Introduction
Impaired insulin sensitivity and glucose tolerance have been reported in α-synucleopathies such as Parkinson’s disease. The prevalence of impaired glucose tolerance ranges from 50–80% in this population. Studies using national survey data found higher rates of type 2 diabetes mellitus in adults with Parkinson disease compared with those without this condition.[6, 21] Neurodegenerative conditions and states of insulin resistance such as obesity shared common pathophysiological pathways. Both induced pro-inflammatory cytokines, mitochondrial dysfunction and have documented impaired signaling to a hormone very similar to insulin, the insulin growth factor 1 (IGF-1). This is a neurotropic peptide involved in brain development, maturation, function and neuroprotection.[27]
Multiple system atrophy (MSA) is another progressive neurodegenerative movement disorder. It is characterized by autonomic failure, neurogenic orthostatic hypotension, parkinsonism, cerebellar, and urological dysfunction.[9] In MSA patients, pathological α-synuclein is distributed to glial cells in the basal ganglia and cortex, and these patients have impaired IGF-1 signaling in the brain which is associated with disease progression.[17] It is unknown, however, whether MSA patients have reduced insulin sensitivity. Similarly, there has been a paucity of metabolic data in patients with pure autonomic failure (PAF) a condition characterized by α-synuclein deposits in peripheral autonomic nerves. Therefore, the purpose of this study was to test the hypothesis that insulin sensitivity is impaired in MSA and PAF patients compared with healthy subjects. As a secondary objective, we determined whether residual autonomic activity and the presence of supine hypertension contributed to insulin sensitivity in these patients.
Methods
Study Participants
We studied 19 patients with probable MSA, 26 patients with PAF and 8 healthy controls of similar age. MSA and PAF patients were recruited from the Autonomic Dysfunction Center at Vanderbilt University Medical Center. All patients had severe autonomic failure defined as an absence of autonomic reflexes with a standardized autonomic function tests.[4] Exclusion criteria included history of diabetes mellitus following the American Diabetes Association guidelines.[2] Healthy controls were recruited from the Vanderbilt community, were nonsmokers, and were excluded if pregnant, had evidence of systemic illness, or were taking medications known to interfere with regulation of blood pressure or blood volume. Patients and healthy controls were placed on a low-monoamine caffeine-free diet containing 150mEq sodium and 70mEq potassium per day, for at least 3 days before evaluation. The Vanderbilt Institutional Review Board approved this study, and all participants provided written informed consent.
Screening
MSA and PAF patients were admitted to the Clinical Research Center at Vanderbilt University. Medications known to stimulate the autonomic nervous system (i.e., midodrine), affect blood pressure (i.e. anti-hypertensives), or blood volume (e.g., fludrocortisone) were discontinued for at least 5 half-lives before admission. Healthy controls were studied on an outpatient basis. A full medical history, physical examination, and standardized autonomic function tests were conducted for all subjects. The autonomic function tests were performed to evaluate the integrity of autonomic reflex arcs and to confirm the diagnosis of autonomic failure using the 2010 American Autonomic Society criteria.[7] Patients with MSA were diagnosed based on Gilman’s criteria.[9] Patients were diagnosed with supine hypertension if the average supine blood pressure obtained during a 12-hour blood pressure monitoring was equal or above 150/90 mm Hg as previously defined.[12]
Blood Analyses
Plasma glucose was measured with a glucose analyzer (YSI Life Sciences, Yellow Springs, OH). Plasma insulin concentrations were determined by radioimmunoassay (Millipore, St. Charles, MO). Plasma norepinephrine (NE) was determined by high-performance liquid chromatography with electrochemical detection.[10]
Measurement of insulin sensitivity
