Abstract
Duplications of the Xq28 region are the most frequent chromosomal aberrations observed in patients with intellectual disability (ID), especially in males. These duplications occur by variable mechanisms, including interstitial duplications mediated by segmental duplications in this region and terminal duplications (functional disomy) derived from translocation with other chromosomes. The most commonly duplicated region includes methyl CpG-binding protein 2 gene (MECP2), which has a minimal duplicated size of 0.2 Mb. Patients with MECP2 duplications show severe ID, intractable seizures and recurrent infections. Duplications in the telomeric neighboring regions, which include GDP dissociation inhibitor 1 gene (GDI1) and ras-associated protein RAB39B gene (RAB39B), are independently associated with ID, and many segmental duplications located in this region could mediate these frequently observed interstitial duplications. In addition, large duplications, including MECP2 and GDI1, induce hypoplasia of the corpus callosum. Abnormalities observed in the white matter, revealed by brain magnetic resonance imaging, are a common finding in patients with MECP2 duplications. As primary sequence analysis cannot be used to determine the region responsible for chromosomal duplication syndrome, finding this region relies on the collection of genotype–phenotype data from patients.
Introduction
Widespread application of chromosomal microarray testing has identified many new, contiguous gene syndromes.1 The accumulation of genotype and phenotype data of patients has narrowed the chromosomal regions responsible for common phenotypic features, and detailed examinations have isolated the genes responsible for certain human disorders. In particular, many X-chromosomal regions have been analyzed to investigate their relationship with X-linked intellectual disability (ID). For example, Froyen et al.2 reported a de novo microdeletion of Xp11.4 in which the calcium/calmodulin-dependent serine protein kinase (MAGUK family) gene (CASK; MIM #300172) is located. Najm et al.3 identified two additional deletions involving CASK in patients with mental retardation and microcephaly with pontine and cerebellar hypoplasia (MICPCH; MIM #300749). Furthermore, by screening 46 individuals with MICPCH, these authors identified two nucleotide alterations. These data suggest that CASK is involved in the pathogenesis of MICPCH, and mutations of this gene suggest that CASK is clinically significant in X-linked human disorders.4 Mutations in the Cdc42 guanine nucleotide exchange factor (GEF) 9 gene (ARHGEF9; MIM #300429) have been identified using the same strategy. Shimojima et al.5 identified a small deletion on Xq11.2 in patients with severe ID and epilepsy. Among the three deleted genes, ARHGEF9 was of particular interest, and subsequent screening for nucleotide variations identified a nonsense mutation in this gene in 23 male patients with similar manifestations. Thus, it is now recognized that this gene is involved in X-linked ID and epilepsy (early infantile epileptic encephalopathy 8 (EIEE8); MIM #300607). This two-step approach, which involves narrowing down the chromosomal region followed by nucleotide screening, has identified multiple genes involved in human disorders. Currently, the use of next-generation sequencing has accelerated the identification of such genes.
This strategy has been applied to genes involved in pathogenesis in cases of haploinsufficiency because it is easy to confirm the involvement of genes in a phenotype when loss-of-function mutations are identified. In comparison, this strategy cannot be applied to gene mutations with dominant negative (gain-of-function) effects or to genes associated with pathogenesis in cases of copy number gain. For example, one of the gene regions that this strategy cannot be applied to is the Down syndrome-critical region because the genes responsible for Down syndrome cannot be identified through screening of nucleotide sequences.6,7 The isolation of genes or gene regions responsible for human disorders associated with gene-dosage-gain requires the collection of overlapping genotype and phenotype data from patients. Experimental animals would also be helpful to confirm the biological effects of the gene-dosage-gains.8
The most commonly reported chromosomal regions with copy number gains are Xq28 regions involving the methyl CpG-binding protein 2 gene (MECP2; MIM #300005).9 Indeed, we have previously reported seven patients with Xq28 duplications.10,11 In this review, we will discuss recent advances in the research on Xq28 genes that are responsible for ID when duplicated.
