Abstract
Biochemical, genetic, and animal studies in recent years have established a critical role for the adipokine Acrp30/adiponectin in controlling whole-body metabolism, particularly by enhancing insulin sensitivity in muscle and liver, and by increasing fatty acid oxidation in muscle. We describe a widely expressed and highly conserved family of adiponectin paralogs designated as C1q/tumor necrosis factor-α-related proteins (CTRPs) 1–7. In the present study, we focus on mCTRP2, the mouse paralog most similar to adiponectin. At nanomolar concentrations, bacterially produced mCTRP2 rapidly induced phosphorylation of AMP-activated protein kinase, acetyl-CoA carboxylase, and mitogen-activated protein kinase in C2C12 myotubes, which resulted in increased glycogen accumulation and fatty acid oxidation. The discovery of a family of adiponectin paralogs has implications for understanding the control of energy homeostasis and could provide new targets for pharmacologic intervention in metabolic diseases such as diabetes and obesity.
Adipose tissue plays an active role in monitoring and controlling whole-body metabolism by secreting a variety of bioactive molecules collectively termed adipokines (1). Acrp30/adiponectin is one such adipokine discovered in a screen to identify novel secreted proteins induced upon adipocyte differentiation (2). Adiponectin is composed of four distinct domains: a signal peptide at the N terminus, a short variable region, a collagenous domain, and a C-terminal globular domain homologous to C1q. The crystal structure of adiponectin globular domain reveals a striking resemblance to the structure of tumor necrosis factor (TNF)-α (3). Adiponectin belongs to a growing family of proteins, all of which contain a C-terminal globular C1q-like domain of ≈135 aa. Most of them also contain a variable number of “Gly-X-Y” (where X and Y represent any amino acid) collagenous repeats. Including the seven adiponectin paralogs described here, there are currently 25 proteins belonging to the C1q/TNF-α superfamily. Among these proteins are the Siberian chipmunk hibernating proteins HP20, 25, and 27; serum levels of these three proteins are dramatically reduced during hibernation (4).
Adiponectin is expressed exclusively by differentiated adipocytes and its expression is induced ≈100-fold during adipocyte differentiation (2). Serum levels of adiponectin correlate inversely with insulin sensitivity. In healthy adults, adiponectin circulates in serum at a high concentration (1.9–17.0 μg/ml). Adiponectin levels are significantly reduced in a variety of obese and insulin-resistant states in mice (5), monkeys (6), and humans (7). Diabetic humans with coronary artery disease have even lower plasma levels of adiponectin than those without (8). Mutations and polymorphisms in the adiponectin gene are associated with reduced serum levels of adiponectin, in part due to effects on protein secretion, multimerization, and/or stability (9–11). Weight loss, caloric restriction, or thiazolidinedione treatment increase adiponectin levels in human (12–14) and mice (15, 16), and this increase correlates with increased insulin sensitivity.
Injection of a proteolytically generated globular C-terminal form of recombinant adiponectin into mice significantly reduced levels of plasma-free fatty acids and glucose after a high-fat meal (17). Furthermore, long-term administration of adiponectin to mice fed a high-fat diet caused profound weight loss by enhancing free fatty acid oxidation in muscles without affecting food intake. Subsequently, Yamauchi et al. (18) demonstrated that recombinant adiponectin could restore insulin sensitivity to insulin-resistant obese (ob/ob), diabetic (db/db), or lipoatrophic mice by increasing β-oxidation of fatty acids in muscle. Full-length, but not the globular C-terminal domain of adiponectin produced in mammalian cells, enhanced the ability of insulin to suppress gluconeogenesis and glucose release by primary rat hepatocytes (19). Many recent studies (summarized in ref. 20) have confirmed and extended these initial findings and corroborated the current notion that adiponectin acts as an insulin sensitizer in vivo, exerting its effects on the liver to suppress glucose output while acting on muscle to increase glucose uptake and fatty acid oxidation.
Differences in activity initially attributed to full-length or globular adiponectin can be ascribed to the different oligomeric forms of adiponectin (11, 21–23). In serum, adiponectin exists as trimers, hexamers, and high molecular weight species and the proportion of these oligomeric forms changes according to metabolic status and disease states (24). These different oligomeric forms possess distinct signaling properties; hexameric and high molecular weight forms of adiponectin induce NF-κB activation, whereas the trimeric forms of adiponectin induce AMP-activated protein kinase (AMPK) activation in muscle (21, 22). In muscle, AMPK activation results in increased glucose uptake (25, 26) and glycogen accumulation (27). In addition, activated AMPK phosphorylates and inhibits acetylCoA carboxylase (ACC), leading to decreased fatty acid synthesis and a concomitant increase in β-oxidation of fatty acid (26). Beyond its role in controlling glucose and lipid metabolism, additional functions of adiponectin have been suggested, including antiinflammatory (28, 29), antiatherosclerotic (30), and pro- (31) or antiangiogenic (32) properties.
Here, we describe a family of adiponectin paralogs. We show that at least one paralog, mouse CTRP2 (mCTRP2), possesses similar biologic properties as adiponectin in enhancing glycogen accumulation and fatty acid oxidation in C2C12 myotubes by activating the AMPK signaling pathway. We discuss important implications for metabolic control orchestrated by adiponectin and its paralogs.
Materials and Methods
Cloning of Acrp30/Adiponectin Paralogs. Several human and mouse ESTs that encode novel adiponectin-like proteins were identified from National Center for Biotechnology Information GenBank databases on the basis of significant homology to the globular C1q domain of adiponectin. These ESTs were identical/similar to seven human cDNAs deposited in GenBank by Zymogenetics. These seven adiponectin paralogs were designated as C1q/TNF-α related proteins (CTRPs) 1–7. The GenBank accession nos. for mCTRPs1–7 are NM_019959, XM_126141, NM_030888, NM_026161, NM_145613, NM_028331, and NM_175425, respectively. The GenBank accession nos. for human (h)CTRPs 1–7 are NM_198594, NM_031908, NM_181435, NM_031909, NM_015645, NM_031910, and NM_031911, respectively.