The Homeostasis Model Assessment (HOMA2) was used to determine insulin sensitivity. This measurement takes into account variations in hepatic and peripheral glucose resistance, increases in the insulin secretion curve for plasma glucose concentrations above 180 mg/dl, and the contribution of circulating proinsulin.[15] The model estimates steady state beta cell function (%B) and insulin sensitivity (%S), as percentages of a normal reference population, using fasting plasma glucose and insulin values.[19] We used a HOMA2 calculator available online (https://www.dtu.ox.ac.uk/homacalculator/)
Spectral analysis of heart rate and blood pressure variability
Continuous blood pressure was measured using NEXFIN device (BMEYE, Amsterdam, Netherlands) and continuous ECG was measured using the VITAL-GUARD 450c monitor (Ivy Biomedical Systems, Brandford, CT, USA). Data was obtained while the patient was resting in the supine posture, in a quiet environment for at least 5 minutes. Physiological data were recorded using the WINDAQ data acquisition system (DI220, DATAQ, Akron, OH, 14 Bit, 1000Hz), and were processed off-line using a custom written software in PV-Wave language (PV-wave, Visual Numerics Inc., Houston, TX), developed by one of the authors (AD). Detected beat-to-beat values of R-R intervals (RRI) and blood pressure were interpolated and low pass filtered (cutoff 2 Hz). Data segments of at least 180 seconds were used for spectral analysis. Linear trends were removed and power spectral density was estimated with the FFT-based Welch algorithm. The total power (TP) and the power in the low (LF: 0.04 to <0.15 Hz), and high (HF: 0.15 to < 0.40 Hz) frequency ranges were calculated according to the Task Force recommendations.[1]
Measurements of baroreflex sensitivity
Cross spectra, coherence and transfer function analysis were used to capture inter-relationships between R-R interval and systolic blood pressure (SBP). The baroreflex gain was determined as the mean magnitude value of the transfer function in the low frequency band, with negative phase and squared coherence value greater than 0.5.
Statistical Analysis
The differences of patient’s demographic and clinical variables between groups (MSA, PAF and controls) were assessed based on descriptive statistics such as medians and quantiles for continuous variables and proportions for categorical variables. For continuous outcomes, we used non-parametric Kruskal-Wallis tests to determine differences among groups and Mann-Whitney U test for pairwise comparisons. Multiple linear regression was used to test for overall linear relations between the dependent variable (insulin sensitivity) and independent variables (age, presence of supine hypertension, sympathetic and parasympathetic activity), and to assess the significance of these relations after adjustments for each covariate. Residual analysis was performed to assess the adequacy of the model fit. Logarithmic transformations of insulin sensitivity, sympathetic activity (LFSBP) and parasympathetic activity (HFRRI) were used to normalize the data and improve model fit. LFSBP and HFRRI were measured in mm Hg2 and ms2, respectively. All tests were two-tailed, and a p-value of <0.05 was considered significant. Analyses were performed using SPSS for Windows (version 22.0; SPSS Inc., Chicago, IL, USA).
Results
Patient’s characteristics
The clinical characteristics of study participants are shown in Table 1. Weight, height, body mass index (BMI), and fasting glucose levels were similar among groups. Age, fasting insulin and supine and upright NE were significantly different among groups, Table 1. Pairwise comparison showed that patients with MSA were 10 years younger compared to PAF patients (0.012), and had elevated fasting insulin levels compared to healthy controls (P=0.034). Both MSA and PAF patients had lower supine NE levels compared to healthy controls (P=0.008; P<0.001, respectively). Similarly, upright NE was lower in MSA and PAF patients compared to healthy controls (P<0.001; P<0.001, respectively). As expected PAF patients had lower supine and upright plasma NE compared with MSA (P=0.004; P=0.049, respectively).
Table 1.