MECP2 Duplication
Since its first description,12 Rett syndrome (MIM #312750) has been known as a female-specific neurological disorder characterized by developmental regression, characteristic hand movements, autistic features and post-natal microcephaly.13 In 1999, the MECP2 gene was shown to be responsible for Rett syndrome.14 Multiple pathogenic mutations have been identified in patients with Rett syndrome,15 and de novo MECP2 mutations have been observed in females with typical manifestations of this syndrome.16 This finding indicates a dominant X-linked trait and lethality in hemizygous males17 and excludes aneuploidy of the X-chromosome in males (e.g., 47,XXY males)18,19 and specific MECP2 nucleotide changes that lead to milder affects in females.20
Conversely, MECP2 duplication syndrome is a male-specific disorder associated with severe ID, intractable seizures and recurrent infections that lead to early death.21 This clinical condition was first recognized as Lubs-type X-linked mental retardation syndrome (MIM #300260),22 and its genetic etiology was identified as chromosomal duplications in the MECP2 region.17,23 Varying sizes of the MECP2 duplications (0.2–4.0 Mb) have been identified.24–26 Overlapping duplications narrowed down the shortest region overlapped in which MECP2 and interleukin-1 receptor-associated kinase 1 gene (IRAK1; MIM #300283) were included (Figure 1).27 A twofold increase in MECP2 expression was identified in patients compared with normal controls.23 Thus, MECP2, but no other contiguous genes in the duplicated region, is thought to be responsible for the neurological features of this condition.27 MECP2 duplication syndrome is inherited as an X-linked recessive trait. Nearly all mothers of MECP2 duplication patients are carriers of the duplication but do not develop severe ID due to skewed X-chromosome inactivation (XCI). Heterozygous females with random XCI, but not females with functional Xq28 disomy derived from translocation between autosomal chromosomes, show clinical manifestations of MECP2 duplication syndrome but with a milder phenotype.10,11 As Ramocki et al.27 suggested that the majority of female carriers display neuropsychiatric symptoms before the birth of an affected son, careful follow-up of the families is required.
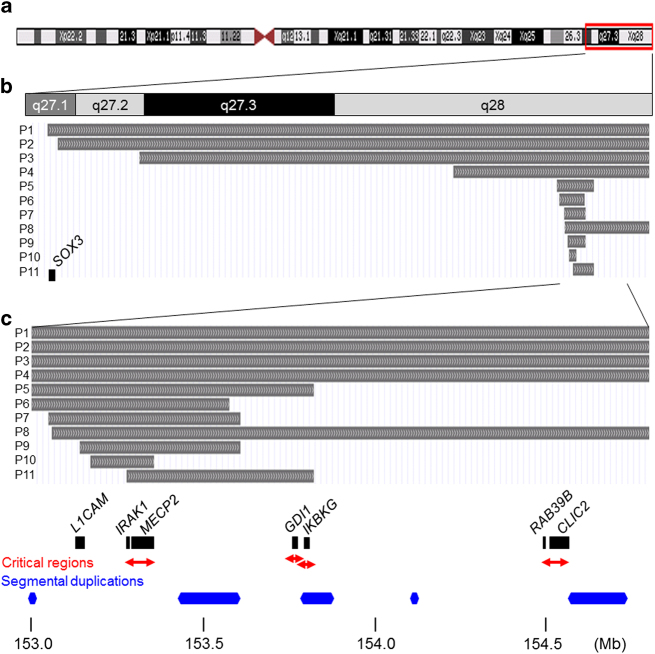
Figure 1.
The genome map around Xq28. (a) A scheme of X chromosome downloaded from the UCSC genome browser. (b) Duplication regions identified in 11 patients are integrated by custom track and shown by grey bars. (c) Xq28 region is expanded. Examples of relevant genes are shown by black rectangles. Critical regions for distinct clinical features and segmental duplication regions are shown by red arrows and blue hexagons.
Although MECP2 is related to genetic causes, there is a discrepancy in the XCI statuses of female patients carrying MECP2 mutations (Rett syndrome) and those with MECP2 duplications. Nearly all female patients with Rett syndrome show de novo mutations of MECP2 and do not show skewed XCI. Conversely, MECP2 duplication syndrome is associated with the X-linked recessive trait and skewed XCI in female carriers, which suggests that overexpression of neighboring genes in the duplicated region, rather than MECP2 itself, may induce negative selection in the early embryo, leading to a preferential XCI.28 Alternatively, embryonic damage may be more severe in cases of MECP2 duplication compared with MECP2 nucleotide changes, although this remains controversial.
