Abstract
We tested the hypothesis that altered sympathetic baroreceptor control to the vessels (svBRS) and disrupted coupling between blood pressure (BP) fluctuations and muscle sympathetic activity (MSNA) discharge pattern in the low frequency band (LF, around 0.1Hz) precede vasovagal syncope. Seven healthy males underwent ECG, BP, respiratory, and MSNA recordings at baseline (REST) and during a 15-minute 80° head-up tilt followed by a −10 mmHg step wise increase of lower body negative pressure up to presyncope. Spectral and coherence analyses of systolic arterial pressure (SAP) and MSNA variability provided the indexes of vascular sympathetic modulation, LFSAP, and of the linear coupling between MSNA and SAP in the low frequency band (around 0.1 Hz), K2MSNA-SAP(LF). svBRS was assessed as the slope of the regression line between MSNA and diastolic arterial pressure (DAP). Data were analyzed at REST, during asymptomatic and presyncope periods of tilt. svBRS declined during presyncope period compared to REST and asymptomatic tilt. The presyncope period was characterized by a decrease of RR interval, LFMSNA, LFSAP, and K2MSNA-SAP(LF) values compared to the asymptomatic one, whereas MSNA burst rate was unchanged. The reduction of svBRS producing an altered coupling between MSNA and SAP variability at 0.1 Hz, may provoke circulatory changes leading to presyncope.
1. Introduction
In healthy humans, standing is associated with pooling of about 500–800 ml blood in venous capacitance vessels below the heart leading to a drop of central venous pressure, a reduction of cardiac output, and a potential decrease of blood pressure (BP) (Mosqueda-Garcia et al 1997, Diedrich and Biaggioni 2004). This response reduces afferent baroreceptor traffic to the brain stem (Mosqueda-Garcia et al 1997) by unloading cardiopulmonary and arterial baroreceptors, eventually resulting in reflex sympathetic activation. During 75° head-up tilt, a mild diastolic arterial pressure increase, a marked enhancement of heart rate (HR), plasma norepinephrine, and neural sympathetic traffic to the vessels (muscle sympathetic nerve activity, MSNA) have been observed in healthy volunteers (Furlan et al 2000). In healthy subjects, upright HR, BP, and MSNA spontaneous fluctuations are characterized by a period of 10 seconds (0.1 Hz) and a strict coupling among these variables fluctuations at 0.1 Hz as assessed by the coherence function (Furlan et al 2000). When blood pressure declines, MSNA and HR increase and when blood pressure increases, MSNA and HR diminish. Orthostatic tolerance in healthy volunteers requires intact baroreceptor regulation to tune cardiovascular variables and the sympathetic vasomotor tone (Furlan et al 2000). By contrast, impaired baroreflex regulation in patients with afferent baroreflex failure (Robertson et al 1993), profoundly disrupts rhythmic spontaneous HR, BP, and MSNA oscillations and in their coupling promoting orthostatic intolerance and syncope (Heusser et al 2005, Furlan et al 2001). In addition, subjects characterized by a low baroreflex sensitivity (BRS) are more likely to experience syncope (Mosqueda-Garcia et al 1997). Of interest, in some patients affected by baroreflex failure, orthostatic hypertension has also been observed during the earlier stage of the disease (Robertson 2011, Heusser et al 2005).
Arterial baroreflex control of heart rate (BRS) can be evaluated by assessing heart rate changes in response to modifications in systolic arterial pressure (Bertinieri et al 1988, Blaber et al 1995, Porta et al 2000). In addition, baroreflex MSNA control can be assessed by relating MSNA changes to preceding variations in diastolic arterial pressure (Sundlof and Wallin 1978, Kienbaum et al 2001, Keller et al 2006, Hart et al 2010). Notably, the DAP-MSNA relationship proved to be more effective in evaluating sympathetic baroreceptor gain than the SAP-MSNA relationship (Sundlof and Wallin 1978).
