Summary
Objective
Assess cognitive effects of adjunctive perampanel in adolescents.
Methods
In this double‐blind study (ClinicalTrials.gov identifier: NCT01161524), patients aged 12 to <18 years with partial‐onset seizures despite receiving 1–3 antiepileptic drugs were randomized (2:1) to perampanel or placebo. Perampanel was increased weekly in 2‐mg increments to 8–12 mg/day (6‐week titration; 13‐week maintenance). Changes in neuropsychological outcomes were assessed at end of maintenance: Cognitive Drug Research (CDR) System Global Cognition Score (primary end point), five CDR System domain T‐scores (secondary end points), letter fluency, category fluency, and Lafayette Grooved Pegboard Test (LGPT).
Results
One hundred thirty‐three patients were randomized. In the full analysis set, there were no differences of perampanel (n = 79) vs. placebo (n = 44) in CDR System Global Cognition Score (least squares mean change, −0.6 vs. 1.6; p = 0.145), Quality of Working Memory (1.1 vs. 2.0; p = 0.579), or Power of Attention (−6.9 vs. −2.7; p = 0.219). There were small differences with perampanel vs. placebo in other CDR System domains: improvements in Quality of Episodic Memory (3.0 vs. −1.2; p = 0.012), and worsening in Continuity of Attention (−3.3 vs. 1.6; p = 0.013) and Speed of Memory (0.3 vs. 7.0; p = 0.032). Letter fluency, category fluency, and LGPT were not significantly different between groups. The most frequent adverse events with perampanel were dizziness (30.6%) and somnolence (15.3%).
Significance
Perampanel did not differ from placebo in the global cognitive score, two of five subdomains, and four other cognitive measures. Perampanel was worse on two and better on one subdomain.
Keywords: Adolescent, Antiepileptic drugs, Cognition, Partial seizures, Perampanel
Key Points.
Adolescents with inadequately controlled partial‐onset seizures (n = 133) were randomized to adjunctive perampanel or placebo under double‐blind conditions
Perampanel was increased weekly in 2 mg increments, to a target of 8–12 mg/day (6‐week titration; 13‐week maintenance)
The primary outcome was change in cognition from baseline to end of treatment, as assessed by a standardized computerized test battery
Adjunctive perampanel did not significantly differ from placebo in in the global cognitive score, two of five subdomains, and four other cognitive measures, but was worse on two and better on one subdomain
Antiepileptic drugs (AEDs) can produce adverse cognitive and behavioral side effects, potentially compounding effects of epilepsy on learning and development.1, 2, 3 Thus, neuropsychological profiles of AEDs are important considerations for treatment selection, particularly in children and adolescents.
The Cognitive Drug Research (CDR) System is a set of automated tests of cognitive function,4 available in >60 languages, and validated across several clinical populations.5, 6, 7 The CDR System has been used widely in clinical research, including studies involving children and adolescents.8, 9, 10, 11, 12, 13, 14, 15 Previously, the CDR System has demonstrated differences in the cognitive effects of carbamazepine versus remacemide in a phase III trial16: measures of attention were significantly impaired by carbamazepine, relative to remacemide, after 8 weeks of treatment in patients aged 12–75 years with newly diagnosed epilepsy16 (effects consistent with previous observations1, 3, 17, 18). Notably, prior to dosing, study patients showed impairments on the CDR System Power of Attention domain compared with healthy volunteers, with a Cohen's d effect size of 0.63 in those aged 12–17 years.15
Perampanel is an α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazole‐propionic acid (AMPA) receptor antagonist19 approved in the United States and Europe for the adjunctive treatment of partial‐onset seizures, with or without secondary generalization, and primary generalized tonic–clonic seizures in patients with epilepsy aged 12 years and older.20, 21 There have been no prior studies that have formally assessed the cognitive effects of perampanel. The present 19‐week study used the CDR System to examine the cognitive effects of adjunctive perampanel in adolescent patients with inadequately controlled partial‐onset seizures.
Methods
Standard protocol approvals, registrations, and patient consents
This trial (Eisai Inc. protocol E2007‐G000‐235; ClinicalTrials.gov identifier: NCT01161524) was conducted at 39 centers across 11 countries in North America, Europe, Asia, and Australia. The trial was performed in accordance with the Declaration of Helsinki, ICH‐E6 Guideline CPMP/ICH/135/95, European Directives 2005/28/EC and 2001/20/EC, the U.S. Code of Federal Regulations, and the Pharmaceutical Affairs Law for studies conducted in Japan. The trial protocol, amendments, and informed consent were reviewed by national regulatory authorities in each country and independent ethics committees or institutional review boards for each site. Before participation, all patients gave written assent and their legal guardian gave written informed consent.
Patients
Patients were aged 12 to <18 years, with an intelligence quotient (IQ) ≥7022 and a diagnosis of partial‐onset seizures according to the 1981 International League Against Epilepsy (ILAE) Classification of Epileptic Seizures.23 Normal interictal electroencephalography results were allowed if other ILAE criteria were met and a progressive cause of epilepsy was ruled out. Patients had at least one partial‐onset seizure during the previous 4 weeks, despite a stable regimen of 1–3 AEDs, which could include one enzyme‐inducing AED (carbamazepine or phenytoin).