Overlapping EST clones that correspond to mCTRP1, mCTRP2, and mCTRP7 were obtained from the I.M.A.G.E. Consortium. PCR was used to clone the entire coding region of mCTRP1, mCTRP2, and mCTRP7.
RT-PCR Analysis of CTRP Transcripts in Mouse Tissues. A semiquantitative PCR approach was used to screen multiple-tissue cDNA panels (Clontech) and RNA isolated from adipose tissue derived from ob/ob mice or control mice for the presence of CTRP1–7 transcripts. The oligonucleotides used in these transcript studies and the sizes of the resulting PCR products are summarized in Table 2, which is published as supporting information on the PNAS web site.
COS Cell Transfection. C-terminal hemagglutinin (HA)-tagged mCTRP1, mCTRP2, mCTRP7, and adiponectin constructs were generated by PCR and cloned into the mammalian expression vector pcDNA3.1 TOPO (Invitrogen). COS-7 cells were cultured in DMEM containing 10% FCS. Transient transfections were performed in COS-7 cells by using SuperFect reagent (Qiagen, Valencia, CA). Twenty-four hours after transfection, cells were washed and then cultured in serum-free Opti-MEM I medium (GIBCO) for another 24 h before the conditioned medium and cell pellets were collected for Western blot analysis by using an anti-HA monoclonal antibody (Covance). A sample of the conditioned medium or cell lysate from each transfectant was incubated with PNGaseF (New England Biolabs) to determine the presence of N-linked glycans.
Generation of Recombinant mCTRP2 in Escherichia coli. Recombinant mCTRP2 and its C-terminal globular “head” were produced by cloning full-length or truncated forms of the mCTRP2 construct, tagged with the FLAG epitope at the N terminus, into pTrcHis TOPO vector (Invitrogen), and maintained in E. coli. strain TOP10. The N-terminal His6-tagged fusion protein was produced in E. coli, isolated from the lysed bacterial pellet by nickel-affinity column with Probond resin (Invitrogen), eluted with imidazole-containing buffer, and dialyzed against PBS. Detoxi-Gel endotoxin-removing gel (Pierce) was used to remove potential endotoxin contaminants.
Gel Filtration Analysis of mCTRP2. Purified recombinant FLAG-tagged mCTRP2 or homogenized mouse lung lysate were loaded into an Akta FPLC and fractionated either through a 16/60 or a 10/30 Superdex 200 column (Amersham Pharmacia Biosciences) in PBS. The collected fractions were subjected to Western blot analysis by using anti-FLAG monoclonal antibody (Sigma) for recombinant mCTRP2. A rabbit polyclonal anti-peptide serum directed against the N-terminal variable region of mCTRP2 (NH2-AFARRDFQKGGPQLVC-COOH) was used to detect endogenous mCTRP2.
Western Blot Analysis. C2C12 myotubes were cultured in DMEM containing 2% horse serum. On day 6 or 7, the cells were stimulated with recombinant mCTRP2 before cells were lysed in RIPA buffer containing phosphatase inhibitors (Sigma) and a protease inhibitor mixture (Roche Applied Science). Cleared lysates were fractionated by SDS/PAGE and blotted onto poly (vinylidene difluoride) membranes (Bio-Rad) for Western blot analysis, by using rabbit antibodies recognizing phospho-AMPKα (Thr-172), phospho-ACC (Ser-79), phospho-p44/42 mitogen-activated protein kinase (MAPK) (Thr-202 and Tyr-204), phospho-IκB-α (Ser-32), total AMPKα, or total p44/42 MAPK, at 1:1,000 dilution (all from Cell Signaling Technology, Beverly, MA).
Oleic Acid Oxidation. The oleate oxidation assay was performed as described (17). Briefly, C2C12 myotubes (day 6 or 7) were cultured in DMEM (GIBCO) containing 2% horse serum in 25-cm2 cell culture flasks (Corning). One hour before the experiment, the medium was removed and 1 ml of preincubation medium (DMEM/12 mM glucose/4 mM glutamine/25 mM Hepes/1% FFA-free BSA/0.25 mM oleate) was added. At the start of the oxidation experiment, [1-14C]oleic acid [1 μCi/ml (1 Ci = 37 GBq), American Radiolabeled Chemicals, St. Louis] was added, and cells were incubated for 90 min at 37°C in the absence or presence of 4 μg/ml mCTRP2. The incubation flasks were sealed with a rubber septum from which a center well carrying a piece of Whatman paper was suspended. After the incubation period, 1 ml of 35% perchloric acid was injected into the flask with a syringe, and 0.45 ml of 3 M NaOH was injected onto the Whatman paper in the center well. After a 60-min collection period, the Whatman paper was removed from the center well, and the amount of 14C radioactivity was determined by liquid scintillation counting.
Glycogen Content Analysis. C2C12 myotubes (day 6) were cultured in DMEM (GIBCO) containing 2% horse serum. Media were replaced with DMEM containing 1% horse serum 1 day before the experiment. C2C12 myotubes were stimulated with 4 μg/ml of full-length mCTRP2 or g-mCTRP2 (globular head only) or gAdiponectin (17) for 16 h. Media were aspirated, cells were washed once with ice-cold PBS containing 0.9 mM CaCl2 and 0.5 mM MgCl2, and then scraped off the plate. Cell pellets were boiled for 15 min in 0.2 ml of 3 M KOH. Cell lysates were spotted onto Whatman filter papers (2.4 × 2.4 cm) and dried. Glycogen was precipitated onto the filter papers by immersing the filters in cold 66% ethanol. Filters were washed three times, 20 min each, in cold 66% ethanol and dried. Dried filters were placed into a six-well plate. A total of 1.2 ml amyloglucosidase buffer (pH 4.7) containing 0.2 mg/ml amyloglucosidase (Roche Applied Science) was added to each well. Glycogen digestions were carried out overnight at room temperature with moderate rocking. The recovered digests (≈0.7 ml) were transferred to microfuge tubes and dried in a speed-vac to ≈0.1 ml. Glucose oxidase reagent (Sigma, 1 ml) was added to each tube. An aliquot of the reaction (1 ml) was then transferred to disposable cuvettes and the absorbance at 505 nm was measured. The protein concentrations of the original cell lysates after boiling were determined by using BCA assay (Pierce). The glycogen content in each sample was normalized to total protein.