Data about the changes in the MSNA before syncope are to some extent controversial. Some authors (Mosqueda-Garcia et al 1997, Morillo et al 1997, Jardine et al 2002) described a marked vascular sympathetic withdrawal up to neural silence before syncope. Others, such as Vaddadi and colleagues (Vaddadi et al 2010) found that, in a number of cases, MSNA does not disappear through the faint, suggesting that mechanisms other than a simple decrease of the vascular sympathetic activity might be involved in the vasovagal syncope.
In the present study we tested the hypothesis that the period just preceding orthostatic syncope may be characterized by an altered baroreflex control of sympathetic vasomotion leading to a disruption of the linear coupling between BP fluctuation and MSNA discharge pattern at 0.1 Hz.
2. Methods
2.1. Experimental protocol
As part of the European Space Agency Medium-Term-Bedrest Study (ClinicalTrials.gov Identifier: NCT01655979) (Buehlmeier et al 2014), seven healthy male volunteers (33±1 years, BMI 23.5±0.2 kg/m2) underwent ECG, beat by beat BP (Finapres Medical Systems, Ohmeda), respiratory activity (Electrobioimpedance Amplifier, Biopac System, Inc.), and MSNA recordings (Nerve Traffic Analyzer (model 662C-3; University of Iowa Bioengineering, Iowa City, IA) in supine position (REST) and during fifteen minutes 80° head-up tilt. In the absence of orthostatic intolerance symptoms or signs, an additional three minutes of −10 mmHg stepwise increase of lower body negative pressure was applied until signs of presyncope were evoked.
Pre-syncope symptoms were pallor, lightheadedness, blurred vision, sweating, nausea. Pre-syncope signs were increase of RR interval >80% and/or decrease of SAP >40% compared to head-up tilt. At the onset of one of the pre-syncope symptoms and signs the test was interrupted by the doctor in charge who was acting as a third party.
MSNA was recorded from the peroneal nerve of the right leg as detailed elsewhere (Mosqueda-Garcia 1996). Briefly, multiunit recordings of postganglionic sympathetic activity were obtained by placing a tungsten electrode in a fascicle of the right peroneal nerve, posterior to the fibular head. A reference electrode was inserted subcutaneously, close by the recording needle.
All the subjects gave their written informed consent. The protocol adhered to the principles of the Declaration of Helsinki and was approved by the Ethical Review Board of the European Space Agency.
2.2. Data Analysis
ECG, BP, respiratory activity, and MSNA were digitized at 500 Hz by an analog-to-digital converter (AT-MIO 16E2; National Instruments) and recorded with BNC-2110 data acquisition system and LabVIEW 7.0 software (National Instruments, Austin, Texas) for the off-line analysis.
The raw nerve signal was band-pass filtered (700 – 2,000 Hz), amplified (1000 × 99.9), rectified, and integrated (time constant of 0.1 s) by a nerve traffic analysis system (662C-3, University of Iowa). Then, sympathetic bursts were detected by a running threshold methodology that updated the burst detection threshold on a beat-to-beat basis to follow baseline wandering and modifications of MSNA burst amplitude (Diedrich et al 2009). The threshold was calculated by evaluating the sympathetic burst minimum value and the difference between the maximum and minimum value inside each cardiac cycle. The minimum value plus a 30% of the difference between the maximum and minimum value provided the running threshold. To account for the conduction time of about 1.3 s from aortic and carotid baroreceptors to sympathetic changes, the MSNA burst was searched in a temporal window ranging from 0.9 to 1.7 s and starting from the QRS complex (Wallin et al 1994, Hamner and Taylor 2001, Diedrich et al 2009). The MSNA value corresponding to each cardiac beat was derived as the integral of the MSNA signal inside a specific cardiac interval divided by its duration and assessed on a beat-to-beat basis, thus obtaining the MSNA series (Pagani et al 1997, Furlan et al 2000). Systolic arterial pressure (SAP) was computed as the maximum of the BP in a given heart period approximated as the temporal distance between two successive R-wave peaks detected in the ECG. Diastolic arterial pressure (DAP) was computed as the minimum of the arterial pressure following SAP. The temporal occurrences of the MSNA burst and DAP were also stored.
