Abstract
Removal of the carotid body (CB) improves animal models of hypertension (HTN) and heart failure, presumably via withdrawal of chemoreflex-induced sympathetic activation. The effect of CB tumor (CBT) resection on blood pressure (BP) in subjects with HTN is unknown. We conducted a retrospective analysis of 20 subjects with HTN (BP≥140/90 mmHg or use of antihypertensives) out of 134 who underwent CBT resection. Short-term (from 3 months before to the first reading after 30 days from surgery) and long-term (slope of the regressions on time over the entire follow up) changes in BP and heart rate (HR) were ascertained and adjusted for covariates (interval between readings, total follow up, number of readings and changes in therapy). Age and duration of HTN were 56±4 and 9±5 years. Adjusted short-term decreases in systolic (SBP: −9.9±3.1, p<0.001) and pulse pressures (PP: −7.9±2.7, p<0.002) were significant and correlated with their respective long-term changes (SBP: r=0.47, p=0.047; PP: r=0.54, p=0.019). Also, there was a strong relationship between adjusted short-term changes in SBP and PP (r=0.64, p<0.004). Out of 12 subjects with concordant decreases in short- and long-term BP changes, 6 (50% of responders or 33% of the total) had short-term falls of SBP ≥10 mmHg and of PP ≥ 5mmHg. To our knowledge this study is the first to show that unilateral CBT resection is associated with sustained reduction of BP in subjects with HTN. Hence, we suggest that targeted removal of the CB chemoreflex conceivably has a role in the therapy of human HTN.
Keywords: carotid body tumors, essential hypertension, pulse pressure, chemoreflex, sympathetic nervous system
INTRODUCTION
The carotid bodies (CBs) are bilateral ovoid organs of 1.5–7.0 mm located at the carotid bifurcation and innervated by a parasympathetic (glossopharyngeal nerve) and sympathetic (superior cervical ganglion) nerve plexus. They are chemosensors for arterial oxygen, carbon dioxide, blood pH, blood glucose and blood flow 1, 2 via Type II glomus cells derived from the neural crest, and relay their information to the medulla oblongata, particularly for the control of respiration in response to hypoxia.
Activation of CB chemoreceptive cells is a powerful stimulator of the sympathetic nervous system and their intermittent or chronic overactivity has been linked to development and progression of cardiovascular diseases such as hypertension (HTN) and heart failure (CHF) 3. In animal models, resection of the CB or inhibition of its output by hyperoxia improves HTN, CHF and diabetes mellitus (DM) 4–7. In humans, the size of the CB correlates with the prevalence of sympathetically mediated diseases such as HTN, CHF and DM 8, 9, CB hypersensitivity relates to increased mortality in CHF 10, and hyperoxia improves hemodynamic characteristics of CHF 11; hence, CB resection has been attempted as a possible treatment for patients with advanced CHF 12.
In human essential hypertensive subjects: a) the CBs are hypersensitive to chemical stimuli compared to those of their normotensive counterparts 13, b) surgical CB removal reduces blood pressure (BP) in subjects with comorbid asthma 3, 14 and c) hyperoxia reduces BP in otherwise untreated subjects 15, 16. A pivotal single-arm, unblinded trial testing the effects of surgical and interventional CB resection on human resistant HTN is ongoing in Europe (clinicaltrials.gov identifier: NCT01745172/NCT02099851).
Tumors of the CB (CBTs) are rare (prevalence of 1–2 per 100,000), usually benign and only rarely catecholamine-secreting (1–5% of the cases 17, 18). Surgical resection of non-functional tumors removes the stimulatory effect of the chemoreflex on the sympathetic nervous system but is associated with opposing effects on sympathetic regulation by concomitant damage of the baroreflex. We took advantage of a large series of patients who underwent CBT resection by one of the authors (JLN) to assess the net effect of this intervention on BP in a subset of patients with pre-existing HTN.
MATERIAL AND METHODS
We conducted a retrospective review of the medical records of 134 patients with uni- or bilateral resection of CBTs between 1990–2012 at the Vanderbilt’s Head and Neck Surgical Department. IRB approval was obtained from the Vanderbilt University (IRB 131501). Only 20 subjects met the entry criterion of preceding HTN, defined as BP≥140/90 mmHg or use of antihypertensive drugs. Two of these subjects were excluded from analyses because of inadequate follow-up data. Two subjects had a subsequent surgical excision of a contralateral CBT. In these subjects, the study was limited to data after the first procedure, for uniformity among all subjects and to avoid including data in patients with baroreflex failure, a complication of bilateral CBT resection. Sixteen of the remaining 18 subjects were on antihypertensive medications, whereas the other two were not, despite meeting blood pressure criteria for hypertension. Clinical and BP data were obtained by review of Vanderbilt’s medical record, records of the patients’ primary care providers and telephone interviews. The average of all sphygmomanometric seated BPs obtained during the 3 months preceding surgery was used as the baseline BP. Short-term changes in systolic (ΔSBPst), diastolic (ΔDBPst), pulse pressures (ΔPPst) and heart rates (ΔHRst) were calculated as the subtraction of this baseline from the first BP or HR reading obtained at least 30 days after the surgical procedure. BP readings obtained before 30 days from the surgical date were purposely discarded to minimize confounding effects of immediate post-operative BP variability. Long-term changes in BP and HR (ΔSBPlt, ΔDBPlt, ΔPPlt and ΔHRlt) were estimated by the slope of the regression of these BPs and HRs on time, using all data available for the entire period of follow-up in each individual patient and are reported as the changes per year (mmHg/yr or bpm/year).
