Abstract
The Mycobacterium tuberculosis Rv2686c-Rv2687c-Rv2688c operon, encoding an ABC transporter, conferred resistance to ciprofloxacin and, to a lesser extent, norfloxacin, moxifloxacin, and sparfloxacin to Mycobacterium smegmatis. The resistance level decreased in the presence of the efflux pump inhibitors reserpine, carbonyl cyanide m-chlorophenylhydrazone, and verapamil. Energy-dependent efflux of ciprofloxacin from M. smegmatis cells containing the Rv2686c-Rv2687c-Rv2688c operon was observed.
The intrinsic and acquired multidrug resistance play an important role in the insurgence of tuberculosis (21). Cumulative research efforts into the study of antibiotic resistance mechanisms have implicated efflux pumps as a major underlying factor in intrinsic resistance (15).
Efflux pumps, by which bacteria export various molecules outside the cell, can be classified on the basis of the energy source, which can be either the transmembrane proton gradient or the hydrolysis of ATP (12). The involvement of proton gradient-dependent transporters in drug resistance has been described in several mycobacterial species (1, 5-7, 11, 17, 18, 20). The ABC transporters are part of the ATP-dependent transporter class and are functionally related to the human P-glycoprotein (MDR1), which is involved in the multidrug resistance shown by tumor cells (19). In antibiotic-producing actinomycetes, ABC transporters are involved in the secretion of the antibiotic through the cell membrane and contribute to self-resistance to the produced antibiotic (13). Only a few ATP-dependent drug transporters have been characterized in other bacteria, which mediate the resistance to structurally different compounds, including fluoroquinolones (8-10, 14). In Mycobacterium tuberculosis, the genes encoding the predicted ABC transporters occupy about 2.5% of the genome (3). Nevertheless, most of these putative ABC transporters have not been characterized yet. To date, only one ABC transporter involved in drug resistance has been described for M. tuberculosis (4). Beside the predicted ABC antimicrobial transporters, the putative nucleotide binding domain involved in uptake of inorganic phosphate, which mediates fluoroquinolone efflux, has been described in Mycobacterium smegmatis (2).
In this paper we demonstrate that the Rv2686c-Rv2687c-Rv2688c genes from M. tuberculosis encode an ABC transporter responsible for fluoroquinolone efflux.
Analysis of the products of the M. tuberculosis Rv2686c-Rv2687c-Rv2688c operon as a possible ABC drug transporter.
We analyzed the M. tuberculosis hypothetical ABC drug transporters, described by Braibant et al. (3), for the presence of transmembrane segments (TMS) by the TMHMM tool (http://www.cbs.dtu.dk/services/TMHMM-2.0) and for gene organization according to the sequences retrieved from the Tuberculist web server (http://genolist.pasteur.fr/TubercuList/). We focused our attention on the Rv2686c-Rv2687c-Rv2688c operon. The 5′ and 3′ ends of the Rv2687c open reading frame overlap, respectively, with the stop codon of Rv2688c and the translation start codon of Rv2686c, which suggests that the three genes may be cotranscribed. Rv2686c and Rv2687c have six TMS, whereas Rv2688c protein does not have any predicted TMS, but it possesses a nucleotide binding domain and is likely involved into ATP hydrolysis.
A BLAST analysis performed on the M. smegmatis sequence data, available at the website http://www.tigr.org, showed the presence of three genes homologous to M. tuberculosis Rv2686c, Rv2687c, and Rv2688c with the same operon-like organization (data not shown).
Expression of M. tuberculosis Rv2686c-Rv2687c-Rv2688c in M. smegmatis and analysis of the antibiotic resistance profile.