Clinical characteristics
| Parameter | MSA | PAF | Healthy controls | P value |
|---|---|---|---|---|
| N | 19 | 26 | 8 | |
| Gender (Females) | 5 | 14 | 5 | |
| Age (years) | 59 (56 to 66) | 71 (62 to 74) | 60 (57 to 67) | 0.031* |
| Height (cm) | 175 (170 to 180) | 172 (165 to 178) | 160 (159 to 175) | 0.122 |
| Weight (Kg) | 89 (65 to 94) | 71 (59 to 86) | 73 (63 to 88) | 0.167 |
| Body Mass Index (kg/m2) | 26 (23 to 30) | 25 (23 to 26) | 27 (24 to 29) | 0.185 |
| Fasting Glucose (mg/dL) | 95 (92 to 100) | 93 (89 to 97) | 92 (88 to 95) | 0.360 |
| Fasting Insulin (μU/mL) | 12 (7 to 18) | 9 (5 to 12) | 6 (4 to 9) | 0.050* |
| Supine NE (pg/mL) | 123 (94 to 210) | 69 (46 to 11) | 287 (212 to 368) | <0.001* |
| Upright NE (pg/mL) | 222 (116 to 303) | 138 (61 to 221) | 744 (553 to 817) | <0.001* |
| Alkaline phosphatase (U/L) | 61 (36 to 87) | 77 (43 to 111) | 58 (43 to 73) | 0.062 |
| Aspartate transaminase (U/L) | 22 (16 to 29) | 23 (12 to 34) | 22 (16 to 28) | 0.367 |
| Alanine transaminase (U/L) | 18 (8 to 28) | 18 (10 to 25) | 16 (6 to 27) | 0.918 |
Values represent median (interquartile range, IQR). MSA, multiple system atrophy; PAF, pure autonomic failure; NE, norepinephrine.
P<0.05
None of our patients had renal failure. However, there were significant differences in BUN and creatinine levels among groups (P<0.001; P<0.001, respectively). PAF patients had elevated creatinine levels compared with healthy controls 1.12 mg/dL (IQR 0.90 to 1.23) versus 0.73 mg/dL (IQR 0.65 to 0.83), P<0.001. Similarly, MSA patients had elevated creatinine levels compared with healthy controls versus 0.96 mg/dL (IQR 0.90 to 1.18) versus 0.73 mg/dL (IQR 0.65 to 0.83), P=0.005. There were no differences in creatinine levels between PAF and MSA (P=0.174).
Cardiovascular and autonomic function
Patients with MSA and PAF had higher supine SBP compared with healthy controls (P=0.001); 47% of MSA, and 70% of PAF patients met the definition of supine hypertension (average SBP/DBP ≥150/90 mm Hg) during an overnight blood pressure monitoring. The results of the autonomic function tests are presented in Table 2. MSA and PAF patients had a profound decrease in SBP upon standing −83 mmHg (IQR −92 to −42) and −79 mm Hg (IQR −90 to−58). These patients had a greater decrease in systolic blood pressure (SBP) during phase II of the Valsalva maneuver compared to healthy controls, and the SBP overshoot during phase IV was absent; the pressor responses to isometric handgrip exercise or pain stimulus (cold pressor test) were impaired, and sinus arrhythmia (S/A) was markedly reduced indicating impaired parasympathetic responses. Thus, autonomic testing indicated severe sympathetic and parasympathetic involvement in autonomic failure patients.
Table 2.
Autonomic Function Tests
| Parameter | MSA | PAF | Healthy controls | P value |
|---|---|---|---|---|
| Supine SBP, mm Hg | 15153 (137 to 168) | 151 (139 to 170) | 118 (107 to 133) | 0.001** |
| Supine DBP, mm Hg | 88 (79 to 95) | 85 (73 to 91) | 83 (74 to 83) | 0.083 |
| Supine HR, bpm | 70 (66 to 77) | 71 (61 to 77) | 66 (62 to 76) | 0.640 |
| Upright SBP, mmHg | 82 (74 to 99) | 81 (71 to 101) | 110 (103 to 138) | 0.003** |
| Upright DBP, mmHg | 54 (45 to 62) | 52 (43 to 60) | 79 (74 to 87) | 0.001** |
| Upright HR, bpm | 86 (68 to 91) | 79 (74 to 91) | 82 (70 to 84) | 0.703 |
| S/A ratio (normal >1.2) | 1.1 (1.0 to 1.1) | 1.0 (1.0 to 1.1) | 1.3 (1.2 to 1.5) | <0.001** |
| Valsalva phase II, mmHg | −72 (−87 to −55) | −61 (−78 to −45) | −16 (−24 to −1) | <0.001** |
| Valsalva phase IV, mmHg | −36 (−42 to −28) | −42 (−48 to −32) | 15 (14 to 22) | <0.001** |
| Valsalva ratio | 1.1 (1.1 to 1.2) | 1.1 (1.0 to 1.1) | 1.5 (1.4 to 1.7) | <0.001** |
| Hyperventilation, mmHg | −16 (−28 to −10) | −31 (−52 to −13) | 0.5 (−5 to 5) | <0.001** |
| Cold- Pressor, mmHg | 1 (−2 to 12) | 8 (−3 to 16) | 17 (8 to 24) | 0.030* |
| Handgrip, mmHg | 3 (−3 to 8) | 7 (−6 to 15) | 17 (8 to 26) | 0.017* |
Values represent median (interquartile range, IQR). MSA, multiple system atrophy; PAF, pure autonomic failure; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; ∆SBP, orthostatic change in SBP; ∆DBP, orthostatic change in DBP; and ∆HR, orthostatic change in HR. S/A represents sinus arrhythmia ratio calculated by the maximum divided by the minimum heart rate values during controlled breathing.