Results
Identification and Analysis of Acrp30/Adiponectin Paralogs. By using the adiponectin cDNA sequence to interrogate the mouse and human ESTs and genomic databases, we identified a highly conserved family of proteins homologous to Acrp30/adiponectin (Fig. 1). All these proteins share a similar modular organization to adiponectin and contain four distinct domains: a signal peptide at the N terminus, a short variable region, a collagenous domain, and a C-terminal globular domain that is homologous to complement protein C1q. The C-terminal globular domain is thought to be a functional domain that may interact with other proteins or receptors. Because all these proteins contain a C1q-like globular domain, and because the known crystal structure of mouse adiponectin globular domain shows striking homology to TNF-α, these proteins were designated as CTRPs 1–7. mCTRP3 is identical to the recently described TGF-β induced protein CORS26 (33).
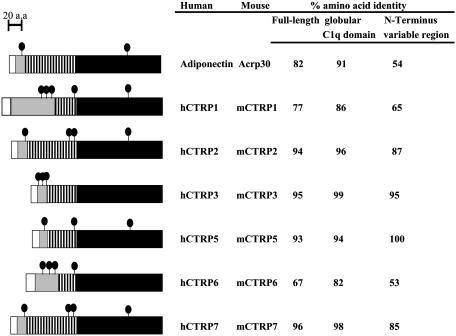
Fig. 1.
Identification of a family of Acrp30/adiponectin paralogs. The adiponectin paralogs, CTRPs 1–7, were identified by searching GenBank EST and genomic databases with the adiponectin cDNA sequence. The predicted amino acid sequences of all of the CTRPs share a similar modular organization to adiponectin and consist of four distinct domains; a signal peptide (white), a short variable region (gray), a collagenous domain with various length of Gly-X-Y repeats (hatched), and a C-terminal globular domain homologous to complement C1q (black). The predicted signal peptides of mCTRP1, mCTRP2, mCTRP3, mCTRP5, mCTRP6, and mCTRP7 consist of 25, 15, 22, 15, 20, and 16 aa, respectively. The short variable regions of mCTRP1, mCTRP2, mCTRP3, mCTRP5, mCTRP6, and mCTRP7 consist of 73, 24, 22, 14, 58, and 21 aa, respectively. There are a total of 14, 34, 23, 23, 14, and 34 Gly-X-Y (X and Y refer to any amino acid) repeats in the collagenous domain of mCTRP1, mCTRP2, mCTRP3, mCTRP5, mCTRP6, and mCTRP7, respectively. The predicted globular domain of mCTRP1, mCTRP2, mCTRP3, mCTRP5, mCTRP6, and mCTRP7 consists of 143, 146, 135, 147, 142, and 152 aa, respectively. • indicates cysteine residues; cysteine residues in the signal peptides are not shown because they are not part of the mature proteins. hCTRPs and their corresponding mouse orthologs are highly conserved. The numbers on the right refer to the percent amino acid identity between human and mouse orthologs when comparing the full-length protein (first column), the C-terminal globular domain (second column), or the N-terminal variable region (third column).
The family of CTRPs is highly conserved during evolution (Fig. 6, which is published as supporting information on the PNAS web site). mCTRPs and their corresponding human orthologs share 53–100% amino acid identity in their short N-terminal variable regions and 82–99% amino acid identity in their C-terminal globular domains (Fig. 1.). The C-terminal globular domains of paralogous CTRPs are also conserved, ranging from 27% to 73% amino acid identity between each other and among other proteins of the C1q/TNF-α superfamily (Table 1, and Table 3, which is published as supporting information on the PNAS web site). Of the seven CTRPs, the globular domains of mCTRP2 and mCTRP7 share the highest degree of amino acid identity (42–43%) to adiponectin. Structure-based alignment between adiponectin, complement C1q, and TNF family members (TNF-α, TNF-β, and CD40L) reveals four highly conserved residues (Tyr-161, Gly-159, Phe-237, and Leu-242 in adiponectin), which is important in the packing of the protomer's hydrophobic core (3). These residues are conserved in all CTRPs (Fig. 7, which is published as supporting information on the PNAS web site).
Table 1. Comparison of the amino acid sequences of the C-terminal globular domains of adiponectin and CTRPs.
| Acrp30 | mCTRP1 | mCTRP2 | mCTRP3 | mCTRP4 | mCTRP5 | mCTRP6 | mCTRP7 | |
|---|---|---|---|---|---|---|---|---|
| Acrp30 | 100 | |||||||
| mCTRP1 | 30 | 100 | ||||||
| mCTRP2 | 42 | 33 | 100 | |||||
| mCTRP3 | 31 | 27 | 27 | 100 | ||||
| mCTRP4 | 30 | 35 | 31 | 31 | 100 | |||
| mCTRP5 | 41 | 29 | 38 | 28 | 35 | 100 | ||
| mCTRP6 | 34 | 64 | 31 | 32 | 38 | 32 | 100 | |
| mCTRP7 | 43 | 31 | 73 | 29 | 33 | 40 | 32 | 100 |
Numbers indicate percent amino acid identities.