GDI1 Duplications
The GDP dissociation inhibitor 1 gene (GDI1; MIM #300104) is located on the telomeric neighboring region of the shortest region overlapped of MECP2 duplication syndrome (Figure 1) and was identified as a gene responsible for ID because it was mutated in male patients.29 Heterozygous females manifested milder phenotypes, indicating an X-linked, semidominant inheritance. In 2007, microduplications, including GDI1, were independently reported with MECP2 duplication by Froyen et al.2 and Madrigal et al.30 Vandewalle et al.31 reported the clinical entity associated with microduplications, including GDI1 as a new duplication syndrome, and concluded that increased levels of GDI1 are related to ID. The more increased copy number of GDI1 correlated with more severe clinical phenotypes, including a Dandy–Walker malformation.31
IKBKG Duplications
The kappa light polypeptide gene enhancer in B cells, inhibitor kinase gamma (IKBKG; MIM #300248), is located in the telomeric neighboring region of GDI1 (Figure 1). Mutations in this gene lead to incontinentia pigmenti.32 A variety of distinct syndromes, including immunodeficiency with or without hypohidrotic ectodermal dysplasia, osteopetrosis, and lymphedema, are allelic disorders.33 Van Asbeck et al.34 reported a female patient with a de novo duplication of the IKBKG region in which GDI1 was not included. This patient showed progressive macrocephaly, recurrent infections, ectodermal dysplasia, among others, but no ID. The XCI pattern was random in this patient.
RAB39B Duplications
Giannandrea et al.35 identified mutations in the ras-associated protein RAB39B gene (RAB39B; MIM #300774) in patients with X-linked mental retardation associated with autism, epilepsy and macrocephaly. El-Hattab et al.36 identified recurrent microduplications involving RAB39B in male patients with cognitive impairment and behavioral abnormalities, including hyperactivity and aggressiveness. The duplication sizes observed in four unrelated patients were consistent at approximately 0.5 Mb and surrounded by segmental duplications (Figure 1). In each case, the mother was a non-symptomatic carrier with a skewed XCI. Vanmarsenille et al.37 identified a twofold increase in the expression level of RAB39B in the lymphocytes of patients compared with controls and confirmed decreased neuronal branching in Rab39b overexpressing mice. Andersen et al.38 reported a family with ID associated with an unbalanced inversion between Xp and Xq that resulted in duplication of the terminal region of Xq28. The duplicated region did not include MECP2 but included RAB39B and the chloride intracellular channel 2 gene (CLIC2; MIM #300138). These results suggest that RAB39B may be responsible for the microduplication syndrome involving this region.
Incidence of Xq28 Duplication
Currently, we have performed microarray-based comparative genomic hybridization analysis on 1250 patients with ID with or without other manifestations, such as congenital malformations, epilepsy or autistic features, in accordance with the previously described method.7 Two hundred thirteen patients showed one of the pathogenic genomic copy number aberrations (detection ratio of 17%). Among the identified aberrations, the most frequently observed copy number gain was the Xq28 duplication. In addition to the seven previously reported patients, four new patients presented Xq28 duplications, including MECP2 (Table 1), resulting in a frequency of Xq28 duplications of 0.9%, which is comparable to the data reported by Lugtenberg et al.39 Furthermore, two of the patients were female.10,11
Table 1. Summary of the aberration regions in the patients with Xq28 duplications.
| Age | Gender | Type | Region |
Start
a
|
Stop
a
|
References | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Maximum | Minimum | Minimum | Maximum | ||||||||
| Patient 1 | 3 years | M | Terminal; inverted X | MECP2+GDI1+RAB39B | 139 433 084 | // | 139 531 883 | 155 270 560 | Shimada et al.10 (Pt 3) | ||
| Patient 2 | 2 years | M | Terminal; t(X;Y) | MECP2+GDI1+RAB39B | 139 743 195 | // | 139 801 223 | 155 270 560 | Shimada et al.11 (Pt 2) | ||
| Patient 3 | 4 years | M | Terminal; t(3;X) | MECP2+GDI1+RAB39B | 141 727 608 | // | 141 931 114 | 155 270 560 | New | ||
| Patient 4 | 13 years | F | Terminal; t(12;X) | MECP2+GDI1+RAB39B | 150 097 794 | // | 150 153 607 | 155 270 560 | Shimada et al.11 (Pt 1) | ||
| Patient 5 | 7 years | M | Interstitial | MECP2+GDI1 | 152 819 509 | // | 152 857 869 | 153 822 717 | // | 153 822 717 | New |
| Patient 6 | 5 years | F | Interstitial | MECP2 | 152 916 694 | // | 152 916 694 | 153 576 940 | // | 153 595 528 | Shimada et al.10 (Pt 4) |
| Patient 7 | 20 years | M | Interstitial | MECP2 | 153 032 004 | // | 153 049 224 | 153 609 163 | // | 153 628 132 | Shimada et al.10 (Pt 1) |
| Patient 8 | 2 years | M | Terminal; tandem | MECP2+GDI1+RAB39B | 153 032 004 | // | 153 059 079 | 155 270 560 | New | ||
| Patient 9 | 14 years | M | Interstitial | MECP2 | 153 083 345 | // | 153 140 483 | 153 609 163 | // | 153 628 132 | Shimada et al.10 (Pt 2) |
| Patient 10 | 5 years | M | Interstitial | MECP2 | 153 140 483 | // | 153 177 776 | 153 357 772 | // | 153 406 233 | Shimada et al.10 (Pt 3) |
| Patient 11 | 14 years | M | Interstitial | MECP2+GDI1 | 153 246 671 | // | 153 277 239 | 153 822 717 | // | 153 877 929 | New |
Abbreviations: F, female; GDI1, GDP dissociation inhibitor 1 gene; Interstitial, interstitial duplication; M, male; MECP2, methyl CpG-binding protein 2 gene; Pt, patient; t, translocation; Terminal, terminal duplication.
