Abstract
Patients with treatment-resistant arterial hypertension exhibited profound reductions in single sympathetic vasoconstrictor fiber firing rates following renal nerve ablation. In contrast, integrated multi-unit muscle sympathetic nerve activity (MSNA) changed little or not at all. We hypothesized that conventional MSNA analysis may have missed single fiber discharges, thus, obscuring sympathetic inhibition following renal denervation. We studied patients with difficult to control arterial hypertension (age 45–74 years) before, 6 (n=11), and 12 months (n=8) following renal nerve ablation. Electrocardiogram, respiration, brachial, and finger arterial blood pressure (BP), as well as the MSNA raw MSNA signal were analyzed. We detected MSNA action potential spikes using 2 stage kurtosis wavelet denoising techniques to assess mean, median, and maximum spike rates for each beat-to-beat interval. Supine heart rate and systolic BP did not change at 6 (ΔHR: −2±3 bpm; ΔSBP: 2±9 mmHg) or at 12 months (ΔHR: −1±3 mmHg, ΔSBP: −1±9 mmHg) after renal nerve ablation. Mean burst frequency and mean spike frequency at baseline were 34±3 bursts per minute and 8±1 spikes per sec. Both measurements did not change at 6 months (−1.4±3.6 bursts/minute; −0.6±1.4 spikes per sec) or at 12 months (−2.5±4.0 bursts/minute; −2.0±1.6 spikes per sec) following renal nerve ablation. After renal nerve ablation, BP decreased in 3 out of 11 patients. BP and MSNA spike frequency changes were not correlated (slope=−0.06; p=0.369). Spike rate analysis of multi-unit MSNA neurograms further suggests that profound sympathetic inhibition is not a consistent finding following renal nerve ablation.
Keywords: blood pressure, arterial hypertension, renal nerve ablation, sympathetic nerve traffic
Introduction
Physiological studies suggest that signals generated in the kidney, conveyed through afferent renal nerves to the brain, promote arterial hypertension via sympathetic activation.[8] Local renal injury elicited by phenol injection produced sustained neurogenic hypertension in rats.[32, 33] Efferent muscle sympathetic nerve activity (MSNA) recordings have been indispensable in translating these findings from animals to patients. For example, removal of the diseased native kidney attenuated MSNA in renal transplant recipients.[16] Investigations in patients with resistant hypertension treated with catheter-based renal nerve ablation suggest that afferent renal nerves may also regulate MSNA and blood pressure in the absence of overt renal disease. In one patient treated with catheter-based renal nerve ablation, MSNA had decreased approximately 27% at week six and 66% at month 12 following the intervention.[26] However, subsequent studies showed no or much smaller MSNA changes following catheter-based renal nerve ablation.[5, 12, 18, 31] All these investigations recorded multiple efferent sympathetic nerve fibers analyzing the rectified and integrated nerve signal. In contrast, MSNA single fiber discharges massively decreased following catheter-based renal nerve ablation.[17] Possibly, integrated multi-fiber MSNA assessment obscured important information contained in MSNA raw signals, particularly discharges occurring outside regular bursts. Given that each action potential is coupled to quantal norepinephrine release, we reasoned that changes in single fiber activity would be of limited physiological relevance unless the total number of discharges is altered profoundly. We applied novel wavelet-based methodology [6, 10] to test the hypothesis that renal nerve ablation substantially reduces the number of discharges in the MSNA raw signal in patients with drug resistant hypertension. Because clinical trials over up to two years had suggested a delayed clinical response, we conducted measurements at about 6 and 12 months.
Methods
Patients
We studied 12 patients (11 men and 1 woman) with difficult to control arterial hypertension referred for catheter based renal nerve ablation into an open clinical trial (NCT01355055). Patients had to have uncontrolled essential hypertension despite treatment with ≥3 antihypertensive medications at full doses, including a diuretic. The mean number of antihypertensive drugs was 7±2. Patient characteristics were published earlier[5] together with the results of integrated MSNA analysis after 6 months following renal nerve ablation. Eight patients agreed to undergo microneurography again during follow up about 12 months following renal nerve ablation. All patients were judged to be compliant by their treating physician. In addition, we checked compliance with the antihypertensive drug regimen through phone interviews before each study visit. Medication and body mass index kept constant during the study. Participants were excluded if they had secondary hypertension. The local research internal review board (IRB) ethics committees approved the study. Written informed consent was obtained.
Catheter-based renal nerve ablation
The right femoral artery was punctured (Seldinger technique) after local anesthesia and a 6-French sheath was placed. Four to eight radiofrequency applications along both main renal arteries were applied (max. 8 Watts, max. 75°C for 2 min.) using the Symplicity Catheter System (Ardian, CA, USA) as described previously.[5] Operators were all well experienced in the technique and each has performed >12 such procedures in patients, according to published guidelines.
Cardiovascular measurements
The study protocol at 12 months after renal nerve ablation was exactly the same as 6 months after renal nerve ablation.[5] During testing, patients remained in the supine position. An ECG was continuously recorded (Niccomo, Medis GmbH, Germany). Noninvasive finger blood pressure recording was used (Finometer, Finapres Medical Systems, NL) and adjusted against brachial oscillometric blood pressure measurements (Dinamap, Critikon, USA).