Because of the retrospective nature of the study, there was large variability in time intervals between BP and HR readings, number of readings, duration of follow up, and changes of medications during the short-term and long-term periods. Therefore, both the short-term BP changes and the long-term slopes were adjusted by use of covariate analyses. For short-term data, the covariates were: a) the interval between baseline and post-operative readings, and b) the change in therapy during this interval. For long-term data, the covariates were: a) the total duration of follow-up, b) the total number of readings during the study, and c) the change in therapy from the beginning to the end of follow-up. Changes in therapy were quantified using a treatment intensity score for the baseline and each follow up visit, based on a combination of maximum recommended daily dose for each medication according to the April 2014 Monthly Prescribing Reference 19 and equipotency of different antihypertensive agents (supplemental Table S1).
Descriptive data are presented as means ± SEM, percentages or medians and quartiles as appropriate. Deltas of parameters before and after tumor resection were analyzed by paired Student’s t-tests. Correlations between parameters were assessed by Pearson’s correlation coefficients and simple linear regression analyses. Covariate analysis was carried out with multivariate regression for data in all subjects, entering the covariates as regressors in the form of a matrix (deviations from the mean for the employed continuous covariates). The beta coefficients for the covariates and the matrix covariates for each individual patient were used for calculation of adjusted y as previously reported 20.
RESULTS
Table 1 shows the baseline clinical characteristics of the 18 subjects analyzed in the study. Age was 56±2 years, with an almost equal gender distribution (44% male and 56% female). Fourteen subjects were on antihypertensive therapy; baseline SBP and DBP were 141±3/83±3 mmHg and duration of known HTN was 9±2 years. BMI was 32.5±3, with 12 out of 18 subjects (66.7%) exceeding the cutoff for diagnosis of obesity (30 Kg/m2). Following resection the average BMI increased to 33.4±3.1 (p <0.002). Seven subjects had significant cardiovascular or metabolic comorbidities, which are listed in Table 1. Glomus tissue was confirmed histologically in all resected specimens. Fifty-six percent of CBTs were located on the right side of the neck. Catecholamine-secreting CBTs were suspected in four subjects but excluded with measurement of plasma catecholamines or their metabolites. There was large variability in the interval between the baseline and the first BP and HR measurements for assessment of the short-term responses (mean±SEM 266±101 days, with median 138 and inter-quartile range 68–225 days) and also in the total duration of follow-up (mean±SEM 3.1±0.6 years, with median 3.2 and inter-quartile range 0.8–4.3 years).
TABLE 1.
Clinical Characteristics of the subjects
| Patient ID# | Age (yrs) | Gender (F/M) | SBP/DBP (mm Hg) | Duration of HTN (yrs) | BMI (Kg/m2) | Comorbidities |
|---|---|---|---|---|---|---|
| 1 | 64 | F | 140/77 | 5 | 30.4 | CAD/CHF/DM |
| 2 | 47 | F | 121/76 | 5 | 41.2 | DM/OSA |
| 3 | 72 | F | 133/78 | - | 33.0 | CHF/DM/CKD |
| 4 | 61 | M | 129/80 | 12 | 25.2 | |
| 5 | 66 | F | 149/84 | 1 | 24.6 | |
| 6 | 54 | M | 136/82 | 1 | 40.3 | |
| 7 | 44 | F | 151/102 | 0.5 | 22.0 | |
| 8 | 73 | F | 167/83 | 3 | 32.9 | CHF |
| 9 | 52 | F | 130/84 | 5 | 28.4 | |
| 10 | 46 | M | 157/84 | - | 27.2 | |
| 11 | 61 | M | 141/93 | 8 | 30.1 | |
| 12 | 52 | F | 139/57 | 7 | 47.7 | |
| 13 | 46 | M | 149/90 | 5 | 27.8 | |
| 14 | 46 | M | 134/87 | 10 | 37.0 | DM |
| 15 | 46 | F | 152/100 | - | 32.8 | |
| 16 | 66 | M | 117/61 | 30 | 38.6 | CAD/DM/CKD/OSA |
| 17 | 54 | F | 147/93 | 31 | 32.0 | CAD/CHF |
| 18 | 56 | M | 144/84 | - | 40.9 |
SBP: systolic blood pressure, DBP: systolic blood pressure, BMI: body mass index, CAD: coronary artery disease, CHF: congestive heart failure, DM: diabetes mellitus type 2, OSA: obstructive sleep apnea, CKD: chronic kidney disease.