In the present study tonic MSNA refers to the post-ganglionic sympathetic activity as assessed in bursts/minute or bursts/100 beats by a time domain analysis. Phasic MSNA refers to the pattern of the post-ganglionic sympathetic discharge activity assessed by a frequency domain analysis, i.e. power spectrum analysis of burst discharge variability.
Autoregressive spectrum analysis of SAP and MSNA variability, provided the power of the low frequency fluctuations (0.1 Hz) of SAP (LFSAP) (Diedrich et al 2003, Barbic et al 2007) and MSNA discharge variability (LFMSNA) (Pagani et al 1997, Furlan et al 2000). The coefficients of the autoregressive model and the variance of the white noise were estimated via Levinson-Durbin recursion (Kay and Marple 1981). The number of coefficients p was chosen according to the Akaike’s figure of merit in the range from 8 to 14. The squared coherence function (K2) quantified the amount of linear coupling between oscillatory components centered at the same frequency in different signal variabilities. In particular, cross-spectral analysis allowed the calculation of K2MSNA-SAP, computed as the ratio of the MSNA-SAP square cross-spectrum modulus divided to the product of the power spectra of MSNA and SAP series (Furlan et al 2000). K2MSNA-SAP was sampled in correspondence of the central frequency of the SAP components detected in the LF band, K2MSNA-SAP(LF) (Porta et al 2000, Furlan et al 2000). Cross-spectrum and power spectra involved in the calculation of K2MSNA-SAP were estimated via bivariate autoregressive approach. The coefficient of the bivariate autoregressive model were identified via traditional least squares method and the model order was fixed to 10 (Porta et al 2009)
Analyses were performed during baseline in supine position (REST) and during two different periods of orthostatic stimulus: the asymptomatic tilt (T1) and the period of tilt just preceding the tilt interruption, (T2) because of the onset of pre-syncope (see experimental protocol). The time series length of REST, asymptomatic tilt and tilt pre-syncope comprised 300 consecutive beats. The stationarity of the selected sequence was tested according to Magagnin et al (Magagnin et al 2011) over the original series after linear detrending. If the test for the steadiness of mean and variance was not fulfilled, a new selection was carried out again until the fulfillment of the prerequisites for restricted weak stationarity (Magagnin et al 2011). Testing the stationarity of the mean is necessary even after linear detrending. Indeed, the cardiovascular variability trends are more complex and are not fully addressed by a simple linear approach.
2.3. Assessment of baroreflex control of sympathetic vasomotion (svBRS)
The method for the assessment of svBRS examines how DAP value relates to the occurrence of a MSNA burst accounting for the baroreflex latency (Hart et al 2010). DAP values were grouped into bins of 1 mmHg and the percentage of times we detected a MSNA burst associated to the considered values of DAP were counted. Linear regression analysis was performed in the plane reporting MSNA burst incidence values on the y-axis and DAP values on the x-axis (Figure 1). The slope of the regression line was taken as an estimate of svBRS gain provided that the correlation coefficient (rsvBRS) was significant with p<0.05. Being the slope a negative value, the higher the svBRS gain the more negative is the slope. On the contrary when the slope tends to 0, also the svBRS gain decrease. Therefore, a flattening of the DAP-MSNA relationship implies loss of sympathetic vasomotor coupling.
Figure 1.
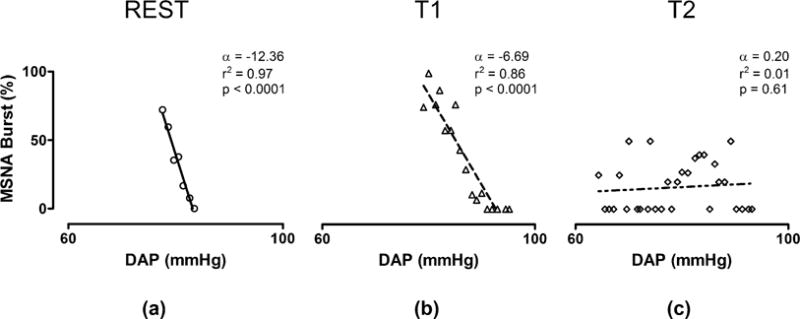
Sympathetic baroreflex sensitivity assessment in a typical volunteer during REST (a), asymptomatic tilt T1 (b) and presyncope period T2 (c). α: slope of linear regression (svBRS); DAP: diastolic arterial pressure.