Microneurography
A unipolar tungsten electrode with uninsulated tip diameter 1 to 5 µm and shaft diameter 200 µm (Frederick Haer and Co, Bowdoinham, MA, USA) was inserted into the muscle nerve fascicles of the peroneal nerve at the fibular head for multi-unit recordings. Satisfactory recordings of muscle sympathetic nerve activity were defined by (1) heart pulse synchronicity; (2) facilitation during Valsalva straining and suppression during the hypertensive overshoot after release; (3) increases in response to breath-holding; and (4) no change during tactile or auditory stimulation. Raw nerve activity was amplified with a total gain of 100 000, band pass filtered from 0.7 to 2 kHz (662C-3 Nerve Traffic Analysis System, University of Iowa, Iowa City, USA) and recorded at 5000 Hz, 14 bit resolution using the Windaq data acquisition system (DI-720, DATAQ Instruments, Akron OH). The lag nerve signal of about −1.3 sec was corrected before further processing.
Spike Detection with Kurtosis and Stationary Wavelet Transform
Action potential spikes were detected in the raw neurogram recordings using two-stage kurtosis wavelet denoising [6, 7, 9, 10]. This technique applies translation invariant stationary wavelet transform (SWT) and mother wavelet Symlet 7, which has been shown to improve sympathetic spike detection [6, 7]. The raw neurogram was decomposed into 3 bands of wavelet coefficients. Levels 2 and 3 were included for further analysis since these levels have the most dynamic response to the sympathetic activation (Figure 1A). Local Kurtosis was calculated for each level of activation (Figure 1B). A kurtosis value of around 3 indicates an ideal Gaussian distribution. Signal episodes with spike activity have usually higher kurtosis values. We extracted regions dominated by normally distributed noise identified as section with kurtosis level less than 4 (Figure 1C). Then we calculated an amplitude threshold from all coefficients with an absolute value less than 4 times the standard deviation of the identified noise regions (threshold T2 and T3 for levels 2 and 3 respectively). The thresholds were applied and values were set to zero at each wavelet decomposition level (Figure 1D). The de-noised signal was reconstructed using the inverse SWT (Figure 1E). Action potentials were detected in the denoised MSNA signal using a simple peak detection scheme that locates maxima above 99% of the signal energy in a 3 milliseconds time-window [9]. The binary spike train was converted to a spike rate series by convolving with a Gaussian filter with a 3 Hz cutoff frequency [29]. The cutoff frequency of 3 Hz was selected to ensure to include cardiogenic pulsating component of maximal 180 beats per minute. The resultant instantaneous spike rate series was linearly interpolated to the original sample frequency. Finally, spike frequency parameters for each heart beat were estimated using mean, median, and maximum spike frequency for each beat to beat interval and smoothed with a sliding median filter over three heart beats.
Figure 1.
Illustration of the spike detection method. Two stage kurtosis wavelet denoising technique for single spike detection. SWT= stationary wavelet transform, ISWT= inverse stationary wavelet transform
MSNA recorded during pharmacological sympathetic inhibition with clonidine infusion (maximum 2 µg/kg) before renal nerve ablation in five patients served as positive control intervention for single spike frequency analysis.
Statistical analysis
Data are expressed as mean values ± SEM. One-way ANOVA for repeated measures was used to test for differences. The post test was computed only if the overall p was <0.05 (Dunnett post hoc test, GraphPad-Prizm 5.0). The relationship between changes in mean spike frequency and arterial pressure as well as between burst frequency and mean spike frequency was assessed by linear regression analysis. A value for p<0.05 was considered significant. This study is registered with ClinicalTrials.gov, number NCT01355055.
