Abstract
Objective
Identify the relative importance of factors that impact parents' attitudes towards use of their child's dried newborn blood spots for research purposes.
Methods
Respondents were parents aged 18 and older with at least one child aged 17 or younger born in Indiana visiting an urban pediatrics clinic. They were asked to rate the acceptability of hypothetical scenarios involving the research use of blood spots. Three pieces of information varied between the scenarios: 1) who would be conducting the research; 2) whether the child's identity would be linked to the spots; and 3) whether and how often the parents' consent would be sought before the research began.
Results
506 predominantly Black and low-income parents completed the survey. The conjoint analysis model showed good fit (Pearson's R=0.998, p<0.001). The rank order of factors affecting parents' attitudes was: 1) consent (importance score=64.9), 2) whether the child's identity was linked to the spot (importance score=19.4), 3) affiliation of the researcher using the spots (importance score=14.6). Respondents preferred being asked for their consent each time their children's spots would be used. They preferred that the children's identity not be linked to the spots and that the research is conducted by university researchers, though these issues had less impact on attitudes than consent.
Conclusions
Parents strongly prefer that consent be sought for each use of their children's spots. These findings have implications for future research and policymaking decisions.
Keywords: residual blood spots, dried blood spots, newborn blood spots, newborn screening, bioethics, conjoint analysis
Introduction
Beginning in the 1960s, routine newborn screening began in the U.S.1,2 Since 1965, when it first started screening for phenylketonuria (PKU), the Indiana State Department of Health has collected newborn blood spots and now tests them for nearly four dozen conditions3,4. Blood spots are collected for each child, and the residual dried blood spots (DBS) are stored for use in the event that positive findings must be confirmed or that screening must be repeated5. Residual DBS are stored indefinitely in Indiana and may be used for quality assurance after screening, but are not released for research purposes6.
DBS are potentially useful in studies of conditions believed to be genetic or acquired in utero, studies of in utero environmental toxin exposure, and other research on biological conditions7-11. In Indiana, for example, given the state's strong electronic medical records systems, e.g., the Indiana Network for Patient Care12, DBS of Indiana-born children could be linked with their electronic medical records to identify early markers of conditions not currently screened for at birth. Moreover, using DBS is feasible and potentially cost-effective compared to collecting fresh blood specimens10.
Many states in the U.S. do not explicitly regulate the use of DBS for research purposes, and state policies surrounding the retention and use of DBS vary greatly13. Litigation focusing on DBS research use in the U.S. has varied in outcome. For example, Texas now has an opt-in policy where parents can both disallow their child's DBS for use in research and insist that the specimens be destroyed (specifically pertaining to spots collected on or after June 1, 2012)14,15. Researchers have long investigated attitudes towards children's participation in a variety of research16-18, and, more specifically, the use of pediatric biospecimens in research19,20. Other work has focused on attitudes towards using DBS in research21-25. Consistently-emerging concerns of respondents in previous research involving use of biomaterial include: anonymity/identifiability of the person whose material it is; the researcher using the biospecimen; and whether and how frequently parent consent is sought, among other factors21-25.
Policymakers have expressed a need for information concerning parental attitudes surrounding the research use of DBS in order to formulate acceptable policies governing their use26. However, little is known about the relative weight that parents place on the factors affecting their attitudes. Here we address the relative importance of three key factors in driving parental attitudes, providing needed information for the development of state policy and institutional research practices.
Our hypothesis was that parents differently weight concerns about the use of their children's DBS. These concerns included: 1) whether consent is sought, 2) the affiliation of the researcher using the DBS, and 3) whether their children's identity is linked to the DBS. We chose these factors based on existing work21-25 and hypothesized that the issue of consent was likely to emerge as the most significant concern.
Methods
Survey Development
We employed conjoint analysis, a multivariate technique commonly used in marketing research and increasingly in health-related research, including work on attitudes about biobanking27. Conjoint analysis is often used to determine respondent preferences when multiple features or attributes impact a decision28-30. We chose a full-profile, ratings-based design. The survey items were generated using SPSS 17®31.
A full-factorial, within-subjects, repeated measures design produces 12 scenarios (3 levels of consent X 2 levels of identity linkage X 2 levels of who is conducting research). However, we were interested in conceptualizing respondents' ratings as relative preferences, and the conjoint analysis approach aligns with this objective. This approach produced eight experimental survey items. We then strengthened the design by including four additional holdout items. Participants' responses to these holdout items are used as a conjoint analysis model validation check. This resulted in a total of 12 orthogonal survey items. We included a 13th duplicate item as an index of intra-respondent reliability as has been done by other investigators 32.