Genomic positions refer to build19.
Patient 3 was a 4-year-old boy born at 37 weeks gestation and with a birth weight of 2268 g (10–50th centile). Generalized fetal edema secondary to severe anemia was noted. The patient had distinctive features, including synophrys, telecantus, flat nasal bridge, tented mouth, cleft palate, absent right thumb, bilateral radiohumeral synostosis and hypoplastic scrotum. This patient was diagnosed with Diamond–Blackfan anemia. Owing to these abnormalities, conventional chromosomal analysis was performed and showed 46,XY,add(3)(q27). Finally, microarray-based comparative genomic hybridization analysis and subsequent fluorescence in situ hybridization analyses confirmed an unbalanced translocation associated with functional disomy of Xq28. The deleted region (chr3: 197 052 877–198 022 430) included the ribosomal protein L35a gene (RPL35A; MIM #180468), which is responsible for Diamond–Blackfan anemia (patient no. 71).40 The developmental milestones were severely delayed in this patient: no neck control, no rolling over and no meaningful words. Neurological examination showed generalized hypotonia, and this patient never experienced epilepsy.
Patient 5 was a 7-year-old boy with a birth weight of 2,450 g (10–50th centile) at 37 weeks of gestation. Poor sucking due to generalized hypotonia was noted from early infancy. This patient had a history of multiple infections beginning from the first year of life. He began to suffer epileptic seizures from 1 year of age. Although he had been able to feed himself, he gradually lost the ability to eat from the age of 4 years. Thereafter, he was fed by tube, and a Nissen fundoplication was performed at the age of 5 years. At present, he is bedridden. He has never used meaningful words.
Patient 8 was aged 2 years and 9 months and had a birth weight of 2,422 g (3–10th centile), length of 46 cm (3–10th centile) and an occipito-frontal circumference of 32.5 cm (10–25th centile). His development was delayed, with head control and rolling over observed at 5 months, sitting alone at 13 months, standing with support at 24 months and walking with support at 33 months. He has made no eye contact and has exhibited intractable epilepsy since the first year of life.
Patient 11 was a 14-year-old male student at a school for disabled children. His height was 164.9 cm (50–75th centile), weight was 60.1 kg (75–90th centile) and occipito-frontal circumference was 60.0 cm (>97th centile), indicating macrocephaly. He has intractable epilepsy associated with myoclonic seizures and spasms. His gait is ataxic, there are no meaningful words, and he requires support during his daily life.
The Xq28 duplication patterns of the 11 patients from our laboratory were classified into two patterns, interstitial and terminal duplications, in 6 and 5 patients, respectively (Table 1). Interstitial duplications including MECP2 were further classified into two types: a pure MECP2 duplication in four patients and a MECP2+GDI1 duplication in two patients. The terminal duplications were further classified into two types: functional Xq28 disomy derived from unbalanced translocations in four patients and pure terminal duplications (no translocation) in one patient (Table 1), which indicates the existence of variable patterns of Xq28 duplications. In cases of interstitial duplications, the distal ends overlap with segmental duplications (Figure 1), which indicates that these interstitial duplications are mediated by multiple segmental duplications in this region.25
Previously, we reported abnormal findings in the white matter that were confirmed by brain magnetic resonance imaging) and suggested this as a common manifestation among patients with MECP2 duplications.10,11 This finding was supported by those of Reardon et al.41 Four new patients with Xq28 also showed T2-weighted high intensity in the white matter (Figure 2). Dilatation of bilateral ventricles was also commonly observed.
Figure 2.