2.4. Statistical Analysis
Continuous variables are expressed as mean ±standard deviation. The normality of data was tested via Kolmogorov-Smirnov test. One-way ANOVA for repeated measures followed by Holm–Sidak’s post hoc test were used. The level of significance was set at 5%. SigmaPlot 11 (Systat Software Inc., Chicago, IL, USA) was used for statistical analysis. Sample size was calculated with PS Power and Sample Size Calculator Software for Windows (http://biostat.mc.vanderbilt.edu/wiki/Main/PowerSampleSize).
3. Results
In all seven volunteers the tilt maneuver required an additional three minute lasting period of −10 mmHg stepwise increase of lower body negative pressure to evoke the symptoms and signs of presyncope. Specifically, the presyncope was induced by a −10 mmHg of LBNP in three subjects, −30 mmHg in other three individuals and −40 mmHg in one subject. Table 1 summarizes the mean values of the hemodynamics, cardiovascular autonomic parameters, and svBRS index assessed during REST, T1, and T2 periods. During T1 (asymptomatic tilt) we observed a significant decrease of RR interval and an increase in MSNA burst rate compared to REST, as expected. SAP values were unchanged in the presence of a marked increase of LFSAP as previously observed (Peterson et al 2000, Furlan et al 2000). Compared to T1, the presyncope period (T2) was characterized by an additional significant decrease of RR intervals and by a further increase of MSNA. LFMSNA, LFSAP and K2 MSNA-SAP (LF) significantly decreased during T2 compared to T1.
Table 1.
Hemodynamics, autonomic parameters and sympathetic baroreceptor index assessed during rest (REST), asymptomatic tilt (T1) and the period of tilt just preceding syncope (T2). Values are expressed as mean ± standard deviation.
| REST | T1 | T2 | |
|---|---|---|---|
| RR [ms] | 871 ± 106 | 628 ± 89* | 459 ± 99#§ |
| SAP [mmHg] | 126 ± 10 | 131 ± 13 | 112 ± 15 |
| RESP [cycles/min] | 17 ± 2 | 16 ± 1 | 18 ± 1 |
| MSNA [burst/min] | 17 ± 4 | 29 ± 4* | 34 ± 9§ |
| MSNA [bursts/100 beats] | 24 ± 2 | 31 ± 2 | 25 ± 2 |
| LFMSNA [n.u.] | 35.7 ± 7.6 | 45.1 ± 11.2 | 21.5 ± 18.1# |
| LFSAP [mmHg2] | 5.1 ± 2.5 | 38.8 ± 21.0* | 14.0 ± 7.7#§ |
| K2MSNA-SAP(LF) | 0.78 ± 0.20 | 0.91 ± 0.05 | 0.75 ± 0.16# |
| svBRS [%*mmHg−1] | −6.1 ± 3.5 | −4.4 ± 1.2 | −0.4 ± 0.9#§ |
| rsvBRS | −0.83 ± 0.17 | −0.82 ± 0.17 | −0.13 ± 0.31#§ |
p <0.05 REST vs T1
p <0.05 T1 vs T2
p <0.05 REST vs T2. RR: RR interval; SAP: systolic arterial pressure; MSNA: muscle sympathetic nerve activity; LF: low frequency; K2: squared coherence function; svBRS: sympathetic baroreflex sensitivity; rsvBRS: correlation coefficient.
At REST and during T1 significant negative relationships between MSNA and DAP values were present, as indicated by svBRS index values. Conversely, T2 was characterized by a loss of coherence between MSNA and DAP and by a much less negative slope of the regression line (svBRS), thus indicating a reduced baroreflex control of MSNA during pre-syncope. Figure 1 shows the changes in svBRS during REST, asymptomatic tilt (T1), and presyncope tilt (T2) in a representative example. Note that the DAP-MSNA relationship was lost during T2 (Fig. 1,c), that is just before syncope. The mean svBRS and correlation coefficient reported in Tab.1 were computed over the entire group of subjects independently of the significance of the individual linear regression.
