 4:29-35. [Google Scholar]
- 4.Champagne, D. E., O. Koul, M. B. Isman, G. G. E. Scudder, and G. H. N. Towers. 1992. Biological activity of limonoids from Rutales. Phytochemistry 31:377-394. [Google Scholar]
- 5.DeCarvalho, A. C. V., C. P. Ndi, A. Tsopmo, P. Tane, J. Ayafor, J. D. Conolly, and J. L. Teem. 2002. A novel natural product compound enhances cAMP-regulated chloride conductance of cells expressing CFTRΔF508. Mol. Med. 8:75-87. [PMC free article] [PubMed] [Google Scholar]
- 6.Desjardin, R. E., C.-J. Canfield, J. D. Haynes, and J. D. Chulay. 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 16:710-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huffman, M. A. 1997. Current evidence for self-medication in primates: a multidisciplinary perspective. Yearb. Phys. Anthropol. 40:171-200. [Google Scholar]
- 8.Huffman, M. A., and M. Seifu. 1989. Observations of illness and consumption of a possibly medicinal plant Vernonia amygdalina (Del.), by a wild chimpanzee in the Mahale Mountains National Park, Tanzania. Primates 30:51-63. [Google Scholar]
- 9.Janzen, D. H. 1978. Complication in interpreting the chemical defenses of trees against tropical arboreal plant-eating vertebrates, p. 73-84. In G. G. Montgomery (ed.), The ecology of arboreal folivores. Smithsonian Institution Press, Washington, D.C.
- 10.Jisaka, M., H. Ohigashi, T. Takagaki, T. Nozaki, T. Tada, M. Hirota, R. Irie, M. A. Huffman, T. Nishida, M. Kajie, and K. Koshimizu. 1992. Bitter steroid glucosides, vernoniosides A1, A2 and A3, and related B1 from a possible medicinal plant, Vernonia amygdalina, used by wild chimpanzees. Tetrahedron 48:625-632. [Google Scholar]
- 11.Joshi, S. P., S. R. Rojatkar, and B. A. Nasagampagi. 1998. Antimalarial activity of Neem (Azadirachta indica). J. Med. Aromatic Plant Sci. 20:1000-1002. [Google Scholar]
- 12.Khalid, S. A., A. Farouk, T. G. Geary, and J. B. Jensen. 1986. Potential antimalarial candidates from African plants: an in vitro approach using Plasmodium falciparum. J. Ethnopharmacol. 15:201-209. [DOI] [PubMed] [Google Scholar]
- 13.Khalid, S. A., G. M. Friedrichsen, A. Kharazmi, G. T. Theander, C. E. Olsen, and S. B. Christensen. 1998. Limonoids from Khaya senegalensis. Phytochemistry 49:1769-1772. [DOI] [PubMed] [Google Scholar]
- 14.Koshimizu, K., H. Ohigashi, M. A. Huffman, T. Nishida, and H. Takasaki. 1993. Physiological activities and the active constituents of potentially medicinal plants used by wild chimpanzees of the Mahale Mountains, Tanzania. Int. J. Primatol. 14:345-356. [Google Scholar]
- 15.MacKinnon, S., T. Durst, J. T. Arnason, C. Angerhofer, J. Pezzuto, P. E. Sanchez-Vindas, L. J. Poveda, and M. Gbeaessor. 1997. Antimalarial activity of tropical Meliaceae extracts and gedunin derivatives. J. Nat. Prod. 60:336-341. [DOI] [PubMed] [Google Scholar]
- 16.Omar, S., J. Zhang, S. MacKinnon, D. Leaman, T. Durst, B. J. R. Philogene, J. T. Arnason, P. E. Sanchez-Vindas, L. Poveda, P. A. Tamez, and J. M. Pezzuto. 2003. Traditionally-used antimalarials from the Meliaceae. Curr. Top. Med. Chem. 3:133-139. [DOI] [PubMed] [Google Scholar]
- 17.Trager, W., and J. B. Jensen. 1976. Human malarial parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 18.Wrangham, R. W., and J. Goodall. 1989. Chimpanzee use of medicinal leaves, p. 22-37. In P. G. Heltne and L. A. Marquardt (ed.), Understanding chimpanzees. Harvard University Press, Cambridge, Mass.
- 19.Wrangham, R. W. 1995. Leaf-swallowing by chimpanzees and its relationship to tapeworm infection. Am. J. Primatol. 37:297-304. [DOI] [PubMed] [Google Scholar]
- 20.Wrangham, R. W., and T. Nishida. 1983. Aspilia spp. leaves: a puzzle in the feeding behavior of wild chimpanzees. Primates 24:276-282. [Google Scholar]
- 21.Wrangham, R. W., and P. G. Waterman. 1983. Condensed tannins in fruits eaten by chimpanzees. Biotropica 15:217-222. [Google Scholar]
- 22.Wrangham, R. W., N.-L. Conklin-Brittain, and K. D. Hunt. 1998. Dietary response of chimpanzees and cercopithecines to seasonal variation in fruit abundance. I. Antifeedants. Int. J. Primatol. 19:946-970. [Google Scholar]