To characterize the Rv2686c-Rv2687c-Rv2688c operon, the entire operon and each gene were amplified from M. tuberculosis H37Rv genomic DNA by PCR utilizing the primers reported in Table 1. The PCR products were cloned into pGEM-T Easy vector and sequenced. The operon and the single open reading frames were then cloned into pSODIT-2 shuttle expression vector to generate the plasmids pMP178 (Rv2686c-Rv2687c-Rv2688c/pSODIT-2), pM45 (Rv2686c/pSODIT-2), pM57 (Rv2687c/pSODIT-2) and pM70 (Rv2688c/pSODIT-2). Plasmid DNAs, isolated from Escherichia coli by the alkaline lysis method (16), were introduced into M. smegmatis mc2155 by electroporation, and the resistance of the transformed strains to different compounds was analyzed. M. smegmatis strains were grown in Middlebrook 7H9 broth and Middlebrook 7H11 agar supplemented with 10% Middlebrook oleic acid-albumin-dextrose-catalase enrichment and 0.2% glycerol. The MICs of the following antibiotics were determined by twofold serial dilution, either by plate dilution or by microdilution in liquid medium: gentamicin, rifampin, streptomycin, lincomycin, acriflavine, ciprofloxacin, lomefloxacin, ofloxacin, norfloxacin, sparfloxacin, moxifloxacin, levofloxacin, chloramphenicol, rhodamine 123, ethambutol, isoniazid, doxorubicin, erythromycin, tetraphenylphosphonium, and tetracycline. The ciprofloxacin resistance level showed by M. smegmatis was eightfold the MIC for the wild type when overexpressing the entire operon (pMP178) and fourfold the MIC for the wild type when overexpressing the Rv2686c gene (pM45). The norfloxacin resistance level was about twofold the MIC for the wild type, irrespective of whether the bacteria expressed the full-length operon or the Rv2686c gene alone (Table 2). The increased MICs for M. smegmatis cells expressing the Rv2686c gene could be due to possible interactions of the Rv2686c protein with the homologous host proteins, which result in a functional transporter. Finally, the resistance level to the newer fluoroquinolones, moxifloxacin and sparfloxacin, of M. smegmatis expressing the operon was twofold the MIC for the wild type. No significant changes in the MICs of the other tested drugs were observed (Table 2).
TABLE 1.
Primers used in this studya
| Primer | Nucleotide sequence | Purpose |
|---|---|---|
| RG360 | 5′-TTATCGATATGACGGCGCTCAA-3′ | Sense primer for Rv2686c-Rv2687c-Rv2688c operon and Rv2688c gene |
| RG330 | 5′-TTATCGATTCACGCGTGCTTAG-3′ | Antisense primer for Rv2686c-Rv2687c-Rv2688c operon and Rv2686c gene |
| RG357 | 5′-CAATCGATGTGAGAGCGATATC-3′ | Sense primer for Rv2686c gene |
| RG359 | 5′-TAATCGATATGACCCGGTTGGT-3′ | Sense primer for Rv2687c gene |
| RG358 | 5′-CTATCGATTCACAGCACACCCG-3′ | Antisense primer for Rv2687c gene |
| RG361 | 5′-TTATCGATTCATGTCAGCTGCCT-3′ | Antisense primer for Rv2688c gene |
The underlined sequence indicates the ClaI restriction site.
TABLE 2.
MICs of drugs shown by M. smegmatis mc2155 cells transformed with plasmids pMP178 (Rv2686c-Rv2687c-Rv2688c/pSODIT-2), pM45 (Rv2686c/pSODIT-2), pM57 (Rv2687c/ pSODIT-2), pM70 (Rv2688c/pSODIT-2), and pSODIT-2a
| Drug (μg/ml) |
M. smegmatis cells containing:
|
||||
|---|---|---|---|---|---|
| pSODIT-2 | pMP178 | pM45 | pM57 | pM70 | |
| Ciprofloxacin | 0.12 | 0.96 | 0.48 | 0.24 | 0.12 |
| Norfloxacin | 2.5 | 5 | 5 | 2.5 | 2.5 |
| Moxifloxacin | 0.08 | 0.16 | |||
| Sparfloxacin | 0.07 | 0.14 | |||
| Levofloxacin | 0.5 | 0.5 | |||
| Lomefloxacin | 0.4 | 0.4 | |||
| Ofloxacin | 0.25 | 0.25 | |||
| Gentamicin | 0.6 | 0.6 | |||
| Rifampin | 128 | 128 | |||
| Streptomycin | 1.2 | 1.2 | |||
| Chloramphenicol | 15 | 15 | |||
| Acriflavine | 25 | 25 | |||
| Rhodamine 123 | 5 | 5 | |||
| Ethambutol | 1.5 | 1.5 | |||
| Isoniazid | 32 | 32 | |||
| Doxorubicin | 20 | 20 | |||
| Erythromycin | 30 | 30 | |||
| Tetraphenylphosphonium | 1 | 1 | |||
| Tetracycline | 0.5 | 0.5 | |||
| Lincomycin | 32 | 32 | |||
The MICs were confirmed in three different experiments.