Results
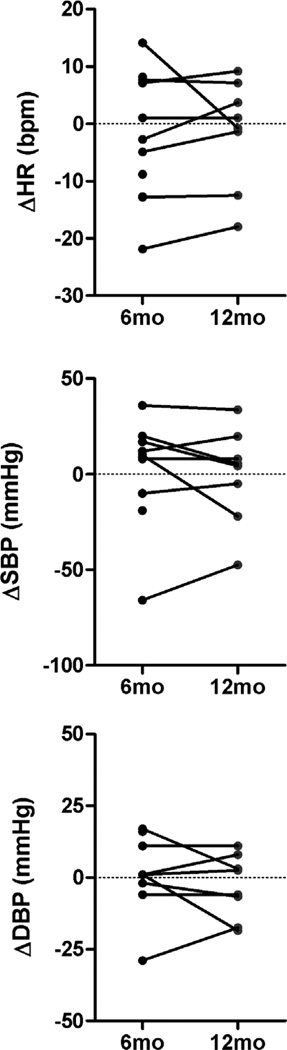
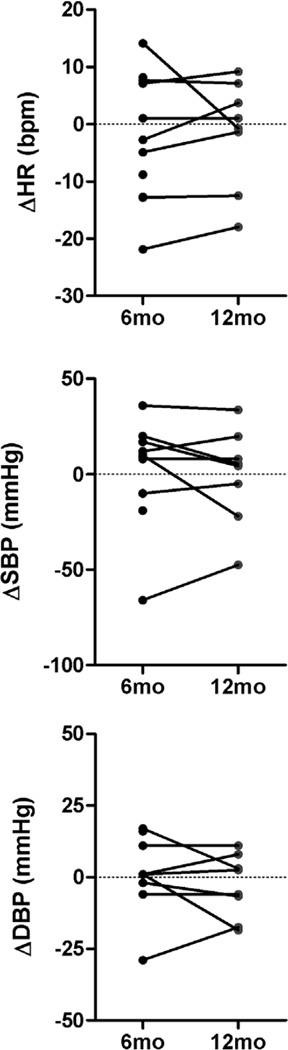
Supine heart rate and systolic BP did not change at 6 months (ΔHR: −2±3 bpm; ΔSBP: 2±9 mmHg) or at 12 months (ΔHR: −1±3 mmHg, ΔSBP: −1±9 mmHg) following renal nerve ablation. Absolute group mean values are presented in table 1. Individual changes in supine blood pressure and heart rate data obtained 6 months and 12 months following catheter-based renal nerve ablation (Figure 2) illustrate large inter individual variability. Supine blood pressure decreased in 3 out of 11 patients only.
Table 1.
Group mean values of heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP), and muscle sympathetic nerve activity (MSNA) before (baseline), 6 months, and 12 months after renal nerve ablation
| Parameter | Baseline | 6 Months | 12 Months |
|---|---|---|---|
| N | 11 | 11 | 8 |
| HR (bpm) | 62 ± 11 | 60 ± 9 | 60 ± 11 |
| SBP (mm Hg) | 156 ± 27 | 157 ± 22 | 157 ± 23 |
| DBP (mm Hg) | 86 ± 12 | 85 ± 12 | 82 ± 6 |
| MSNAf (bursts/minute) | 34 ± 8 | 32 ± 11 | 33 ± 14 |
| MSNAi (bursts/100 beats) | 57 ± 16 | 55 ± 17 | 55 ± 20 |
| Mean spike frequency (spikes/second) | 8 ± 3 | 7 ± 3 | 10 ± 5 |
| Median spike frequency (spikes/second) | 5 ± 4 | 4 ± 3 | 6 ± 4 |
| Maximum spike frequency (spikes/second) | 27 ± 10 | 25 ± 10 | 30 ± 9 |
N, number of patients; MSNAf, burst frequency; MSNAi, burstincidence.
Figure 2.
Individual changes in supine blood pressure and heart rate data obtained 6 months and 12 months following catheter-based renal nerve ablation compared to baseline measurements.
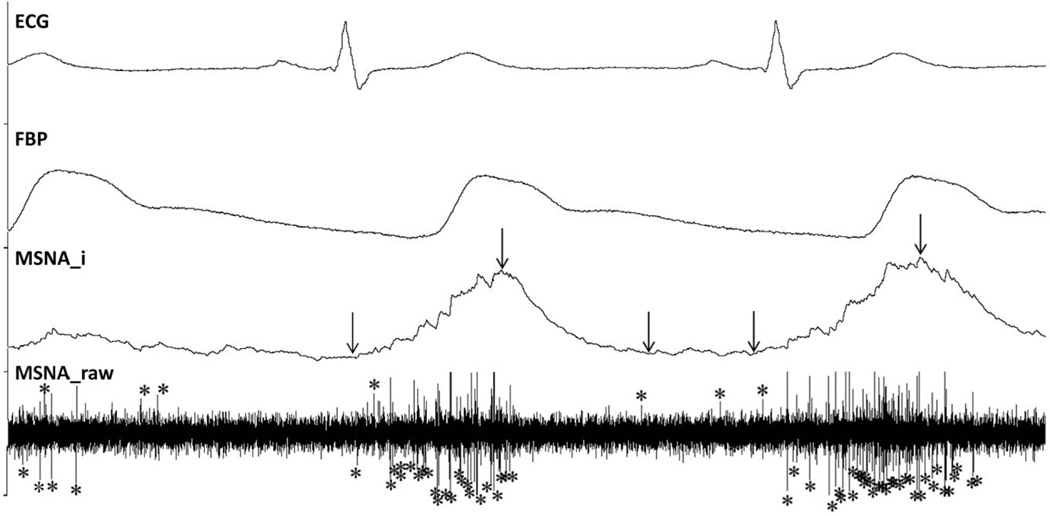
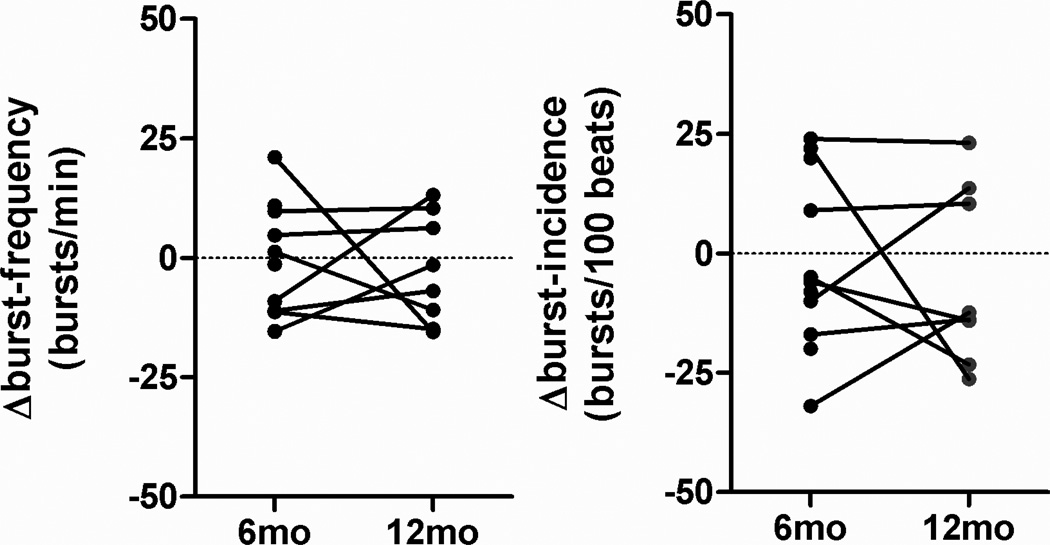
Figure 3 shows representative integrated and raw MSNA tracings. The signal to noise ratio of the raw nerve signal is sufficiently high such that single MSNA spikes can be visually discriminated. Several MSNA spikes occurred outside discernible bursts in the integrated microneurogram and are, therefore, not considered using conventional MSNA analysis techniques. Individual changes in burst frequency and burst incidence calculated from the integrated MSNA signal 6 months and at 12 months following renal nerve ablation are illustrated in figure 4. Both measurements changed little or not at all at 6 months (−1.4±4.3 bursts/minute; −2.1±5.6 bursts per 100 beats) and at 12 months (−2.54±4.0 bursts/minute; −5.3±6.5 bursts per 100 beats) following renal nerve ablation.