The paper-based survey's 13 hypothetical scenarios followed the same pattern of information delivery: 1) who (academicians or a pharmaceutical company) would be conducting the research using the DBS, 2) whether the child's identity would be linked to his/her DBS, and 3) whether the parent's consent would be sought once for all research (one-time, blanket consent), every time the child's DBS would be used for research, or not at all. Respondents were asked to rate each of the scenarios via an 11-point semantic differential scale, anchored at “Completely Unacceptable” (0) and “Completely Acceptable” (100). There were five different survey versions with the order of the 13 scenarios randomized to minimize order-effects. Figure 1 reflects survey item wording.
Figure 1.
Survey item wording. Researcher affiliation, identity linkage, and consent type were varied across the 13 scenarios (see Table 2 for listing of variable combinations reflected in each scenario).
Pilot testing of the survey with 15 English speaking adults confirmed that the survey was understandable and clear. The survey and research protocol were exempted from review by the Indiana University Institutional Review Board.
Study Population, Recruitment, and Procedure
Individuals visiting one of three urban public pediatric clinics or one private pediatric clinic in and around the Indianapolis metropolitan area were approached by a research assistant (RA) from a pediatric research network, a university research group with permission to collect data in clinics affiliated with the Indiana University School of Medicine. Recruitment occurred between June 2011 and March 2012. The IRB waived informed consent for this study. The RA first ensured respondent eligibility through brief screening questions. Eligibility criteria included English fluency, 18 years of age or older, and parent or primary caregiver to at least one child 17 years of age or younger born in the state of Indiana. The RA then asked eligible respondents to keep his or her child in mind when completing the survey. For respondents with multiple children 17 or younger born in Indiana, the RA flipped a coin to determine whether the respondent should focus on the oldest or youngest child born in Indiana as the referent child when completing the survey.
The RA explained the study and asked the respondents to complete a few questions, including ages and sexes of children, whether they recalled the referent child receiving a heelstick before leaving the hospital when the baby was born, and whether they had heard of newborn screening. The RA then provided respondents with general information about newborn screening (the “heelstick”) in Indiana, as well as the storage of the DBS. The RA then informed participants they would rate the acceptability of 13 unique scenarios, each with different information that described fictional (“what-if”) research situations that were not actually taking place, followed by some general demographic questions. The RA reiterated that the survey was anonymous and that the scenarios depicted in the survey were not real.
Respondents completed surveys in 12-15 minutes, primarily while in clinic waiting rooms before appointments. Occasionally, respondents and their children were called back for their appointments before they completed their surveys. When this occurred, respondents were instructed to return the survey to the RA when their appointments finished. Respondents received a $5 gift card for completing the survey.
An RA scanned the completed surveys. Optical character recognition software identified responses, recorded them to a database, and the RA reviewed each survey's scanned responses to ensure data accuracy.
Data Analysis
Survey responses were analyzed using conjoint analysis. Conjoint analysis produces importance scores, denoting which factors most strongly impact preferences/attitudes, and part-worth utility values, denoting respondents' preferences (here, preferences for what are acceptable research situations). Conjoint analysis also produces Pearson's R and Kendall's tau. The Pearson's R coefficient is an indicator of fit between the conjoint analysis model (i.e., predicted responses) and respondents' observed (i.e., actual) responses to the eight experimental survey items. The Kendall's tau coefficient for the four holdout items was generated; this is the coefficient denoting how well the observed (i.e., actual) responses correlate with the predicted responses as produced by the holdout items. This Kendall's tau for holdouts serves as a model validation index. We also report the correlation for the redundant survey items as a reliability check. All analyses were conducted using SPSS 17®31.
First, we report overall importance scores for the factors, which denote the impact of each factor (researcher affiliation, identity linkage, and consent) upon acceptability ratings. Specifically, these importance scores denote the percentage of respondents' preferences that are explained by the factors we included in our study, and, therefore, sum to 100 (give or take due to rounding).
Next we examined the part-worth utility values, which sum to zero for each factor. While importance scores indicate how strongly a factor drives one's preferences, part-worth utilities convey the preferences associated for the specific attribute levels within those factors (e.g., whether the respondent exhibited a preference for university researchers versus drug company researchers using the DBS).