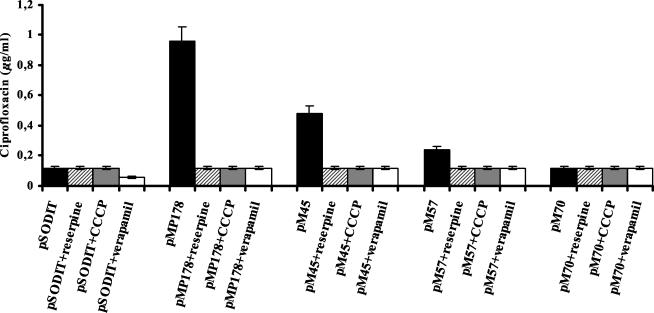
The MICs of ciprofloxacin and norfloxacin were also determined in the presence of either the known mammalian P-glycoprotein inhibitor reserpine (12 μg/ml), the calcium blocker verapamil (40 μg/ml), a potent inhibitor of a wide range of multidrug efflux pumps, or the proton uncoupler carbonyl cyanide m-chlorophenylhydrazone (CCCP; 15 μg/ml), in order to evaluate the effects of alterations in the proton gradient on resistance levels. The presence of the efflux pump inhibitors reserpine, verapamil, and CCCP reduced ciprofloxacin resistance to the same level as that of the control strain, transformed with the pSODIT-2 expression vector (Fig. 1). The observation that known inhibitors of ABC transporters (reserpine and verapamil) are able to inhibit this activity indicates that the efflux is driven by ATP. The effect of CCCP is likely due to the depletion of the intracellular pool of ATP, an indirect effect of H+ gradient uncoupling.
FIG. 1.
Effect of reserpine, verapamil, and CCCP on MICs of ciprofloxacin for M. smegmatis cells carrying different recombinant clones. The results are the averages of three replicates, and error bars indicate standard deviations.
Efflux of ciprofloxacin outside the bacterial cells.
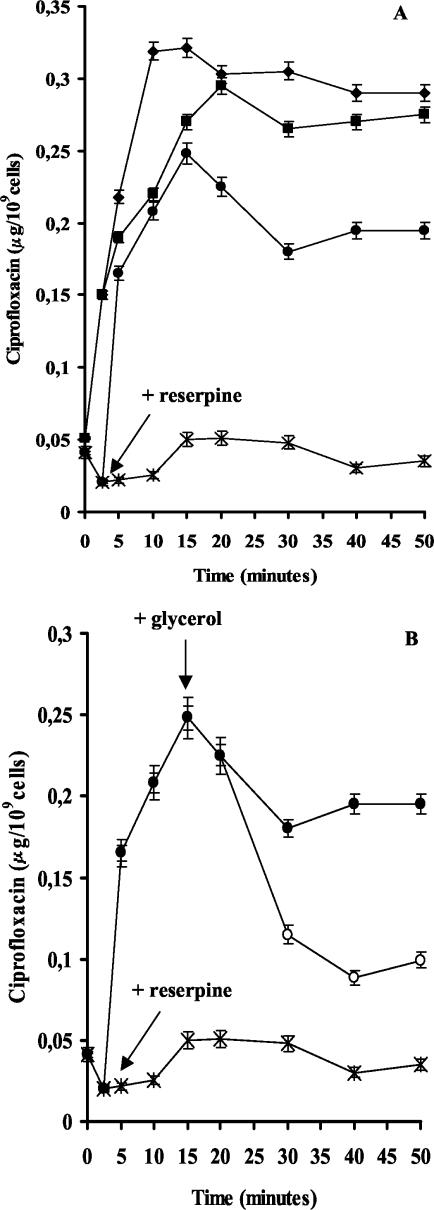
To determine whether the resistance to fluoroquinolones was due to an active drug efflux mechanism, the accumulation of ciprofloxacin in M. smegmatis mc2155 cells was monitored by a fluorimetric method, as previously described (5). As shown in Fig. 2A, accumulation of ciprofloxacin was rapid, achieved a steady-state level within 2 to 3 min of incubation, and was approximately 60% lower in cells harboring the pMP178 recombinant plasmid.
FIG.2.