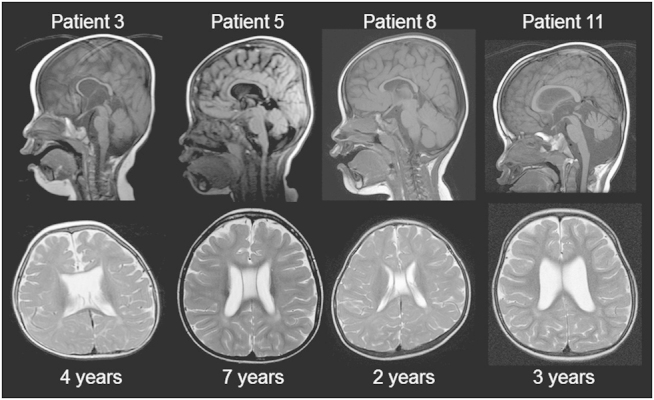
Brain magnetic resonance imaging (MRI) findings of new patients with Xq28 duplications. Sagittal T1 (up) and axial T2 (bottom)-weighted images are shown. All patients showed hypoplasia of the corpus callosum and T2 signal high intensities in the deep white matter. Three patients (other than patient 8) showed atrophies of the cerebellum and bilateral dilatations of the lateral ventricles, indicating age-dependent progression. Patient 3 showed a translucent septal defect, and patients 5 and 8 showed a verga cavity.
Genotype–Phenotype Correlation
The shortest region overlapped observed in patients with MECP2 duplications associated with the typical phenotypic features of MECP2 duplication syndrome included MECP2 and the neighboring IRAK1, with a size of 0.2 M,24 which was supported by our study (Figure 1). Although Velinov et al.42 suggested that the distal Xq28 region beyond MECP2 did not contribute to additional features, Honda et al.43 suggested that duplications of the GDI1 region modified the clinical features in patients with MECP2 duplications associated with hypoplasia of the corpus callosum. In this review, two new patients (patients 5 and 11) showed interstitial duplications beyond the shortest region overlapped of MECP2 duplication syndrome that included the GDI1 region. Careful observation of the patients’ magnetic resonance images showed hypoplasia of the corpus callosum, as suggested by Honda et al.,43 although there were no definite differences in the severity of clinical symptoms in patients with a MECP2+GDI1 duplication compared with patients with pure MECP2 duplications.
Patients with terminal duplication of Xq28 had significantly more severe neurological manifestations compared with patients with pure MECP2 duplications. Four patients with interstitial duplications involving MECP2, but not RAB39B, could temporarily walk. However, patients with terminal duplications of Xq28 were immobile, which is a response derived from the integrated effects of the RAB39B region.
The maximum interstitial duplications observed in patients manifesting the phenotypic features of MECP2 duplication syndrome involved a 4-Mb region.26 In comparison, some patients showed large terminal Xq duplications beyond Xq27,44–47 although most of the analyzed duplications had conventional G-banding levels. The largest duplication presented in this review was observed in patient 1, and a 139-Mb region in Xq27.1 was at the most proximal end. Furthermore, the neurological features in patient 1 were the most severe among the 11 patients studied because this patient showed severe hypotonia from early infancy and required continuous tube feeding. There were many episodes of life-threatening infection. In comparison with the second largest duplication that was observed in patient 2, the Y-box 3 gene (SOX3; MIM #313430) in the sex-determining region was located in the additional duplicated region in patient 1. This gene is related to X-linked ID and hypopituitarism,48 and duplication of this region is related to similar phenotypes, indicating dosage sensitivity.49 Therefore, the involvement of SOX3 in the duplicated region could affect clinical severity in patients with Xq terminal duplications.
Conclusions
The chromosomal patterns of Xq28 duplications and their clinical relevance have been discussed in this review. A review of the scientific evidence indicates that duplicated regions of the Xq28 chromosome that are responsible for ID can be separated into three distinct regions: MECP2, GDI1 and RAB39B. Furthermore, SOX3 may have modifier effects for severe ID. To establish the clinical significance of gene duplications, further analysis of genotype–phenotype data from these patients is required.
The authors declare no conflict of interest.