P<0.05,
P<0.01
The results for spectral analysis of blood pressure and heart rate variability are shown in Table 3. Indices of sympathetic activity (LFSBP, mmHg2), and parasympathetic activity (HFRRI, ms2) were different among groups. MSA and PAF patients had lower sympathetic and parasympathetic activity compared to healthy controls, Table 3.
Table 3.
Spectral analysis of blood pressure and heart rate
| Parameter | MSA | PAF | Healthy controls | P value |
|---|---|---|---|---|
| LF SBP, mmHg2 | 1.9 (0.8 to 4.7) | 1.1 (0.6 to 2.7) | 5.8 (3.3 to 7.9) | 0.010* |
| BRS, ms/mmHg | 5.5 (2.5 to 9.6) | 3.3 (1.7 to 8.3) | 7.2 (4.9 to 9.4) | 0.098 |
| LF RRI, ms2 | 42.9 (20.7 to 143.5) | 19.1 (3.2 to 77.3) | 320.1 (201.4 to 352.5) | <0.001** |
| HF RRI, ms2 | 15.9 (6.8 to 32.4) | 11.8 (4.9 to 28.2) | 102.6 (64.5 to 310.3) | 0.008** |
Values represent median (interquartile range, IQR). RRI, R-R interval; SBP, systolic blood pressure; DBP, diastolic blood pressure; BRS, baroreflex slope; LF, low frequency; HF, high frequency.
P<0.05,
P<0.01
Insulin Sensitivity and Beta cell Function Measurements
There was a statistically significant difference in insulin sensitivity measured by HOMA 2 index among groups (P=0.048). Patients with MSA have reduced insulin sensitivity compared to healthy controls, 64% (IQR, 43 to 117) versus 139% (IQR, 83 to 212), P=0.032, respectively. However, there were no differences in insulin sensitivity between MSA versus PAF, 64% (IQR (43 to 117) versus 88%, IQR (67 to 148), P=0.103, respectively; or between PAF versus healthy controls 88% (IQR, 67 to 148) vs 139%, IQR (83 to 212), P=0.150, respectively, Figure 1. Beta cell function was similar among groups (P=0.103).
Figure 1.
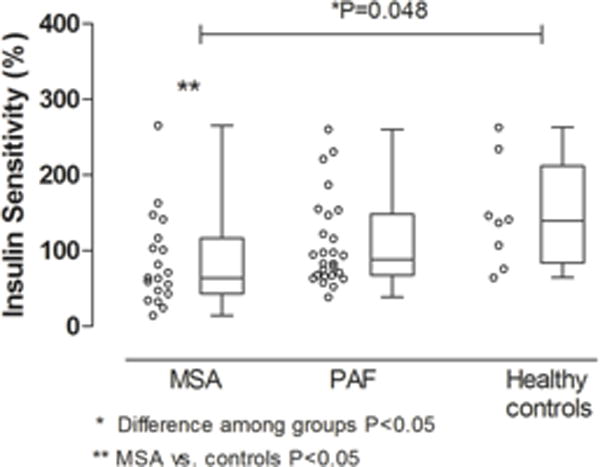
Difference in insulin sensitivity between patients with Multiple System Atrophy (MSA), Pure Autonomic Failure (PAF) and healthy controls.