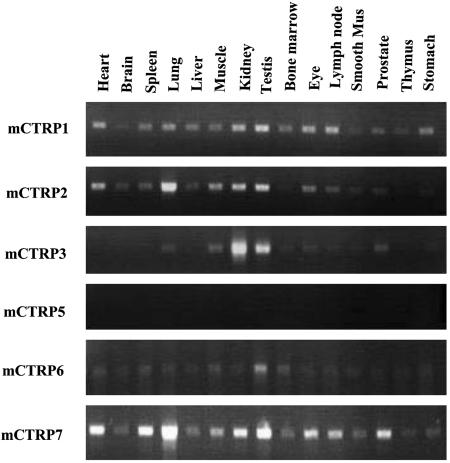
The genes that encode CTRPs are scattered throughout the mouse genome, and range in size from ≈2 to 30 kb (Fig. 8, which is published as supporting information on the PNAS web site). Similar to all proteins in the C1q/TNF-α family, the last exon of each CTRP gene encodes the entire C-terminal globular domain. Unlike adiponectin, whose expression is restricted to adipose tissue, RT-PCR analysis revealed that CTRPs are widely expressed in a variety of adult mouse tissues (Fig. 2). Mouse adipose tissue also express mCTRP1, mCTRP3, and mCTRP7 transcripts, albeit at lower levels compared with adiponectin. Interestingly, RT-PCR analysis also revealed that mCTRP1 transcripts are expressed at higher levels in adipose tissues derived from obese (ob/ob) mice compared with control mice (Fig. 9, which is published as supporting information on the PNAS web site).
Fig. 2.
Expression profile of mCTRP transcripts in adult mouse tissues. A semiquantitative PCR method was used to screen BALB/c mouse cDNA panels to identify tissues that express mCTRP1, mCTRP2, mCTRP3, mCTRP5, mCTRP6, and mCTRP7. Mus, muscle.
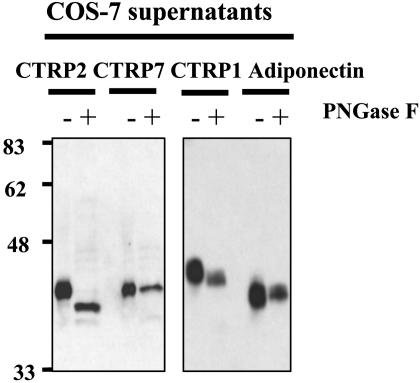
Posttranslational Modifications of CTRPs. All CTRPs possess a predicted signal peptide. When full-length constructs encoding C-terminal HA-tagged mCTRP1, mCTRP2, and mCTRP7 were transfected into COS7 cells, the resultant proteins could be detected in the conditioned media of transfected cells, suggesting that these proteins are secreted. Among the CTRPs, mCTRP1, mCTRP2, mCTRP6, and mCTRP7 contain 1, 1, 2, and 1 potential N-linked glycosylation sites conforming to the consensus motif N-X-S/T, respectively. Peptide:N-glycosidase treatment revealed that the secreted mCTRP2 protein contains N-linked glycans (Fig. 3).
Fig. 3.
Analysis of posttranslational modifications of mCTRP1, mCTRP2, mCTRP7, and adiponectin. COS-7 cells were transfected with expression vectors encoding C-terminal HA-tagged mCTRP1, mCTRP2, mCTRP7, or adiponectin. Conditioned media from transfected cells were collected 48 h later. An aliquot of each sample was left untreated (–) or treated with PNGaseF (+) before being analyzed by SDS/PAGE and probed for mCTRP1, mCTRP2, mCTRP7, and adiponectin by using an anti-HA antibody. PNGaseF removes all N-linked glycans.
In adiponectin, four lysines (residues 68, 71, 80, and 104) located in the collagenous domain within the motif GXKGE(D) are sequentially hydroxylated and glycosylated (34). The glycosides attached to each of these four hydroxylated lysines are thought to be glucosylgalactosyl groups. A total of 1, 1, 3, 1, 2, and 5 GXKGE(D) motifs occur within the collagenous domain of mCTRP1, mCTRP2, mCTRP3, mCTRP5, mCTRP6, and mCTRP7, respectively, suggesting that these proteins may undergo similar posttranslational modifications.
Adiponectin contains two cysteine residues; Cys-22 in the N-terminal variable region, and Cys-138 in the C-terminal globular domain. The crystal structure showed that Cys-138 is buried within the C-terminal globular domain (3). Although not required for trimer formation, site-directed mutagenesis revealed that Cys-22 is critical for adiponectin to assemble into hexameric and higher molecular weight forms through disulfide bonds (22). All CTRPs contain 1–3 cysteine residues within their N-terminal variable region (Fig. 1). When expressed in COS7 cells, the secreted mCTRP1, mCTRP2, and mCTRP7 proteins exist as higher-order oligomers as revealed by native gel electrophoresis (data not shown). The exact number of subunits comprising these higher order oligomer forms remains to be determined.
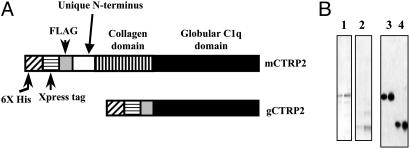
Production of Recombinant mCTRP2. Of the seven CTRPs, mCTRP2 is most similar to adiponectin. Therefore, in the present study we focused on characterizing mCTRP2. We purified recombinant full-length mCTRP2 as well as the isolated mCTRP2 globular head, produced in E. coli (Fig. 4). Gel filtration chromatographic analysis revealed that purified recombinant mCTRP2 and the endogenous mCTRP2 from homogenized mouse lung lysate assembled into higher-order oligomeric forms, similar to those of adiponectin (data not shown). Bacterial-produced mCTRP2 was used throughout the present study to address its biologic function.
Fig. 4.
Generation and purification of recombinant CTRP2 and its globular C1q domain in bacteria. (A) Constructs encoding full-length or globular domain of CTRP2 were expressed in E. coli bacteria. Recombinant CTRP2 and its globular domain were tagged with a His6 Xpress epitope and a FLAG epitope at the N terminus. (B) Recombinant proteins were purified by nickel-affinity column chromatography. The purity of the recombinant proteins was assessed by Coomassie blue staining of SDS/PAGE gels (lanes 1 and 2). The identities of the purified proteins were confirmed by Western blot analysis using anti-FLAG antibody (lanes 3 and 4).