Figure 3.
Original tracings of ECG, finger blood pressure (FBP), integrated (MSNA_i) and raw multi-fiber MSNA signals. The signal to noise ratio in the raw nerve signal is sufficiently high such that single sympathetic spikes can be discriminated. Several MSNA spikes occurred outside discernible bursts in the integrated MSNA signal. Arrows mark the beginning, maximum and the end of a burst in the integrated nerve signal. Stars mark single spikes.
Figure 4.
Individual changes in burst frequency and burst incidence calculated from the integrated MSNA signal at 6 months and at 12 months following renal nerve ablation compared to baseline measurements.
Similarly to a previous study [12], we divided patients into groups with MSNA incidence changes above and below the median value of −6 bursts per 100 heart beats at six months. In the group above the median, integrated MSNA changed 14±5.4 bursts/100 bpm and SBP changed −14.5±18 mm Hg (n=5). In the group below the median, integrated MSNA changed −15.5±4.0 bursts/100 bpm and SBP changed 12.5±7.4 mm Hg (n=6).
Individual changes in mean, median and maximum beat to beat MSNA spike frequency 6 months and at 12 months following renal nerve ablation are shown in figure 5. Mean spike frequency did not change significantly at 6 (−0.6±1.6 spikes/sec) and at 12 months (−2.0±1.6 spikes/sec) after the procedure. A reduction in beat-to-beat mean MSNA spike frequency occurred in a subset of patients. Absolute group mean values of MSNA measurements are summarized in table 1.
Figure 5.
Individual changes in mean, median and maximum beat to beat spike frequency calculated directly from the MSNA neurogram at 6 months and at 12 months after renal nerve ablation compared to baseline measurements.
MSNA spike frequency decreased from 7±1 spikes/sec before to 4±1 spikes/sec during clonidine infusion in 4 patients, which served as a positive control intervention.