CBT resection resulted in a significant short-term mean reduction in unadjusted ΔSBPst of −9.9±3.7 mmHg, p<0.005, and ΔPPst −7.9±3.3 mmHg, p<0.01 in the 18 subjects. In contrast, there were no significant changes in ΔDBPst −2.0±2.4 mmHg, ns, or ΔHRst 1.4±2.7 bpm, ns. Individual adjusted values (covariate analyses) for the short-term data sustained minor changes, with the exception of a few patients with the shortest and longest intervals from surgery to the first recording of BP or with the shortest and longest total periods of study duration. Mean adjusted short-term changes in BP and HR did not change for any parameter; only minor changes in variances occurred, as expected from the covariate analyses. Therefore, the statistical results for the changes in adjusted ΔSBPst, ΔDBPst, ΔPPst and ΔHRst remained unmodified compared to those of the unadjusted values.
Changes in unadjusted long-term ΔSBPlt −4.6±3.5 mmHg, and ΔPPlt −0.4±2.7 mmHg/yr were of smaller magnitude than their short-term counterparts with the same variability; hence, they were not significant. Those for ΔHRlt remained not significant (−0.1±4.2), whereas those for diastolic BP (ΔDBPlt −4.2±2.4, p=0.037) became significant over the long-term. Again, the covariate analysis did not change the means or significance for these long-term parameters. The values for the adjusted short and long-term BP changes are given in Table 2.
TABLE 2.
Adjusted short- and long-term changes in blood pressure produced by removal of carotid body tumors
| Pt ID# | ΔSBPst (mmHg) | ΔSBPlt (mmHg/yr) | ΔDBPst (mmHg) | ΔDBPlt (mmHg/yr) | ΔPPst (mmHg) | ΔPPlt (mmHg/yr) |
|---|---|---|---|---|---|---|
| 1 | −31.7 | −8.8 | −5.8 | −2.9 | −26.0 | −6.0 |
| 2 | 5.2 | 0.6 | 1.1 | 4.4 | 4.0 | −3.4 |
| 3 | 11.8 | 11.0 | 1.6 | −0.1 | 10.3 | 10.9 |
| 4 | 7.1 | 6.0 | 4.7 | 0.1 | 2.4 | 6.4 |
| 5 | −16.0 | −2.0 | −19.4 | −12.8 | 3.2 | 12.7 |
| 6 | −6.6 | −0.1 | −1.0 | −2.1 | −5.6 | 1.6 |
| 7 | −25.6 | −2.1 | −15.6 | −1.6 | −10.0 | −1.0 |
| 8 | −17.2 | −23.6 | −2.8 | −2.9 | −13.9 | −22.8 |
| 9 | −0.1 | −3.3 | 1.6 | −6.1 | −1.7 | 2.3 |
| 10 | −20.6 | −30.9 | −0.7 | −9.2 | −19.8 | −21.1 |
| 11 | −2.0 | −8.4 | −2.7 | −13.9 | 0.6 | 3.9 |
| 12 | 2.6 | −3.9 | 19.0 | 3.4 | −16.3 | −7.7 |
| 13 | −7.7 | −21.9 | −9.4 | −22.0 | 1.7 | 1.0 |
| 14 | −27.3 | 0.0 | −12.8 | −4.0 | −14.6 | 3.8 |
| 15 | −20.8 | −16.5 | −4.9 | −8.6 | −15.9 | −7.2 |
| 16 | −10.1 | −6.0 | 21.0 | −5.7 | −31.2 | −1.3 |
| 17 | −19.9 | −12.5 | −11.1 | −2.5 | −8.8 | −9.0 |
| 18 | 0.2 | 39.0 | 1.5 | 10.7 | −1.4 | 29.2 |
Short-term changes in systolic (ΔSBPst), diastolic (ΔDBPst) and pulse (ΔPPst) pressures are those from baseline to the first reading after 30 days from the surgical procedure. Long-term changes are the yearly changes in SBPlt, DBPlt and PPlt derived from the slopes of the regressions of these BPs over time for the entire period of study in each patient. Data are adjusted by the covariate analyses described in Methods.