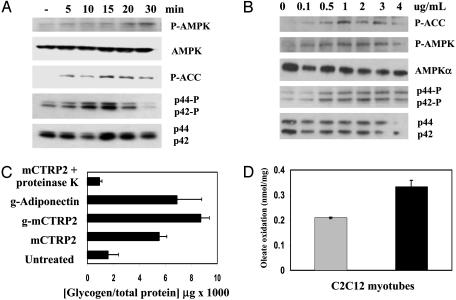
mCTRP2 Induces AMPK, ACC, and p44/42 MAPK Phosphorylation in C2C12 Myotubes. Adiponectin induces glucose uptake, glycogen accumulation, and fatty acid oxidation in cultured C2C12 myotubes and in isolated extensor digitorum longus muscle by activating the AMPK signaling pathway (26). To determine whether mCTRP2 has similar activities, C2C12 myotubes were stimulated with 4 μg/ml of bacterial produced full-length mCTRP2 for different times. As shown in Fig. 5A, within 5 min mCTRP2 rapidly induced the phosphorylation of AMPK on Thr-172 of the α2-subunit and its downstream target, ACC, on Ser-79. p44/42 MAPK was also phosphorylated within 10 min in C2C12 myotubes. These time courses suggest that the phosphorylation events in C2C12 myotubes are directly stimulated by mCTRP2 and are not a result of secondary effects induced by mCTRP2. Moreover, mCTRP2-induced phosphorylation of AMPK, ACC, and p44/42 MAPK in C2C12 myotubes are specific, because other signaling molecules such as IκB, Janus kinase 3, protein kinase D/PKCμ, c-Jun N-terminal kinase-1, p38 MAPK, and insulin receptor substrate-1 are not phosphorylated in these cells after mCTRP2 addition (data not shown). Lipopolysaccharide induces IκB phosphorylation in C2C12 through toll-like receptor-4. We detected no IκB phosphorylation in C2C12 myotubes stimulated with mCTRP2, confirming the absence of potential lipopolysaccharide contamination in our mCTRP2 preparation. Importantly, mCTRP2 induced AMPK, ACC, and p44/42 MAPK phosphorylation in C2C12 myotubes in a dose-dependent manner (Fig. 5B). An mCTRP2 concentration of 0.5 μg/ml (corresponding to 2 nM) mCTRP2 was sufficient to induce AMPK, ACC, and p44/42 MAPK phosphorylation in C2C12 myotubes within 15 min.
Fig. 5.
Effects of mCTRP2 on C2C12 myotubes. (A) Differentiated C2C12 myotubes were untreated or treated with 4 μg/ml of recombinant mCTRP2 for 5, 10, 15, 20, and 30 min. Western blot analyses were carried out on cleared cell lysates by using antibodies specific for phospho-ACC (Ser-79), phospho-AMPK (Thr 172), total AMPK-α, phospho-p44/42 MAPK (Thr-202/Tyr-204), and total p44/42 MAPK. (B) Differentiated C2C12 myotubes were stimulated with recombinant mCTRP2 proteins (0–4 μg/ml) for 15 min, and Western blot analyses were carried out on these cell lysates. (C) Differentiated C2C12 myocytes were treated for 16 h with 4 μg/ml of recombinant g-adiponectin (globular domain only; positive control), CTRP2 (full-length), gCTRP2 (globular domain only), or mCTRP2 predigested with proteinase K. The amount of glycogen in these cells was determined and normalized to total cellular protein. (D) [1-14C]oleate oxidation was measured in differentiated C2C12 myotubes. The formation of 14CO2 was determined in cells incubated for 90 min in the absence (gray) or presence (black) of 4 μg/ml mCTRP2. Incubation with mCTRP2 led to a statistically significant increase in oleate oxidation (P = 0.0023, Student's t test). Each experiment was performed in triplicate.
mCTRP2 Induces Glycogen Accumulation and Fatty Acid Oxidation in C2C12 Myotubes. Phosphorylation of Thr-172 of the α2-subunit activates AMPK, whereas AMPK phosphorylation of ACC inhibits its activity. In muscle, AMPK activation led to increased glucose uptake and glycogen accumulation, whereas inactivation of ACC led to increased fatty acid oxidation (26). To investigate the consequence of AMPK and ACC phosphorylation induced in differentiated C2C12 myotubes by mCTRP2, we measured the change in glycogen content and the alterations in fatty acid oxidation. As shown in Fig. 5C, C2C12 myotubes stimulated with 4 μg/ml of full-length mCTRP2, globular head of mCTRP2 (g-mCTRP2), or bacteria produced gAdiponectin (positive control) for 16 h showed a 6- to 8-fold increase in glycogen content, presumably due to increased glucose uptake as a result of AMPK activation (27). The effect on increased glycogen accumulation was abolished when mCTRP2 was digested with proteinase K, again suggesting that its effect on C2C12 myotubes is not due to proteolytically resistant lipopolysaccharide contaminants.
To ascertain the effect of mCTRP2 on fatty acid oxidation in C2C12 myotubes, the conversion of radiolabeled [14C]oleic acid to 14CO2 was measured in the presence or absence of 4 μg/ml mCTRP2. As shown in Fig. 5D, mCTRP2 significantly increased by 60% oleic acid oxidation in C2C12 myotubes.
Discussion
Here, we describe a highly conserved family of proteins homologous to Acrp30/adiponectin. Seven of these widely expressed adiponectin paralogs, designated as CTRP1–7, share a similar modular organization as adiponectin. We focused our characterization on mCTRP2, which is most similar in sequence to adiponectin. We showed that at nanomolar concentration and in a dose-dependent manner bacterially produced recombinant mCTRP2 rapidly induced phosphorylation and presumably activation of AMPK and MAPK, and phosphorylation and inactivation of ACC in cultured C2C12 myotubes. Activation of AMPK presumably resulted in the increased glycogen accumulation and fatty acid oxidation we observed in C2C12 myotubes after mCTRP2 addition.