Patients were excluded if they were pregnant or breastfeeding, or had the following: primary generalized‐onset epilepsy or Lennox‐Gastaut syndrome; a history of psychogenic nonepileptic seizures in the previous 5 years; a history of status epilepticus requiring hospitalization in the previous 12 months, or seizure clusters; an unstable psychiatric diagnosis or any comorbidities that could affect cognitive function; any progressive central nervous system (CNS) disease; epilepsy surgery scheduled in the following 6 months; participated in previous trials involving perampanel; multiple drug allergies or a severe reaction to AEDs; used phenobarbital, primidone, or benzodiazepines within 4 weeks of the study; felbamate within 8 weeks (unless they were receiving a stable dose and had been taking felbamate for at least 2 years, without hepatic or bone marrow dysfunction), or vigabatrin within 5 months; any history of vigabatrin‐associated clinically significant abnormalities in visual perimetry testing; used any other drugs that affected the CNS where the dose had not been stabilized for at least 4 weeks; a vagus nerve stimulator (VNS) implanted within 5 months of the study (if implanted earlier, VNS counted as one of the 1–3 permitted AEDs; stimulatory parameters must not have changed within 4 weeks of the study).
Study design
This was a randomized, double‐blind, placebo‐controlled, parallel‐group phase II study. Following a 1‐week baseline, patients were randomized (2:1) to receive once‐daily oral perampanel (Fycompa, Eisai Co. Ltd, Kawashima, Japan) or matching placebo tablets during a 6‐week titration period and 13‐week maintenance period. Patients were assigned to perampanel or placebo using a computer‐generated randomization scheme. Patients and study personnel were blinded to treatment during the randomized period.
Perampanel was started at 2 mg/day and uptitrated weekly, in 2 mg increments, to a target dose range of 8–12 mg/day. According to investigator judgment, patients experiencing intolerable adverse events could have dose reductions, with subsequent increases if tolerability improved. Patients not tolerating perampanel 2 mg/day discontinued treatment.
Patients completing the maintenance period could receive perampanel in an open‐label extension. Alternatively, patients participated in a 4‐week follow‐up period after stopping treatment.
Neuropsychological assessments
The CDR System was included in the Pediatric Investigational Plan for perampanel, which was approved by the Pediatric Committee of the European Medicines Agency. It is an automated system, with task stimuli presented on the screen of a notebook computer for patients to provide responses using a simple response box containing buttons marked “YES” and “NO.” The CDR System was selected as the primary assessment tool for this study as a means to standardize methodology, and because it had validated language versions appropriate for each of the 11 countries in which the study was run. In addition, it has been used previously to detect cognitive effects of AEDs in pediatric patients with epilepsy.15
In the present study, cognitive assessments using the CDR System were conducted at week 0 (baseline, prior to study drug administration), week 10, and the end of treatment (week 19, or last observation carried forward for nine patients who underwent a final assessment at the time of early termination). Nine tests were administered at each time point: three to assess attention, information processing, and vigilance (simple reaction time, choice reaction time, and digit vigilance), three to assess verbal episodic memory (immediate word recall, delayed word recall, and word recognition), two to assess articulatory and spatial working memory (numeric working memory and spatial working memory), and one to assess nonverbal episodic memory (picture recognition). Tests were administered by a trained individual, using standardized instructions given prior to each computerized test, and could be completed within 20 min. The CDR System has >50 parallel forms of the various tests, ensuring that different stimuli are used each time a patient performs a task with the System.4 Patients performed all tests twice during the screening period prior to the baseline visit, to ensure familiarity and help overcome training effects.24
The primary outcome was change in the CDR System Global Cognition Score from baseline to end of treatment. Key secondary end points were changes in the five core CDR System domains: (1) Power of Attention (a measure of focused attention and information processing, derived from speed scores for simple reaction time, choice reaction time, and digit vigilance); (2) Continuity of Attention (a measure of sustained attention, derived from accuracy scores for choice reaction time and digit vigilance); (3) Quality of Episodic Memory (a measure of the ability to encode, store, and retrieve verbal and nonverbal episodic information derived from accuracy scores for immediate word recall, delayed word recall, word recognition, and picture recognition); (4) Quality of Working Memory (a measure of the ability to hold numeric and spatial information in the working memory derived from sensitivity scores for numeric working memory and spatial working memory); and (5) Speed of Memory (a measure of the time needed to retrieve information from episodic and working memory derived from speed scores for numeric working memory, spatial working memory, word recognition, and picture recognition).25 Z‐scores were calculated for each domain using normative data from the CDR System database for the age range of the study population. Specifically, Z‐scores were calculated by subtracting each patient's domain score from the normative population mean of that domain and dividing the result by the standard deviation (SD) of the normative population mean. Z‐scores were converted into T‐scores by multiplying by 50 and adding 50. Power of Attention and Speed of Memory T‐scores were also multiplied by −1, so that for all domains, greater T‐scores reflected superior cognitive function. T‐scores ranged from 0 to 100, with a mean of 50 and an SD of 10. The CDR System Global Cognition Score was created by adding the T‐scores for the five domains.
Additional secondary end points included changes in language and manual dexterity from baseline to end of treatment. Language was assessed using the Controlled Oral Word Association Test letter fluency test (in which patients list as many words as they can in 1 min that start with a given letter), and a category fluency test (in which patients list as many words as they can in 1 min that relate to a given topic)26; numbers of correct items were summarized, with improvements reflected by increased scores. Manual dexterity was assessed using the Lafayette Grooved Pegboard Test (LGPT)26; time to complete the LGPT was reported for each hand, with improvements reflected by reductions in time.