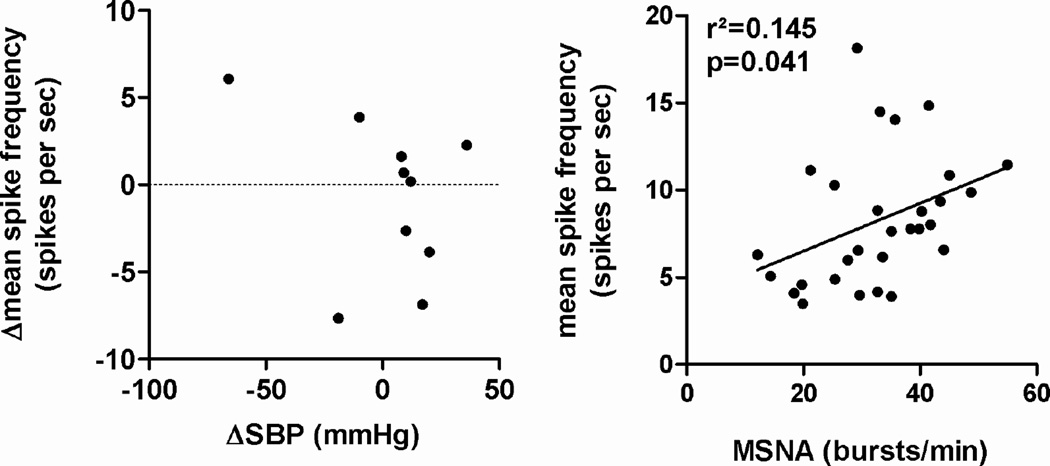
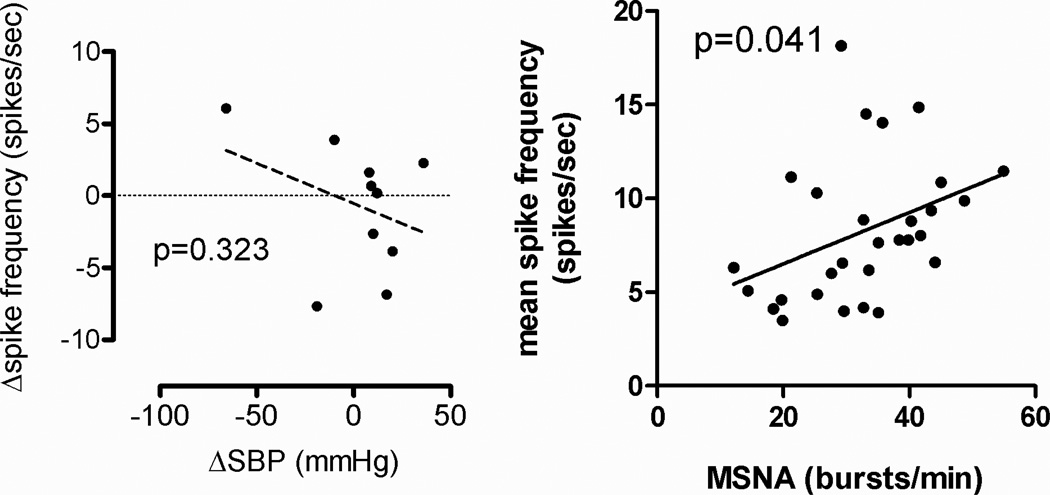
Reductions in supine BP following renal nerve ablation were not associated with reductions in MSNA spike rate (slope=−0.06; p=0.323, figure 6, left). MSNA burst frequency per minute and beat-to-beat mean spike frequency for the pooled measurements showed a positive linear relationship (p=0.041, figure 6, right).
Figure 6.
Linear regression analysis between changes in supine systolic blood pressure and changes in mean beat to beat spike frequency at 6 months after renal denervation (left). Linear regression analysis between MSNA burst frequency and mean beat to beat spike frequency based on the pooled data from baseline, 6, and 12 months after renal nerve ablation (right).
Discussion
The main finding of our study is that profound reductions in spike rate in the MSNA raw signal are not a consistent response 6 or 12 months following catheter-based renal nerve ablation. Moreover, we observed a strong correlation between MSNA spike rate and integrated MSNA measurements. Similarly to the present analysis, studies assessing integrated MSNA observed modest or no changes following catheter-based renal nerve ablation.[5, 18, 31] Finally, changes in blood pressure and integrated MSNA [5, 31] or MSNA spike rate were not related to each other.
Microneurography is commonly considered the best methodology to assess efferent sympathetic vasoconstrictor activity in human beings. Yet, the methodology is far from being perfect. Commonly, MSNA is recorded in nerve fascicles of the peroneal nerve. The assumption that MSNA measurements are qualitatively and quantitatively representative for systemic sympathetic responses may not always be true. Nevertheless, microneurography has been an important tool in clinical research. Indeed, integrated MSNA carries prognostic information in heart failure patients [1] and predicts cardiovascular organ damage [21]. Moreover, integrated MSNA has been applied to elucidate human physiology and pathophysiology [27] and to study cardiovascular autonomic responses to pharmacological [30] and non-pharmacological treatments [13, 19]. Overall, integrated multi-fiber MSNA is better scientifically and clinically validated than other ways to assess MSNA. The fact that catheter-based renal nerve ablation did not elicit relevant reductions in integrated MSNA in several studies cannot be discounted.