Results
Of the 593 potential respondents approached, 521 (88%) agreed to participate. The 72 (12% of 593) non-participants were ineligible, uninterested, or indicated they did not have time. Of the 521 who agreed to participate, 506 (97%) respondents completed the survey, and 15 (3%) either did not complete it or returned a survey to the RA with missing pages.
Respondents ranged in age from 18-63 with a mean age of 32 years. The referent child about whom the survey was completed ranged in age from newborn to 17 years of age with a mean age of 5.7 years. Referent children were predominantly male (51%). Respondents were predominantly Black or African American, not Hispanic or Latino, female, and were mother to the referent child. The referent child was most likely a Medicaid recipient. Other descriptive demographic data, including political affiliation, income, and religiosity are noted in Table 1.
Table 1. Characteristics of parent respondents and the referent child.
| Mean | Min | Max | ||
|---|---|---|---|---|
| Ages | Parent Age | 32 | 18 | 63 |
| Referent Child Age | 5.7 | 0 | 17 | |
| Characteristic | N | % (of N responding) | ||
| Parent Race (N=487) | Black or African American | 298 | 61 | |
| White | 169 | 35 | ||
| Other | 20 | 4 | ||
| Parent Ethnicity (N=452) | Hispanic/Latino | 19 | 4 | |
| Not Hispanic/Latino | 433 | 96 | ||
| Referent Child's Health Care Payer Source (N=483) | Public (Medicaid) | 300 | 62 | |
| Child Covered by Parent's Policy | 172 | 36 | ||
| Other/Uninsured | 11 | 2 | ||
| Parent Income Level (N=486) | Less than $10,000 | 149 | 31 | |
| $10,000-$25,000 | 130 | 27 | ||
| $25,000-$50,000 | 88 | 18 | ||
| $50,000-$75,000 | 31 | 6 | ||
| More than $75,000 | 88 | 18 | ||
| Parent Political Affiliation (N=473) | Democrat | 250 | 53 | |
| Independent | 156 | 33 | ||
| Republican | 67 | 14 | ||
| Parent Political Views (N=468) | Very Liberal | 131 | 28 | |
| Moderate | 221 | 47 | ||
| Conservative | 116 | 25 | ||
| Does Parent Consider Self Spiritual or Religious (N=494) | Yes | 325 | 66 | |
| No | 54 | 11 | ||
| Somewhat | 115 | 23 | ||
| Respondent Relationship to Referent Child (N=501) | Mother | 419 | 84 | |
| Father | 72 | 14 | ||
| Other | 10 | 2 | ||
Over three-quarters of respondents (75%) indicated that they recalled their children receiving a heelstick when they were born. However, fewer respondents indicated that they had heard of newborn screening (60%).
We first calculated overall acceptability ratings for each of the 13 scenarios. Table 2 presents the scenarios and their overall acceptability ratings. In the scenario rated as least acceptable the researcher affiliation was a drug company, the child's identity was linked to the DBS, and consent was not sought (M = 28.6; SD = 36.4). In the scenario rated as most acceptable, the researcher affiliation was a university, the child's identity was not linked to the DBS, and consent was sought for each and every use of the DBS (M = 78; SD = 29.7). Of note, just over 2% (12/506) of respondents found research use of DBS “completely unacceptable” across all scenarios, and nearly 9% (45/506) found it “completely acceptable” across all scenarios. We also conducted an Analysis of Variance (ANOVA) to determine whether demographic characteristics impacted acceptability ratings. We did not find any statistically significant effects of respondent race, respondent ethnicity, child and respondent sex, child and respondent age, or parents' awareness of newborn screening (all Fs<3.0, all ps>.09, ns).
Table 2. Average acceptability ratings of survey scenarios.
| Scenario | Researcher Affiliation | Identity Linkage | Consent | Mean Acceptability Rating | SD |
|---|---|---|---|---|---|
| 1 | University | N | Repeated | 78 | 29.7 |
| 2* | Drug Co. | N | Repeated | 76.4 | 31.4 |
| 3* | University | Y | Repeated | 69.4 | 35.1 |
| 4 | Drug Co. | Y | Repeated | 68 | 35.7 |
| 5* | University | N | One-time | 67.4 | 36.1 |
| 6 | Drug Co. | N | One-time | 61.4 | 37.3 |
| 7 | University | Y | One-time | 59.3 | 38.2 |
| 8** | University | Y | One-time | 59 | 38.7 |
| 9* | Drug Co. | Y | One-time | 57.7 | 38.7 |
| 10 | University | N | No | 38.2 | 39.9 |
| 11 | Drug Co. | N | No | 34.4 | 39.8 |
| 12 | University | Y | No | 30.4 | 37.3 |
| 13 | Drug Co. | Y | No | 28.6 | 36.4 |
Scenario ratings were provided on a scale anchored at 0 (completely unacceptable) to 100 (completely acceptable) in increments of 10. Order presentation was randomized between participants in five different survey versions.