AMPK Activation Leads to Increased Glycogen Deposition and Fatty Acid Oxidation. The insulin sensitizing effect of adiponectin on liver and skeletal muscle is due primarily to its ability to activate AMPK (26, 35, 36). Here, we show that recombinant mCTRP2 rapidly activates AMPK in cultured C2C12 myotubes and that this change is associated with increased glycogen accumulation, presumably due to increased glucose uptake. Alternatively, mCTRP2-induced fatty acid oxidation in myotubes could lead to secondary inhibition of glycolysis and stimulation of glycogen synthesis (37). AMPK is a fuel-sensing enzyme that gauges the energy status of a cell (38). It is activated by elevated AMP/ATP ratios, LKB-1 (39), an upstream kinase, and by other cellular pathways that respond to stress. Activated AMPK phosphorylates a variety of intracellular proteins to increase ATP levels (38). AMPK effects on metabolism have been extensively studied in skeletal muscle, where its activation contributes to increased glucose transport (40), glycogen accumulation (27), and fatty acid oxidation (27, 41, 42). Adiponectin stimulates glucose uptake in muscle via an AMPK-dependent pathway (26). Diabetic patients have decreased levels of adiponectin (7) and ≈50% lower rate of glycogen synthesis in muscles (43). The fact that both adiponectin and mCTRP2 induce glycogen accumulation in C2C12 myotubes, presumably through AMPK activation, raises the possibility that mCTRP2 may lower glucose levels in vivo.
Adiponectin, like mCTRP2, also increases fatty acid oxidation in muscle and in cultured C2C12 myotubes (26). Activated AMPK phosphorylates and inhibits ACC, the rate-limiting enzyme that generates malonyl-CoA from ACC. Malonyl-CoA is an allosteric inhibitor of carnitine palmitoyl transferase-I that facilitates import of long-chain fatty acyl-CoA molecules into mitochondria where they are oxidized. Phosphorylation of ACC on Ser-79 by AMPK leads to decreased production of malanoyl-CoA and a net increase in fatty acid oxidation. Here, we showed that mCTRP2 rapidly induced the phosphorylation of ACC on Ser-79 in C2C12 myotubes, resulting in increased fatty acid oxidation. Because muscle represents ≈25% of total body weight of humans, a moderate increase in fatty acid oxidation translates into a significant overall energy expenditure.
The higher circulating levels of free fatty acids found in diabetic patients interfere with signaling pathway involved in glucose transport (43). Overexpression of an adiponectin transgene in obese (ob/ob) or diabetic (db/db) mice ameliorates insulin resistance in part through stimulating glucose uptake and fatty acid oxidation in muscle (28). The ability of mCTRP2 to increase fatty acid oxidation in C2C12 myotubes raises the possibility of its therapeutic potential as an insulin sensitizer.
The Target Tissues of CTRP. Most studies suggested that adiponectin acts on peripheral tissues, principally liver and muscle, to control glucose and lipid metabolism (44), but adiponectin can also act centrally in the hypothalamus to decrease body weight by stimulating energy expenditure (45). Unlike adiponectin, whose expression is restricted to adipose tissue, CTRPs are widely expressed by many different adult mouse tissues. We showed that cultured C2C12 myotubes are responsive to mCTRP2. The question remains whether in vivo CTRPs exert their biologic effects in a paracrine and/or autocrine fashion. All CTRPs possess a predicted signal peptide and hence are predicted to be secreted proteins. Indeed, we showed that mCTRP1, mCTRP2, and mCTRP7 are secreted when constructs encoding these proteins were transfected into COS-7 cells. CTRP3/CORS26 is also a secreted protein (33). Unlike adiponectin, we failed to detect mCTRP2 in mouse serum (data not shown), and thus mCTRP2 may exert its effects in vivo in an autocrine fashion.
CTRP May Compensate for Loss of Adiponectin. Three separate lines of adiponectin–/– mice have recently been generated (46–48) and differences in genetic background may contribute to their different phenotypes. What is common in all three adiponectin–/– mouse lines is the absence of insulin resistance when they are maintained on a normal diet, suggesting that adiponectin is dispensable for maintaining normal glucose and lipid metabolism under this condition. One possible explanation for this finding is that mCTRP2 or other CTRPs is able to substitute for adiponectin function in vivo. Our discovery of a family of adiponectin paralogs allows us to address the issue of functional redundancy.
In summary, we identified a family of adiponectin paralogs. One such protein, mCTRP2, exhibited similar structural and biological properties to adiponectin. Elucidation of the function of this family of proteins will likely yield insights into the control of energy homeostasis and may provide a therapeutic target for metabolic diseases such as obesity and diabetes.
Supplementary Material
Acknowledgments
This work was supported in part by U.S. Public Health Service Grant R37DK47618 (to H.F.L.) and mentor-based grants from the American Diabetes Association (to H.F.L.). T.-S.T. is supported by a fellowship from the Ares-Serono Foundation and the American Diabetes Association. C.H. was supported by a training grant from the National Institutes of Health to the Division of Respiratory Diseases at The Children's Hospital and is the recipient of a Charles Hood Award from The Medical Foundation. G.W.W. is supported by a fellowship from the Ruth L. Kirschstein National Service Award.
Abbreviations: TNF, tumor necrosis factor; CTRP, C1q/TNF-α-related protein; mCTRP, mouse CTRP; hCTRP, human CTRP; AMPK, AMP-activated protein kinase; HA, hemagglutinin; ACC, acetyl-CoA carboxylase; MAPK, mitogen-activated protein kinase.