Integrated multi-fiber MSNA recordings may not capture all the information contained in the raw signal. Over the years, scientists interested in the physiology of human sympathetic control developed new ways to record and to analyze such signals. Single fiber MSNA have been useful in assessing discharge characteristics of individual sympathetic vasoconstrictor fibers.[15] Using this technique baroreceptor influences were shown to affect firing probability, onset latency, and number of spikes in a burst of individual vasoconstrictor fibers.[23] Single fiber MSNA recordings also showed distinct abnormalities in patients with heart failure and with respiratory diseases [11, 22, 24], thus, providing insight in the mechanisms raising sympathetic activity in these patients. Single fiber MSNA recordings also suggested that abnormalities in sympathetic control may precede overt increases in integrated multifiber MSNA in arterial hypertension.[14] The observation that single fiber MSNA firing rates decreased 37% while integrated multifiber MSNA burst incidence decreased only 8% following catheter-based renal nerve ablation is thought provoking.[17]
Dissociation between integrated and single fiber activity following renal nerve ablation [17] could indicate that there is, as suggested by the authors, a substantial reduction in sympathetic activity. Perhaps, integrated multifiber MSNA analysis does not capture all single fiber discharges. Figure 2 suggests that this idea is not completely off the mark. Another possible explanation is that while the discharge pattern of individual vasoconstrictor fibers is altered, overall sympathetic activity (i.e., total number of discharges in a vascular bed) remains unchanged. Wavelet-based analysis of high-fidelity MSNA raw signals is an excellent approach to address these mechanisms.[6, 10] The methodology counts the number of single fiber discharges (spikes) in MSNA raw signals independently of synchronized burst activity. The methodology was shown to reliably detect changes in sympathetic activity elicited by baroreflex and chemoreflex mechanisms [4, 25] or diseases, such as heart failure[34]. In the present study, we observed no consistent reduction in wavelet-based MSNA measurements following catheter-based renal nerve ablation. The observation further strengthens the hypothesis that catheter-based renal nerve ablation does not result in a consistent and substantial reduction in sympathetic nerve traffic. Our study does not exclude that the firing pattern of individual fibers is altered which could, conceivably, affect coupling between electrical nerve activity and neurotransmitter release.[28] However, the number of action potentials travelling along sympathetic vasoconstrictor fibers determines quantal norepinephrine release. We suggest that under physiological conditions, total number of sympathetic fiber discharges may be clinically more important than individual firing characteristics.
The main weakness of our study – and of current applied approaches for renal nerve ablation in general - is lack of a clinical readout for successful renal nerve ablation. In earlier studies, the norepinephrine-spillover technique refined by Esler et al. was used in patients undergoing renal nerve ablation.[20] This method is based on the use of radioisotopes, a technique that for various reasons we were not able to employ. Our measurements and concomitant norepinephrine-spillover studies should be done if possible; however, we did not have this option. Another important limitation is the relatively small sample size of our study. We cannot exclude subtle MSNA changes. Yet, we are confident that the previously reported massive changes in single fiber discharge [17] do not translate into equally large changes in efferent sympathetic vasomotor traffic. A recent MSNA study with more frequent measurements following renal nerve ablation at 15 days, 1 month, 3 months and 6 did not detect a temporal, qualitative, or quantitative relationship between BP and MSNA responses.[12] Indeed, blood pressure reduction preceded reductions in MSNA. [12]
Provided that the renal nerve ablation procedure actually led to renal denervation, these findings challenge the idea that renal afferent traffic tonically augments centrally generated sympathetic activity in the absence of overt renal disease. It is also possible that catheter based renal nerve ablation is not always sufficient to interrupt renal afferent transmission. Moreover, renal afferent and efferent innervation may regrow [3]. Catheter-based renal nerve ablation did not lower blood pressure in SYMPLICITY HTN3 [2] compared to rigorous controls. The observation damped the enthusiasm for the procedure among hypertension specialists. Nevertheless, the experimental evidence that renal innervation contributes to arterial hypertension is strong. Instead of abandoning the concept of catheter-based renal nerve ablation, the scientific community should seek a better understanding of how the procedure actually works. Patients who are more or less likely to respond should be identified. Meanwhile, devices should be improved and methods to monitor nerve ablation success should be developed. Clearly, this and previous studies suggests that current methodologies are not sufficient to lower systemic sympathetic activity.[5, 18, 31] Before we know more, catheter-based renal nerve ablation should not be applied outside clinical studies.
Acknowledgments
We are grateful to Bahlmann E. and Groen I. for technical assistance.
Sources of Funding:
The Deutsche Forschungsgemeinschaft supported Jordan J. and Brinkmann J. (JO 284/6-1). The German Space Agency supported Tank J. and Heusser K. (50WB1117). The National Institutes of Health (NIH) Autonomic Cardiovascular Regulation supported Diedrich A (P01 HL56693).
Abbreviations
- HR
heart rate
- BP
blood pressure
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- MSNA
muscle sympathetic nerve activity
- SWT
stationary wavelet transform
Footnotes
Conflicts of Interest: None to be declared.