Safety assessments
Throughout the study, treatment‐emergent adverse events (TEAEs), hematology, blood chemistry, urine values, and vital signs were monitored, and electrocardiography studies and physical examinations were performed. TEAEs were graded as mild (discomfort noticed, but no disruption of normal daily activity), moderate (discomfort sufficient to reduce or affect normal daily activity), or severe (incapacitating, with inability to work or to perform normal daily activity). Serious TEAEs included those that were life‐threatening, or resulted in hospitalization, persistent or significant disability/incapacity, or death.
Other assessments
Further end points relating to safety (growth and development outcomes, photosensitivity, and TEAEs of special interest), efficacy, pharmacokinetics, behavior, and quality of life will be reported separately.
Statistical analyses
The a priori primary end point was the change in the CDR System Global Cognition Score from baseline to end of treatment. Analyses of the individual CDR system domain scores are secondary end points meant to evaluate sensitivity of the primary end point. Letter fluency test, category fluency test, and LGPT are additional cognitive tests. The study is sufficiently powered to show the effect in the primary end point of Global cognitive score. All other end points are supportive. Hence no adjustment for multiplicity was planned. The other end points also offer important safety assessments; therefore, assessing these variables without multiplicity adjustment is a more conservative approach, which is appropriate for safety measures.
It was preplanned that all neuropsychological assessments would be based on the full analysis set (FAS) for cognition, which comprised all randomized patients who received study drug, had baseline cognition data, and had at least one CDR System domain assessment at or after week 10. For additional information, CDR System Global Cognition Scores are also presented for the per‐protocol population (all patients in the FAS who sufficiently complied with the protocol) and completers’ population (all randomized patients who completed the study and were assessed at week 19).
Changes in CDR System scores were analyzed by analysis of covariance (ANCOVA), with baseline score and age as covariates, and gender, treatment, and region as factors (region based on pooling of countries according to their geographic location: Europe, Asia, or North America). A two‐sided 95% confidence interval (CI) was created on the least squares (LS) mean for the difference between the placebo and perampanel groups. A difference of five T‐score units in the CDR System Global Cognition Score was prespecified as clinically meaningful. This is comparable to the effect of 0.08 g/dL alcohol (legal limit for driving in the United Kingdom and U.S.A.) on the CDR System Global Cognition Score (4.4 units), but more conservative than the effects of lorazepam 1 mg (9.4 units) or lorazepam 2 mg (17.7 units).27, 28
The power calculation identified that a sample size of 117 patients was required (with an additional 10% allowed to account for study dropouts). This provided 80% power to detect a difference of five T‐score units in CDR System Global Cognition Score between placebo and perampanel, using a between‐group t‐test at a 5% two‐sided level of significance. Based on this sample size, it was assumed that a two‐sided 95% CI for the difference in mean would extend by <3.5 units on each side from the observed difference in mean.
Changes in letter and category fluency were compared for the two treatment arms post hoc by ANCOVA, with baseline scores as covariates. Changes in time to complete the LGPT test were compared post hoc by log‐rank test.
The safety analysis set consisted of all randomized patients who received study drug and had at least one postdose safety assessment.
Results
Patient disposition, demographics, and treatment
The first patient was enrolled in September 2010, and the last patient visit occurred in June 2013. Of 154 screened patients, 133 were randomized and received treatment (placebo, n = 48; perampanel, n = 85), and 119 completed the study (placebo, n = 43 [89.6% of randomized and treated patients]; perampanel, n = 76 [89.4% of randomized and treated patients]; Fig. 1). There were 76 patients aged 12 to <15 years and 57 patients aged 15 to <18 years. Patient demographics and baseline characteristics were largely balanced between groups (Table 1). Although not statistically significant, possible exceptions were that, compared with the placebo group, more perampanel‐treated patients had an IQ above 115 (12.5% vs. 21.2%) and more had a history of seizures with secondary generalization (39.6% vs. 51.8%) (p = 0.171 and p = 0.169, respectively, based on post hoc Cochran‐Mantel‐Haenszel tests, adjusted for region).
Figure 1.
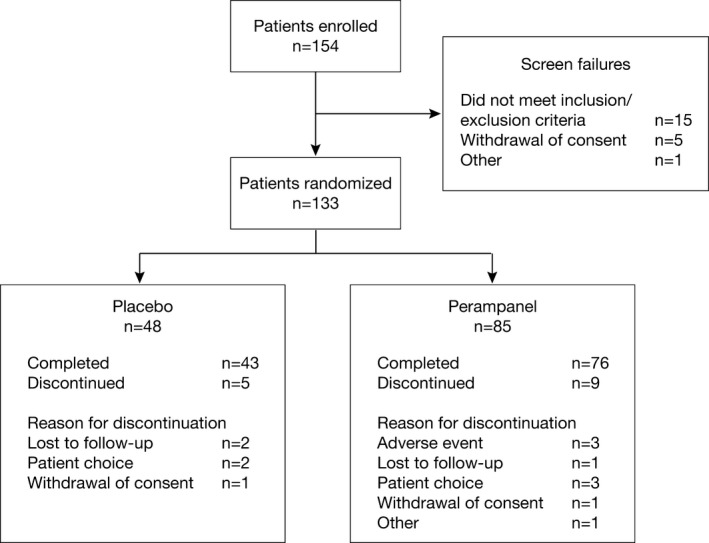
Patient disposition.
Table 1.