4. Discussion
The results of the present study indicate that the presyncope phase of tilt (T2) was characterized by a high sympathetic activity to the heart compared to the asymptomatic phase of tilt (T1), as suggested by the significant reduction of heart period in T2 compared to T1. In addition, during T2 the MSNA burst frequency values remained unchanged or even slightly increased compared to asymptomatic phase of tilt. This finding is in keeping with previous observations by Vaddadi et al (Vaddadi et al 2010) indicating a persistent MSNA during vasovagal syncope, but diverges from results of Mosqueda-Garcia et al. (Mosqueda-Garcia et al 1997) and Morillo et al (Morillo et al 1997) who observed a sympathetic neural silence just before syncope. It has to be pointed out however, that in these latter studies subjects underwent a complete loss of consciousness and MSNA recordings were continued up to recovery whereas in our study the tilt and lower body negative pressure maneuvers were stopped as soon as pre-syncope symptoms and physical signs began, thus, none of the volunteers lost consciousness. Differences in syncope timing might explain, at least partially, differences between studies.
The tilt test maneuver followed by a progressively increasing LBNP till pre-syncope was first developed by el-Bedawi and Hainsworth for addressing orthostatic tolerance in healthy volunteers (el-Bedawi and Hainsworth 1994).
Remarkably, we observed profoundly increased tonic MSNA activity during T2 with a concomitant progressive trend towards decreased SAP compared to T1.
The analysis of the spontaneous LF fluctuations of SAP and MSNA during T2 indicates a decline of the power of such oscillations compared to asymptomatic tilt. In particular, as to MSNA variability, this observation reflects the loss of 0.1 Hz rhythmicity in sympathetic discharge activity, which, however, was still present in presyncope phase of tilt. We observed a shift from prevailing 0.1 Hz fluctuations (LF) toward ~0.2 Hz, respiratory mediated, oscillations in MSNA discharge activity. The observation was linked to a marked reduction in the linear relationship between SAP and MSNA oscillations in the LF band as suggested by a decrease of K2MSNA-SAP(LF) during T2.
It is important to point out that modifications in the respiratory frequency before syncope might have affected the venous return thus making our observations a function of the respiratory activity rather than of the circulatory brain stem network. However, this was not the case. Indeed, respiratory frequency was unchanged in all the three experimental conditions, in keeping with previous observations by Furlan and colleagues (Furlan et al 1998) who used a similar protocol.
In keeping with these observations, a similar decrease of the LFMSNA before syncope was previously described by Kamiya et al (Kamiya et al 2005). In this context a progressive decrease of blood pressure was also evident, suggesting the likely inability of the vasculature smooth muscles to adequately respond to sympathetic vasoconstrictor stimuli. It is important to highlight that the coupling strength was not influenced by the reduced values of SAP during presyncope, because the coherence is a normalized function, ranging from 0 to 1.
That a specific neural modulatory activity at 0.1 Hz is required to induce an optimal vasomotor response, i.e. an appropriate vasoconstriction, has been suggested by human studies using electrical stimulation of sympathetic fibers (Stauss et al 1998) and by previous investigations using an orthostatic stimulus (Furlan et al 2000). Study in healthy subjects with infrequent bursts showed that the lowest systolic pressure value occurred about 4.3 sec before the onset of the sympathetic burst and that the following systolic pressure values peaked about 5sec after the occurrence of the sympathetic bursts. This suggests that a rhythmic neural firing characterized by a period of about 9–10 sec defines a preferred physiological frequency of the sympathetic baroreceptor control of the vessels (Diedrich et al 2013). Cardiopulmonary and arterial baroreceptor mechanisms appear to play a major role in maintaining such a common 0.1 Hz oscillatory pattern in heart rate, blood pressure and post-ganglionic sympathetic discharge activity variability (Furlan et al 2000). Indeed, in patients with Parkinson’s disease and orthostatic hypotension a remarkable reduction was observed in the LFSAP both at rest and during tilt compared to healthy controls (Barbic et al 2007). A decrease in the LF component of RR variability was observed before syncope by using a time variant spectrum analysis approach (Furlan et al 2001). Finally, a significant orthostatic intolerance and hypotension, in spite of high level of plasma catecholamines and MSNA during tilt, were observed in patients with cardiac baroreflex failure following neck radiotherapy because of a rhinopharyngeal cancer (Furlan et al 2001).