References
- 1.Barretto AC, Santos AC, Munhoz R, Rondon MU, Franco FG, Trombetta IC, et al. Increased muscle sympathetic nerve activity predicts mortality in heart failure patients. Int J Cardiol. 2009;135(3):302–307. doi: 10.1016/j.ijcard.2008.03.056. [DOI] [PubMed] [Google Scholar]
- 2.Bhatt DL, Kandzari DE, O'Neill WW, D'Agostino R, Flack JM, Katzen BT, et al. A Controlled Trial of Renal Denervation for Resistant Hypertension. N Engl J Med. 2014 doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- 3.Booth LC, Nishi EE, Yao ST, Ramchandra R, Lambert GW, Schlaich MP, et al. Reinnervation of Renal Afferent and Efferent Nerves at 5.5 and 11 Months After Catheter-Based Radiofrequency Renal Denervation In Sheep. Hypertension. 2014 doi: 10.1161/HYPERTENSIONAHA.114.04176. [DOI] [PubMed] [Google Scholar]
- 4.Breskovic T, Steinback CD, Salmanpour A, Shoemaker JK, Dujic Z. Recruitment pattern of sympathetic neurons during breath-holding at different lung volumes in apnea divers and controls. Auton Neurosci. 2011;164(1–2):74–81. doi: 10.1016/j.autneu.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Brinkmann J, Heusser K, Schmidt BM, Menne J, Klein G, Bauersachs J, et al. Catheter-based renal nerve ablation and centrally generated sympathetic activity in difficult-to-control hypertensive patients: prospective case series. Hypertension. 2012;60(6):1485–1490. doi: 10.1161/HYPERTENSIONAHA.112.201186. [DOI] [PubMed] [Google Scholar]
- 6.Brychta RJ, Shiavi R, Robertson D, Diedrich A. Spike detection in human muscle sympathetic nerve activity using the kurtosis of stationary wavelet transform coefficients. J Neurosci Methods. 2007;160(2):359–367. doi: 10.1016/j.jneumeth.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brychta RJ, Tuntrakool S, Appalsamy M, Keller NR, Robertson D, Shiavi RG, et al. Wavelet methods for spike detection in mouse renal sympathetic nerve activity. IEEE Trans Biomed Eng. 2007;54(1):82–93. doi: 10.1109/TBME.2006.883830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dibona GF. Neural control of the kidney: past, present, and future. Hypertension. 2003;41(3 Pt 2):621–624. doi: 10.1161/01.HYP.0000047205.52509.8A. [DOI] [PubMed] [Google Scholar]
- 9.Diedrich A, Charoensuk W, Brychta RJ, Ertl AC, Shiavi R. Analysis of raw microneurographic recordings based on wavelet de-noising technique and classification algorithm: wavelet analysis in microneurography. IEEE Trans Biomed Eng. 2003;50(1):41–50. doi: 10.1109/TBME.2002.807323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diedrich A, Charoensuk W, Brychta RJ, Ertl AC, Shiavi R. Analysis of raw microneurographic recordings based on wavelet de-noising technique and classification algorithm: wavelet analysis in microneurography. IEEE Trans Biomed Eng. 2003;50(1):41–50. doi: 10.1109/TBME.2002.807323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elam M, McKenzie D, Macefield V. Mechanisms of sympathoexcitation: single-unit analysis of muscle vasoconstrictor neurons in awake OSAS subjects. J Appl Physiol (1985) 2002;93(1):297–303. doi: 10.1152/japplphysiol.00899.2001. [DOI] [PubMed] [Google Scholar]
- 12.Grassi G, Seravalle G, Brambilla G, Trabattoni D, Cuspidi C, Corso R, et al. Blood pressure responses to renal denervation precede and are independent of the sympathetic and baroreflex effects. Hypertension. 2015;65(6):1209–1216. doi: 10.1161/HYPERTENSIONAHA.114.04823. [DOI] [PubMed] [Google Scholar]
- 13.Grassi G, Seravalle G, Colombo M, Bolla G, Cattaneo BM, Cavagnini F, et al. Body weight reduction, sympathetic nerve traffic, and arterial baroreflex in obese normotensive humans. Circulation. 1998;97(20):2037–2042. doi: 10.1161/01.cir.97.20.2037. [DOI] [PubMed] [Google Scholar]
- 14.Greenwood JP, Stoker JB, Mary DA. Single-unit sympathetic discharge : quantitative assessment in human hypertensive disease. Circulation. 1999;100(12):1305–1310. doi: 10.1161/01.cir.100.12.1305. [DOI] [PubMed] [Google Scholar]
- 15.Hallin RG, Torebjork HE. Single unit sympathetic activity in human skin nerves during rest and various manoeuvres. Acta Physiol Scand. 1974;92(3):303–317. doi: 10.1111/j.1748-1716.1974.tb05749.x. [DOI] [PubMed] [Google Scholar]
- 16.Hausberg M, Kosch M, Harmelink P, Barenbrock M, Hohage H, Kisters K, et al. Sympathetic nerve activity in end-stage renal disease. Circulation. 2002;106(15):1974–1979. doi: 10.1161/01.cir.0000034043.16664.96. [DOI] [PubMed] [Google Scholar]