Patient demographics and baseline characteristics (safety analysis set)
| Placebo (n = 48) | Perampanel (n = 85) | Total (n = 133) | |
|---|---|---|---|
| Mean age, years (SD) | 14.3 (1.9) | 14.3 (1.7) | 14.3 (1.8) |
| Female, n (%) | 20 (41.7) | 33 (38.8) | 53 (39.8) |
| Mean height, cm (SD) | 161.2 (12.2) | 161.8 (11.1) | 161.6 (11.5) |
| Mean weight, kg (SD) | 55.9 (17.3) | 54.7 (17.1) | 55.2 (17.1) |
| Mean BMI, kg/m2 (SD) | 21.1 (4.4) | 20.6 (4.9) | 20.8 (4.7) |
| Race, n (%) | |||
| White | 28 (58.3) | 48 (56.5) | 76 (57.1) |
| Black | 1 (2.1) | 3 (3.5) | 4 (3.0) |
| Asian | 19 (39.6) | 32 (37.6) | 51 (38.3) |
| Other | 0 (0.0) | 2 (2.4) | 2 (1.5) |
| Country, n (%) | |||
| Australia | 1 (2.1) | 0 (0.0) | 1 (0.8) |
| Belgium | 2 (4.2) | 2 (2.4) | 4 (3.0) |
| Czech Republic | 1 (2.1) | 0 (0.0) | 1 (0.8) |
| Hungary | 7 (14.6) | 17 (20.0) | 24 (18.0) |
| India | 12 (25.0) | 23 (27.1) | 35 (26.3) |
| Republic of Korea | 3 (6.3) | 3 (3.5) | 6 (4.5) |
| Latvia | 8 (16.7) | 11 (12.9) | 19 (14.3) |
| Poland | 2 (4.2) | 4 (4.7) | 6 (4.5) |
| Spain | 2 (4.2) | 7 (8.2) | 9 (6.8) |
| Thailand | 4 (8.3) | 6 (7.1) | 10 (7.5) |
| U.S.A. | 6 (12.5) | 12 (14.1) | 18 (13.5) |
| Mean IQ score (SD) | 100.5 (12.9) | 101.6 (14.7) | 101.2 (14.0) |
| IQ score, group, n (%) | |||
| 70–84 | 5 (10.4) | 12 (14.1) | 17 (12.8) |
| 85–115 | 37 (77.1) | 55 (64.7) | 92 (69.2) |
| >115 | 6 (12.5) | 18 (21.2) | 24 (18.0) |
| Seizure type, n (%) | |||
| Simple partial without motor signs | 9 (18.8) | 12 (14.1) | 21 (15.8) |
| Simple partial with motor signs | 15 (31.3) | 31 (36.5) | 46 (34.6) |
| Complex partial | 35 (72.9) | 59 (69.4) | 94 (70.7) |
| Partial with secondary generalization | 19 (39.6) | 44 (51.8) | 63 (47.4) |
| Generalized seizures | 1 (2.1)a | 0 | 1 (0.8) |
| Unclassified epileptic seizures | 0 | 2 (2.4) | 2 (1.5) |
| Number of concomitant AEDs (%) | |||
| 1 | 18 (37.5) | 35 (41.2) | 53 (39.8) |
| 2 | 23 (47.9) | 35 (41.2) | 58 (43.6) |
| 3 | 7 (14.6) | 15 (17.6) | 22 (16.5) |
| Most commonly administered concomitant AEDs (>10% of patients), n (%) | |||
| Valproic acid | 23 (47.9) | 33 (38.8) | 56 (42.1) |
| Levetiracetam | 9 (18.8) | 37 (43.5) | 46 (34.6) |
| Lamotrigine | 12 (25.0) | 18 (21.2) | 30 (22.6) |
| Oxcarbazepine | 11 (22.9) | 16 (18.8) | 27 (20.3) |
| Carbamazepine | 10 (20.8) | 13 (15.3) | 23 (17.3) |
| Topiramate | 8 (16.7) | 13 (15.3) | 21 (15.8) |
| Lacosamide | 5 (10.4) | 10 (11.8) | 15 (11.3) |
BMI, body mass index; IQ, intelligence quotient; SD, standard deviation.
This patient had a medical history of both complex partial and generalized seizures but with complex partial seizures as the primary diagnosis.
Most patients received treatment for >18 weeks with placebo (85.4%) or perampanel (85.9%). The median/mean (SD) daily dose of perampanel was 6.0/6.2 (1.1) mg during the titration period and 10.0/9.6 (2.1) mg during the maintenance period. The dose received for the longest duration was 8–12 mg in most cases (83.5%).
Neuropsychological outcomes
In the FAS, LS mean change in CDR System Global Cognition Score between baseline and end of treatment was 1.6 with placebo (n = 44) and −0.6 with perampanel (n = 79; Table 2). These outcomes were not statistically significantly different (difference in LS mean −2.2, 95% confidence interval [CI] −5.2 to 0.8; p = 0.145). Furthermore, the mean difference between the placebo and perampanel groups was less than the prespecified level of five T‐score units and was therefore not considered clinically meaningful. In the per‐protocol population, the LS mean change in CDR System Global Cognition Score was 2.1 with placebo (n = 44) and 0.4 with perampanel (n = 72; difference in LS mean −1.7, 95% CI −4.7 to 1.3; p = 0.269). In the completers’ population, the LS mean change in CDR System Global Cognition Score was 1.4 with placebo (n = 40) and −0.4 with perampanel (n = 74), which approached statistical significance (difference in LS mean −1.7, 95% CI −3.5 to 0.0; p = 0.050), but remained <5 T‐score units.