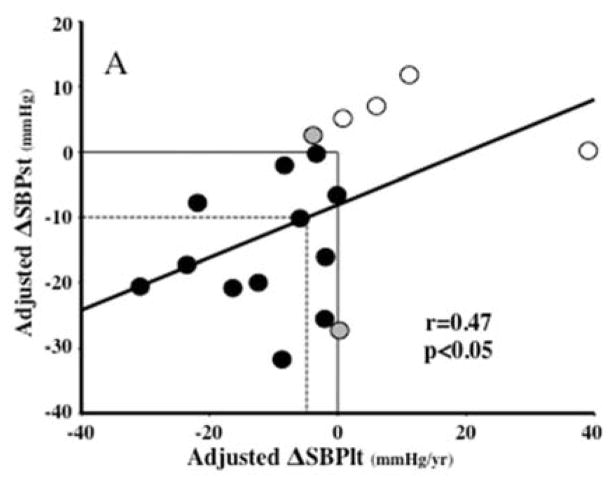
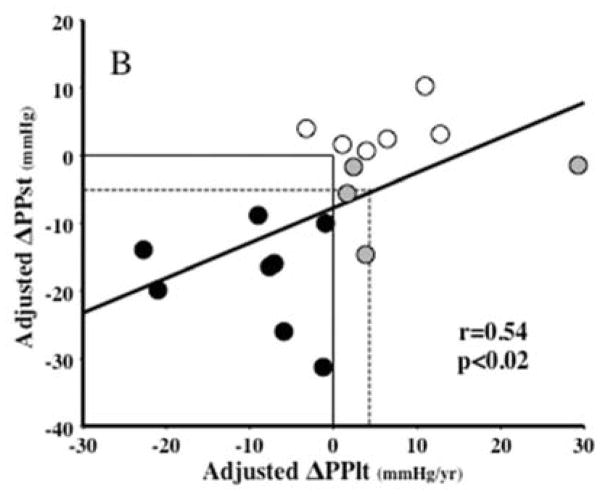
Correlation analyses showed trends for positive relationships between the short- and long-term unadjusted changes in SBP (r=0.38, p=0.12), DBP (r=0.37, p=0.13), PP (r=0.44, p=0.07) and HR (r=0.45, p=0.06), but none reached statistical significance. In contrast, after adjustment for covariates, these relationships became significant for SBP (r=0.47, p<0.05; Figure 1A), PP (r=0.54, p<0.02; Figure 1B) and HR (r=0.53, p<0.03; not shown), but not for DBP (r=0.36, p=0.13; not shown). Table 2 and Figure 1A show that 16 out of the 18 patients had a concordant change (same directional change for short- and long-term observations) in adjusted ΔSBP; 12 sustaining decreases (black dots) and 4 sustaining increases (white dots) in both parameters. Furthermore, out of the 12 with concordant decreases, 6 (50% of responders or 33% of the total group) had ΔSBPst ≥10 mmHg, possibly clinically significant. The dotted lines on Figure 1A encompass these 6 subjects, and show that a ΔSBPst ≥10 mmHg corresponded to a ΔSBPlt ≥4.8 mmHg/yr. Analogously, Table 2 and Figure 1B show that 14 out of the 18 patients had a concordant change in adjusted short-term and long-term ΔPP; 8 sustaining decreases (black dots) and 6 sustaining increases (white dots) in both parameters. The other four patients exhibited an initial reduction in PP that was not sustained on a long-term basis (grey dots). Despite this, the overall regression of short-term ΔPP on long-term ΔPP was statistically the strongest. Six of the 10 subjects encompassed by the dotted lines in this panel (representing ΔPPst −5 mmHg or greater) were the same six subjects with short-term ΔSBPst ≥ 10 mmHg in panel A.
Figure 1.
Regressions of the short-term changes in systolic (panel A, ΔSBPst in mmHg) and pulse (Panel B, ΔPPst) pressures on the long-term slopes of these parameters (ΔSBPlt and ΔPPlt, mmHg) over the entire period of follow up, all adjusted for covariates as explained in Methods. In Panel A, black dots represent the 12 subjects with concomitant decreases and white dots the 4 subjects with concomitant increases in ΔSBPst and ΔSBPlt. The two subjects represented by gray dots had minor increases in ΔSBPst but minor decreases in long-term slopes. The dotted-line square encompasses 6 subjects with reductions in SBPst ≥10 mmHg. The vertical projection from the intersection between the ΔSBPst= −10 mmHg and the regression line to the x axis shows that an SBPst reduction of 10 mmHg predicts a slope of −4.8 mmHg/yr. In panel B, 14 out of the 18 patients had a concordant change in adjusted ΔPPst and ΔPPlt; 8 sustaining decreases (black dots) and 6 sustaining increases (white dots). The other four patients exhibited an initial reduction in PP that was not sustained on a long-term basis (grey dots). Six of the 10 subjects encompassed by the dotted lines in this panel (representing an −5 mmHg ΔPPst) were the same six subjects with SBPst fall ≥10 mmHg in panel A. R and p represent the Pearson’s correlation coefficients and their statistical significance. The regression lines are for all data analyzed together.
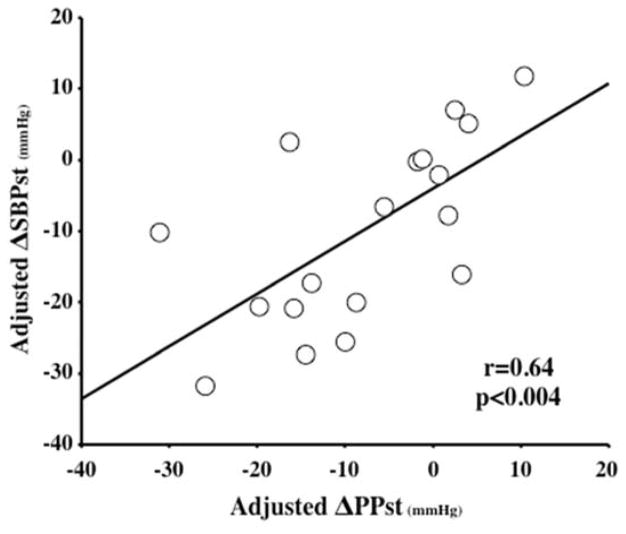
The totality of these observations suggests that initial BP responses to CBT excision tend to be sustained and are predominantly characterized by significant reductions in SBP and PP. A strong relationship between adjusted short-term ΔSBP and ΔPP is shown in Figure 2 (r=0.64, p<0.004) and suggests that the primary effect of CBT excision may be a reduction in PP, supported by the lack of change in short-term DBP.
Figure 2.