Denotes that scenario was a holdout item for model validation.
Scenario #8 duplicated scenario #7 as index of response reliability.
For the conjoint analysis, data for 432 out of a total of 506 respondents were usable. Of the unusable data (N=74), 45 respondents indicated all scenarios were acceptable, 12 respondents indicated all were unacceptable, 8 indicated all in the mid-range of the response scale, and 9 failed to respond to all scenarios. Conjoint analysis requires that all survey items have responses and that there be some variability among the responses (to denote preferences).
Table 3 contains the fit indices generated by the conjoint analysis. The model showed strong fit with a high Pearson's R by indicating that the predictive conjoint analysis model strongly fits the responses to the eight experimental survey items. The Kendall's tau for holdouts also suggests that the fit between the actual ratings provided by respondents on the eight experimental survey items and the predicted ratings generated by the four holdout model validation items is very strong. The duplicated survey item showed a strong correlation of .87, indicating high response reliability.
Table 3. Indices of conjoint analysis model fit.
| Fit Index | Value | Significance |
|---|---|---|
| Pearson's R for Conjoint Model | .998 | p < .001 |
| Kendall's tau for Conjoint Model | .929 | p = .001 |
| Kendall's tau for Conjoint Model Holdouts | 1.000 | p = .02 |
| Pearson's R for Duplicated Scenario Validation Check | .874 | p < .001 |
Figure 2 presents the importance scores. Consent was the most important factor in respondents' ratings of acceptability of the scenarios (average importance score of 64.9). This was followed by identity linkage (average importance score of 19.4), and lastly, the affiliation of the researcher using the DBS (average importance score of 14.6).
Figure 2.
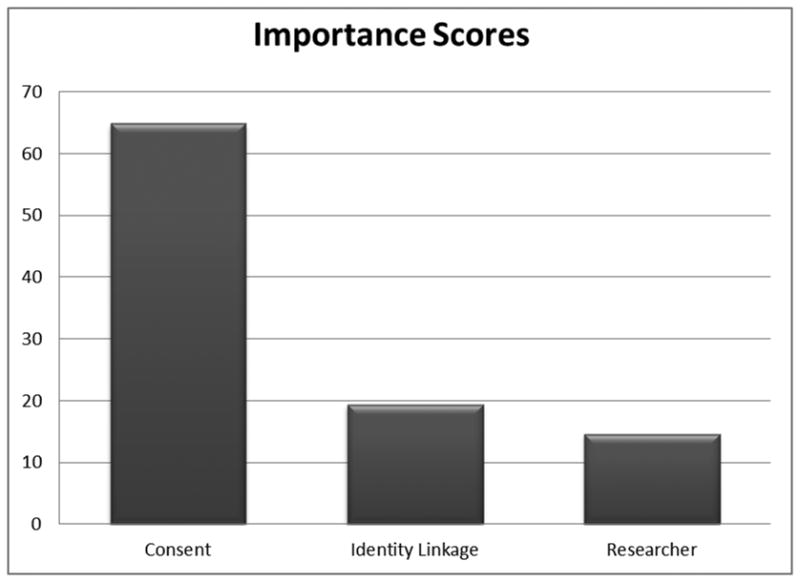
Importance scores depicting extent to which these factors accounted for scenario acceptability ratings.