Table 2.
Changes in CDR System Global Cognition Score and domain T‐scores between baseline and end of treatmenta (full analysis set)
| Placebo (n = 44) | Perampanel (n = 79) | Difference in LS mean (95% CI) for perampanel vs. placebo | |
|---|---|---|---|
| CDR System Global Cognition Score | |||
| Baseline mean score (SD) | 41.2 (10.7) | 40.8 (13.0) | – |
| End of treatment mean score (SD) | 42.2 (11.8) | 39.7 (13.5) | – |
| LS mean change (SE) | 1.6 (1.3) | −0.6 (1.0) | −2.2 (−5.2 to 0.8); p = 0.145 |
| Power of Attention | |||
| Baseline mean score (SD) | 24.7 (19.4) | 24.9 (23.9) | – |
| End of treatment mean score (SD) | 22.0 (25.6) | 17.9 (24.6) | – |
| LS mean change (SE) | −2.7 (3.0) | −6.9 (2.3) | −4.2 (−11.0 to 2.6); p = 0.219 |
| Continuity of Attention | |||
| Baseline mean score (SD) | 53.1 (9.1) | 52.8 (7.5) | – |
| End of treatment mean score (SD) | 54.1 (6.4) | 50.7 (9.2) | – |
| LS mean change (SE) | 1.6 (1.2) | −1.7 (0.9) | −3.3 (−6.0 to −0.7); p = 0.013 |
| Quality of Episodic Memory | |||
| Baseline mean score (SD) | 51.6 (12.9) | 52.1 (14.4) | – |
| End of treatment mean score (SD) | 50.9 (12.7) | 55.5 (15.1) | – |
| LS mean change (SE) | −1.2 (1.5) | 3.0 (1.1) | 4.2 (0.9–7.5); p = 0.012 |
| Quality of Working Memory | |||
| Baseline mean score (SD) | 52.2 (8.6) | 50.7 (11.4) | – |
| End of treatment mean score (SD) | 53.4 (9.9) | 52.4 (9.4) | – |
| LS mean change (SE) | 2.0 (1.5) | 1.1 (1.2) | −1.0 (−4.4 to 2.5); p = 0.579 |
| Speed of Memory | |||
| Baseline mean score (SD) | 24.2 (23.0) | 23.4 (28.1) | – |
| End of treatment mean score (SD) | 29.6 (20.8) | 22.2 (34.2) | – |
| LS mean change (SE) | 7.0 (2.7) | 0.3 (2.1) | −6.6 (−12.7 to −0.6); p = 0.032 |
CDR System, Complete Drug Research System; CI, confidence interval; LS, least squares; SD, standard deviation; SE, standard error.
Statistical comparisons based on analysis of covariance, with baseline score and age as covariates, and region, treatment, and gender as factors.
Higher scores indicate better cognitive function.
Similarly, there were no apparent differences between placebo and perampanel in terms of changes in the T‐scores of the CDR System domains of Power of Attention or Quality of Working Memory (FAS; Table 2). However, there were small but significant differences in terms of Continuity of Attention (increased with placebo, but reduced with perampanel), Quality of Episodic Memory (reduced with placebo, but increased with perampanel), and Speed of Memory (compared with placebo, there was a smaller reduction over time with perampanel).
There were minimal changes in letter and category fluency scores, and time to complete the LGPT test, between baseline and end of treatment in both the placebo and perampanel groups (FAS; Table 3). Differences between the treatment groups were not statistically significant.
Table 3.
Changes in letter and category fluency scores,a and time to complete the Lafayette Groove Pegboard Test,b between baseline and end of treatment (full analysis set)
| Placebo (n = 44) | Perampanel (n = 79) | Perampanel vs. placebo | |
|---|---|---|---|
| Letter fluency score | |||
| Baseline, n | 44 | 76 | – |
| Mean score (SD) | 23.4 (9.5) | 28.7 (12.8) | – |
| End of treatment, n | 44 | 76 | – |
| Mean score (SD) | 24.0 (10.6) | 29.3 (13.6) | – |
| LS mean for change (SE) | 0.206 (1.069) | 0.854 (0.808) | Difference in LS mean (95% CI): 0.648 (−2.034, 3.330); p = 0.633 |
| Category fluency score | |||
| Baseline, n | 44 | 76 | – |
| Mean score (SD) | 14.4 (5.15) | 15.4 (4.6) | – |
| End of treatment, n | 44 | 76 | – |
| Mean score (SD) | 14.7 (5.4) | 14.8 (4.4) | – |
| LS mean for change (SE) | 0.113 (0.490) | −0.447 (0.372) | Difference in LS mean (95% CI): −0.560 (−1.782, 0.661); p = 0.365 |
| LGPT: dominant hand | |||
| Baseline, n | 44 | 75 | – |
| Mean time, s (SD) | 86.8 (37.7) | 85.0 (22.1) | – |
| End of treatment, n | 44 | 79 | – |
| Mean time, s (SD) | 77.6 (19.9) | 84.9 (20.2) | – |
| Change | −9.2 (28.8) | 0.2 (17.2) | p = 0.143 |
| LGPT: nondominant hand | |||
| Baseline, n | 44 | 74 | – |
| Mean time, s (SD) | 99.5 (47.1) | 105.9 (46.5)c | – |
| End of treatment, n | 43 | 79 | – |
| Mean time, s (SD) | 91.7 (34.7) | 102.4 (37.8)c | – |
| Change | −7.7 (28.8) | −2.5 (25.0) | p = 0.109 |
CI, confidence interval; LGPT, Lafayette Groove Pegboard Test; LS, least squares; s, second; SD, standard deviation; SE, standard error.