In the present study we quantified the baroreflex control of the sympathetic nerve activity to the vessels. The results showed an important decrease in the svBRS as well as an absence of correlation between DAP and MSNA during T2, that is the presyncope phase of tilt. The finding suggests that svBRS impairment may play a role in the pathogenesis of neural-mediated syncope, in keeping with previous observations using different approaches based on pheniylephrine and nitroprusside ev administration to elicit blood pressure changes (Mosqueda-Garcia et al 1997, Morillo et al 1997).
5. Conclusion
In conclusion, the onset of vasovagal syncope seems to be preceded by a decrease of the svBRS and reduced coupling between spontaneous blood pressure variability and the sympathetic discharge activity pattern to the vessels at 0.1 Hz. These observations may add a valuable insight into the complex autonomic changes underlying vasovagal syncope. Future studies including a higher number of subjects are needed to confirm our novel observations.
Acknowledgments
This work was supported by the European Space Agency grant AO-06-BR-18.
A. R. Zamunér was founded by a ‘sandwich’ doctoral scholarship, CAPES Foundation grant BEX 12833/13-4.
We acknowledge Wolfram Sies for technical support and Judith Buehlmeier for the organization and constructive criticisms.
References
- Barbic F, Perego F, Canesi M, Gianni M, Biagiotti S, Costantino G, Pezzoli G, Porta A, Malliani A, Furlan R. Early abnormalities of vascular and cardiac autonomic control in Parkinson’s disease without orthostatic hypotension. Hypertension. 2007;49:120–126. doi: 10.1161/01.HYP.0000250939.71343.7c. [DOI] [PubMed] [Google Scholar]
- Bertinieri G, Di Rienzo M, Cavallazzi A, Ferrari AU, Pedotti A, Mancia G. Evaluation of baroreceptor reflex by blood pressure monitoring in unanesthetized cats. Am J Physiol. 1988;254:H377–H383. doi: 10.1152/ajpheart.1988.254.2.H377. [DOI] [PubMed] [Google Scholar]
- Blaber AP, Yamamoto Y, Hughson RL. Methodology of spontaneous baroreflex relationship assessed by surrogate data analysis. Am J Physiol. 1995;268:H1682–H1687. doi: 10.1152/ajpheart.1995.268.4.H1682. [DOI] [PubMed] [Google Scholar]
- Buehlmeier J, Mulder E, Noppe A, Frings-Meuthen P, Angerer O, Rudwill F, Biolo G, Smith SM, Heer M. A combination of whey protein and potassium bicarbonate supplements during head-down-tilt bed rest: Presentation of a multidisciplinary randomized controlled trial (MEP study) Acta Astronautica. 2014;95:82–91. [Google Scholar]
- el-Bedawi KM, Hainsworth R. Combined head-up tilt and lower body suction: a test of orthostatic tolerance. Clin Auton Res. 1994;4:1–2. 41–47. doi: 10.1007/BF01828837. [DOI] [PubMed] [Google Scholar]
- Diedrich A, Biaggioni I. Segmental orthostatic fluid shifts. Clin Auton Res. 2004;14:146–147. doi: 10.1007/s10286-004-0188-9. [DOI] [PubMed] [Google Scholar]
- Diedrich A, Crossman AA, Beightol LA, Tahvanainen KU, Kuusela TA, Ertl AC, Eckberg DL. Baroreflex physiology studied in healthy subjects with very infrequent muscle sympathetic bursts. J Appl Physiol (1985) 2013;114:203–210. doi: 10.1152/japplphysiol.00509.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrich A, Jordan J, Tank J, Shannon JR, Robertson R, Luft FC, Robertson D, Biaggioni I. The sympathetic nervous system in hypertension: assessment by blood pressure variability and ganglionic blockade. J Hypertens. 2003;21:1677–1686. doi: 10.1097/00004872-200309000-00017. [DOI] [PubMed] [Google Scholar]