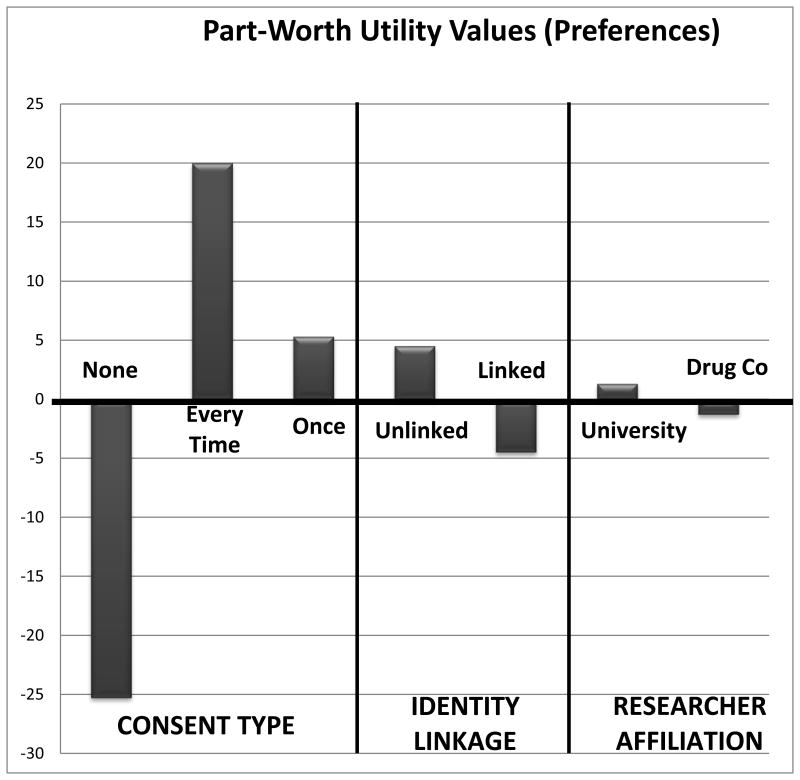
Analyses of the part-worth utility values for each of the factors' levels indicate that, focusing on the issue of consent, respondents find it unacceptable (i.e., do not prefer) when consent is not sought for research use of their child's DBS (utility = -25.3). They prefer that their consent be sought for each and every study that would use their child's DBS (utility = 20), followed by their consent being sought one time for all studies that would use their child's DBS (5.3). Regarding the issue of identity linkage, respondents prefer that their child's identity not be linked to his or her DBS (utility = 4.5), compared to when their child's identity would be linked (utility = -4.5). Finally, respondents prefer that the research using their child's DBS be conducted by university researchers (utility = 1.3), compared to researchers from a drug company (utility = -1.3). These part-worth utilities are presented in Figure 3.
Figure 3.
Part-worth utilities values conveying preferences for the attribute levels within each factor. Values below the baseline of 0 indicated non-preferred (unacceptable) attribute levels and values above the baseline of 0 indicate preferred (acceptable) attribute levels.
Discussion
This predominantly minority, low-income study sample overwhelmingly conveyed that parents do not find acceptable (i.e., do not prefer) situations in which their consent would not be sought in order to use their children's DBS in research. They preferred that their consent be sought for every use of the child's DBS. Though not as strong an influence on attitudes, respondents preferred that the child's identity not be linked to the DBS and the research be conducted by university researchers. Most respondents report that they recall their children receiving the heelstick at birth, but over a third does not know what newborn screening is. This echoes calls to educate parents about newborn screening practices33.
This work contributes to the body of literature on parent preferences concerning the research use of their child's biomaterial. Specifically, this research echoes previous work conducted in Michigan highlighting the emphasis parents place on providing their consent to research use of their children's spots21, but we have quantified the high level of importance parents place on consent relative to other concerns. This work also echoes public attitude assessments surrounding the research use of DBS, in which the opportunity to provide one's permission emerges as a critical theme in both qualitative and quantitative inquiry24,25.
Limitations and Future Directions
Although this study contributes to the understanding of parents' preferences concerning the research use of their children's biomaterial, several limitations should be noted. First, we only studied three factors that have emerged as important in previous literature (consent, identity linkage, and researcher affiliation), though many others may impact parents' attitudes surrounding the research use of DBS21-25.
Another limitation is that respondents rated the acceptability of hypothetical scenarios. ‘Real’ requests to use their children's DBS could produce different results. It is also possible that respondents, especially when completing the survey in a busy, clinic setting, may not have fully understood the information conveyed in the 13 hypothetical scenarios. No respondents in our study sample, nor any of the 15 respondents to the pilot test, indicated that they had difficulty understanding the information being conveyed in the scenarios or the general information presented by the RA regarding newborn screening or blood spot storage. We used the terms “newborn screening” and “heelstick” to gauge participants' awareness and recall of newborn screening. Some individuals, however, might be more likely to use terms like PKU or sickle cell screening. Further, some respondents' participation was interrupted by being called back for an appointment (N=26, 5% of 506), and there is the possibility that this could have imparted some response bias.