Correlation between the adjusted short term changes in systolic (ΔSBPst) and pulse (ΔPPst) pressures. R and p represent the Pearson’s correlation coefficient and its statistical significance. The regression line is for all data analyzed together.
DISCUSSION
The main observation of our retrospective study is that despite interindividual variability, the overall response to unilateral carotid body tumor (CBT) removal was a significant short-term reduction in PP and SBP. Over the long term (slopes of BP over time) the magnitude of these changes was smaller with analogous variability, hence they did not reach statistical significance. However, concordance between short- and long-term responses and their strong correlations indicate that the effect of CBT removal on BP is sustained over time.
The idea that the CBs may have a role in cardiovascular control originated in the published studies of CB resection in humans (also known as glomectomy), carried out for the treatment of dyspnea in subjects with asthma or long-term obstructive lung disease, and supported by recent work in animals 3. In 1961, Nakayama et al reported that CB resection resulted in marked reduction of BP in a subset of 29 asthma patients who had HTN; the effect was observed within few days and sustained for 6 months 14. A prompt effect of glomectomy in reducing BP of subject with severe COPD and HTN was also noted by Winters and Whipp 21.
It is widely accepted that the autonomic nervous system participates in the pathophysiology of HTN and other cardiovascular diseases 22, 23. The CB mediated peripheral chemoreflex is enhanced in patients with essential HTN 24, 25 and animal models of HTN 26, as sympathetic tone is 27, 28. Indeed, peripheral chemoreceptor reflex has been shown to be elevated in other sympathetically mediated diseases in humans, like CHF 10, 29. In animal models of HTN 4, 7, 30 there is compelling evidence for a CB contribution to cardiovascular control. For example, in spontaneously hypertensive rats, carotid sinus denervation restored autonomic balance through a reduction in sympathetic outflow and blunted the genesis and progression of HTN 4, 5. The antihypertensive effect was rapid (within 1–2 days) but, interestingly, only present following bilateral and not unilateral ablation. Despite the inevitable surgical damage to the mechanoreceptors and associated afferents, baroreceptor function improved. Also, in humans, brief exposure to oxygen lowers SBP, presumably due to sympathetic effects subsequent to inactivation of the chemoreflex 16.
Extensive surgeries performed for removal of bilateral CBTs are often complicated by baroreflex failure owing to baroreceptor damage after adventitial stripping. This leads to undesired hypertension with wide blood pressure variability 31–34. Before our study, it was conceivable that removal of unilateral CBTs might also produce baroreflex damage, counteracting or abolishing any beneficial effect that denervation of the chemoreflex might have on the blood pressure of hypertensive patients. It is therefore encouraging that we did not confirm such possibility, since we observed short-term and long-term improvements in BP in 12 patients (67%). Our observation predicts that a more targeted approach to unilateral or bilateral removal of the CBs in hypertensive subjects (as opposed to extensive surgical resection of tumors) may have greater antihypertensive effect owing to preservation of the baroreflex and perhaps allowing for bilateral interventions. Out of our 12 patients exhibiting blood pressure reduction, 6 (33% of total) had a clinically significant short-term SBP reduction of >10 mmHg. This was associated with a long-term BP reduction of 4.8 mmHg/yr.
Maybe of more importance was the decrease in brachial artery PP. PP is a surrogate of vascular structure and left ventricular (LV) mass 35, 36 and a major determinant of cardiovascular outcomes 37–39. Because we observed reductions of PP without change in HR or DBP, the effect of CBT resection on SBP could have only been mediated by diminished stroke volume or improvement in the elastic properties of conduit vessels 40, 41, an issue that remains to be investigated with hemodynamic measurements. Analogously, the long-term (but not short term) reduction in DBP supports the speculation that initial changes in the pulsatile characteristics of BP leads to long-term autoregulation or remodeling of small arterioles.
Finally, the blood pressure changes we observed occurred despite the high likelihood of some degree of baroreceptor damage during surgery and an overall increase in BMI thereafter. A continuous and gradual decline in SBP and PP makes it unlikely that the effect was due to removal of undetected catecholamine secretion by surgery 42. Our observations are consistent with an effect on CB in autonomic regulation of the cardiovascular system and are reminiscent of those after renal denervation 43, presumably due to effects on sympathetic outflow 44. They support the worthiness of ongoing studies on removal or ablation of the CB as a treatment for HTN and other sympathetically mediated diseases, some of which have already produced encouraging results 12.
Limitations of our study include the retrospective design, varied times at follow up and lack of systematic exclusion of functional (secretory) CBTs; which would be required if a prospective trial of this rare disease were feasible. Lack of a control group does not permit us to entirely exclude that changes in blood pressure in our subjects merely reflected regression to the mean. Prospective research on the effect of carotid body denervation on blood pressure will require a sham procedure, not ethically feasible in patients with tumors but permissive if testing use of new devices in a hypertensive population. Patients at our institution were tested for catecholamine-secreting CBTs only if there was clinical suspicion by the surgical/medical team. In the small number of tested cases, the workup was negative. However, we cannot exclude that a subject might have had improvement in HTN owing to resection of an undetected functionally secreting CBT, as proposed by de Franciscis et al. in a cohort of normotensive patients 42. A more likely interpretation for our results, observed in a group of hypertensive subjects is that reduction of PP and SBP was mediated by removal of the sympathetic stimulatory effects of the CB, not by undetected secretory function. Our data suggests that there is hyperactivity of the CB in HTN, making it conceivable that interventions to suppress it may have a therapeutic effect, a contention to be proved in prospective trials. Also, some but not all of our subjects responded with blood pressure reduction, suggesting that chemoreflex function may differ among hypertensive subjects. It follows that clinical trials targeting the CB for treatment of hypertension may require testing of chemoreflex function as an attempt at prospectively identifying responders vs not responders.