References
- 1.Rajala, M. W. & Scherer, P. E. (2003) Endocrinology 144, 3765–3773. [DOI] [PubMed] [Google Scholar]
- 2.Scherer, P. E., Williams, S., Fogliano, M., Baldini, G. & Lodish, H. F. (1995) J. Biol. Chem. 270, 26746–26749. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro, L. & Scherer, P. E. (1998) Curr. Biol. 8, 335–338. [DOI] [PubMed] [Google Scholar]
- 4.Takamatsu, N., Ohba, K., Kondo, J., Kondo, N. & Shiba, T. (1993) Mol. Cell. Biol. 13, 1516–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu, E., Liang, P. & Spiegelman, B. M. (1996) J. Biol. Chem. 271, 10697–10703. [DOI] [PubMed] [Google Scholar]
- 6.Hotta, K., Funahashi, T., Bodkin, N. L., Ortmeyer, H. K., Arita, Y., Hansen, B. C. & Matsuzawa, Y. (2001) Diabetes 50, 1126–1133. [DOI] [PubMed] [Google Scholar]
- 7.Hotta, K., Funahashi, T., Arita, Y., Takahashi, M., Matsuda, M., Okamoto, Y., Iwahashi, H., Kuriyama, H., Ouchi, N., Maeda, K., et al. (2000) Arterioscler. Thromb. Vasc. Biol. 20, 1595–1599. [DOI] [PubMed] [Google Scholar]
- 8.Arita, Y., Kihara, S., Ouchi, N., Takahashi, M., Maeda, K., Miyagawa, J., Hotta, K., Shimomura, I., Nakamura, T., Miyaoka, K., et al. (1999) Biochem. Biophys. Res. Commun. 257, 79–83. [DOI] [PubMed] [Google Scholar]
- 9.Kondo, H., Shimomura, I., Matsukawa, Y., Kumada, M., Takahashi, M., Matsuda, M., Ouchi, N., Kihara, S., Kawamoto, T., Sumitsuji, S., et al. (2002) Diabetes 51, 2325–2328. [DOI] [PubMed] [Google Scholar]
- 10.Vasseur, F., Helbecque, N., Dina, C., Lobbens, S., Delannoy, V., Gaget, S., Boutin, P., Vaxillaire, M., Lepretre, F., Dupont, S., et al. (2002) Hum. Mol. Genet. 11, 2607–2614. [DOI] [PubMed] [Google Scholar]
- 11.Waki, H., Yamauchi, T., Kamon, J., Ito, Y., Uchida, S., Kita, S., Hara, K., Hada, Y., Vasseur, F., Froguel, P., et al. (2003) J. Biol. Chem. 278, 40352–40363. [DOI] [PubMed] [Google Scholar]
- 12.Yang, W. S., Lee, W. J., Funahashi, T., Tanaka, S., Matsuzawa, Y., Chao, C. L., Chen, C. L., Tai, T. Y. & Chuang, L. M. (2001) J. Clin. Endocrinol. Metab. 86, 3815–3819. [DOI] [PubMed] [Google Scholar]
- 13.Maeda, N., Takahashi, M., Funahashi, T., Kihara, S., Nishizawa, H., Kishida, K., Nagaretani, H., Matsuda, M., Komuro, R., Ouchi, N., et al. (2001) Diabetes 50, 2094–2099. [DOI] [PubMed] [Google Scholar]
- 14.Yu, J. G., Javorschi, S., Hevener, A. L., Kruszynska, Y. T., Norman, R. A., Sinha, M. & Olefsky, J. M. (2002) Diabetes 51, 2968–2974. [DOI] [PubMed] [Google Scholar]
- 15.Moore, G. B., Chapman, H., Holder, J. C., Lister, C. A., Piercy, V., Smith, S. A. & Clapham, J. C. (2001) Biochem. Biophys. Res. Commun. 286, 735–741. [DOI] [PubMed] [Google Scholar]
- 16.Combs, T. P., Wagner, J. A., Berger, J., Doebber, T., Wang, W. J., Zhang, B. B., Tanen, M., Berg, A. H., O'Rahilly, S., Savage, D. B., et al. (2002) Endocrinology 143, 998–1007. [DOI] [PubMed] [Google Scholar]
- 17.Fruebis, J., Tsao, T. S., Javorschi, S., Ebbets-Reed, D., Erickson, M. R., Yen, F. T., Bihain, B. E. & Lodish, H. F. (2001) Proc. Natl. Acad. Sci. USA 98, 2005–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamauchi, T., Kamon, J., Waki, H., Terauchi, Y., Kubota, N., Hara, K., Mori, Y., Ide, T., Murakami, K., Tsuboyama-Kasaoka, N., et al. (2001) Nat. Med. 7, 941–946. [DOI] [PubMed] [Google Scholar]
- 19.Berg, A. H., Combs, T. P., Du, X., Brownlee, M. & Scherer, P. E. (2001) Nat. Med. 7, 947–953. [DOI] [PubMed] [Google Scholar]
- 20.Pajvani, U. B. & Scherer, P. E. (2003) Curr. Diab. Rep. 3, 207–213. [DOI] [PubMed] [Google Scholar]
- 21.Tsao, T. S., Murrey, H. E., Hug, C., Lee, D. H. & Lodish, H. F. (2002) J. Biol. Chem. 277, 29359–29362. [DOI] [PubMed] [Google Scholar]
- 22.Tsao, T. S., Tomas, E., Murrey, H. E., Hug, C., Lee, D. H., Ruderman, N. B., Heuser, J. E. & Lodish, H. F. (2003) J. Biol. Chem. 278, 50810–50817. [DOI] [PubMed] [Google Scholar]
- 23.Pajvani, U. B., Hawkins, M., Combs, T. P., Rajala, M. W., Doebber, T., Berger, J. P., Wagner, J. A., Wu, M., Knopps, A., Xiang, A. H., et al. (2004) J. Biol. Chem. 279, 12152–12162. [DOI] [PubMed] [Google Scholar]