References
- Weise A, Mrasek K, Klein E, Mulatinho M, Llerena JC Jr , Hardekopf D et al. Microdeletion and microduplication syndromes. J Histochem Cytochem 2012; 60: 346–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froyen G, Van Esch H, Bauters M, Hollanders K, Frints SG, Vermeesch JR et al. Detection of genomic copy number changes in patients with idiopathic mental retardation by high-resolution X-array-CGH: important role for increased gene dosage of XLMR genes. Hum Mutat 2007; 28: 1034–1042. [DOI] [PubMed] [Google Scholar]
- Najm J, Horn D, Wimplinger I, Golden JA, Chizhikov VV, Sudi J et al. Mutations of CASK cause an X-linked brain malformation phenotype with microcephaly and hypoplasia of the brainstem and cerebellum. Nat Genet 2008; 40: 1065–1067. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Okamoto N, Chinen Y, Takanashi J, Makita Y, Hata A et al. Novel intragenic duplications and mutations of CASK in patients with mental retardation and microcephaly with pontine and cerebellar hypoplasia (MICPCH). Hum Genet 2012; 131: 99–110. [DOI] [PubMed] [Google Scholar]
- Shimojima K, Sugawara M, Shichiji M, Mukaida S, Takayama R, Imai K et al. Loss-of-function mutation of collybistin is responsible for X-linked mental retardation associated with epilepsy. J Hum Genet 2011; 56: 561–565. [DOI] [PubMed] [Google Scholar]
- Patterson D. Molecular genetic analysis of Down syndrome. Hum Genet 2009; 126: 195–214. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Shimojima K, Nishizawa T, Matsuo M, Ito M, Imai K. Clinical manifestations of the deletion of Down syndrome critical region including DYRK1A and KCNJ6. Am J Med Genet A 2011; 155A: 113–119. [DOI] [PubMed] [Google Scholar]
- Yamakawa K. Towards the understanding of Down syndrome using mouse models. Congenit Anom (Kyoto) 2012; 52: 67–71. [DOI] [PubMed] [Google Scholar]
- Shao L, Shaw CA, Lu XY, Sahoo T, Bacino CA, Lalani SR et al. Identification of chromosome abnormalities in subtelomeric regions by microarray analysis: a study of 5,380 cases. Am J Med Genet A 2008; 146A: 2242–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada S, Okamoto N, Hirasawa K, Yoshii K, Tani Y, Sugawara M et al. Clinical manifestations of Xq28 functional disomy involving MECP2 in one female and two male patients. Am J Med Genet A 2013; 161A: 1779–1785. [DOI] [PubMed] [Google Scholar]
- Shimada S, Okamoto N, Ito M, Arai Y, Momosaki K, Togawa M et al. MECP2 duplication syndrome in both genders. Brain Dev 2013; 35: 411–419. [DOI] [PubMed] [Google Scholar]
- Rett A. Ueber ein eigenartiges hirnatrophisches Syndrom bei Hyperammoniamie in Kindesalter. Wien Med Wochenschr 1966; 116: 723–726. [PubMed] [Google Scholar]
- Hagberg B, Aicardi J, Dias K, Ramos O. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett's syndrome: report of 35 cases. Ann Neurol 1983; 14: 471–479. [DOI] [PubMed] [Google Scholar]
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet 1999; 23: 185–188. [DOI] [PubMed] [Google Scholar]
- Philippe C, Villard L, De Roux N, Raynaud M, Bonnefond JP, Pasquier L et al. Spectrum and distribution of MECP2 mutations in 424 Rett syndrome patients: a molecular update. Eur J Med Genet 2006; 49: 9–18. [DOI] [PubMed] [Google Scholar]
- Matijevic T, Knezevic J, Slavica M, Pavelic J. Rett syndrome: from the gene to the disease. Eur Neurol 2009; 61: 3–10. [DOI] [PubMed] [Google Scholar]
- Van Esch H, Bauters M, Ignatius J, Jansen M, Raynaud M, Hollanders K et al. Duplication of the MECP2 region is a frequent cause of severe mental retardation and progressive neurological symptoms in males. Am J Hum Genet 2005; 77: 442–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moog U, Smeets EE, van Roozendaal KE, Schoenmakers S, Herbergs J, Schoonbrood-Lenssen AM et al. Neurodevelopmental disorders in males related to the gene causing Rett syndrome in females (MECP2). Eur J Paediatr Neurol 2003; 7: 5–12. [DOI] [PubMed] [Google Scholar]