The relationship between beta cell function and insulin sensitivity among different groups of patients (MSA, PAF and controls) is presented in figure 2. For comparison, we also included insulin sensitivity and beta cell function data obtained in 115 moderate obese subjects with a median BMI of 36.1 IQR (33.3 to 40.7) kg/m2. Even though patients with MSA were lean, their reduced insulin sensitivity was comparable to obese subjects. In MSA and obese subjects, beta cell function was elevated to compensate for the decrease in insulin sensitivity.
Figure 2.
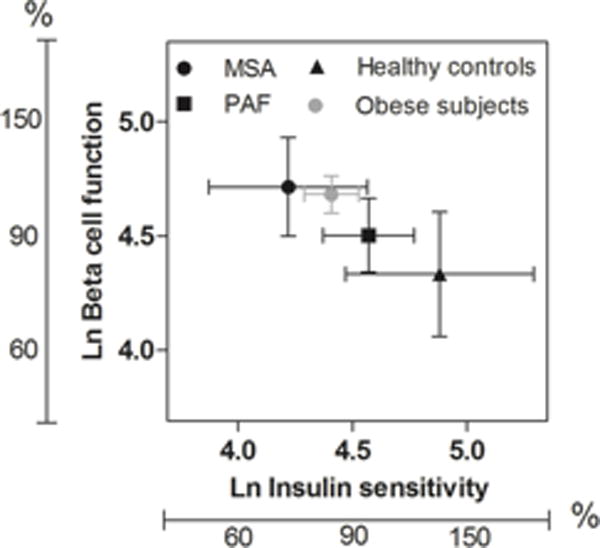
Relationship between insulin sensitivity % and beta cell function % in patients with Multiple System Atrophy (MSA), Pure Autonomic Failure (PAF), moderate obese subjects and healthy controls. Graphic represents mean and 95% CI.
Effect of residual autonomic activity and other variables on insulin sensitivity
We assessed the relationship between the logarithm of insulin sensitivity and age, the presence of supine hypertension, and sympathetic and parasympathetic activity (as measured by log (LFSBP) and log (HFRRI), respectively). Insulin sensitivity was positively correlated with age (r=0.248, P=0.043) and parasympathetic activity [log HFRRI (r=0.311, P=0.015)], but negatively correlated with presence of supine hypertension (r=−0.260, P=0.036) and sympathetic activity [log LF SBP, (r= −0.177, P=0.112)], Table 4. Adjusted R2 for this model was 0.32, F(4, 44)=6.253, P<0.001, indicating that approximately 32% of the variability in insulin sensitivity was explained by our model. As shown in Table 4, two predictors suppressed insulin sensitivity: sympathetic activity (log LF SBP) and the presence of supine hypertension (yes=1; no=0).
Table 4.
Predicting insulin sensitivity (N=44)
| Predictor | r | β | 95% CI for P | P value |
|---|---|---|---|---|
| Age, years | 0.248 | 0.28* | 0.009, 0.047 | 0.006 |
| Supine Hypertension (1=yes; 0=no) | −2.600 | −0.43* | −0.793, −0.076 | 0.019 |
| ln LFSBP, mm Hg2 | −0.177 | −0.17* | −0.328, −0.013 | 0.035 |
| ln HFRRI, ms2 | 0.311 | 0.13* | 0.036, 0.229 | 0.008 |
P<0.05,
P<0.01
Our model indicated a 24% increase in insulin sensitivity per one-year increase in age, a 43% reduction in insulin sensitivity if the patient had supine hypertension, a 17% reduction in insulin sensitivity for 1 % increase in LFSBP (sympathetic activity), and a 13% improvement in insulin sensitivity for 1% increase in HFRRI (parasympathetic activity).