- 24.Combs, T. P., Berg, A. H., Rajala, M. W., Klebanov, S., Iyengar, P., Jimenez-Chillaron, J. C., Patti, M. E., Klein, S. L., Weinstein, R. S. & Scherer, P. E. (2003) Diabetes 52, 268–276. [DOI] [PubMed] [Google Scholar]
- 25.Kurth-Kraczek, E. J., Hirshman, M. F., Goodyear, L. J. & Winder, W. W. (1999) Diabetes 48, 1667–1671. [DOI] [PubMed] [Google Scholar]
- 26.Tomas, E., Tsao, T. S., Saha, A. K., Murrey, H. E., Zhang, Cc. C., Itani, S. I., Lodish, H. F. & Ruderman, N. B. (2002) Proc. Natl. Acad. Sci. USA 99, 16309–16313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merrill, G. F., Kurth, E. J., Hardie, D. G. & Winder, W. W. (1997) Am. J. Physiol. 273, E1107–E1112. [DOI] [PubMed] [Google Scholar]
- 28.Yamauchi, T., Kamon, J., Waki, H., Imai, Y., Shimozawa, N., Hioki, K., Uchida, S., Ito, Y., Takakuwa, K., Matsui, J., et al. (2003) J. Biol. Chem. 278, 2461–2468. [DOI] [PubMed] [Google Scholar]
- 29.Ouchi, N., Kihara, S., Funahashi, T., Matsuzawa, Y. & Walsh, K. (2003) Curr. Opin. Lipidol. 14, 561–566. [DOI] [PubMed] [Google Scholar]
- 30.Okamoto, Y., Kihara, S., Ouchi, N., Nishida, M., Arita, Y., Kumada, M., Ohashi, K., Sakai, N., Shimomura, I., Kobayashi, H., et al. (2002) Circulation 106, 2767–2770. [DOI] [PubMed] [Google Scholar]
- 31.Ouchi, N., Kobayashi, H., Kihara, S., Kumada, M., Sato, K., Inoue, T., Funahashi, T. & Walsh, K. (2004) J. Biol. Chem. 279, 1304–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brakenhielm, E., Veitonmaki, N., Cao, R., Kihara, S., Matsuzawa, Y., Zhivotovsky, B., Funahashi, T. & Cao, Y. (2004) Proc. Natl. Acad. Sci. USA 101, 2476–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maeda, T., Abe, M., Kurisu, K., Jikko, A. & Furukawa, S. (2001) J. Biol. Chem. 276, 3628–3634. [DOI] [PubMed] [Google Scholar]
- 34.Wang, Y., Xu, A., Knight, C., Xu, L. Y. & Cooper, G. J. (2002) J. Biol. Chem. 277, 19521–19529. [DOI] [PubMed] [Google Scholar]
- 35.Yamauchi, T., Kamon, J., Minokoshi, Y., Ito, Y., Waki, H., Uchida, S., Yamashita, S., Noda, M., Kita, S., Ueki, K., et al. (2002) Nat. Med. 8, 1288–1295. [DOI] [PubMed] [Google Scholar]
- 36.Combs, T. P., Pajvani, U. B., Berg, A. H., Lin, Y., Jelicks, L. A., Laplante, M., Nawrocki, A. R., Rajala, M. W., Parlow, A. F., Cheeseboro, L., et al. (2004) Endocrinology 145, 367–383. [DOI] [PubMed] [Google Scholar]
- 37.Ruderman, N. B., Ross, P. S., Berger, M. & Goodman, M. N. (1974) Biochem. J. 138, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hardie, D. G. (2003) Endocrinology 144, 5179–5183. [DOI] [PubMed] [Google Scholar]
- 39.Woods, A., Johnstone, S. R., Dickerson, K., Leiper, F. C., Fryer, L. G., Neumann, D., Schlattner, U., Wallimann, T., Carlson, M. & Carling, D. (2003) Curr. Biol. 13, 2004–2008. [DOI] [PubMed] [Google Scholar]
- 40.Hayashi, T., Hirshman, M. F., Fujii, N., Habinowski, S. A., Witters, L. A. & Goodyear, L. J. (2000) Diabetes 49, 527–531. [DOI] [PubMed] [Google Scholar]
- 41.Ruderman, N. B., Saha, A. K., Vavvas, D. & Witters, L. A. (1999) Am. J. Physiol. 276, E1–E18. [DOI] [PubMed] [Google Scholar]
- 42.Winder, W. W. & Hardie, D. G. (1999) Am. J. Physiol. 277, E1–E10. [DOI] [PubMed] [Google Scholar]
- 43.Shulman, G. I. (2000) J. Clin. Invest. 106, 171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsao, T. S., Lodish, H. F. & Fruebis, J. (2002) Eur. J. Pharmacol. 440, 213–221. [DOI] [PubMed] [Google Scholar]
- 45.Qi, Y., Takahashi, N., Hileman, S. M., Patel, H. R., Berg, A. H., Pajvani, U. B., Scherer, P. E. & Ahima, R. S. (2004) Nat. Med. 10, 524–529. [DOI] [PubMed] [Google Scholar]
- 46.Kubota, N., Terauchi, Y., Yamauchi, T., Kubota, T., Moroi, M., Matsui, J., Eto, K., Yamashita, T., Kamon, J., Satoh, H., et al. (2002) J. Biol. Chem. 277, 25863–25866. [DOI] [PubMed] [Google Scholar]
- 47.Maeda, N., Shimomura, I., Kishida, K., Nishizawa, H., Matsuda, M., Nagaretani, H., Furuyama, N., Kondo, H., Takahashi, M., Arita, Y., et al. (2002) Nat. Med. 8, 731–737. [DOI] [PubMed] [Google Scholar]
- 48.Ma, K., Cabrero, A., Saha, P. K., Kojima, H., Li, L., Chang, B. H., Paul, A. & Chan, L. (2002) J. Biol. Chem. 277, 34658–34661. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.