- Topcu M, Akyerli C, Sayi A, Toruner GA, Kocoglu SR, Cimbis M et al. Somatic mosaicism for a MECP2 mutation associated with classic Rett syndrome in a boy. Eur J Hum Genet 2002; 10: 77–81. [DOI] [PubMed] [Google Scholar]
- Orrico A, Lam C, Galli L, Dotti MT, Hayek G, Tong SF et al. MECP2 mutation in male patients with non-specific X-linked mental retardation. FEBS Lett 2000; 481: 285–288. [DOI] [PubMed] [Google Scholar]
- Friez MJ, Jones JR, Clarkson K, Lubs H, Abuelo D, Bier JA et al. Recurrent infections, hypotonia, and mental retardation caused by duplication of MECP2 and adjacent region in Xq28. Pediatrics 2006; 118: e1687–e1695. [DOI] [PubMed] [Google Scholar]
- Lubs H, Abidi F, Bier JA, Abuelo D, Ouzts L, Voeller K et al. XLMR syndrome characterized by multiple respiratory infections, hypertelorism, severe CNS deterioration and early death localizes to distal Xq28. Am J Med Genet 1999; 85: 243–248. [DOI] [PubMed] [Google Scholar]
- Meins M, Lehmann J, Gerresheim F, Herchenbach J, Hagedorn M, Hameister K et al. Submicroscopic duplication in Xq28 causes increased expression of the MECP2 gene in a boy with severe mental retardation and features of Rett syndrome. J Med Genet 2005; 42: e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Gaudio D, Fang P, Scaglia F, Ward PA, Craigen WJ, Glaze DG et al. Increased MECP2 gene copy number as the result of genomic duplication in neurodevelopmentally delayed males. Genet Med 2006; 8: 784–792. [DOI] [PubMed] [Google Scholar]
- Carvalho CM, Zhang F, Liu P, Patel A, Sahoo T, Bacino CA et al. Complex rearrangements in patients with duplications of MECP2 can occur by fork stalling and template switching. Hum Mol Genet 2009; 18: 2188–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton-Smith J, Walters S, Hobson E, Burkitt-Wright E, Smith R, Toutain A et al. Xq28 duplication presenting with intestinal and bladder dysfunction and a distinctive facial appearance. Eur J Hum Genet 2009; 17: 434–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramocki MB, Tavyev YJ, Peters SU. The MECP2 duplication syndrome. Am J Med Genet A 2010; 152A: 1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo S, Monfort S, Rosello M, Orellana C, Oltra S, Armstrong J et al. De novo interstitial triplication of MECP2 in a girl with neurodevelopmental disorder and random X chromosome inactivation. Cytogenet Genome Res 2011; 135: 93–101. [DOI] [PubMed] [Google Scholar]
- Bienvenu T, des Portes V, Saint Martin A, McDonell N, Billuart P, Carrie A et al. Non-specific X-linked semidominant mental retardation by mutations in a Rab GDP-dissociation inhibitor. Hum Mol Genet 1998; 7: 1311–1315. [DOI] [PubMed] [Google Scholar]
- Madrigal I, Rodriguez-Revenga L, Armengol L, Gonzalez E, Rodriguez B, Badenas C et al. X-chromosome tiling path array detection of copy number variants in patients with chromosome X-linked mental retardation. BMC Genomics 2007; 8: 443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandewalle J, Van Esch H, Govaerts K, Verbeeck J, Zweier C, Madrigal I et al. Dosage-dependent severity of the phenotype in patients with mental retardation due to a recurrent copy-number gain at Xq28 mediated by an unusual recombination. Am J Hum Genet 2009; 85: 809–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smahi A, Courtois G, Vabres P, Yamaoka S, Heuertz S, Munnich A et al. Genomic rearrangement in NEMO impairs NF-kappaB activation and is a cause of incontinentia pigmenti. the International Incontinentia Pigmenti (IP) Consortium. Nature 2000; 405: 466–472. [DOI] [PubMed] [Google Scholar]
- Zonana J, Elder ME, Schneider LC, Orlow SJ, Moss C, Golabi M et al. A novel X-linked disorder of immune deficiency and hypohidrotic ectodermal dysplasia is allelic to incontinentia pigmenti and due to mutations in IKK-gamma (NEMO). Am J Hum Genet 2000; 67: 1555–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Asbeck E, Ramalingam A, Dvorak C, Chen TJ, Morava E. Duplication at Xq28 involving IKBKG is associated with progressive macrocephaly, recurrent infections, ectodermal dysplasia, benign tumors, and neuropathy. Clin Dysmorphol 2014; 23: 77–82. [DOI] [PubMed] [Google Scholar]