(A) Effect of the addition of reserpine on the accumulation of ciprofloxacin by M. smegmatis cells carrying the Rv2686c-Rv2687c-Rv2688c-containing plasmid (*, no reserpine addition; •, addition of reserpine) or expression vector pSODIT-2 (⧫, no reserpine addition; ▪, addition of reserpine). (B) Effect of the addition of reserpine and glycerol on the accumulation and efflux of ciprofloxacin by M. smegmatis cells carrying the Rv2686c-Rv2687c-Rv2688c-containing plasmid (*, no reserpine addition; •, addition of reserpine; ○, addition of glycerol). The addition of glycerol had no effect on the level of ciprofloxacin accumulation in cells not treated with reserpine (data not shown). The arrows indicate the time of addition of reserpine and glycerol. The results are the averages of three replicates, and error bars indicate standard deviations.
To determine whether the accumulation of ciprofloxacin was energy dependent, reserpine was added to cells 2.5 min after the addition of ciprofloxacin. As shown in Fig. 2A, accumulation increased to levels close to the values shown by cells transformed with pSODIT-2, a finding that suggests that reserpine inhibits the efflux pump. In contrast, reserpine had no significant effect on the level of ciprofloxacin accumulation in the control strain carrying the cloning vector pSODIT-2 (Fig. 2A). Overall, these results demonstrate that the reserpine-sensitive resistance to ciprofloxacin is likely due to reduced accumulation of this fluoroquinolone and that this transporter pumps out ciprofloxacin by an energy-dependent process. To determine whether the readily available source of energy could lead to antibiotic efflux, glycerol was added 17.5 min after the addition of reserpine. Resistant cells rapidly eliminated ciprofloxacin (Fig. 2B), whereas the sensitive ones did not extrude significant amounts of the drug (data not shown).
These results strongly indicate that the Rv2686c-Rv2687c-Rv2688c proteins actively pump out ciprofloxacin, probably by using ATP hydrolysis as an energy source. To our knowledge, this is the first example of an ABC transporter which mediates single fluoroquinolone resistance in M. tuberculosis.
One member of the ABC transporter, involved in inorganic phosphate transport and whose mRNA overexpression is correlated to high-level fluoroquinolone resistance, has already been characterized in M. smegmatis (2). In contrast, an ABC transporter involved in fluoroquinolone resistance has never been described for M. tuberculosis to date. Resistance to fluoroquinolones in 42 to 85% of clinical isolates of M. tuberculosis has been shown to be associated with mutations in a 120-bp region in the gyrA gene encoding DNA gyrase, the fluoroquinolone target (22). Active drug efflux mediated by the Rv2686c-Rv2687c-Rv2688c proteins could account for the fluoroquinolone resistance in the remaining isolates. Further characterization of this pump, and the study of the mutant phenotype after gene inactivation, will be useful to determine the role of these proteins in the fluoroquinolone resistance.
Acknowledgments
This research was supported by European Union research project “Quality of life and management of living resources” (contract no. QLK2-CT-2000-01761) and by FAR 2003 and 2004 (University of Pavia).
REFERENCES
- 1.Ainsa, J. A., M. C. Blokpoel, I. Otal, D. B. Young, K. A. De Smet, and C. Martin. 1998. Molecular cloning and characterization of Tap, a putative multidrug efflux pump present in Mycobacterium fortuitum and Mycobacterium tuberculosis. J. Bacteriol. 180:5836-5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatt, K., S. K. Banerjee, and P. K. Chakraborti. 2000. Evidence that phosphate specific transporter is amplified in a fluoroquinolone resistant Mycobacterium smegmatis. Eur. J. Biochem. 267:4028-4032. [DOI] [PubMed] [Google Scholar]
- 3.Braibant, M., P. Gilot, and J. Content. 2000. The ATP binding cassette (ABC) transport systems of Mycobacterium tuberculosis. FEMS Microbiol. Rev. 24:449-467. [DOI] [PubMed] [Google Scholar]