Higher scores indicate better language skills; statistical comparisons based on analysis of covariance, with baseline score as a covariate.
Lower scores (shorter times) indicate better manual dexterity; statistical comparisons based on log‐rank test.
One patient was unable to complete the test in 300 s, and so a time of 300 s was recorded.
Safety outcomes
The safety analysis set included 133 patients (placebo, n = 48; perampanel, n = 85). Perampanel was generally well tolerated, with no clinically important mean changes in laboratory values, vital signs, or electrocardiography parameters. The most frequently reported TEAEs with perampanel were dizziness and somnolence (Table 4).
Table 4.
Summary of adverse events (safety analysis set)
| Adverse event, n (%) | Placebo (n = 48) | Perampanel (n = 85) |
|---|---|---|
| Any TEAE | 31 (64.6) | 68 (80.0) |
| TEAEs occurring in ≥5% of patients | ||
| Dizziness | 7 (14.6) | 26 (30.6) |
| Somnolence | 2 (4.2) | 13 (15.3) |
| Headache | 7 (14.6) | 9 (10.6) |
| Fatigue | 1 (2.1) | 8 (9.4) |
| Aggression | 1 (2.1) | 7 (8.2) |
| Irritability | 1 (2.1) | 6 (7.1) |
| Weight increased | 0 (0.0) | 5 (5.9) |
| Convulsion | 5 (10.4) | 4 (4.7) |
| Nasopharyngitis | 3 (6.3) | 4 (4.7) |
| Upper respiratory tract infection | 3 (6.3) | 4 (4.7) |
| Insomnia | 3 (6.3) | 3 (3.5) |
| Treatment‐related adverse events | 20 (41.7) | 58 (68.2) |
| Severe TEAEs | 1 (2.1) | 5 (5.9) |
| Serious TEAEs | 2 (4.2) | 5 (5.9) |
| Deaths | 0 (0.0) | 0 (0.0) |
| TEAEs requiring inpatient hospitalization or prolongation of existing hospitalization | 2 (4.2) | 2 (4.2) |
| TEAEs leading to study drug dose adjustment | 2 (4.2) | 24 (28.2) |
| Discontinuation | 0 (0.0) | 3 (3.5) |
| Dose increase | 0 (0.0) | 2 (2.4) |
| Dose reduction | 2 (4.2) | 20 (23.5) |
TEAE, treatment‐emergent adverse event.
Aggressive behavior was reported in one of 48 placebo‐treated patients (2.1%) and 7 of 85 perampanel‐treated patients (8.2%). Three perampanel‐treated patients had aggression requiring treatment modification (dose reduced from 8 to 6 mg due to moderate aggression, n = 2; dose increased from 8 to 10 mg when aggression changed in intensity from moderate to mild, n = 1), and two had serious aggression (aggression treated separately; patients recovered with no change to study treatment), although no cases necessitated discontinuation. No homicidal ideation/threats or suicidal ideation/behaviors were reported.
Discussion
Although AED treatment is a known potential contributor to cognitive dysfunction, cognition studies in pediatric epilepsy have been inadequate.2 For example, topiramate has been associated with cognitive impairment in adults,29 and treatment‐limiting cognitive impairment/sedation in a retrospective study of children and adolescents,30 yet there have been no objective assessments of cognitive function in a pediatric population treated with this AED. Nonetheless, some recent pediatric studies using appropriate measures of cognition have been reported. One of the first demonstrated no difference between adjunctive placebo and levetiracetam in the Leiter International Performance Scale‐Revised on Attention and Memory, in children aged 4–16 years with partial‐onset seizures.31
In the present 19‐week study, adjunctive perampanel did not have any significant overall cognitive effect in adolescent patients with inadequately controlled partial‐onset seizures. Specifically, compared with placebo, adjunctive perampanel resulted in a nonsignificant LS mean difference of −2.2 (95% CI −5.2 to 0.8; p = 0.145) in the change in CDR System Global Cognition Score from baseline to end of treatment, which was less than the five T‐score units postulated to be clinically meaningful.
The individual CDR System domain scores suggested possible benefits for Quality of Episodic Memory and possible worsening of Continuity of Attention and Speed of Memory with perampanel compared with placebo, although these differences were small. Nonetheless, the possible beneficial effect of perampanel on episodic memory is novel compared with other AEDs, and warrants further investigation to confirm this finding and identify the underlying mechanism. There were no significant differences between perampanel and placebo in the other CDR System domains (Power of Attention and Quality of Working Memory).
Measures of language (letter and category fluency) and manual dexterity (LGPT) were also not significantly different for placebo and perampanel. However, it should be noted that statistical analyses of these measures were conducted post hoc, and the study was not powered to investigate the statistical significance of these comparisons.