- Giannandrea M, Bianchi V, Mignogna ML, Sirri A, Carrabino S, D'Elia E et al. Mutations in the small GTPase gene RAB39B are responsible for X-linked mental retardation associated with autism, epilepsy, and macrocephaly. Am J Hum Genet 2010; 86: 185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hattab AW, Fang P, Jin W, Hughes JR, Gibson JB, Patel GS et al. Int22h-1/int22h-2-mediated Xq28 rearrangements: intellectual disability associated with duplications and in utero male lethality with deletions. J Med Genet 2011; 48: 840–850. [DOI] [PubMed] [Google Scholar]
- Vanmarsenille L, Giannandrea M, Fieremans N, Verbeeck J, Belet S, Raynaud M et al. Increased dosage of RAB39B affects neuronal development and could explain the cognitive impairment in male patients with distal Xq28 copy number gains. Hum Mutat 2014; 35: 377–383. [DOI] [PubMed] [Google Scholar]
- Andersen EF, Baldwin EE, Ellingwood S, Smith R, Lamb AN. Xq28 duplication overlapping the int22h-1/int22h-2 region and including RAB39B and CLIC2 in a family with intellectual and developmental disability. Am J Med Genet A 2014; e-pub ahead of print 3 April 2014; doi: 10.1002/ajmg.a.36524. [DOI] [PubMed]
- Lugtenberg D, Kleefstra T, Oudakker AR, Nillesen WM, Yntema HG, Tzschach A et al. Structural variation in Xq28: MECP2 duplications in 1% of patients with unexplained XLMR and in 2% of male patients with severe encephalopathy. Eur J Hum Genet 2009; 17: 444–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu M, Sato-Otsubo A, Morio T, Takagi M, Toki T, Terui K et al. Extensive gene deletions in Japanese patients with Diamond-Blackfan anemia. Blood 2012; 119: 2376–2384. [DOI] [PubMed] [Google Scholar]
- Reardon W, Donoghue V, Murphy AM, King MD, Mayne PD, Horn N et al. Progressive cerebellar degenerative changes in the severe mental retardation syndrome caused by duplication of MECP2 and adjacent loci on Xq28. Eur J Pediatr 2010; 169: 941–949. [DOI] [PubMed] [Google Scholar]
- Velinov M, Novelli A, Gu H, Fenko M, Dolzhanskaya N, Bernardini L et al. De-novo 2.15 Mb terminal Xq duplication involving MECP2 but not L1CAM gene in a male patient with mental retardation. Clin Dysmorphol 2009; 18: 9–12. [DOI] [PubMed] [Google Scholar]
- Honda S, Hayashi S, Nakane T, Imoto I, Kurosawa K, Mizuno S et al. The incidence of hypoplasia of the corpus callosum in patients with dup (X)(q28) involving MECP2 is associated with the location of distal breakpoints. Am J Med Genet A 2012; 158A: 1292–1303. [DOI] [PubMed] [Google Scholar]
- Vasquez AI, Rivera H, Bobadilla L, Crolla JA. A familial Xp+ chromosome, dup (Xq26.3-->qter). J Med Genet 1995; 32: 891–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammer EJ, Punglia DR, Fuchs AE, Rowe AG, Cotter PD. Inherited duplication of Xq27.2-->qter: phenocopy of infantile Prader-Willi syndrome. Clin Dysmorphol 2001; 10: 141–144. [DOI] [PubMed] [Google Scholar]
- Goodman BK, Shaffer LG, Rutberg J, Leppert M, Harum K, Gagos S et al. Inherited duplication Xq27-qter at Xp22.3 in severely affected males: molecular cytogenetic evaluation and clinical description in three unrelated families. Am J Med Genet 1998; 80: 377–384. [DOI] [PubMed] [Google Scholar]
- Akiyama M, Kawame H, Ohashi H, Tohma T, Ohta H, Shishikura A et al. Functional disomy for Xq26.3-qter in a boy with an unbalanced t(X;21)(q26.3;p11.2) translocation. Am J Med Genet 2001; 99: 111–114. [DOI] [PubMed] [Google Scholar]
- Laumonnier F, Ronce N, Hamel BC, Thomas P, Lespinasse J, Raynaud M et al. Transcription factor SOX3 is involved in X-linked mental retardation with growth hormone deficiency. Am J Hum Genet 2002; 71: 1450–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon NM, Ross SA, Morgan T, Belsky JL, Hol FA, Karnes PS et al. Array comparative genomic hybridisation analysis of boys with X linked hypopituitarism identifies a 3.9 Mb duplicated critical region at Xq27 containing SOX3. J Med Genet 2004; 41: 669–678. [DOI] [PMC free article] [PubMed] [Google Scholar]