- Diedrich A, Porta A, Barbic F, Brychta RJ, Bonizzi P, Diedrich L, Cerutti S, Robertson D, Furlan R. Lateralization of expression of neural sympathetic activity to the vessels and effects of carotid baroreceptor stimulation. Am J Physiol Heart Circ Physiol. 2009;296:H1758–H1765. doi: 10.1152/ajpheart.01045.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlan R, Magatelli R, Palazzolo L, Rimoldi A, Colombo S, Porta A. Orthostatic intolerance: different abnormalities in the neural sympathetic response to a gravitational stimulus. Auton Neurosci. 2001;90:83–88. doi: 10.1016/S1566-0702(01)00271-5. [DOI] [PubMed] [Google Scholar]
- Furlan R, Porta A, Costa F, Tank J, Baker L, Schiavi R, Robertson D, Malliani A, Mosqueda-Garcia R. Oscillatory patterns in sympathetic neural discharge and cardiovascular variables during orthostatic stimulus. Circulation. 2000;101:886–892. doi: 10.1161/01.cir.101.8.886. [DOI] [PubMed] [Google Scholar]
- Hamner JW, Taylor JA. Automated quantification of sympathetic beat-by-beat activity, independent of signal quality. J Appl Physiol (1985) 2001;91:1199–1206. doi: 10.1152/jappl.2001.91.3.1199. [DOI] [PubMed] [Google Scholar]
- Hart EC, Joyner MJ, Wallin BG, Karlsson T, Curry TB, Charkoudian N. Baroreflex control of muscle sympathetic nerve activity: a nonpharmacological measure of baroreflex sensitivity. Am J Physiol Heart Circ Physiol. 2010;298:H816–H822. doi: 10.1152/ajpheart.00924.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heusser K, Tank J, Luft FC, Jordan J. Baroreflex failure. Hypertension. 2005;45:834–839. doi: 10.1161/01.HYP.0000160355.93303.72. [DOI] [PubMed] [Google Scholar]
- Jardine DL, Melton IC, Crozier IG, English S, Bennett SI, Frampton CM, Ikram H. Decrease in cardiac output and muscle sympathetic activity during vasovagal syncope. Am J Physiol Heart Circ Physiol. 2002;282:H1804–H1809. doi: 10.1152/ajpheart.00640.2001. [DOI] [PubMed] [Google Scholar]
- Kay SM, Marple SL. Spectrum analysis: a modern perspective. Proc IEEE. 1981;69:1380–1418. [Google Scholar]
- Kamiya A, Hayano J, Kawada T, Michikami D, Yamamoto K, Ariumi H, Shimizu S, Uemura K, Miyamoto T, Aiba T, Sunagawa K, Sugimachi M. Low-frequency oscillation of sympathetic nerve activity decreases during development of tilt-induced syncope preceding sympathetic withdrawal and bradycardia. Am J Physiol Heart Circ Physiol. 2005;289:H1758–H1769. doi: 10.1152/ajpheart.01027.2004. [DOI] [PubMed] [Google Scholar]
- Keller DM, Cui J, Davis SL, Low DA, Crandall CG. Heat stress enhances arterial baroreflex control of muscle sympathetic nerve activity via increased sensitivity of burst gating, not burst area, in humans. J Physiol. 2006;573:445–451. doi: 10.1113/jphysiol.2006.108662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienbaum P, Karlssonn T, Sverrisdottir YB, Elam M, Wallin BG. Two sites for modulation of human sympathetic activity by arterial baroreceptors? J Physiol. 2001;531:861–869. doi: 10.1111/j.1469-7793.2001.0861h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magagnin V, Bassani T, Bari V, Turiel M, Maestri R, Pinna GD, Porta A. Non-stationarities significantly distort short-term spectral, symbolic and entropy heart rate variability indices. Physiol Meas. 2011;32:1775–1786. doi: 10.1088/0967-3334/32/11/S05. [DOI] [PubMed] [Google Scholar]