CONCLUSIONS
This is the first study to show that CBT resection is associated with a sustained reduction of BP in a subset of patients with comorbid HTN, perhaps through a primary effect on PP. Because concomitant baroreceptor damage by CBT surgery most likely leads to underestimation of the depressor effect of chemoreflex disruption, development of a targeted removal of the CB chemoreflex may conceivably have a role in the therapy of human HTN. Future well-designed prospective trials are needed to test the hypothesis that CB removal improves autonomic balance and thus reduces BP via effects on cardiac output or conduit artery compliance.
Supplementary Material
SUPPLEMENTAL TABLE 1 Medication Score
Highlights.
We study the effects of carotid body tumor resection on patients with essential hypertension.
First study to show that unilateral carotid body tumor removal is associated with a significant short-term reduction in systolic blood pressure and pulse pressure.
Concordance between short- and long-term responses and their strong correlations indicate that the effect of carotid body tumor removal on blood pressure is sustained over time.
Acknowledgments
SOURCES OF FUNDING
Supported in part by P01 HL056693 (D. Robertson)
Footnotes
CONFLICT OF INTEREST
Marat Fudim is a former employee and stockholder of Cibiem Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marshall JM. Peripheral chemoreceptors and cardiovascular regulation. Physiological reviews. 1994;74:543–594. doi: 10.1152/physrev.1994.74.3.543. [DOI] [PubMed] [Google Scholar]
- 2.Wehrwein EA, Curry TB, Basu A, Rizza RA, Basu R, Joyner MJ. Do the carotid bodies modulate hypoglycemic counterregulation and baroreflex control of blood pressure in humans? Advances in experimental medicine and biology. 2012;758:129–135. doi: 10.1007/978-94-007-4584-1_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paton JF, Sobotka PA, Fudim M, Engleman ZJ, Hart EC, McBryde FD, Abdala AP, Marina N, Gourine AV, Lobo M, Patel N, Burchell A, Ratcliffe L, Nightingale A. The Carotid Body as a Therapeutic Target for the Treatment of Sympathetically Mediated Diseases. Hypertension. 2012;61:5–13. doi: 10.1161/HYPERTENSIONAHA.111.00064. [DOI] [PubMed] [Google Scholar]
- 4.McBryde FD, Abdala AP, Hendy EB, Pijacka W, Marvar P, Moraes DJ, Sobotka PA, Paton JF. The carotid body as a putative therapeutic target for the treatment of neurogenic hypertension. Nature communications. 2013;4:2395. doi: 10.1038/ncomms3395. [DOI] [PubMed] [Google Scholar]
- 5.Abdala ANM, Gourine A, Paton J. Peripheral chemoreceptor inputs contribute to the development of high blood pressure in spontaneously hypertensive rats Annual meeting of Physiological Society; July 12; England. 2011. [Google Scholar]
- 6.Del Rio R, Marcus NJ, Schultz HD. Carotid chemoreceptor ablation improves survival in heart failure: rescuing autonomic control of cardiorespiratory function. Journal of the American College of Cardiology. 2013;62:2422–2430. doi: 10.1016/j.jacc.2013.07.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ribeiro MJ, Sacramento JF, Gonzalez C, Guarino MP, Monteiro EC, Conde SV. Carotid body denervation prevents the development of insulin resistance and hypertension induced by hypercaloric diets. Diabetes. 2013;62:2905–2916. doi: 10.2337/db12-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cramer JA, Wiggins RH, Fudim M, Engelman ZJ, Sobotka PA, Shah LM. Carotid body size on CTA: correlation with comorbidities. Clinical radiology. 2014;69:e33–36. doi: 10.1016/j.crad.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 9.Nair S, Gupta A, Fudim M, Robinson C, Ravi V, Hurtado-Rua S, Engelman Z, Lee KS, Phillips CD, Sista AK. CT angiography in the detection of carotid body enlargement in patients with hypertension and heart failure. Neuroradiology. 2013;55:1319–1322. doi: 10.1007/s00234-013-1273-3. [DOI] [PubMed] [Google Scholar]
- 10.Ponikowski P, Chua TP, Anker SD, Francis DP, Doehner W, Banasiak W, Poole-Wilson PA, Piepoli MF, Coats AJ. Peripheral chemoreceptor hypersensitivity: an ominous sign in patients with chronic heart failure. Circulation. 2001;104:544–549. doi: 10.1161/hc3101.093699. [DOI] [PubMed] [Google Scholar]
- 11.Ponikowski P, Chua TP, Piepoli M, Ondusova D, Webb-Peploe K, Harrington D, Anker SD, Volterrani M, Colombo R, Mazzuero G, Giordano A, Coats AJ. Augmented peripheral chemosensitivity as a potential input to baroreflex impairment and autonomic imbalance in chronic heart failure. Circulation. 1997;96:2586–2594. doi: 10.1161/01.cir.96.8.2586. [DOI] [PubMed] [Google Scholar]