In addition, perampanel was well tolerated by adolescent patients, with a safety profile consistent with the findings of a subgroup analysis of pooled phase III data from adolescent patients.32, 33 Aggressive behavior was reported in 2.1% (1/48) of placebo‐treated patients and 8.2% (7/85) of perampanel‐treated patients. Although dose reduction was required in some patients, none required discontinuation, and no homicidal ideation/threats or suicidal ideation/behaviors were reported. Similar findings were observed across three phase III randomized, double‐blind, placebo‐controlled studies in patients with partial‐onset seizures, with aggression reported in 8.2% (8/98) of adolescent patients treated with perampanel and none (0/45) treated with placebo.34
Limitations of the study include the relatively limited neuropsychological battery. In addition, although the study was of sufficient duration to allow habituation to AED effects as opposed to acute‐dose studies, it was not long enough to assess potential long‐term effects on factors such as school performance. The results from the open‐label long‐term extension to this study will be reported separately. Another limitation is that the sensitivity of the CDR to AEDs was established in a monotherapy trial. An adjunctive trial may be less sensitive to identify cognitive effects of AEDs because the baseline medications may produce adverse cognitive effects resulting in floor effects. However, differential effects were seen on three of the five CDR subdomains. Further studies may be useful to explore cognitive effects using more detailed neuropsychological batteries, and to confirm the possible benefits of perampanel on episodic memory.
Overall, it is important to recognize that the effectiveness of an AED is a combination of efficacy and tolerability. One of the major factors determining tolerability is the frequency and severity of drug‐induced adverse cognitive effects, as such effects can have a detrimental impact on patient quality of life.35 Indeed, these drug‐related effects can outweigh the positive effects of seizure reduction, short of achieving complete seizure freedom. The finding of a favorable cognitive profile for perampanel is, therefore, important in this regard. Additional studies are needed to compare perampanel with other AEDs.
Conflict of Interest
Dr. Meador has received research support from the National Institutes of Health, the Patient‐Centered Outcomes Research Institute, and UCB Pharma. The Epilepsy Study Consortium pays Dr. Meador's university for his research consultant time related to Eisai, GW Pharmaceuticals, NeuroPace, Novartis, Supernus, Upsher‐Smith Laboratories, UCB Pharma, and Vivus Pharmaceuticals. Dr. Yang, Dr. Laurenza, and Mr. Kumar are employees of Eisai Inc. Dr. Piña‐Garza has been a consultant for Eisai, Lundbeck, Supernus, and UCB, and a speaker for Cyberonics, Eisai, Supernus, and UCB. He has also participated in research funded by Eisai, Cyberonics, and UCB Pharma. Dr. Wesnes was an employee of Bracket, the company that supplied the CDR System, at the time that this study was conducted. Now, for over a year, he has run his own consultancy company, Wesnes Cognition Ltd, which specializes in cognitive assessments in clinical trials. The authors confirm that they have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Acknowledgments
Study funded by Eisai Inc. Hannah FitzGibbon, of Complete Medical Communications, Macclesfield, United Kingdom, assisted with the technical editing of the manuscript and preparation of tables and figures, also funded by Eisai Inc.
Biography
Kimford J. Meador is professor of neurology and neurological sciences at Stanford University.

References
- 1. Rudzinski LA, Meador KJ. Epilepsy and neuropsychological comorbidities. Continuum 2013;19:682–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marsh ED, Brooks‐Kayal AR, Porter BE. Seizures and antiepileptic drugs: does exposure alter normal brain development? Epilepsia 2006;47:1999–2010. [DOI] [PubMed] [Google Scholar]
- 3. Meador KJ. Cognitive effects of epilepsy and its treatments In Wyllie E, Cascino GD, Gidal BE, Goodkin HP. (Eds) Wyllie's treatment of epilepsy: principles & practice. Philadelphia, PA: Lippincott Williams & Wilkins, 2014:989–994. [Google Scholar]
- 4. Wesnes KA. The value of assessing cognitive function in drug development. Dialogues Clin Neurosci 2000;2:183–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blacker CV, Greenwood DT, Wesnes KA, et al. Effect of galantamine hydrobromide in chronic fatigue syndrome: a randomized controlled trial. JAMA 2004;292:1195–1204. [DOI] [PubMed] [Google Scholar]
- 6. Fearn SJ, Pole R, Wesnes K, et al. Cerebral injury during cardiopulmonary bypass: emboli impair memory. J Thorac Cardiovasc Surg 2001;121:1150–1160. [DOI] [PubMed] [Google Scholar]
- 7. McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double‐blind, placebo‐controlled international study. Lancet 2000;356:2031–2036. [DOI] [PubMed] [Google Scholar]
- 8. Ingwersen J, Defeyter MA, Kennedy DO, et al. A low glycaemic index breakfast cereal preferentially prevents children's cognitive performance from declining throughout the morning. Appetite 2007;49:240–244. [DOI] [PubMed] [Google Scholar]
- 9. Lloyd A, Brett D, Wesnes K. Coherence training in children with attention‐deficit hyperactivity disorder: cognitive functions and behavioral changes. Altern Ther Health Med 2010;16:34–42. [PubMed] [Google Scholar]