Although not demographically representative of Indiana parents, which are predominantly White34, this study sample captures individuals who could potentially be asked to provide consent about research use of their children's DBS. Moreover, the importance to parents of providing consent for DBS research use aligns with previous findings, based on data collected from demographically different respondents21,25.
Lastly, we did not collect data specifically asking about prematurity, neonatal intensive care unit admissions, or other health conditions present during infancy. Presence of health conditions could impact the likelihood of and/or parental recall of newborn screening.
Future directions could build upon this work by eliciting parental preferences for the timing and structure of the consent process. For example, do they prefer an “opt-in” or “opt-out” structure? Do they prefer to be asked at the time of the screening or prior to the baby's arrival? Moreover, future studies could focus on the situations in which identity linkage or consent waivers might be permissible, as well as parental preferences for being informed of study results involving their child's DBS.
Conclusions
Policymakers, those leading biobanking initiatives, and researchers using minors' biomaterial in their work might benefit from taking parents' preferences into consideration when developing protocols for the use of children's biomaterial, especially consent policies. Though logistic problems with seeking consent for DBS research use might seem overwhelming, such consent is not necessarily unattainable35. Public preferences are critically important for policymakers to keep in mind when developing governance policies; it is also imperative that policymakers understand that preferences can vary depending on the parameters of DBS use. For example, if the DBS are being used in research identifying treatable illnesses, perhaps parents would want their children's identities linked to their specimens so that they could be notified.
Further, democratic deliberation approaches that have been employed in biobanking-related research36,37 and involve intensive education efforts and ongoing discourse between citizens and those responsible for policymaking, might render different sentiments from parents regarding use of their children's biomaterial. However, many individuals from whom consent would be sought for DBS use would not likely be able to participate in long-term, ongoing, intensive education and dialogue about biomaterial use. This study's objective was to measure attitudes from these individuals not undergoing a democratic deliberation process.
Lastly, states that have already implemented governance policies on this topic, especially if it does not involve seeking consent from parents for use of DBS, might benefit from a re-evaluation of such policy.
What's New.
Compared to researcher affiliation and identity linkage of a child to a blood spot, consent protocol emerged as the strongest factor impacting Indiana parents' attitudes about using dried blood spots for research. This has implications for biospecimen use policy.
Acknowledgments
Funding: This work was funded in part by a Ruth L. Kirschstein National Research Service Award (NRSA) T32 #5T32HS017588-04 (KSH); the Indiana University Center for Bioethics Program in Predictive Health Ethics Research (PredictER) (KSH, EMM); UL1RR025761-01 (NIH/NCRR) (EMM); and by Children's Health Services Research.
Special thanks to Gregory Zimet, PhD, the Decision Sciences Working Group, and the Indiana University Center for Bioethics for their input on the design, analyses, and data interpretation for this study. Special thanks to the Pediatric Research Network (PResNet) for data collection and to Katherine Schwartz, JD, MPA, for manuscript feedback. The first author had full access to all of the study data and takes responsibility for the integrity of the data and accuracy of the data analysis.
Abbreviations
- PKU
Phenylketonuria
- DBS
Dried Blood Spot
- RA
Research Assistant
Footnotes
Financial Disclosures: none
Conflicts of Interest: none
References
- 1.Guthrie R, Whitney S. Health Educ Welfare. Washington, DC: US Dept; 1964. Phenylketonuria: detection in the newborn infant as a routine hospital procedure. Childrens' Bureau Publ.# 419. [Google Scholar]
- 2.Therrell B, Adams J. Newborn screening in North America. Journal of inherited metabolic disease. 2007;30(4):447–465. doi: 10.1007/s10545-007-0690-z. [DOI] [PubMed] [Google Scholar]
- 3.Indiana State Department of Health. Newborn Screening home. http://www.in.gov/isdh/20215.htm.
- 4.Indiana State Department of Health. Maternal and Children's Special Health Care Services. Genomics and Newborn Screening Program. An Introduction to Indiana's Newobrn Screening Program. www.in.gov/isdh/files/Introduction_to_Newborn_Screening.ppt.