Discussion
The main new finding of this study was that patients with MSA, a form of primary autonomic failure characterized by central autonomic compromise with preserved peripheral noradrenergic nerves, have reduced insulin sensitivity compared with healthy controls. Furthermore, the reduced insulin sensitivity observed in lean MSA is comparable to subjects with moderate obesity. In contrast, no differences were found in insulin sensitivity in patients with peripheral autonomic impairment or PAF compared with healthy controls. The factors that significantly contributed to the decrease in insulin sensitivity in MSA were the presence of residual sympathetic activity, as measured by low frequency variability of systolic blood pressure. Impaired insulin sensitivity in MSA was also associated with the presence of supine hypertension, which we have previously shown is also due to residual sympathetic activity.[23] On the other hand, parasympathetic activity, as assessed by high frequency heart rate variability and age, had a favorable effect on insulin sensitivity.
Several explanations may account for the reduced insulin sensitivity in MSA patients; it may be possible that the reduced insulin sensitivity observed in MSA is related to a decrease in physical activity, a sedentary lifestyle or severity of the disease. It is well-known that patients with autonomic failure become hypotensive on standing[3] and during exercise.[26] Furthermore, patients with MSA developed pyramidal and extrapyramidal symptoms with severe rigidity and decreased postural reflexes. An adopted protective behavior against fainting or falls has been to decrease physical activity. Of note, in a previous study,[24] we reported a significant (82% reduction) in physical activity in autonomic failure patients as measured by an actigraphy monitor compared with age-matched normal volunteers.
It is well known that the autonomic nervous system plays a significant role in the regulation of glucose homeostasis. Metabolically active tissues such as adipose, muscle, liver and pancreas are innervated by parasympathetic and sympathetic fibers.[16] Previous studies have shown that acute stimulation of the sympathetic nervous system with lower body negative pressure reduces insulin sensitivity.[11] Conversely, acute and chronic sympathetic inhibition has been shown to improve insulin sensitivity.[8, 13] It may possible that the unrestrained residual sympathetic activity in MSA contributes to reduced insulin sensitivity. We previously reported that the residual sympathetic activity in MSA still modulates some metabolic function such as energy expenditure,[24] autonomic/endocrine circadian rhythm[20] and blood pressure.[23] The residual sympathetic activity could negatively influence insulin sensitivity by decreasing glucose delivery to target tissue through blood flow mechanism. Indeed, in our cohort, the presence of supine hypertension,[23] induced by unrestrained residual sympathetic tone in MSA, and measurements of sympathetic activity (LFsbp) significantly predicted the decrease in insulin sensitivity.
In our cohort, residual parasympathetic activity had a favorable effect on insulin sensitivity. It has been reported that parasympathetic activity is reduced in chronic inflammatory conditions associated with impaired insulin sensitivity such as obesity. Weight loss resulting from increased physical activity, dieting or bariatric surgery reduces inflammation, ameliorates the metabolic complications, and improves the heart rate variability (a measurement of parasympathetic tone).[14, 17]
It is noteworthy that our results showed that aging had a favorable effect on insulin sensitivity. This is unexpected considering previous studies that reported an inverse association between age and insulin sensitivity.[5, 25]In our cohort, MSA patients were 10 years younger than PAF patients, this age imbalance may contribute to this observation.
Our findings raised several questions. For instance, in patients with Parkinson’s disease, concurrent diabetes mellitus can accelerate progression of both motor and cognitive symptoms.[22] Current randomized clinical trials with drugs approved for the treatment of type II diabetes mellitus (pioglitazone and exenatide) are being conducted to determine their neuroprotective effects. If proven successful, similar trials could be conducted in MSA, a disease characterized by a rapidly progressive neurodegeneration.
Alternatively, because of the association between increased parasympathetic activity and insulin sensitivity, one could postulate that interventions that increase parasympathetic tone, such as central acetylcholinesterase inhibitors and/or vagal stimulators, may have a beneficial effect on insulin sensitivity and possibly neuroprotection in MSA patients.
Limitations
Our study has several limitations. We did not measure body composition. In a previous publication, we did not observed significant difference in body composition between autonomic failure patients and healthy controls.[24] The gold standard for evaluation of insulin sensitivity is the hyperinsulinemic euglycemic clamp. This technique, however, cannot be safely applied in patients with autonomic failure because of the risk of hypotension during insulin infusion.[18] Finally, PAF patients have a tendency to reduced insulin sensitivity. The lack of significant difference may be explained by the small sample size. PAF, however, is a rare disease, with unknown prevalence in the general population.