- 17.Hering D, Lambert EA, Marusic P, Walton AS, Krum H, Lambert GW, et al. Substantial reduction in single sympathetic nerve firing after renal denervation in patients with resistant hypertension. Hypertension. 2013;61(2):457–464. doi: 10.1161/HYPERTENSIONAHA.111.00194. [DOI] [PubMed] [Google Scholar]
- 18.Hering D, Marusic P, Walton AS, Lambert EA, Krum H, Narkiewicz K, et al. Sustained sympathetic and blood pressure reduction 1 year after renal denervation in patients with resistant hypertension. Hypertension. 2014;64(1):118–124. doi: 10.1161/HYPERTENSIONAHA.113.03098. [DOI] [PubMed] [Google Scholar]
- 19.Heusser K, Tank J, Engeli S, Diedrich A, Menne J, Eckert S, et al. Carotid baroreceptor stimulation, sympathetic activity, baroreflex function, and blood pressure in hypertensive patients. Hypertension. 2010;55(3):619–626. doi: 10.1161/HYPERTENSIONAHA.109.140665. [DOI] [PubMed] [Google Scholar]
- 20.Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373(9671):1275–1281. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 21.Lambert E, Sari CI, Dawood T, Nguyen J, McGrane M, Eikelis N, et al. Sympathetic nervous system activity is associated with obesity-induced subclinical organ damage in young adults. Hypertension. 2010;56(3):351–358. doi: 10.1161/HYPERTENSIONAHA.110.155663. [DOI] [PubMed] [Google Scholar]
- 22.Macefield VG, Rundqvist B, Sverrisdottir YB, Wallin BG, Elam M. Firing properties of single muscle vasoconstrictor neurons in the sympathoexcitation associated with congestive heart failure. Circulation. 1999;100(16):1708–1713. doi: 10.1161/01.cir.100.16.1708. [DOI] [PubMed] [Google Scholar]
- 23.Macefield VG, Wallin BG, Vallbo AB. The discharge behaviour of single vasoconstrictor motoneurones in human muscle nerves. J Physiol. 1994;481(Pt 3):799–809. doi: 10.1113/jphysiol.1994.sp020482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Millar PJ, Murai H, Floras JS. Paradoxical muscle sympathetic reflex activation in human heart failure. Circulation. 2015;131(5):459–468. doi: 10.1161/CIRCULATIONAHA.114.010765. [DOI] [PubMed] [Google Scholar]
- 25.Salmanpour A, Shoemaker JK. Baroreflex mechanisms regulating the occurrence of neural spikes in human muscle sympathetic nerve activity. J Neurophysiol. 2012;107(12):3409–3416. doi: 10.1152/jn.00925.2011. [DOI] [PubMed] [Google Scholar]
- 26.Schlaich MP, Sobotka PA, Krum H, Lambert E, Esler MD. Renal sympathetic-nerve ablation for uncontrolled hypertension. N Engl J Med. 2009;361(9):932–934. doi: 10.1056/NEJMc0904179. [DOI] [PubMed] [Google Scholar]
- 27.Schobel HP, Fischer T, Heuszer K, Geiger H, Schmieder RE. Preeclampsia -- a state of sympathetic overactivity. N Engl J Med. 1996;335(20):1480–1485. doi: 10.1056/NEJM199611143352002. [DOI] [PubMed] [Google Scholar]
- 28.Sjoblom-Widfeldt N, Nilsson H. Sympathetic transmission in small mesenteric arteries from the rat: influence of impulse pattern. Acta Physiol Scand. 1990;138(4):523–528. doi: 10.1111/j.1748-1716.1990.tb08880.x. [DOI] [PubMed] [Google Scholar]
- 29.Szucs A. Applications of the spike density function in analysis of neuronal firing patterns. J Neurosci Methods. 1998;81(1–2):159–167. doi: 10.1016/s0165-0270(98)00033-8. [DOI] [PubMed] [Google Scholar]
- 30.Tank J, Schroeder C, Diedrich A, Szczech E, Haertter S, Sharma AM, et al. Selective impairment in sympathetic vasomotor control with norepinephrine transporter inhibition. Circulation. 2003;107(23):2949–2954. doi: 10.1161/01.CIR.0000072786.99163.FE. [DOI] [PubMed] [Google Scholar]
- 31.Vink EE, Verloop WL, Siddiqi L, van Schelven LJ, Liam OP, Blankestijn PJ. The effect of percutaneous renal denervation on muscle sympathetic nerve activity in hypertensive patients. Int J Cardiol. 2014;176(1):8–12. doi: 10.1016/j.ijcard.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 32.Ye S, Gamburd M, Mozayeni P, Koss M, Campese VM. A limited renal injury may cause a permanent form of neurogenic hypertension. Am J Hypertens. 1998;11(6 Pt 1):723–728. doi: 10.1016/s0895-7061(98)00030-2. [DOI] [PubMed] [Google Scholar]
- 33.Ye S, Zhong H, Yanamadala S, Campese VM. Oxidative stress mediates the stimulation of sympathetic nerve activity in the phenol renal injury model of hypertension. Hypertension. 2006;48(2):309–315. doi: 10.1161/01.HYP.0000231307.69761.2e. [DOI] [PubMed] [Google Scholar]
- 34.Zubin MP, Breskovic T, Shoemaker JK, Olson TP, Johnson BD, Eterovic D, et al. Firing patterns of muscle sympathetic neurons during short-term use of continuous positive airway pressure in healthy subjects and in chronic heart failure patients. Respir Physiol Neurobiol. 2013;187(2):149–156. doi: 10.1016/j.resp.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]