- 5.Olney R, Moore C, Ojodu J, Lindegren M, Hannon W. Storage and use of residual dried blood spots from state newborn screening programs. The Journal of pediatrics. 2006;148(5):618–622. doi: 10.1016/j.jpeds.2005.12.053. [DOI] [PubMed] [Google Scholar]
- 6.Babies First Test. Indiana. [May 31, 2012];Genetic Alliance Funded by Health Resource and Service Administration (HRSA) Maternal and Child Health Bureau: Genetic Services Branch Quality Assessment of the Newborn Screening System. 2012 http://www.babysfirsttest.org/newborn-screening/about-site.
- 7.Nelson KB, Grether JK, Croen LA, et al. Neuropeptides and neurotrophins in neonatal blood of children with autism or mental retardation. Annals of neurology. 2001 May;49(5):597–606. [PubMed] [Google Scholar]
- 8.Burse VW, DeGuzman MR, Korver MP, et al. Preliminary investigation of the use of dried-blood spots for the assessment of in utero exposure to environmental pollutants. Biochemical and molecular medicine. 1997 Aug;61(2):236–239. doi: 10.1006/bmme.1997.2603. [DOI] [PubMed] [Google Scholar]
- 9.McDade T, Williams S, Snodgrass J. What a drop can do: Dried blood spots as a minimally invasive method for integrating biomarkers into population-based research. Demography. 2007;44(4):899–925. doi: 10.1353/dem.2007.0038. [DOI] [PubMed] [Google Scholar]
- 10.Hollegaard M, Grauholm J, Børglum A, et al. Genome-wide scans using archived neonatal dried blood spot samples. BMC genomics. 2009;10(1):297. doi: 10.1186/1471-2164-10-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hollegaard MV, Grove J, Thorsen P, Norgaard-Pedersen B, Hougaard DM. High-throughput genotyping on archived dried blood spot samples. Genetic testing and molecular biomarkers. 2009 Apr;13(2):173–179. doi: 10.1089/gtmb.2008.0073. [DOI] [PubMed] [Google Scholar]
- 12.McDonald CJ, Overhage JM, Barnes M, et al. The Indiana network for patient care: a working local health information infrastructure. An example of a working infrastructure collaboration that links data from five health systems and hundreds of millions of entries. Health affairs (Project Hope) 2005 Sep-Oct;24(5):1214–1220. doi: 10.1377/hlthaff.24.5.1214. [DOI] [PubMed] [Google Scholar]
- 13.Lewis MH, Goldenberg A, Anderson R, Rothwell E, Botkin J. State laws regarding the retention and use of residual newborn screening blood samples. Pediatrics. 2011 Apr;127(4):703–712. doi: 10.1542/peds.2010-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dobson A. The Texas Newborn Blood Spot Saga Continues. 2010 http://www.genomicslawreport.com/index.php/2010/03/01/the-texas-newborn-blood-spot-saga-continues/ Genomics Law Report.
- 15.Use of Newborn Screening Blood Spots After Completion of Newborn Screening. http://www.dshs.state.tx.us/lab/nbsBloodspotsUse.shtm.
- 16.Clausen JA, Seidenfeld MA, Deasy LC. Parent attitudes toward participation of their children in polio vaccine trials. American journal of public health and the nation's health. 1954 Dec;44(12):1526–1536. doi: 10.2105/ajph.44.12.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caldwell PH, Butow PN, Craig JC. Parents' attitudes to children's participation in randomized controlled trials. J Pediatr. 2003 May;142(5):554–559. doi: 10.1067/mpd.2003.192. [DOI] [PubMed] [Google Scholar]
- 18.Bernhardt BA, Tambor ES, Fraser G, Wissow LS, Geller G. Parents' and children's attitudes toward the enrollment of minors in genetic susceptibility research: implications for informed consent. American journal of medical genetics Part A. 2003 Feb 1;116A(4):315–323. doi: 10.1002/ajmg.a.10040. [DOI] [PubMed] [Google Scholar]
- 19.Neidich AB, Joseph JW, Ober C, Ross LF. Empirical data about women's attitudes towards a hypothetical pediatric biobank. American journal of medical genetics Part A. 2008 Feb 1;146(3):297–304. doi: 10.1002/ajmg.a.32145. [DOI] [PubMed] [Google Scholar]
- 20.Kaufman D, Geller G, Leroy L, Murphy J, Scott J, Hudson K. Ethical implications of including children in a large biobank for genetic-epidemiologic research: a qualitative study of public opinion. American journal of medical genetics Part C, Seminars in medical genetics. 2008 Feb 15;148C(1):31–39. doi: 10.1002/ajmg.c.30159. [DOI] [PubMed] [Google Scholar]
- 21.Tarini B, Goldenberg A, Singer D, Clark S, Butchart A, Davis M. Not without my Permission: Parents' Willingness to Permit Use of Newborn Screening Samples for Research. Public Health Genomics. 2009 doi: 10.1159/000228724. [DOI] [PubMed] [Google Scholar]
- 22.Fleck L, Mongoven A, Marzec S. Stored Bloodspots: Ethical and Policy Challenges. East Lansing, Michigan: Michigan State University Institute for Public Policy and Social Research. Available at: http://ippsr.msu.edu/Publications/HPFleck.pdf2009.