- 12.Niewinski P, Janczak D, Rucinski A, Jazwiec P, Sobotka PA, Engelman ZJ, Fudim M, Tubek S, Jankowska EA, Banasiak W, Hart EC, Paton JF, Ponikowski P. Carotid body removal for treatment of chronic systolic heart failure. International journal of cardiology. 2013;168:2506–2509. doi: 10.1016/j.ijcard.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Trzebski A, Tafil M, Zoltowski M, Przybylski J. Increased sensitivity of the arterial chemoreceptor drive in young men with mild hypertension. Cardiovascular research. 1982;16:163–172. doi: 10.1093/cvr/16.3.163. [DOI] [PubMed] [Google Scholar]
- 14.Nakayama K. Surgical removal of the carotid body for bronchial asthma. Dis Chest. 1961;40:595–604. doi: 10.1378/chest.40.6.595. [DOI] [PubMed] [Google Scholar]
- 15.Izdebska E, Cybulska I, Sawicki M, Izdebski J, Trzebski A. Postexercise decrease in arterial blood pressure, total peripheral resistance and in circulatory responses to brief hyperoxia in subjects with mild essential hypertension. Journal of human hypertension. 1998;12:855–860. doi: 10.1038/sj.jhh.1000716. [DOI] [PubMed] [Google Scholar]
- 16.Sinski M, Lewandowski J, Przybylski J, Zalewski P, Symonides B, Abramczyk P, Gaciong Z. Deactivation of carotid body chemoreceptors by hyperoxia decreases blood pressure in hypertensive patients. Hypertension research: official journal of the Japanese Society of Hypertension. 2014;37:858–862. doi: 10.1038/hr.2014.91. [DOI] [PubMed] [Google Scholar]
- 17.Erickson D, Kudva YC, Ebersold MJ, Thompson GB, Grant CS, van Heerden JA, Young WF., Jr Benign paragangliomas: clinical presentation and treatment outcomes in 236 patients. The Journal of clinical endocrinology and metabolism. 2001;86:5210–5216. doi: 10.1210/jcem.86.11.8034. [DOI] [PubMed] [Google Scholar]
- 18.Erdogan BA, Bora F, Altin G, Paksoy M. Our experience with carotid body paragangliomas. Prague medical report. 2012;113:262–270. doi: 10.14712/23362936.2015.9. [DOI] [PubMed] [Google Scholar]
- 19.http://www.empr.com/.
- 20.Winer BJ. Statistical Principles in Experimental Design. 2. 1971. pp. 787–792. [Google Scholar]
- 21.Winter B, Whipp BJ. Immediate effects of bilateral carotid body resection on total respiratory resistance and compliance in humans. Advances in experimental medicine and biology. 2004;551:15–21. doi: 10.1007/0-387-27023-x_3. [DOI] [PubMed] [Google Scholar]
- 22.Simms AE, Paton JF, Pickering AE, Allen AM. Amplified respiratory-sympathetic coupling in the spontaneously hypertensive rat: does it contribute to hypertension? The Journal of physiology. 2009;587:597–610. doi: 10.1113/jphysiol.2008.165902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Esler M, Kaye D. Sympathetic nervous system activation in essential hypertension, cardiac failure and psychosomatic heart disease. Journal of cardiovascular pharmacology. 2000;35:1–7. doi: 10.1097/00005344-200000004-00001. [DOI] [PubMed] [Google Scholar]
- 24.Sinski M, Lewandowski J, Przybylski J, Bidiuk J, Abramczyk P, Ciarka A, Gaciong Z. Tonic activity of carotid body chemoreceptors contributes to the increased sympathetic drive in essential hypertension. Hypertens Res. 2011;35:487–491. doi: 10.1038/hr.2011.209. [DOI] [PubMed] [Google Scholar]
- 25.Somers VK, Mark AL, Abboud FM. Sympathetic activation by hypoxia and hypercapnia--implications for sleep apnea. Clinical and experimental hypertension Part A, Theory and practice. 1988;10(Suppl 1):413–422. doi: 10.3109/10641968809075998. [DOI] [PubMed] [Google Scholar]
- 26.Tan ZY, Lu Y, Whiteis CA, Simms AE, Paton JF, Chapleau MW, Abboud FM. Chemoreceptor hypersensitivity, sympathetic excitation, and overexpression of ASIC and TASK channels before the onset of hypertension in SHR. Circulation research. 2010;106:536–545. doi: 10.1161/CIRCRESAHA.109.206946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zoccal DB, Simms AE, Bonagamba LG, Braga VA, Pickering AE, Paton JF, Machado BH. Increased sympathetic outflow in juvenile rats submitted to chronic intermittent hypoxia correlates with enhanced expiratory activity. The Journal of physiology. 2008;586:3253–3265. doi: 10.1113/jphysiol.2008.154187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parati G, Esler M. The human sympathetic nervous system: its relevance in hypertension and heart failure. European heart journal. 2012;33:1058–1066. doi: 10.1093/eurheartj/ehs041. [DOI] [PubMed] [Google Scholar]