- Morillo CA, Eckberg DL, Ellenbogen KA, Beightol LA, Hoag JB, Tahvanainen KU, Kuusela TA, Diedrich AM. Vagal and sympathetic mechanisms in patients with orthostatic vasovagal syncope. Circulation. 1997;96:2509–2513. doi: 10.1161/01.cir.96.8.2509. [DOI] [PubMed] [Google Scholar]
- Mosqueda-Garcia R. Microneurography in neurological research. American Academy of Neurology. 1996;2:4–5. [Google Scholar]
- Mosqueda-Garcia R, Furlan R, Fernandez-Violante R, Desai T, Snell M, Jarai Z, Ananthram V, Robertson RM, Robertson D. Sympathetic and baroreceptor reflex function in neurally mediated syncope evoked by tilt. J Clin Invest. 1997;99:2736–2744. doi: 10.1172/JCI119463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani M, Montano N, Porta A, Malliani A, Abboud FM, Birkett C, Somers VK. Relationship between spectral components of cardiovascular variabilities and direct measures of muscle sympathetic nerve activity in humans. Circulation. 1997;95:1441–1448. doi: 10.1161/01.cir.95.6.1441. [DOI] [PubMed] [Google Scholar]
- Petersen ME, Williams TR, Gordon C, Chamberlain-Webber R, Sutton R. The normal response to prolonged passive head up tilt testing. Heart. 2000;84(5):509–514. doi: 10.1136/heart.84.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porta A, Aletti F, Vallais F, Baselli G. Multimodal signal processing for the analysis of cardiovascular variability. Philos Trans A Math Phys Eng Sci. 2009;367:391–409. doi: 10.1098/rsta.2008.0229. [DOI] [PubMed] [Google Scholar]
- Porta A, Baselli G, Rimoldi O, Malliani A, Pagani M. Assessing baroreflex gain from spontaneous variability in conscious dogs: role of causality and respiration. Am J Physiol Heart Circ Physiol. 2000;279:H2558–H2567. doi: 10.1152/ajpheart.2000.279.5.H2558. [DOI] [PubMed] [Google Scholar]
- Robertson D. Orthostatic hypertension: the last hemodynamic frontier. Hypertension. 2011;57:158–159. doi: 10.1161/HYPERTENSIONAHA.110.163485. [DOI] [PubMed] [Google Scholar]
- Robertson D, Diedrich A, Chapleau MW. Editorial on arterial baroreflex issue. Auton Neurosci. 2012;172:1–3. doi: 10.1016/j.autneu.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D, Hollister AS, Biaggioni I, Netterville JL, Mosqueda-Garcia R, Robertson RM. The diagnosis and treatment of baroreflex failure. N Engl J Med. 1993;329:1449–1455. doi: 10.1056/NEJM199311113292003. [DOI] [PubMed] [Google Scholar]
- Stauss HM, Anderson EA, Haynes WG, Kregel KC. Frequency response characteristics of sympathetically mediated vasomotor waves in humans. Am J Physiol. 1998;274:H1277–H1283. doi: 10.1152/ajpheart.1998.274.4.H1277. [DOI] [PubMed] [Google Scholar]
- Sundlof G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol. 1978;274:621–637. doi: 10.1113/jphysiol.1978.sp012170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaddadi G, Esler MD, Dawood T, Lambert E. Persistence of muscle sympathetic nerve activity during vasovagal syncope. Eur Heart J. 2010;31:2027–2033. doi: 10.1093/eurheartj/ehq071. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Burke D, Gandevia S. Coupling between variations in strength and baroreflex latency of sympathetic discharges in human muscle nerves. J Physiol. 1994;474:331–338. doi: 10.1113/jphysiol.1994.sp020025. [DOI] [PMC free article] [PubMed] [Google Scholar]