Acknowledgments
None
Sources of Funding
This work was supported in part by grant P01 HL056693, R01 HL102387, Autonomic Rare Diseases Clinical Research Consortium Grant U54 NS065736, the Vanderbilt Clinical and Translational Science Award grant UL1 RR024975 from the National Center for Research Resources, and the National Institutes of Health. Cyndya A. Shibao is supported by grant K23 HL103976 from the National Institutes of Health, PhRMA Foundation Career Development Award, Doris Duke Clinical Scientist Career Development Award.
Footnotes
Disclosure
CAS and IB are consultants for Lundbeck Pharmaceuticals
Reference List
- 1.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 2.Standards of medical care in diabetes–2010. Diabetes Care. 2010;33(Suppl 1):S11–61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold AC, Shibao C. Current concepts in orthostatic hypotension management. Curr Hypertens Rep. 2013;15:304–312. doi: 10.1007/s11906-013-0362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergström B, Lilja B, Rosberg K, Sundkvist G. Autonomic nerve function test. Reference values in healthy subjects. Clinical Physiology. 1986;6:523–528. doi: 10.1111/j.1475-097x.1986.tb00785.x. [DOI] [PubMed] [Google Scholar]
- 5.Broughton DL, Taylor R. Review: deterioration of glucose tolerance with age: the role of insulin resistance. Age and ageing. 1991;20:221–225. doi: 10.1093/ageing/20.3.221. [DOI] [PubMed] [Google Scholar]
- 6.Chalmanov V, Vurbanova M. Diabetes mellitus in parkinsonism patients. Vutreshni bolesti. 1987;26:68–73. [PubMed] [Google Scholar]
- 7.Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, Cheshire WP, Chelimsky T, Cortelli P, Gibbons CH, Goldstein DS, Hainsworth R, Hilz MJ, Jacob G, Kaufmann H, Jordan J, Lipsitz LA, Levine BD, Low PA, Mathias C, Raj SR, Robertson D, Sandroni P, Schatz I, Schondorff R, Stewart JM, van Dijk JG. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69–72. doi: 10.1007/s10286-011-0119-5. [DOI] [PubMed] [Google Scholar]
- 8.Gamboa A, Okamoto LE, Arnold AC, Figueroa RA, Diedrich A, Raj SR, Paranjape SY, Farley G, Abumrad N, Biaggioni I. Autonomic blockade improves insulin sensitivity in obese subjects. Hypertension. 2014;64:867–874. doi: 10.1161/HYPERTENSIONAHA.114.03738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilman S, Low P, Quinn N, Albanese A, Ben Shlomo Y, Fowler C, Kaufmann H, Klockgether T, Lang A, Lantos P, Litvan I, Mathias C, Oliver E, Robertson D, Schatz I, Wenning G. Consensus statement on the diagnosis of multiple system atrophy American Autonomic Society and American Academy of Neurology. [Review] [26 refs] Clinical Autonomic Research. 1998;8:359–362. doi: 10.1007/BF02309628. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein DS, Cannon RO, 3d, Quyyumi A, Chang P, Duncan M, Brush JE, Jr, Eisenhofer G. Regional extraction of circulating norepinephrine, DOPA, and dihydroxyphenylglycol in humans. J Auton Nerv Syst. 1991;34:17–35. doi: 10.1016/0165-1838(91)90005-n. [DOI] [PubMed] [Google Scholar]
- 11.Henry S, Trueb L, Sartori C, Scherrer U, Jequier E, Tappy L. Effects of a sympathetic activation by a lower body negative pressure on glucose and lipid metabolism. Clinical Physiology. 1998;18:562–569. doi: 10.1046/j.1365-2281.1998.00136.x. [DOI] [PubMed] [Google Scholar]