- 29.Niewinski P, Engelman ZJ, Fudim M, Tubek S, Paleczny B, Jankowska EA, Banasiak W, Sobotka PA, Ponikowski P. Clinical predictors and hemodynamic consequences of elevated peripheral chemosensitivity in optimally treated men with chronic systolic heart failure. Journal of cardiac failure. 2013;19:408–415. doi: 10.1016/j.cardfail.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 30.Abdala AP, McBryde FD, Marina N, Hendy EB, Engelman ZJ, Fudim M, Sobotka PA, Gourine AV, Paton JF. Hypertension is critically dependent on the carotid body input in the spontaneously hypertensive rat. The Journal of physiology. 2012;590:4269–4277. doi: 10.1113/jphysiol.2012.237800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smit AA, Timmers HJ, Wieling W, Wagenaar M, Marres HA, Lenders JW, van Montfrans GA, Karemaker JM. Long-term effects of carotid sinus denervation on arterial blood pressure in humans. Circulation. 2002;105:1329–1335. doi: 10.1161/hc1102.105744. [DOI] [PubMed] [Google Scholar]
- 32.Netterville JL, Reilly KM, Robertson D, Reiber ME, Armstrong WB, Childs P. Carotid body tumors: a review of 30 patients with 46 tumors. The Laryngoscope. 1995;105:115–126. doi: 10.1288/00005537-199502000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Robertson D, Hollister AS, Biaggioni I, Netterville JL, Mosqueda-Garcia R, Robertson RM. The diagnosis and treatment of baroreflex failure. The New England journal of medicine. 1993;329:1449–55. doi: 10.1056/NEJM199311113292003. [DOI] [PubMed] [Google Scholar]
- 34.Timmers HJ, Wieling W, Karemaker JM, Lenders JW. Denervation of carotid baro- and chemoreceptors in humans. The Journal of physiology. 2003;553:3–11. doi: 10.1113/jphysiol.2003.052415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.James MA, Watt PA, Potter JF, Thurston H, Swales JD. Pulse pressure and resistance artery structure in the elderly. Hypertension. 1995;26:301–306. doi: 10.1161/01.hyp.26.2.301. [DOI] [PubMed] [Google Scholar]
- 36.Pannier B, Brunel P, el Aroussy W, Lacolley P, Safar ME. Pulse pressure and echocardiographic findings in essential hypertension. Journal of hypertension. 1989;7:127–132. [PubMed] [Google Scholar]
- 37.Blacher J, Staessen JA, Girerd X, Gasowski J, Thijs L, Liu L, Wang JG, Fagard RH, Safar ME. Pulse pressure not mean pressure determines cardiovascular risk in older hypertensive patients. Archives of internal medicine. 2000;160:1085–1089. doi: 10.1001/archinte.160.8.1085. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell GF, Moye LA, Braunwald E, Rouleau JL, Bernstein V, Geltman EM, Flaker GC, Pfeffer MA. Sphygmomanometrically determined pulse pressure is a powerful independent predictor of recurrent events after myocardial infarction in patients with impaired left ventricular function. SAVE investigators. Survival and Ventricular Enlargement. Circulation. 1997;96:4254–4260. doi: 10.1161/01.cir.96.12.4254. [DOI] [PubMed] [Google Scholar]
- 39.Franklin SS, Khan SA, Wong ND, Larson MG, Levy D. Is pulse pressure useful in predicting risk for coronary heart Disease? The Framingham heart study. Circulation. 1999;100:354–360. doi: 10.1161/01.cir.100.4.354. [DOI] [PubMed] [Google Scholar]
- 40.Okada Y, Galbreath MM, Shibata S, Jarvis SS, VanGundy TB, Meier RL, Vongpatanasin W, Levine BD, Fu Q. Relationship between sympathetic baroreflex sensitivity and arterial stiffness in elderly men and women. Hypertension. 2012;59:98–104. doi: 10.1161/HYPERTENSIONAHA.111.176560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boutouyrie P, Lacolley P, Girerd X, Beck L, Safar M, Laurent S. Sympathetic activation decreases medium-sized arterial compliance in humans. The American journal of physiology. 1994;267:1368–1376. doi: 10.1152/ajpheart.1994.267.4.H1368. [DOI] [PubMed] [Google Scholar]
- 42.de Franciscis S, Grande R, Butrico L, Buffone G, Gallelli L, Scarcello E, Giuseppe Calio F, De Vito D, Compagna R, Amato M, Fugetto F, Gasbarro V, Bruno A, Serra R. Resection of Carotid Body Tumors reduces arterial blood pressure. An underestimated neuroendocrine syndrome. International journal of surgery. 2014;12:S63–67. doi: 10.1016/j.ijsu.2014.05.052. [DOI] [PubMed] [Google Scholar]
- 43.Symplicity HTNI, Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Bohm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376:1903–1909. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 44.Schlaich MP, Sobotka PA, Krum H, Lambert E, Esler MD. Renal sympathetic-nerve ablation for uncontrolled hypertension. The New England journal of medicine. 2009;361:932–934. doi: 10.1056/NEJMc0904179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTAL TABLE 1 Medication Score