- 10. Maldonado EF, Fernandez FJ, Trianes MV, et al. Cognitive performance and morning levels of salivary cortisol and alpha‐amylase in children reporting high vs. low daily stress perception. Span J Psychol 2008;11:3–15. [DOI] [PubMed] [Google Scholar]
- 11. Preece AW, Goodfellow S, Wright MG, et al. Effect of 902 MHz mobile phone transmission on cognitive function in children. Bioelectromagnetics 2005;26(Suppl. 7):S138–S143. [DOI] [PubMed] [Google Scholar]
- 12. Taib MN, Shariff ZM, Wesnes KA, et al. The effect of high lactose‐isomaltulose on cognitive performance of young children. A double blind cross‐over design study. Appetite 2012;58:81–87. [DOI] [PubMed] [Google Scholar]
- 13. Wesnes KA, Pincock C, Richardson D, et al. Breakfast reduces declines in attention and memory over the morning in schoolchildren. Appetite 2003;41:329–331. [DOI] [PubMed] [Google Scholar]
- 14. Wesnes KA, Pincock C, Scholey A. Breakfast is associated with enhanced cognitive function in schoolchildren. An internet based study. Appetite 2012;59:646–649. [DOI] [PubMed] [Google Scholar]
- 15. Wesnes KA, Busner J. Differential effects on attention over 24 weeks of an NMDA antagonist versus carbamazepine in paediatric epilepsy patients. Eur Neuropsychopharmacol 2013;23:S602. [Google Scholar]
- 16. Wesnes KA, Edgar C, Dean AD, et al. The cognitive and psychomotor effects of remacemide and carbamazepine in newly diagnosed epilepsy. Epilepsy Behav 2009;14:522–528. [DOI] [PubMed] [Google Scholar]
- 17. Rapeport WG, Williams SA, Muirhead DC, et al. Absence of a sertraline‐mediated effect on the pharmacokinetics and pharmacodynamics of carbamazepine. J Clin Psychiatry 1996;57(Suppl. 1):20–23. [PubMed] [Google Scholar]
- 18. Lockton JA, Wesnes KA, Yeates N, et al. Remacemide does not affect cognitive function following multiple dosing. J Psychopharmacol 1998;12:A41. [Google Scholar]
- 19. Hanada T, Hashizume Y, Tokuhara N, et al. Perampanel: a novel, orally active, noncompetitive AMPA‐receptor antagonist that reduces seizure activity in rodent models of epilepsy. Epilepsia 2011;52:1331–1340. [DOI] [PubMed] [Google Scholar]
- 20. European Medicines Agency . Fycompa summary of product characteristics, Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002434/WC500130815.pdf. Accessed November 30, 2015.
- 21. Food and Drug Administration . Fycompa Prescribing Information, 2015. Available at:http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202834lbl.pdf. Accessed November 30, 2015.
- 22. Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test. 2nd Ed Minneapolis, MN: American Guidance Service Inc; 2004. [Google Scholar]
- 23. International League Against Epilepsy . Proposal for revised clinical and electroencephalographic classification of epileptic seizures. From the Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia 1981;22:489–501. [DOI] [PubMed] [Google Scholar]
- 24. Wesnes K, Pincock C. Practice effects on cognitive tasks: a major problem? Lancet Neurol 2002;1:473. [DOI] [PubMed] [Google Scholar]
- 25. Wesnes KA, Ward T, McGinty A, et al. The memory enhancing effects of a Ginkgo biloba/Panax ginseng combination in healthy middle‐aged volunteers. Psychopharmacology 2000;152:353–361. [DOI] [PubMed] [Google Scholar]
- 26. Lezak MD, Howieson DB, Bigler ED, et al. Neuropsychological assessment. New York, NY: Oxford University Press; 2012. [Google Scholar]
- 27. O'Neill WM, Hanks GW, White L, et al. The cognitive and psychomotor effects of opioid analgesics. I. A randomized controlled trial of single doses of dextropropoxyphene, lorazepam and placebo in healthy subjects. Eur J Clin Pharmacol 1995;48:447–453. [DOI] [PubMed] [Google Scholar]
- 28. Wesnes KA, Garratt C, Wickens M, et al. Effects of sibutramine alone and with alcohol on cognitive function in healthy volunteers. Br J Clin Pharmacol 2000;49:110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Blum D, Meador K, Biton V, et al. Cognitive effects of lamotrigine compared with topiramate in patients with epilepsy. Neurology 2006;67:400–406. [DOI] [PubMed] [Google Scholar]
- 30. Reith D, Burke C, Appleton DB, et al. Tolerability of topiramate in children and adolescents. J Paediatr Child Health 2003;39:416–419. [DOI] [PubMed] [Google Scholar]
- 31. Levisohn PM, Mintz M, Hunter SJ, et al. Neurocognitive effects of adjunctive levetiracetam in children with partial‐onset seizures: a randomized, double‐blind, placebo‐controlled, noninferiority trial. Epilepsia 2009;50:2377–2389. [DOI] [PubMed] [Google Scholar]
- 32. Renfroe B, Yang H, Williams B, et al. Interim efficacy and safety analysis of adjunctive perampanel in the adolescent population from the extension phase of 3 double‐blind, placebo‐controlled, phase 3 (core) studies in patients with refractory partial‐onset seizures. Neurology 2014;82:P3.271. [Google Scholar]
- 33. Rosenfeld W, Rozentals G, Yang H, et al. Efficacy and safety of adjunctive perampanel in the subgroup of adolescent patients with refractory partial onset seizures included in the 3 double‐blind, placebo‐controlled Phase III clinical trials. Poster E‐18 presented at the 41st Annual Meeting of the Child Neurology Society, 2012.
- 34. Rosenfeld W, Conry J, Lagae L, et al. Efficacy and safety of perampanel in adolescent patients with drug‐resistant partial seizures in three double‐blind, placebo‐controlled, phase III randomized clinical studies and a combined extension study. Eur J Paediatr Neurol 2015;19:435–445. [DOI] [PubMed] [Google Scholar]
- 35. Gilliam F. Optimizing health outcomes in active epilepsy. Neurology 2002;58:S9–S20. [DOI] [PubMed] [Google Scholar]