- 12.Jordan J, Biaggioni I. Diagnosis and treatment of supine hypertension in autonomic failure patients with orthostatic hypotension. Journal of Clinical Hypertension. 2002;4:139–145. doi: 10.1111/j.1524-6175.2001.00516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaaja R, Kujala S, Manhem K, Katzman P, Kibarskis A, Antikainen R, Yliharsila H, Erkkola R, Tuomilehto J. Effects of sympatholytic therapy on insulin sensitivity indices in hypertensive postmenopausal women. Int J Clin Pharmacol Ther. 2007;45:394–401. doi: 10.5414/cpp45394. [DOI] [PubMed] [Google Scholar]
- 14.Karason K, Molgaard H, Wikstrand J, Sjostrom L. Heart rate variability in obesity and the effect of weight loss. The American journal of cardiology. 1999;83:1242–1247. doi: 10.1016/s0002-9149(99)00066-1. [DOI] [PubMed] [Google Scholar]
- 15.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21:2191–2192. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- 16.Marino JS, Xu Y, Hill JW. Central insulin and leptin-mediated autonomic control of glucose homeostasis. Trends Endocrinol Metab. 2011;22:275–285. doi: 10.1016/j.tem.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maser RE, Lenhard MJ, Irgau I, Wynn GM. Impact of surgically induced weight loss on cardiovascular autonomic function: one-year follow-up. Obesity (Silver Spring, Md) 2007;15:364–369. doi: 10.1038/oby.2007.554. [DOI] [PubMed] [Google Scholar]
- 18.Mathias CJ, daCosta DF, Fosbraey P, Christensen NJ, Bannister R. Hypotensive and sedative effects of insulin in autonomic failure. Br Med J. 1987;295:161–163. doi: 10.1136/bmj.295.6591.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 20.Okamoto LE, Gamboa A, Shibao C, Black BK, Diedrich A, Raj SR, Robertson D, Biaggioni I. Nocturnal blood pressure dipping in the hypertension of autonomic failure. Hypertension. 2009;53:363–369. doi: 10.1161/HYPERTENSIONAHA.108.124552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pressley JC, Louis ED, Tang MX, Cote L, Cohen PD, Glied S, Mayeux R. The impact of comorbid disease and injuries on resource use and expenditures in parkinsonism. Neurology. 2003;60:87–93. doi: 10.1212/wnl.60.1.87. [DOI] [PubMed] [Google Scholar]
- 22.Schwab RS. Progression and prognosis in Parkinson’s disease. The Journal of nervous and mental disease. 1960;130:556–566. doi: 10.1097/00005053-196006000-00017. [DOI] [PubMed] [Google Scholar]
- 23.Shannon JR, Jordan J, Diedrich A, Pohar B, Black BK, Robertson D, Biaggioni I. Sympathetically mediated hypertension in autonomic failure. Circulation. 2000;101:2710–2715. doi: 10.1161/01.cir.101.23.2710. [DOI] [PubMed] [Google Scholar]
- 24.Shibao C, Buchowski MS, Chen KY, Yu C, Biaggioni I. Chronic sympathetic attenuation and energy metabolism in autonomic failure. Hypertension. 2012;59:985–990. doi: 10.1161/HYPERTENSIONAHA.111.190157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimokata H, Muller DC, Fleg JL, Sorkin J, Ziemba AW, Andres R. Age as independent determinant of glucose tolerance. Diabetes. 1991;40:44–51. doi: 10.2337/diab.40.1.44. [DOI] [PubMed] [Google Scholar]
- 26.Smith GD, Alam M, Watson LP, Mathias CJ. Effect of the somatostatin analogue, octreotide, on exercise-induced hypotension in human subjects with chronic sympathetic failure. Clinical Science. 1995;89:367–373. doi: 10.1042/cs0890367. [DOI] [PubMed] [Google Scholar]
- 27.Spielman LJ, Little JP, Klegeris A. Inflammation and insulin/IGF-1 resistance as the possible link between obesity and neurodegeneration. Journal of neuroimmunology. 2014;273:8–21. doi: 10.1016/j.jneuroim.2014.06.004. [DOI] [PubMed] [Google Scholar]