- 4.Choudhuri, B. S., S. Bhakta, R. Barik, J. Basu, M. Kundu, and P. Chakrabarti. 2002. Overexpression and functional characterization of an ABC (ATP-binding cassette) transporter encoded by the genes drrA and drrB of Mycobacterium tuberculosis. Biochem. J. 367:279-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Rossi, E., P. Arrigo, M. Bellinzoni, P. A. Silva, C. Martin, J. A. Ainsa, P. Guglierame, and G. Riccardi. 2002. The multidrug transporters belonging to major facilitator superfamily in Mycobacterium tuberculosis. Mol. Med. 8:714-724. [PMC free article] [PubMed] [Google Scholar]
- 6.De Rossi, E., M. C. Blokpoel, R. Cantoni, M. Branzoni, G. Riccardi, D. B. Young, K. A. De Smet, and O. Ciferri. 1998. Molecular cloning and functional analysis of a novel tetracycline resistance determinant, tet(V), from Mycobacterium smegmatis. Antimicrob. Agents Chemother. 42:1931-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Rossi, E., M. Branzoni, R. Cantoni, A. Milano, G. Riccardi, and O. Ciferri. 1998. mmr, a Mycobacterium tuberculosis gene conferring resistance to small cationic dyes and inhibitors. J. Bacteriol. 180:6068-6071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huda, N., E.-W. Lee, J. Chen, Y. Morita, T. Kuroda, T. Mizushima, and T. Tsuchiya. 2003. Molecular cloning and characterization of an ABC multidrug efflux pump, VcaM, in non-O1 Vibrio cholerae. Antimicrob. Agents Chemother. 47:2413-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee, E.-W., M. N. Huda, T. Kuroda, T. Mizushima, and T. Tsuchiya. 2003. EfrAB, an ABC multidrug efflux pump in Enterococcus faecalis. Antimicrob. Agents Chemother. 47:3733-3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin, J., L. O. Michel, and Q. Zhang. 2002. CmeABC functions as a multidrug efflux system in Campylobacter jejuni. Antimicrob. Agents Chemother. 46:2124-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu, J., H. E. Takiff, and H. Nikaido. 1996. Active efflux of fluoroquinolones in Mycobacterium smegmatis mediated by LfrA, a multidrug efflux pump. J. Bacteriol. 178:3791-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKeegan, K. S., M. I. Borges-Walmsley, and A. Walmsley. 2003. The structure and function of drug pumps: an update. Trends Microbiol. 11:21-29. [DOI] [PubMed] [Google Scholar]
- 13.Mendez, C., and J. A. Salas. 2001. The role of ABC transporters in antibiotic-producing organisms: drug secretion and resistance mechanisms. Res. Microbiol. 152:341-350. [DOI] [PubMed] [Google Scholar]
- 14.Poelarends, G. J., P. Mazurkiewicz, M. Putman, R. H. Cool, H. W. Veen, and W. N. Konings. 2000. An ABC-type multidrug transporter of Lactococcus lactis possesses an exceptionally broad substrate specificity. Drug Resist. Updat. 3:330-334. [DOI] [PubMed] [Google Scholar]
- 15.Ryan, B. M., T. J. Dougherty, D. Beaulieu, J. Chuang, B. A. Dougherty, and J. F. Barrett. 2001. Efflux in bacteria: what do we really know about it? Expert Opin. Investig. Drugs 10:1409-1422. [DOI] [PubMed] [Google Scholar]
- 16.Sambrook, J., and D. W. Russell. 2001. Molecular cloning. A laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 17.Sander, P., E. De Rossi, B. Boddinghaus, R. Cantoni, M. Branzoni, E. C. Bottger, H. Takiff, R. Rodriquez, G. Lopez, and G. Riccardi. 2000. Contribution of the multidrug efflux pump LfrA to innate mycobacterial drug resistance. FEMS Microbiol. Lett. 193:19-23. [DOI] [PubMed] [Google Scholar]
- 18.Silva, P. E., F. Bigi, M. de la Paz Santangelo, M. I. Romano, C. Martin, A. Cataldi, and J. A. Ainsa. 2001. Characterization of P55, a multidrug efflux pump in Mycobacterium bovis and Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 45:800-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szabo, D., H. Keyzer, H. E. Kaiser, and J. Molnar. 2000. Reversal of multidrug resistance of tumor cells. Anticancer Res. 20:4261-4274. [PubMed] [Google Scholar]
- 20.Takiff, H. E., M. Cimino, C. M. Musso, T. Weisbrod, R. Martinez, M. B. Delgado, L. Salazar, B. R. Bloom, and W. R. Jacobs, Jr. 1996. Efflux pump of the proton antiporter family confers low-level fluoroquinolone resistance in Mycobacterium smegmatis. Proc. Natl. Acad. Sci. USA 93:362-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Victor, T. C., P. D. van Helden, and R. Warren. 2002. Prediction of drug resistance in M. tuberculosis: molecular mechanisms, tools, and applications. IUBMB Life 53:231-237. [DOI] [PubMed] [Google Scholar]
- 22.Wade, M. M., and Y. Zhang. 2004. Mechanisms of drug resistance in Mycobacterium tuberculosis. Front. Biosci. 9:975-994. [DOI] [PubMed] [Google Scholar]