- 23.Fleck L, Mongoven A, Marzec S. Informing the Debate: Stored Blood Spots Ethical and Policy Challenges. The Institute for Public Policy and Social Research. 2008 [Google Scholar]
- 24.Rothwell E, Anderson R, Goldenberg A, et al. Assessing public attitudes on the retention and use of residual newborn screening blood samples: a focus group study. Social science & medicine (1982) 2012 Apr;74(8):1305–1309. doi: 10.1016/j.socscimed.2011.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Botkin JR, Rothwell E, Anderson R, et al. Public attitudes regarding the use of residual newborn screening specimens for research. Pediatrics. 2012 Feb;129(2):231–238. doi: 10.1542/peds.2011-0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Therrell BL, Jr, Hannon WH, Bailey DB, Jr, et al. Committee report: Considerations and recommendations for national guidance regarding the retention and use of residual dried blood spot specimens after newborn screening. Genetics in medicine : official journal of the American College of Medical Genetics. 2011 Jul;13(7):621–624. doi: 10.1097/GIM.0b013e3182147639. [DOI] [PubMed] [Google Scholar]
- 27.Pullman D, Etchegary H, Gallagher K, et al. Personal privacy, public benefits, and biobanks: A conjoint analysis of policy priorities and public perceptions. Genetics in medicine : official journal of the American College of Medical Genetics. 2011 Sep 26; doi: 10.1097/GIM.0b013e31822e578f. [DOI] [PubMed] [Google Scholar]
- 28.Orme B. Getting started with conjoint analysis. Research Publishers, LLC; 2009. [Google Scholar]
- 29.Orme B. Sawtooth Software Research Paper Series. Vol. 1. Sawtooth Software, Inc; 2002. Interpreting conjoint analysis data; p. 2004. Retrieved November. [Google Scholar]
- 30.SPSS. Conjoint 17.0.2008. Chicago: SPSS Inc; http://support.spss.com/ProductsExt/SPSS/ESD/17/Download/User%20Manuals/English/SPSS%20Conjoint%2017.0.pdf. [Google Scholar]
- 31.SPSS, Inc. SPSS for Windows, Release 17.0.0. Chicago: 2008. [Google Scholar]
- 32.Steele LS, Glazier RH, Lin E, Evans M. Using administrative data to measure ambulatory mental health service provision in primary care. Med Care. 2004 Oct;42(10):960–965. doi: 10.1097/00005650-200410000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Arnold CL, Davis TC, Frempong JO, et al. Assessment of newborn screening parent education materials. Pediatrics. 2006 May;117(5 Pt 2):S320–325. doi: 10.1542/peds.2005-2633L. [DOI] [PubMed] [Google Scholar]
- 34.United States Census Bureau. [March 2, 2013];State & County QuickFacts: Indiana. http://quickfacts.census.gov/qfd/states/18000.html.
- 35.Stegmayr B, Asplund K. Informed consent for genetic research on blood stored for more than a decade: a population based study. BMJ (Clinical research ed) 2002 Sep 21;325(7365):634–635. doi: 10.1136/bmj.325.7365.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molster C, Maxwell S, Youngs L, et al. Blueprint for a deliberative public forum on biobanking policy: were theoretical principles achievable in practice? Health expectations : an international journal of public participation in health care and health policy. 2011 Jun 7; doi: 10.1111/j.1369-7625.2011.00701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Doherty KC, Hawkins AK, Burgess MM. Involving citizens in the ethics of biobank research: Informing institutional policy through structured public deliberation. Social science & medicine (1982) 2012 Jul 28; doi: 10.1016/j.socscimed.2012.06.026. [DOI] [PubMed] [Google Scholar]